Abstract
Cell-cycle induction in hepatocytes protects from prolonged tissue damage after toxic liver injury. Early growth response (Egr)-1−/− mice exhibit increased liver injury after carbon tetrachloride (CCl4) exposure and reduced TNF-α production. Because TNF-α is required for prompt cell-cycle induction after liver injury, here, we tested the hypothesis that Egr-1 is required for timely hepatocyte entry into the cell cycle after CCl4-induced liver injury. Acute liver injury was induced by a single injection of CCl4. Assays were employed to assess indices of the cell cycle in liver after CCl4 exposure. Bromodeoxyuridine incorporation peaked in wild-type mice at 48 h after CCl4 but was reduced by 80% in Egr-1−/− mice. Proliferating-cell nuclear-antigen immunohistochemistry revealed blocks in cell-cycle entry and progression to DNA synthesis in Egr-1-deficient mice 48 h after CCl4. Cyclin D, important for G0/G1 progression, was reduced at baseline and 36 h after CCl4. Cyclin E1, required for G1/S-phase transition, was reduced in Egr-1−/− mice 24 and 48 h after CCl4 exposure and was associated with reduced phosphorylation of the retinoblastoma protein. Proliferation in Egr-1−/− mice was delayed, rather than blocked, because indices of cell-cycle progression were restored 72 h after CCl4 exposure. We concluded that Egr-1 was required for prompt cell-cycle entry (G0- to G1-phase) and G1/S-phase transition after toxic liver injury. These data support the hypothesis that Egr-1 provides hepatoprotection in the CCl4-injured liver, attributable, in part, to timely cell-cycle induction and progression.
Keywords: liver regeneration, PCNA, BrdU
the liver has evolved a robust ability to regenerate after toxin-mediated injury, reflecting its major role in toxin metabolism. Transplant surgeons exploit this striking regenerative capacity, and now living-donor liver transplants are an alternative to whole cadaveric liver transplantation (2). This practice should increase the total number of patients able to receive liver transplants, a critical advance given the burgeoning population of patients requiring liver transplantation (2). However, living-donor morbidity and mortality continue to be issues of considerable concern (2). Therefore, there is a need to expand our understanding of the molecular mechanisms that promote the hepatic repair and regenerative response to improve transplant success for both living donors as well as recipients of small-sized liver transplants.
The profound regenerative capacity of the liver has been extensively studied experimentally using both surgical (partial hepatectomy, PH) and toxin (carbon tetrachloride, CCl4)-mediated liver-injury models (4, 23). Successful liver regeneration requires initiation of the cell cycle in otherwise quiescent hepatocytes (G0 to G1); this promotes replicative competence in hepatocytes when they are exposed to growth factors produced during the progression phase of the cell cycle (G1 through M). Finally growth inhibition (back to G0) occurs after liver mass is restored (4, 23). Many signals important in this progression are known. For example, lipopolysaccharide, complement-activation products, and cytokines, such as tumor necrosis factor (TNF)-α and IL-6, are important mediators of the initiation phase, whereas growth factors, including hepatocyte growth factor and epidermal growth factor, regulate the progression phase (4, 23). Signals, such as those propagated by transforming growth factor (TGF)-β, later terminate the growth process in hepatocytes (4, 23). Perturbations in the liver-regenerative response can have negative effects on outcome. Typically, liver regeneration is delayed, not prevented, in mice deficient in ligands or receptors important to the hepatic regenerative response; this is suggestive of genetic redundancy in the pathways required to restore hepatic function after injury (4, 23).
Early growth response (Egr)-1 is a transcription factor rapidly induced by many growth and differentiation signals (5). Egr-1 is expressed in liver in response to acute CCl4 exposure and PH (12, 18, 24). Egr-1 contributes to the regulation of a large number of genes involved in the regenerative response including TNF-α, cdc20, the phosphatase of regenerating liver-1, and TGF-β (12, 19, 24, 27). To date, no one has examined the role of Egr-1 in liver regeneration after toxin-induced liver injury.
Egr-1 is required for hepatoprotection after acute CCl4 exposure; mice deficient in Egr-1 have reduced expression of TNF-α and other hepatoprotective molecules that play a role in the liver-regenerative response (18). This inadequate hepatoprotection in Egr-1−/− mice exacerbates liver injury (18). Because liver injury is worsened by delay or inhibition of the cell cycle in hepatocytes (15), here we tested the hypothesis that Egr-1 is required for induction of the cell cycle in hepatocytes after acute CCl4 exposure. We found that Egr-1 is required for both induction (G0- to G1-phase) of the cell cycle after CCl4-induced liver injury, as well as prompt G1/S-phase transition during the progression phase of liver regeneration.
MATERIALS AND METHODS
Materials.
CCl4, olive oil, and bromodeoxyuridine (5-bromo-2′deoxyuridine-5′-monophosphate, BrdU) were purchased from Sigma-Aldrich (St. Louis, MO). Buprenorphrine was purchased from Reckitt Benckiser Pharmaceuticals (Richmond, VA). All primers for real-time PCR were synthesized by Integrated DNA Technologies (Coralville, IA). Primary antibodies were purchased from the following companies: BrdU (clone BMC 9318), Roche (Madison, WI); proliferating cell nuclear antigen (PCNA, clone PC10), Chemicon/Millipore (Danvers, MA); Egr-1 (clone 15F7) and phosphoretinoblastoma (pRb, Ser608), cyclin E, Cell Signaling Technology (Danvers, MA).
Mice.
Female wild-type (C57BL/6nTac) mice were purchased from Taconic Farms (Germantown, NY). Egr-1−/− mice were originally developed by Jeffrey Milbrandt (11). Egr-1−/− mice in these studies were bred at the Cleveland Clinic Biological Resource Unit from stock purchased from Taconic Farms. Because of a requirement for Egr-1 in luteinizing hormone-β production required for female fertility (10), Egr-1−/− mice were produced through mating Egr-1+/− females to Egr-1−/− males. Genotype was determined by PCR as described (10). Animals were housed in standard microisolator cages, fed standard laboratory chow, and were maintained on a 12-h:12-h light/dark cycle. All animals received humane care, and all procedures were approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
CCl4 administration and sample collection.
CCl4 was prepared and administered as described (18) except that mice received a single subcutaneous injection of the analgesic, buprenorphine hydrochloride (50 μg/g body wt) 10 min before CCl4 administration. Liver samples were harvested at 24, 36, 48, and 72 h or from olive oil-treated controls (72 h) and snap frozen, preserved in 10% neutral-buffered formalin or submerged in RNAlater (Ambion, Austin, TX), and stored for further analysis.
BrdU administration, immunohistochemistry, and quantification.
Two hours before euthanasia, mice were given a single injection of sterile, endotoxin-free BrdU (50 mg/g body wt, diluted in saline). Five-micrometer paraffin sections were cut, deparaffinized, and rehydrated before DNA denaturation using 2 N hydrochloric acid for 60 min at 37°C. The acid was neutralized using 0.1 M borate buffer, pH 8.5, twice for 5 min. After being further washed in PBS (3 3-min washes), endogenous peroxidase activity was quenched using 3% hydrogen peroxide (H2O2), followed by PBS washes (2 5-min washes). Avidin and biotin were blocked using the Avidin and Biotin Blocking Kit (Vector Laboratories, Burlingame, CA), rinsed quickly in PBS. The sections were further blocked using the Mouse on Mouse IgG Blocking Reagent (MOM Kit, Vector Laboratories) for 1 h at room temperature in a humidified chamber. Following two 5-min PBS washes, the sections were incubated in MOM diluent for 5 min and then incubated with a 1:250 dilution of mouse anti-BrdU monoclonal Ab (mAb), or MOM block alone overnight at 4°C, in a humidified chamber. After two 5-min PBS washes, the biotinylated secondary Ab (provided in the MOM kit) was added to each section and incubated for 10 min. Vectastain ELITE ABC reagent (Vector Laboratories) was added to each section and incubated for 5 min, washed, and then exposed to diaminobenzamidine (DAB) for 2 min. After being washed with running water, the sections were counterstained with hematoxylin for 5 min, washed in running water again for 5 min, dehydrated, and mounted using Permount resinous mounting medium (Sigma-Aldrich). Three to six photomicrographs were taken at ×200 of each section. Images were taken of the periportal and midzone areas (zones 1–2) and always included a vascular structure for orientation of liver architecture; DNA replication did not occur in the necrotic, pericentral areas. Only BrdU-positive hepatocyte nuclei were counted (by a blinded individual) using cell morphology to identify hepatocytes. The analysis was performed on enlarged images using the manual tagging function in Image Pro Plus image analysis software (MediaCybernetics, Bethesda, MD). Data were calculated as percentages of BrdU-positive hepatocyte nuclei of total hepatocyte nuclei.
PCNA immunohistochemistry and quantification.
The staining procedure is as described above for BrdU immunohistochemistry except for the following: antigen retrieval was performed using citrate buffer and a mouse anti-human PCNA mAb used at 1:16,000 dilution. Three to six photomicrographs were taken at ×200 of each section. Cell-cycle stage in hepatocytes was determined by a blinded individual, using specific PCNA staining patterns and cell morphology as described previously (25, 26). The analysis was performed on enlarged images using the manual tagging function in Image Pro Plus image analysis software (MediaCybernetics). Examples of PCNA staining at each cell-cycle stage are provided in Supplemental Fig. S4. (supplemental material for this article is available online at the American Journal of Physiology Gastrointestinal and Liver Physiology website). Data were calculated as percentages of hepatocytes in each cell-cycle stage of total hepatocytes.
Egr-1 immunohistochemistry.
Five-micrometer paraffin sections were cut, deparaffinized, and rehydrated before citrate buffer antigen retrieval, followed by quenching of endogenous peroxidase activity in 3% H2O2 with sodium azide. Sections were blocked in 10% normal goat serum and incubated overnight at 4°C in a humidified chamber, with a 1:200 dilution of rabbit anti-human Egr-1 mAb, or 10% normal goat serum alone. After being washed in PBS twice for 5 min each time, the horseradish peroxidase-conjugated secondary reagent (DAKO polymer; DAKO, Carpinteria, CA) was added to the tissue sections and incubated for 30 min at room temperature in a humidified chamber. After three 5-min washes in PBS, DAB was added to each section, one slide at a time, and incubated for 1 min before submersion in water to stop the peroxidase reaction. Sections were counterstained using hematoxylin (DAKO), washed in water, dehydrated, and then mounted using Permount resinous mounting medium (Sigma-Aldrich). Three to six photomicrographs were taken at ×200 of each slide, which were examined by blinded individuals and scored on the basis of cell type in which positive nuclear staining was found and the anatomical location of that stain in the context of the lobular architecture of the liver.
Hepatic real-time PCR analysis for cell-cycle-regulated genes.
Total liver RNA was isolated and reverse transcribed into cDNA as described (14). Real-time PCR amplification was performed using gene-specific primers (Supplemental Table S1) as described in (14) with one modification: Brilliant II SYBR Green was utilized in place of Brilliant SYBR Green (Agilent Technologies, Santa Clara, CA). The relative amount of hepatic gene-specific mRNA was determined as described (18). Fold changes in gene expression are expressed as fold over wild-type oil (controls) for each gene of interest. Statistical analyses were performed on the ΔCt values [mean Ct gene of interest − mean Ct from 18S (housekeeping gene) from the same mouse].
Liver homogenate preparation, electrophoresis, and Western blotting.
Liver homogenates, polyacrylamide gel electrophoresis, and immunoblotting were performed as described (20).
Statistical analysis.
Values reported are means ± SE. Because of the limited number of Egr-1−/− mice, data were collected from several different experiments. The data were analyzed by the general linear-models procedure (SAS, Carey, NC) followed by least-square-means analysis of differences between groups. Data were log-transformed to obtain a normal distribution, if necessary. Each time point included five wild-type and six Egr-1−/− mice. In all cases, values in graphs with different alphabetical superscripts are significantly different from one another (P < 0.05).
RESULTS
Egr-1 deficiency delays cell-cycle entry and DNA synthesis.
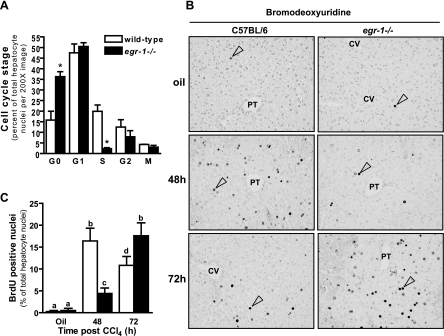
Immunohistochemical detection of PCNA allows for the quantification of cells in different stages of the cell cycle attributable to differences in the staining pattern found at each stage (25, 26). Utilizing this technique, two defects in the hepatocyte cell cycle were observed in Egr-1−/− mice 48 h after CCl4 exposure. First, a 2.3-fold increase in quiescent (G0) hepatocytes was found in livers from Egr-1−/− mice relative to wild-type mice, suggesting a block or delay in cell cycle initiation (Fig. 1A) (See Supplemental Fig. S4 for examples of PCNA staining at each cell-cycle stage). Second, livers from Egr-1−/− mice had nearly ninefold fewer hepatocytes in S-phase compared with wild-type mice, suggesting a block or delay in transition from G1- to S-phase (Fig. 1A). An equivalent percentage of cells was found in the G1-, G2-, and M-phases of the cell cycle in wild-type and Egr-1−/− mice (Fig. 1A).
Fig. 1.
Initiation and progression of the cell cycle are delayed in livers from early growth response (Egr)-1−/− mice after carbon tetrachloride (CCl4) exposure. Mice were given a single intraperitoneal (i.p.) injection of CCl4. A: proliferating cell nuclear antigen was immunolocalized in liver sections from mice at the indicated time points after CCl4 exposure, and cell-cycle stage was quantified by a blinded individual. *Significantly different from wild-type, P < 0.05. B and C: 2 h before euthanasia, mice received an i.p. injection of bromodeoxyuridine (BrdU) and were euthanized at the indicated time points after CCl4 exposure. B: representative BrdU immunohistology, ×200 magnification. PT, portal tract; CV, central vein. Open arrow heads in each image mark an example of a BrdU-positive hepatocyte. C: quantification of BrdU-positive hepatocyte nuclei. The percentage of BrdU-positive hepatocyte nuclei was calculated from 3 (olive-oil controls) or 6 (CCl4-treated mice) nonoverlapping ×200 fields on each slide. Liver sections from 5 wild-type and 6 Egr-1−/− mice were analyzed at each time point. Values with different alphabetical superscripts are significantly different from one another (P < 0.05).
BrdU incorporation into DNA is a specific marker for the S-phase of the cell cycle. BrdU incorporation into hepatocytes was very low in controls (olive oil treated, Fig. 1, B and C) and not increased above baseline at 24 h after CCl4 in wild-type and Egr-1−/− mice (data not shown). In wild-type mice, BrdU incorporation reached a peak at 48 h, was reduced 72 h after CCl4, but remained above olive oil controls (Fig. 1, B and C). In Egr-1−/− mice, BrdU incorporation was also detected at 48 h after CCl4 exposure but was reduced 80% relative to wild-type mice; peak BrdU incorporation in Egr-1−/− mice was observed 72 h after CCl4 exposure (Fig. 1, B and C), a delay of 24 h relative to wild-type mice. There was a spatial and temporal pattern of BrdU incorporation in wild-type mice: BrdU incorporation began in the periportal area (zone 1 to zone 2) at 48 h (Fig. 1B). At 72 h, sparse numbers of BrdU-positive hepatocytes were found in the pericentral area (zone 3, Fig. 1B). This spatial/temporal pattern of BrdU labeling was also detected in Egr-1−/− mice but with a 24-h delay (Fig. 1B).
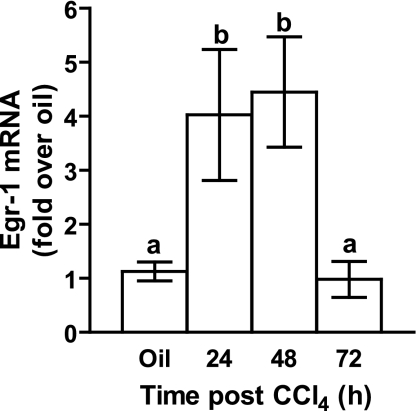
Hepatic Egr-1 induction in response to CCl4.
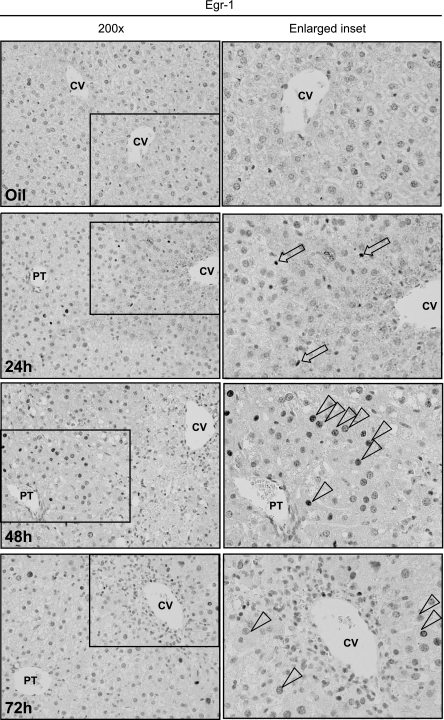
Egr-1, a prototypical immediate early gene, is rapidly and transiently induced in response to CCl4, peaking at 2 h and returning to baseline at 8 h (18). Interestingly, a second, more modest wave of hepatic Egr-1 expression was induced 24 h after CCl4 and remained elevated at 48 h before returning to levels observed in olive-oil controls at 72 h (Fig. 2). The temporal dynamics of this second wave of Egr-1 expression was cell type specific: nuclear Egr-1 was located primarily in nonparenchymal cells (NPC) at 24 h (Fig. 3). At 48 h, the periportal hepatocyte nuclei (zones 1 and 2) were Egr-1 positive (Fig. 3). A few hepatocyte nuclei in the pericentral area (zone 3) were positive for Egr-1 at 72 h after CCl4 (Fig. 3). This temporal/spatial pattern of Egr-1 labeling in hepatocytes paralleled the pattern of BrdU incorporation (Fig. 1, B and C).
Fig. 2.
Hepatic Egr-1 mRNA accumulation in wild-type mice. Egr-1 mRNA accumulation was determined in 5 wild-type mice by real-time PCR after CCl4 exposure at the time points indicated. Values with different alphabetical superscripts are significantly different from one another (P < 0.05).
Fig. 3.
Hepatic Egr-1 protein localization after CCl4 exposure. Wild-type mice received a single CCl4 injection and were euthanized at the indicated time points. Hepatic Egr-1 nuclear protein was determined in by immunohistochemistry. Insets are enlarged and placed to the right of each ×200 image. Open arrows mark Egr-1-positive nonparenchymal cells nuclei, whereas open arrowheads mark Egr-1-positive hepatocyte nuclei. Images are representative of 3 (olive-oil controls) or 6 (CCl4-treated mice) nonoverlapping photomicrographs per slide. Liver sections from 5 wild-type and 6 Egr-1−/− mice were analyzed at each time point.
E- and D-type cyclins are reduced in Egr-1-deficient mice.
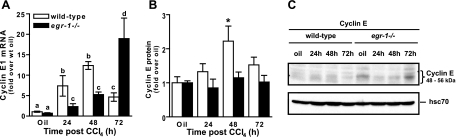
To determine whether reduced BrdU incorporation was attributable to a delay in G1/S transition, we measured hepatic accumulation of the G1/S-specific cyclin, cyclin E1 (16). In wild-type mice, hepatic cyclin E1 mRNA accumulation was induced 24 h after CCl4 exposure and was maintained through 48 h. Expression was reduced at 72 h but still above olive-oil controls (Fig. 4A). In Egr-1−/− mice, cyclin E1 expression was also induced at 24 and 48 h but was lower than wild-type mice at these time points (Fig. 4A). Instead, the greatest accumulation of cyclin E1 mRNA in Egr-1−/− mice was at 72 h (Fig. 4A). Hepatic cyclin E protein was increased twofold above baseline in wild-type mice 48 h after CCl4 exposure and returned to baseline at 72 h (Fig. 4, B and C). In contrast, whereas there was an increase in hepatic cyclin E protein in Egr-1−/− mice at baseline compared with wild-type mice, this amount did increase above baseline 24, 48, or 72 h after CCl4 exposure (Fig. 4, B and C).
Fig. 4.
Hepatic cyclin E expression in wild-type and Egr-1−/− mice. Cyclin E mRNA (A) and protein expression (B and C) was determined at the indicated time points after CCl4 exposure using real-time PCR or immunoblotting, respectively. B: quantification of immunoblot data from 4 separate blots. C: representative cyclin E immunoblot. Four wild-type and 4 Egr-1−/− mice were used for each time point. Values with different alphabetical superscripts are significantly different from one another (P < 0.05). *Significantly different from wild-type mice, P < 0.05.
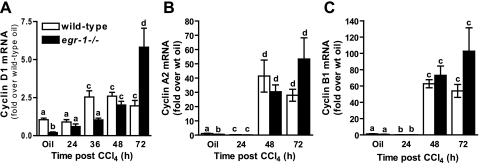
In addition to E-type cyclins, other cyclins are differentially expressed during the cell cycle. Cyclin D1, which is required G1-phase progression (3), was reduced in olive-oil-treated Egr-1−/− mice relative to olive-oil-treated wild-type mice (Fig. 5A) as well as 36 h after CCl4 exposure (Fig. 5A). Cyclin D1 mRNA accumulation was equivalent between genotypes at 48 h, but at 72 h cyclin D1 was greater in Egr-1−/− compared with wild-type mice (Fig. 5A). Cyclin A2, which peaks in the G2-phase of the cell cycle, was reduced in Egr-1−/− mice at baseline; it was equivalent between the genotypes at times after CCl4 exposure (Fig. 5B). Cyclin B1, which peaks at the G2/M transition, was not different between genotypes (Fig. 5C). Hepatic expression of p21, a negative regulator of cyclin E/cdk2 complexes and the G1- to S-phase transition, and p15, a negative regulator of cyclin D/cdk4 complexes and progression through the G1-phase of the cell cycle, were not different in wild-type and Egr-1−/− mice (Supplemental Fig. S1, A and B, respectively).
Fig. 5.
Cyclin D1, A2, and B1 gene expression in wild-type and Egr-1−/− mice. Hepatic mRNA accumulation of cyclins D1 (A), A2 (B), and B1 (C) were determined at 24, 36 (cyclin D1 only), 48 and 72 h after CCl4 exposure, or in livers from olive-oil controls, using real-time PCR. Five wild-type and 6 Egr-1−/− mice were analyzed at each time point. Values with different alphabetical superscripts are significantly different from one another (P < 0.05).
Retinoblastoma phosphorylation is delayed in Egr-1−/− mice.
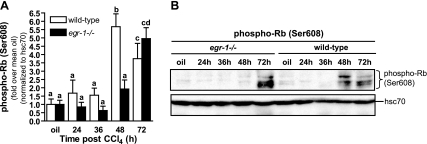
The cell-cycle regulator, Rb, sequesters E2F family members, transcription factors critical for the regulation of genes required for G1/S-phase transition including cyclin E (22), rendering them inactive (17). Once phosphorylated, Rb (pRb) and E2F dissociate, allowing E2F to regulate gene transcription (17). If pRb is required for E2F-mediated transcription of cyclin E, then pRb should be reduced in Egr-1−/− mice. Indeed, at 48 h after CCl4 exposure, the time point of maximal DNA synthesis in wild-type mice, Rb in livers from Egr-1-deficient mice remains unphosphorylated and is not different than olive-oil controls (Fig. 6). However, Rb in livers from wild-type mice is phosphorylated at 48 h after CCl4 (Fig. 6). Rb phosphorylation increased in Egr-1−/− mice 72 h after CCl4 (Fig. 6), coincident with increased BrdU (Fig. 1, B and C) and hepatic cyclin E1 expression (Fig. 4). Collectively, these data revealed a block in G1/S-phase cell cycle progression at 48 h in livers from Egr-1−/− mice in response to CCl4 exposure.
Fig. 6.
Phosphorylation of retinoblastoma (Rb) is delayed in livers from Egr-1−/− mice. Liver homogenates prepared from wild-type and Egr-1−/− mice were utilized to determine the phosphorylation status of Rb at time points after CCl4 exposure in representative immunoblots (A). B: quantification of multiple immunoblot data. Five wild-type and 6 Egr-1−/− mice were analyzed at each time point. Values with different alphabetical superscripts are significantly different from one another (P < 0.05).
DISCUSSION
Here, we identify, for the first time, a critical requirement for Egr-1 in liver regeneration after CCl4-induced liver injury. Our data reveal defects in the initiation phase (G0- to G1-phase transition) of the cell cycle in hepatocytes as well as in the G1/S-phase transition in liver after CCl4 exposure. Expression of cyclin D, important for the transition of hepatocytes from quiescence to replicative competence, was reduced in Egr-1-deficient mice at 36 h and associated with a delay in cell-cycle induction in hepatocytes. Expression of cyclin E1, the G1/S-phase-specific cyclin, and phosphorylation of Rb, required for regulation of cyclin E and progression through the G1/S-phase restriction point (16, 17), were reduced in Egr-1-deficient mice, associated with blockade in progression to S-phase. In addition, this is the first report to identify a dynamic spatial and temporal expression pattern of nuclear Egr-1 localization after acute CCl4 exposure. The Egr-1 expression pattern in hepatocytes paralleled the wave of DNA synthesis, which occurs across the liver lobule, beginning in the periportal area and extending into the pericentral area as time progressed. This dynamic pattern of expression suggests that Egr-1 is of critical importance to the cell cycle in hepatocytes after CCl4 exposure.
Using immunohistochemistry, we identified Egr-1 localization patterns in wild-type mice, which differed with respect to in which cell types Egr-1 was localized and when during the time course of liver repair after CCl4 exposure Egr-1 was observed. Egr-1 was first detected in nuclei of NPC in the hepatic sinusoid 24 h after CCl4. Because proliferation of NPC occurs after proliferation of hepatocytes (23), the early localization of Egr-1 in these cells is most likely associated with Egr-1-regulated cytokine production and not NPC proliferation. Indeed, Egr-1 contributes to the expression of TNF-α in macrophages and is important for liver regeneration (24, 28); when macrophages are depleted using clodronate, TNF-α expression is reduced and liver regeneration delayed (1). In the current and recent (18) studies, Egr-1 nuclear localization in NPC parallels hepatic TNF-α expression, but not NPC BrdU incorporation. However, 48 h after CCl4 exposure, Egr-1 was predominantly localized to hepatocyte nuclei. The presence of Egr-1 in nuclei of hepatocytes 48 and 72 h after CCl4 parallels nuclear BrdU localization and suggests a direct link between Egr-1 and cell-cycle regulation in hepatocytes. Clarification of the specific roles for Egr-1 in NPC vs. hepatocytes over the time course of liver regeneration requires further investigation.
Egr-1 is required for hepatoprotection after acute CCl4-induced liver injury (18). In its absence, liver injury is enhanced twofold 48 h after CCl4 exposure compared with wild-type mice and associated with reduced TNF-α, inducible nitric oxide synthase (iNOS), cyclooxygenase-2, oncostatin M (OSM), phospho- signal transducer and activator of transcription (STAT)3, and phospho-Akt, each of which has hepatoprotective function in wild-type mice (18). Importantly, initial hepatotoxicity of CCl4 is not delayed in Egr-1−/− mice; plasma alanine aminotransferase and aspartate aminotransferase activities are equivalent in wild-type and Egr-1−/− mice at 18 h (18) and at 24 and 36 h (Supplemental Table S2), suggesting that mechanisms of initial liver injury are not different between genotypes. The expression of the immediate early genes, c-jun and c-fos, were also not different between genotypes at 1, 2, or 4 h after CCl4 exposure (Supplemental Fig. S2) suggesting that very early signals generated in the liver are also equivalent between genotypes. Because the direct effects of CCl4-mediated hepatotoxicity wane by 24 h (15), these data support the hypothesis that increased liver injury at 48 h is attributable to mechanisms independent of direct, CCl4-mediated hepatotoxicity.
Because TNF-α, iNOS, OSM, and STAT3 activity are implicated in the initiation phase of the cell cycle during liver regeneration (4, 21, 23), and because induction of the cell cycle protects the liver from prolonged injury secondary to initial hepatotoxin exposure (15), we hypothesized that induction of the cell cycle was inhibited in livers from Egr-1−/− mice. Indeed, using PCNA immunohistochemistry, we found that a greater percentage of hepatocytes from Egr-1−/− mice remained in G0-phase compared with wild-type mice. These data might be explained by reduced TNF-α, a cytokine important for the shift from G0- to G1-phase in hepatocytes during liver regeneration (4, 23), observed in Egr-1−/− mice (18). In addition, hepatic cyclin D mRNA was also reduced in Egr-1−/− mice 36 h after CCl4. These data are consistent with a role for Egr-1 in cyclin D regulation found in other model systems (7, 27). Finally, TNF-α and cyclin D are both reduced in ob/ob (leptin-deficient) mice and associated with a delay in the cell cycle (9). Collectively, these studies support the hypothesis that Egr-1 is important for initiation of the cell cycle after CCl4-induced liver injury.
A defect in cell-cycle progression was also observed in Egr-1-deficient mice. Indeed, there was an 80% reduction in BrdU incorporation, a measure of DNA synthesis, in hepatocytes 48 h after CCl4 exposure; peak BrdU incorporation occurred at 48 h in wild-type mice. Importantly, there was no difference between the number of hepatocyte nuclei counted in sections from wild-type and Egr-1−/− mice (wild-type, 208 ± 21; Egr-1−/− 183 ± 24, P = 0.14). Reduced BrdU incorporation suggested a block in G1- to S-phase transition. Liver regeneration in Egr-1−/− mice was delayed, not prevented. By 72 h after CCl4, indices of cell-cycle progression in Egr-1−/− mice reached or exceed peak levels observed in wild-type mice. Indeed, hepatic mRNA accumulation cyclins A2 and B1 and BrdU incorporation were equivalent to peak levels in wild-type mice, whereas cyclins D1 and E are increased relative to peak expression in wild-type mice. These data are associated with peak TNF-α production in Egr-1−/− mice (18). The exuberant regenerative response detected in Egr-1−/− mice at 72 h, after progression through the delayed G1/S-phase restriction point, likely reflects the increased requirement for hepatocyte proliferation because liver injury in Egr-1−/− mice is more severe than in wild-type mice (18).
Of the cyclins examined, Egr-1 deficiency had the greatest impact on cyclin E1; Egr-1 deficiency was associated with a twofold reduction in hepatic cyclin E1 mRNA at both 24 and 48 h after CCl4. In addition, cyclin E protein expression was not above baseline in livers from Egr-1−/− mice, whereas cyclin E was increased twofold in livers from wild-type mice at 48 h after CCl4 exposure. Cyclin E/CDK2 complexes contribute to the phosphorylation of Rb, liberation of E2F, and expression of S-phase-specific genes, including cyclin E. Therefore, reduced cyclin E and Rb phosphorylation are consistent with the block or delay in G1- to S-phase transition observed in this study. It is not known whether there is a direct role for Egr-1 in the regulation of the cyclin E gene expression. However, the human cyclin E promoter contains many G/C-rich regions (6), a signature associated with Egr-1 DNA-binding and promoter regulation, consistent with this hypothesis. Further work is required to determine whether Egr-1 directly regulates the timely expression of the cyclin E gene in wild-type mice and/or whether perturbation in signals or signaling pathways upstream of cyclin E transcription in Egr-1−/− mice are responsible for reduced expression of cyclin E1 mRNA after CCl4-induced liver injury. Indeed, increased hepatic cyclin E mRNA accumulation at 72 h after CCl4 exposure coincident with Rb phosphorylation and robust BrdU incorporation suggests that regulatory and transcription factors other than Egr-1 are able to induce expression of cyclin E, but with a delay relative to wild-type mice.
PH is another model used to study the hepatic regenerative response in animal models. Using this PH model, Liao et al. identified a different defect in liver regeneration in Egr-1−/− mice. Instead of a G1/S block/delay as observed after CCl4-mediated liver injury and repair, Egr-1−/− mice exhibited a G2/M block after PH (12). Although only a marginal effect on DNA synthesis was observed, reduced Cdc20, a molecule required for the anaphase promoting complex and progression through the mitotic spindle assembly checkpoint, was hypothesized as responsible for perturbations in liver regeneration (12). In contrast to those results, expression of Cdc20 after CCl4 exposure in the present study was not different between genotypes 24 or 48 h after CCl4 exposure and was increased, not reduced, in livers from Egr-1−/− mice at 72 h, consistent with robust hepatocyte proliferation at that time (Supplemental Fig. S3).
Collectively, these data suggest that there are distinct roles for Egr-1 in liver regeneration depending on the nature of the inciting liver injury. The difference between models may reflect differences in mitochondrial status after CCl4 vs. PH and the contribution of cellular energy status and redox state to cell-cycle progression. Indeed, after PH, energy availability and functional parameters in liver are maintained in contrast to profound disturbances in mitochondrial function after CCl4. Uncoupling of oxidative phosphorylation and enhanced oxidative stress contribute to delayed DNA synthesis after CCl4, but not PH (8). Egr-1 is a redox-sensitive transcription factor capable of regulating antioxidant genes such as mitochondrial superoxide dismutase (13). Therefore, antioxidant function could be further reduced after CCl4-induced liver injury in Egr-1−/−mice, relative to wild-type mice. This could exacerbate CCl4-mediated mitochondrial dysfunction and delay DNA synthesis. Therefore, the more subtle effects of Egr-1 deficiency after PH relative to CCl4 exposure likely reflect differences in antioxidant and mitochondrial dysfunction between the two animal models.
In conclusion, our data reveal novel roles for Egr-1 in liver regeneration after hepatotoxin exposure, which contrast with the role of Egr-1 in liver regeneration after partial hepatectomy (12). Understanding the molecular mechanisms responsible for unchecked expansion of toxin-induced tissue injury is of critical importance to the preservation of healthy liver tissue. In particular, improved hepatoprotection or accelerated repair early in the response to a hepatotoxin exposure, such as alcohol exposure, could help reduce subsequent liver injury and fibrosis after chronic exposure. Given the relatively small number of organs available for liver transplant, and complications associated with life after organ transplant, improved viability of a patient's own liver should be of the highest priority. Our studies identify a novel target, Egr-1, to which therapeutic strategies could be aimed that will improve hepatocyte survival and expedite liver regeneration after toxin-induced hepatic injury.
GRANTS
This work was supported by NIH grants F32 AA015833 and K99/R00 AA017918 to M. Pritchard and R01 AA011975 and P20 AA17069 to L. Nagy.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the animal husbandry expertise of Emmanuelle Ogier and Holly Cline and technical assistance of Brian T. Pratt.
REFERENCES
- 1. Abshagen K, Eipel C, Kalff JC, Menger MD, Vollmar B. Loss of NF-κB activation in Kupffer cell-depleted mice impairs liver regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol 292: G1570–G1577, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Brown RS., Jr Live donors in liver transplantation. Gastroenterology 134: 1802–1813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coqueret O. Linking cyclins to transcriptional control. Gene 299: 35–55, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology 43, Suppl 2: S45–S53, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Gashler A, Sukhatme VP. Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol 50: 191–224, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Geng Y, Eaton EN, Picon M, Roberts JM, Lundberg AS, Gifford A, Sardet C, Weinberg RA. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene 12: 1173–1180, 1996 [PubMed] [Google Scholar]
- 7. Guillemot L, Levy A, Raymondjean M, Rothhut B. Angiotensin II-induced transcriptional activation of the cyclin D1 gene is mediated by Egr-1 in CHO-AT(1A) cells. J Biol Chem 276: 39394–39403, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Hernandez-Munoz R, Sanchez-Sevilla L, Martinez-Gomez A, Dent MA. Changes in mitochondrial adenine nucleotides and in permeability transition in two models of rat liver regeneration. Hepatology 37: 842–851, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Leclercq IA, Field J, Farrell GC. Leptin-specific mechanisms for impaired liver regeneration in ob/ob mice after toxic injury. Gastroenterology 124: 1451–1464, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, Milbrandt J. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science 273: 1219–1221, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Lee SL, Tourtellotte LC, Wesselschmidt RL, Milbrandt J. Growth and differentiation proceeds normally in cells deficient in the immediate early gene NGFI-A. J Biol Chem 270: 9971–9977, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Liao Y, Shikapwashya ON, Shteyer E, Dieckgraefe BK, Hruz PW, Rudnick DA. Delayed hepatocellular mitotic progression and impaired liver regeneration in early growth response-1-deficient mice. J Biol Chem 279: 43107–43116, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Maehara K, Oh-Hashi K, Isobe KI. Early growth-responsive-1-dependent manganese superoxide dismutase gene transcription mediated by platelet-derived growth factor. FASEB J 15: 2025–2026, 2001 [DOI] [PubMed] [Google Scholar]
- 14. McMullen MR, Pritchard MT, Wang Q, Millward CA, Croniger CM, Nagy LE. Early growth response-1 transcription factor is essential for ethanol-induced fatty liver injury in mice. Gastroenterology 128: 2066–2076, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehendale HM. Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol Pathol 33: 41–51, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Moroy T, Geisen C. Cyclin E. Int J Biochem Cell Biol 36: 1424–1439, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Poznic M. Retinoblastoma protein: a central processing unit. J Biosci 34: 305–312, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Pritchard MT, Cohen JI, Roychowdhury S, Pratt BT, Nagy LE. Early growth response-1 attenuates liver injury and promotes hepatoprotection after carbon tetrachloride exposure in mice. J Hepatol 53: 655–662, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pritchard MT, Nagy LE. Ethanol-induced liver injury: potential roles for egr-1. Alcohol Clin Exp Res 29, Suppl 11: 146S–150S, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Pritchard MT, Roychowdhury S, McMullen MR, Guo L, Arteel GE, Nagy LE. Early growth response-1 contributes to galactosamine/lipopolysaccharide-induced acute liver injury in mice. Am J Physiol Gastrointest Liver Physiol 293: G1124–G1133, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Rai RM, Lee FY, Rosen A, Yang SQ, Lin HZ, Koteish A, Liew FY, Zaragoza C, Lowenstein C, Diehl AM. Impaired liver regeneration in inducible nitric oxide synthasedeficient mice. Proc Natl Acad Sci USA 95: 13829–13834, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun A, Bagella L, Tutton S, Romano G, Giordano A. From G0 to S phase: a view of the roles played by the retinoblastoma (Rb) family members in the Rb-E2F pathway. J Cell Biochem 102: 1400–1404, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Taub R. Liver regeneration: from myth to mechanism. Nat Rev 5: 836–847, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Taub R, Greenbaum LE, Peng Y. Transcriptional regulatory signals define cytokine-dependent and -independent pathways in liver regeneration. Semin Liver Dis 19: 117–127, 1999 [DOI] [PubMed] [Google Scholar]
- 25. von Montfort C, Beier JI, Kaiser JP, Guo L, Joshi-Barve S, Pritchard MT, States JC, Arteel GE. PAI-1 plays a protective role in CCl4-induced hepatic fibrosis in mice: role of hepatocyte division. Am J Physiol Gastrointest Liver Physiol 298: G657–G666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang T, Fontenot RD, Soni MG, Bucci TJ, Mehendale HM. Enhanced hepatotoxicity and toxic outcome of thioacetamide in streptozotocin-induced diabetic rats. Toxicol Appl Pharmacol 166: 92–100, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Xiao D, Chinnappan D, Pestell R, Albanese C, Weber HC. Bombesin regulates cyclin D1 expression through the early growth response protein Egr-1 in prostate cancer cells. Cancer Res 65: 9934–9942, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J Biol Chem 272: 17795–17801, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.