Abstract
Bone morphogenetic protein (BMP) signaling within the gastrointestinal tract is complex. BMP ligands and their receptors are expressed in both epithelial and mesenchymal compartments, suggesting bidirectional signaling between these two entities. Despite an increasing interest in BMP signaling in gut physiology and pathologies, the distinct contribution of BMP signaling in the epithelium vs. the mesenchyme in gastrointestinal homeostasis remains to be established. We aimed to investigate the role of epithelial BMP signaling in gastric organogenesis, gland morphogenesis, and maintenance of epithelial cell functions. Using the Cre/loxP system, we generated a mouse model with an early deletion during development of BMP receptor 1A (Bmpr1a) exclusively in the foregut endoderm. Bmpr1aΔGEC mice showed no severe abnormalities in gastric organogenesis, gland epithelial proliferation, or morphogenesis, suggesting only a minor role for epithelial BMP signaling in these processes. However, early loss of BMP signaling in foregut endoderm did impact on gastric patterning, leading to an anteriorization of the stomach. In addition, numbers of parietal cells were reduced in Bmpr1aΔGEC mice. Epithelial BMP deletion significantly increased the numbers of chromogranin A-, ghrelin-, somatostatin-, gastrin-, and serotonin-expressing gastric endocrine cells. Cancer never developed in young adult (<100 days) Bmpr1a-inactivated mice although a marker of spasmolytic polypeptide-expressing metaplasia was upregulated. Using this model, we have uncovered that BMP signaling negatively regulates the proliferation and commitment of endocrine precursor cells. Our data also indicate that loss of BMP signaling in epithelial gastric cells alone is not sufficient to induce gastric neoplasia.
Keywords: bone morphogenetic protein, gastric cell lineages, spasmolytic polypeptide-expressing metaplasia
in mouse gut development, the endoderm receives instructive signals to form the primitive gut tube by E8.5-E9.0 (14, 63). Reciprocal interactions of the endoderm with the mesoderm play pivotal roles in these instructive signals and involve many growth factor pathways such as Wnt, Fgfs, Notch, Hh and bone morphogenetic proteins (BMPs) (15, 16, 20, 27, 34, 46, 59). These crucial interactions ultimately pattern the gut tube into its foregut, midgut, and hindgut sections along with their respective functional and cellular particularities. Maintenance of the structural and functional integrity of the gastric epithelium throughout adult life also involves these reciprocal interactions (1, 3, 11, 27, 57, 61).
BMPs are multifunctional growth factors belonging to the transforming growth factor-β (TGF-β) superfamily. BMPs play active roles in many developmental processes, homeostasis, as well as various cellular functions in postnatal and adult animals (6, 37). These morphogenic proteins signal through the serine/threonine kinase receptor subtypes I and II, whereby the type I receptor is activated upon BMP-ligand binding and associates with the type II receptor. This activated receptor complex leads to the transphosphorylation of BMP receptor-regulated Smad (BR-Smad) proteins that include Smad1, Smad5, and Smad8. In turn, these phosphorylated BR-Smads associate with the related protein Smad4 (Co-Smad), a shared partner of the TGF-β superfamily. The BR-Smad/Co-Smad complex then translocates to the nucleus, where it activates or represses transcription of specific targeted genes (6, 35, 37). The expression patterns of the various BMP signaling molecules in adult human and mouse stomach have been previously described (58). Furthermore, using a genetic approach, the relevance of BMP signaling in gastric tumorigenesis has recently been explored in mice (4, 41, 49). BMPs are growth factors known for signaling simultaneously in the epithelial and stromal compartments of the gut (2, 48, 50). Our previous experiments with BMP signaling in the intestine have shown the importance of delineating the specific roles played by this pathway in one cell compartment relative to the other in intestinal organogenesis and maintenance of adult epithelial cell functions (2).
Anatomically, the mouse stomach is subdivided into three major histologically distinct regions. The forestomach, the most proximal region, consists of one-third of the entire organ and is lined with a stratified squamous epithelium. The middle third is comprised of the corpus, whereas the distal third represents the pyloric antrum (32). These latter two regions are composed of mucosal glands lined by a simple columnar epithelium that comprises, at all times, both undifferentiated pluripotent stem cells and differentiated functional epithelial cells. The basic unit of the glandular stomach is the gastric gland, which is divided into four regions, namely pit, isthmus, neck, and base. Pluripotent stem cells and undifferentiated progenitor cells are located in the isthmus of the gland, and mitotic activity is confined to this area. Gastric epithelial cells migrate bidirectionally, either differentiating and moving upward to replace pit-surface mucous-secreting cells or migrating deeper into the glands and differentiating into either mucous neck cells, acid-secreting parietal cells, or, lastly, into pepsinogen-secreting zymogenic cells at the glandular base. However, the latter two cell types are absent from the pyloric antrum, which is mostly composed of surface mucous cells (21–25). Enteroendocrine cells, which secrete hormones such as ghrelin, serotonin, somatostatin, gastrin, and histamine, are found scattered throughout the gastric gland units (45, 48).
Most studies on the potential role of BMP signaling in stomach morphogenesis and epithelial specification have primarily been performed with models where the gastric character of the epithelium is already specified (3, 4, 49, 58). Although these experiments are informative with regard to the requirements for the maintenance of gastric fate in adult life, they provide only minor clues as to their potential roles in gastric patterning and morphogenesis as well as in gastric epithelial lineage specification and cytodifferentiation. Moreover, selective loss of the BMP pathway by one particular cell compartment in relation the other has not been evaluated.
In the present study, we conditionally inactivated BMP receptor 1A (Bmpr1a) in the mouse early gut endoderm to specify the function of the epithelial BMP signaling cascade in gastric organogenesis, morphogenesis, and maintenance of epithelial cell functions. Our results suggest a possible involvement for epithelial BMP signaling in the commitment and determination of the pre-pit and pre-neck epithelial subpopulations targeted to acquire the parietal phenotype. Using this model, we have uncovered that BMP signaling negatively regulates the proliferation and commitment of endocrine precursor cells. Our data also indicate that loss of BMP signaling in epithelial gastric cells alone is not sufficient to incur neoplasia early in life.
MATERIALS AND METHODS
Animals.
129SvEv-Bmpr1afx/fx mice were provided by Dr. Y. Mishina (38), and the C57BL/6 YAC-Foxa3Cre transgenic line was provided by Dr. K. H. Kaestner (31). For this study, the 129SvEv-Bmpr1afx/fx mice were first crossed with the C57BL/6 Foxa3-Cre to generate F1-generation heterozygous animals. F1-generation heterozygous animals were then backcrossed with Bmpr1afx/fx mice to produce F2-generation experimental animals. All experiments were conducted in F2-generation experimental animals. Genomic DNA was isolated using the Spin Doctor genomic DNA kit from Gerard Biotech according to the manufacturer's protocol. All mutations were genotyped according to previously published protocols (31, 38). All experiments were approved by the Animal Research Committee of the Faculty of Medicine and Health Sciences of the University of Sherbrooke.
Tissue collection, RNA extraction, and gene-expression analysis.
Total RNA was isolated and processed using the Totally RNA extraction kit (Ambion). RT-PCR and quantitative real-time PCR were performed as described previously (2). PCR conditions and primer sequences are available upon request.
Tissue preparation and histological staining.
Stomachs from 70- to 90-day-old control littermates and Bmpr1aΔGEC mice were fixed in 4% paraformaldehyde overnight at 4°C, sectioned, and stained [hematoxylin and eosin (H&E) or Alcian blue] as previously described (2, 30).
Isolation of mouse adult gastric epithelium.
RNA was isolated from enriched epithelial or mesenchymal glandular gastric fractions of adult mice by an adaptation of the MatriSperse dissociation method described previously for human intestine (42). Briefly, mice were euthanized, and the stomachs were opened along the greater curvature and rinsed with cold PBS. The sections were further cut in 5-mm pieces and incubated in 5 ml of cold MatriSperse (Becton-Dickinson) in 15-ml tubes at 4°C for 24 h. The epithelial layer was dissociated from the underlying mesenchyme by gentle manual shaking on ice. The epithelial suspension and remaining mesenchyme were collected, centrifuged, and washed with cold PBS. RNA extraction was performed as described above.
Immunofluorescence staining.
Immunofluorescence staining was performed as previously described (2, 30). Nonspecific binding was blocked, and antibodies were diluted in PBS/Tween 20% solution containing 2% BSA and 1% fish gelatin (Sigma-Aldrich). The following antibodies were used at the indicated dilutions: anti-PCNA (1:1,000, Abcam), anti-Bmpr1a (1:75, Abcam), anti-K14 (1:1,000, Novocastra), antichromogranin A (1:1,000, Immunostar), anti-intestinal fatty acid-binding protein (IFABP) [1:5,000, kindly provided by Dr. Gordon (52)], antilysozyme (1:2, DakoCytomation), anti-Griffonia simplicifolica (GSII) (1:200, Invitrogen), anti-Ulex europaeus lectins (UEA-1) (1:200, Sigma), antipepsinogen (1:200, kindly provided by F. Carriere and R. Verger), anti-H/K-ATPase (1:2, MBL), anti-Trefoil factor 2 (TFF2) (1:75, Vector Laboratories), anti-gastric intrinsic factor (GIF) (1:75, Abnova), anti-human epididymis protein 4 (HE4) (1:100, Santa Cruz), antighrelin (1:200, Santa Cruz Biotechnology), antisomatostatin (1:200, Lab Vision), antigastrin (1:100, Chemicon), and antiserotonin (1:200, Immunostar).
Analytical procedures.
Blood glucose values were determined from whole venous blood from either mice fed ad libitum or 14- to 16-h-fasted mice using a glucose monitor (FreeStyle Lite, Abbot). Pancreatic enzymatic activity was assessed after protein extraction from the 90-day-old pancreas (29). Amylase-, lipase-, elastase-, trypsin-, and chymotrypsin- specific activities were determined as previously described (29).
Measurement of basal and stimulated gastric acid secretion.
Gastric acidity values were determined from 16-h-fasted control and Bmpr1aΔGEC mice. Mice were anesthetized by injection of ketamine xylazine (10 μl/10 g body wt), and a central longitudinal incision along the abdomen was performed. The stomach and duodenum were brought to the surface, and three ligatures were made around the gastroesophageal and gastrointestinal junctions to preserve the stomach content. Intraperitoneal injection with PBS (basal acid secretion) or histamine (stimulated acid secretion, 20 mg/kg) was performed on control and Bmpr1aΔGEC mice 30 min before collection of the stomach content. Stomachs were then opened along the greater curvature and rinsed with 2 ml of normal saline (0.9% NaCl, pH 7.0). The collected supernatant was titrated with 0.005 N NaOH to determine the acid content. Gastric acidity was expressed as microequivalents, and values were normalized to kilograms of body weight (61).
Measurement of ghrelin circulating levels.
Circulating serum level of ghrelin was determined from blood collected from the right heart ventricle of 16-h-fasted control and Bmpr1aΔGEC mice. Blood was collected and pretreated with Pefabloc solution to a final concentration of 1 mg/ml. After 30 min at room temperature, all samples were centrifuged at 2,000–3,000 g for 15 min at 4°C. Acidification of the serum samples with HCl to a final concentration of 0.05 N was performed. Mouse active ghrelin serum levels were measured using a rat/mouse ghrelin (active) ELISA kit (Millipore) according to the manufacturer's instructions.
Metabolic analysis.
For metabolic analyses, control and Bmpr1aΔGEC mice (70 to 80 days old) were placed in metabolic cages during the experimentation (7 consecutive days following a 5-day adaptation period). The animals were housed on a reverse light-dark cycle. All groups were fed ad libitum throughout the duration of the study. Control and Bmpr1aΔGEC mice were provided with the same quantities of food and water. Body weight (g), food intake (g), water intake (ml), urine (ml), and feces (g) were measured every day at the same hour.
Quantification of cell number and statistical tests.
Positively stained cells for PCNA, H+/K+-ATPase, chromogranin A, GIF, TFF2, ghrelin, somatostatin, gastrin, or serotonin were counted per glandular axis. The total number of nuclei was quantified using DAPI staining. Statistics were calculated using two-way ANOVA for the percentage of positive cells per glandular axis. All cell count analyses were performed using continuous sections from low-powered fields of well-oriented glandular cross sections in a blind manner on an average of 10 independent fields per animal (n = 3). Forestomach and corpus morphometry analyses were performed using low-powered fields of complete stomach cross sections from both mutant and control animals (n = 6). Morphometry was determined using the MetaMorph v4.6 software (Universal Imaging). Images were imported, and magnification was calibrated by comparison with a stage micrometer (Taiwan Semiconductor Manufacturing measurement slide). Data are expressed as means ± SE or means ± SD. Statistical analysis was performed using the Student's t-test or two-way ANOVA. For metabolic analyses, normality was evaluated with the D'Agostino-Pearson Omnibus K2 test. Water and food intake data were analyzed with the unpaired t-test and urine and feces with the nonparametric Mann-Whitney test. All statistical analyses were carried out using Graph Pad Prism 5 (Graph Pad). Differences were considered significant with a P value of <0.05.
RESULTS
Loss of gastric epithelial Bmpr1a leads to forestomach expansion and squamous epithelial hyperplasia.
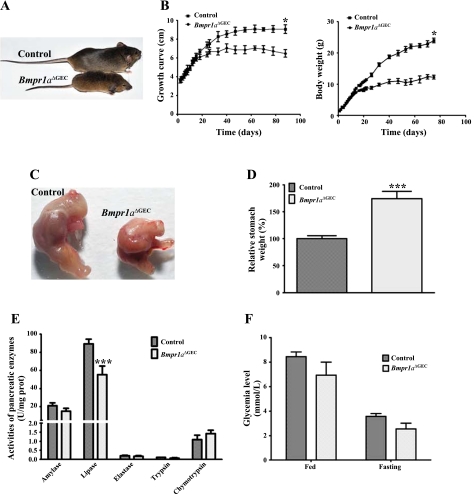
To investigate the functional significance of epithelial BMP signaling in murine gastric epithelium determination and specification, gastric-specific gene ablation of Bmpr1a was performed. Homozygous floxed Bmpr1a mice (Bmpr1afx/fx) (38) were crossed with the YAC-Foxa3-Cre transgenic line, which directs expression of the transgene in foregut endoderm from E8.5 (31). F2-generation conditional knockout mice for Bmpr1a (Bmpr1aΔGEC) were born at the expected Mendelian ratios; these animals were viable at least 90 days of age and suffered from dwarfism (Fig. 1A). Growth and body weight evaluation, from birth to up to 80 days, was subsequently performed (Fig. 1B). Analysis revealed no differences between Bmpr1aΔGEC and control mice up to 20 days after birth with regard to growth and body weight. However, as the Bmpr1aΔGEC mice got older, the difference in their growth curve and weight was significantly more severe (Fig. 1B). At the time of their death, the Bmpr1aΔGEC mice showed a reduction of 28.5% in their growth curve and 48.7% in body weight compared with controls (Fig. 1B). The dwarfism phenotype also affected the size of the stomach (Fig. 1C). Compared with overall animal size, statistical analysis indicated a significant 74% increase in relative weight (Fig. 1D) of the Bmpr1aΔGEC stomach compared with control littermates. Because the YAC-Foxa3-Cre transgenic line drives Cre expression in early foregut endoderm (31), we therefore assessed whether other organs derived from the foregut endoderm were potentially affected by the loss of Bmpr1a and, consequently, would impact on the resulting gastric phenotypes observed. No significant macroscopical phenotypes were observed in the liver (data not shown). Moreover, no significant modulation of pancreatic enzymes was observed in the Bmpr1a mutant, except for lipase (Fig. 1E). Analysis of glycemia levels in both fed and fasted animals revealed no significant modulation of blood glucose levels in Bmpr1aΔGEC compared with control mice (Fig. 1F).
Fig. 1.
Impact of loss of bone morphogenetic protein (BMP) signaling on general physiology of BMP receptor 1a (Bmpr1a)ΔGEC mice. A: photograph of 60-day-old control (top) and Bmpr1aΔGEC mice (bottom). Bmpr1aΔGEC mice were smaller and suffered from dwarfism. During the first 3 wk of life, Bmpr1aΔGEC and control mice followed the same weight and growth curves (B). After 3 wk, Bmpr1aΔGEC mice showed a steady lesser growth curve and gained less body weight than control mice. At the adult stage, Bmpr1aΔGEC mice showed a decrease of 28.5% in their growth curve and 48.7% in weight gain compared with controls (n = 25). C: photograph of 60-day-old control and Bmpr1aΔGEC stomach. Statistical analysis indicated a significant increase of 74% in relative weight of the mutant stomach compared with controls (n = 8) (D). Pancreatic enzyme analysis indicated only a downregulation for lipase activity in the mutant pancreas compared with controls (n = 9) (E). Analysis of glycemia level in fed (n = 5) or fasted (n = 9) animals revealed no significant modulation in blood glucose levels in Bmpr1aΔGEC compared with control mice (F). Student's t-test, *P < 0.05; ***P < 0.0001. Error bars represent SE (B and F) or SD (E).
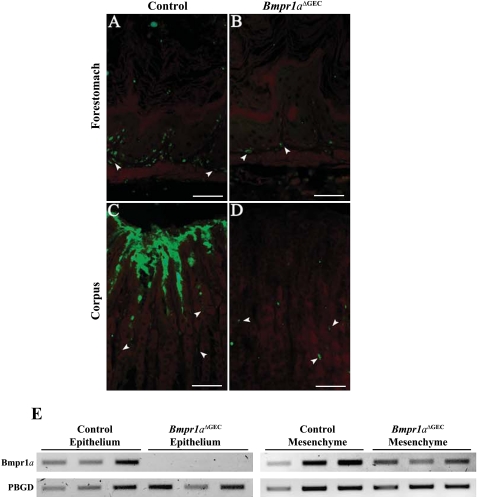
Bmpr1a immunofluorescence in control mice (Fig. 2, A and C) revealed a strong expression in the pit and isthmus regions of the gastric gland unit in the corpus (Fig. 2C) and antrum (data not shown). Expression of Bmpr1a was found in all layers of the pluristratified epithelium of the forestomach (Fig. 2A). Of particular note, the predominant expression pattern of Bmpr1a in the normal glandular stomach was found in regions composed of stem/progenitor cells, differentiating cells, and surface mucous cells. Our analysis confirmed that Bmpr1a expression was lost exclusively in gastric epithelium of the Bmpr1aΔGEC mice but not in subepithelial fibroblasts (Fig. 2, B and D). Semiquantitative RT-PCR analysis was performed on cDNA from MatriSperse-enriched epithelial or mesenchymal gastric fractions of adult mice (Fig. 2E). These results confirmed the loss of Bmpr1a in the epithelial fraction of the Bmpr1aΔGEC compared with control mice. Moreover, because histological visualization of Bmpr1a-expressing fibroblasts can be difficult, this analysis on the mesenchymal fraction confirmed its conserved expression in both control and mutant mice. Given that the mesenchymal fraction was an enrichment and thus not completely exempt of occasional remaining epithelial cells, this may have impacted on the slight decrease observed in Bmpr1a expression in the mutant mesenchymal-enriched fraction. This decrease probably stemmed from the absence of Bmpr1a expression in the remaining epithelium in the mutant mice.
Fig. 2.
Loss of BMP signaling in the gastric epithelium of Bmpr1aΔGEC mice. Immunostaining of Bmpr1a revealed expression of Bmpr1a (in green) in all layers of the pluristratified epithelium of the forestomach in control mice (A). Evans Blue served as counterstain for all immunofluorescences (in red). Loss of Bmpr1a expression was confirmed in the pluristratified epithelium of the forestomach in Bmpr1aΔGEC mice (B). Bmpr1a immunofluorescence in control mice (C) revealed a predominant expression pattern for Bmpr1a in pit and isthmus regions of the gastric gland unit in the corpus. Immunostaining of Bmpr1a confirmed the loss of the receptor exclusively in the gastric epithelium of Bmpr1aΔGEC mice (D) compared with controls (C) (n = 10). Of note, subepithelial fibroblasts in both control (A and C) and mutant (B and D) express Bmpr1a (white arrowheads). Semiquantitative RT-PCR analysis of Bmpr1a expression from MatriSperse-enriched epithelial or mesenchymal gastric fractions of mutant and control adult mice confirmed the loss of Bmpr1a in the epithelial fraction of Bmpr1aΔGEC compared with control mice (n = 7) (E). The conserved expression of Bmpr1a in the mesenchymal fraction in both control and mutant mice was also validated. PBGD, porphobilinogen deaminase.
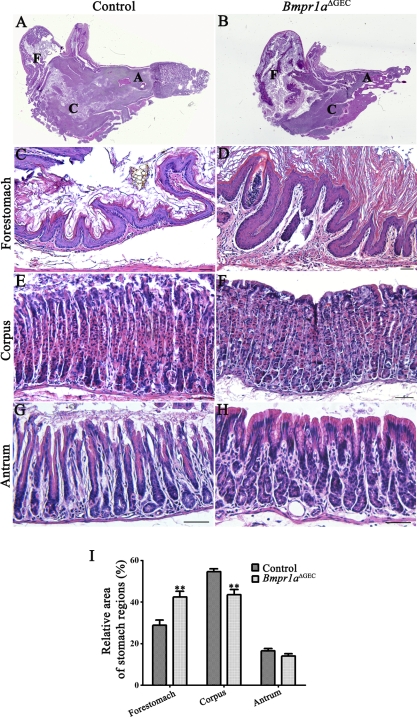
To analyze morphological changes occurring with the loss of BMP signaling early in foregut endoderm, histological analysis with H&E staining was performed on the stomach of control and Bmpr1aΔGEC mice. Histological analysis showed that Bmpr1aΔGEC mice displayed a prominent expansion of the forestomach region (Fig. 3B) with a hyperplasia of the squamous epithelium (Fig. 3D) compared with control littermates (Fig. 3, A and C, respectively). Assessment of the contribution of each region (forestomach, corpus, and pyloric antrum) relative to whole organ weight measured in Bmpr1aΔGEC compared with control littermates revealed a significant 1.47-fold increase of the forestomach and a 1.25-fold decrease of the corpus in Bmpr1aΔGEC mice compared with control counterparts (Fig. 3I). However, Bmpr1aΔGEC mice displayed a normal epithelial glandular architecture in the corpus (Fig. 3F) as well as in the pyloric antrum (Fig. 3H) relative to controls (Fig. 3, E and G, respectively).
Fig. 3.
Loss of epithelial BMP signaling impacts on gastric regionalization but not on glandular architecture. Hematoxylin and eosin (H&E) staining performed on the stomach of mutant and control P90 mice showed a prominent expansion of the forestomach region (B) with an important hyperplasia of squamous epithelium (D) compared with control littermates (A and C, respectively). No significant perturbation of the glandular architecture of the corpus (F) or pyloric antrum (H) was observed in the mutant stomach compared with controls (E and G, respectively). Measurement of the contribution of each region (forestomach, corpus, and pyloric antrum) relative to whole organ weight revealed a significant increase of 1.47-fold of the forestomach and a decrease of 1.25-fold of the corpus in Bmpr1aΔGEC mice compared with controls (n = 6) (I). Two-way ANOVA, **P < 0.005. Error bars represent SE. F, forestomach; C, corpus; A, antrum.
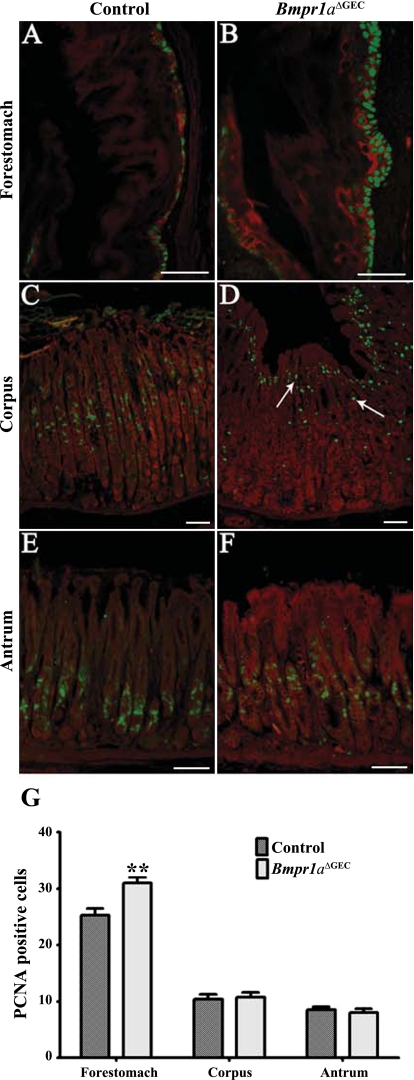
The aforementioned thickening of the squamous epithelium in the forestomach region observed in Bmpr1aΔGEC mice suggested a possible deregulation in proliferation. Proliferation assays with PCNA staining in control animals showed that proliferation occurred in a few basal cells in the forestomach (Fig. 4A), as well as in the isthmus region of the corpus (Fig. 4C) and pyloric antrum (Fig. 4E). Bmpr1aΔGEC mice showed a significant 1.22-fold increase in the number of proliferating basal cells in the forestomach region (Fig. 4, B and G), whereas no modulation was observed in the corpus (Fig. 4, D and G) or in the pyloric antrum (Fig. 4, F and G). Furthermore, proliferative cells in the corpus were partly delocalized from the isthmus region in the controls to the pit region near the surface in Bmpr1aΔGEC mice (Fig. 4D). To better visualize the pluristratified epithelium in the forestomach, a costaining with keratin 14 was performed (red staining) (Fig. 4, A and B). The Evans blue counterstain (red staining) was used for the corpus and antrum (Fig. 4, C–F).
Fig. 4.
Loss of epithelial BMP signaling deregulates cell proliferation in the forestomach. Immunostaining with an anti-PCNA antibody showed proliferating cells (in green) in a few basal cells in the forestomach in control mice (A). Mutant mice displayed abnormal cell proliferation in the forestomach as shown by an increase in the number of labeled proliferating cells (B). Costaining with keratin 14 was used (red staining) to enhance visualization of the forestomach epithelium (A and B). Proliferative cells were found in the isthmus region of the corpus (C) and pyloric antrum (E) in control mice. No modulation was observed in the number of proliferative cells in the corpus (D) or in the pyloric antrum (F) in Bmpr1aΔGEC mice. Delocalization of the proliferative zone from the isthmus region in controls to the pit region near the surface in the corpus was observed in Bmpr1aΔGEC mice (D, white arrows). Evans Blue served as counterstain and is visualized in red in the corpus and antrum (C–F). Statistical analysis of the number of positive PCNA cells confirmed the significant increase in the number of proliferative cells in the forestomach and the absence of modulation in the corpus and pyloric antrum in Bmpr1aΔGEC mice (n = 7) (G). Two-way ANOVA, **P < 0.005. Error bars represent SE.
Epithelial BMP signaling regulates gastric epithelial cell lineage specification.
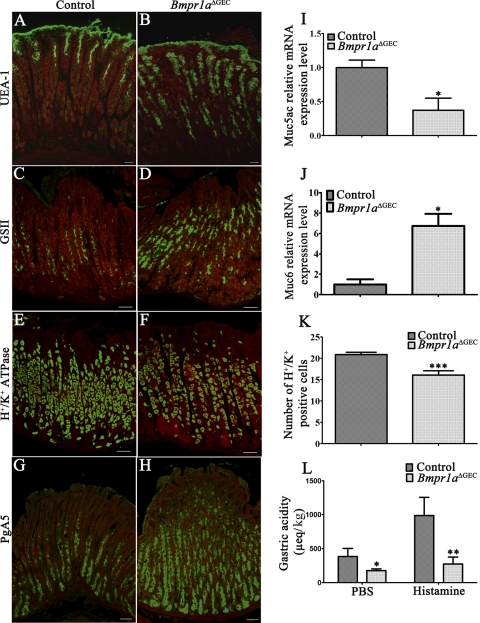
As demonstrated above in Fig. 2C, Bmpr1a is expressed in the isthmus region where progenitor cells are found. Our previous experiments with BMP signaling in the intestine have shown the importance of this signaling pathway in epithelial cell commitment and terminal differentiation (2). We thus hypothesized that its loss could lead to commitment and/or specification defects in the gastric epithelium. Therefore, the adult gastric mucosa of the corpus region of Bmpr1aΔGEC and control mice was stained with specific markers for each major gastric cell type. Pit-surface mucous cells were stained with UEA-1, while mucous neck cells were stained with GSII. An important expansion in both pit-cell and mucous-neck-cell populations was observed in Bmpr1aΔGEC mice (Fig. 5, B and D, respectively) compared with controls (Fig. 5, A and C, respectively). qRT-PCR analyses of Muc5ac and Muc6 expression, expressed by pit and neck mucous cells, respectively, were found to be modulated by the loss of epithelial BMP signaling. Surprisingly, Muc5ac expression was decreased by 2.63-fold (Fig. 5I) despite the observed increase in the pit-cell population, whereas Muc6 expression was increased by sixfold in Bmpr1aΔGEC compared with control mice (Fig. 5J). Immunostaining of H+/K+-ATPase associated with the proton pump of parietal cells was subsequently performed and showed a decrease in number of labeled acid-secreting parietal cells in Bmpr1aΔGEC mice (Fig. 5F) compared with control littermates (Fig. 5E). Parietal cell counts revealed a significant 1.30-fold decrease in parietal cells of glandular units in Bmpr1aΔGEC mice (Fig. 5K). Analysis of neonatal stomachs confirmed that this parietal cell loss in Bmpr1aΔGEC mice was already present at birth (data not shown). In 16-h-fasted mice, basal intragastric acidity was significantly lower by 2.18-fold in Bmpr1aΔGEC mice compared with controls (Fig. 5L). Histamine-induced acid secretion revealed that parietal cells found in Bmpr1aΔGEC mice were functional. However, even following histamine stimulation, intragastric acidity was significantly lower by 3.58-fold in Bmpr1aΔGEC mice compared with controls (Fig. 5L). The pepsinogen-secreting zymogenic cells were normally located in the base region of the gland as shown in control mice (Fig. 5G). An increase in the number of pepsinogen-secreting zymogenic cells was observed in Bmpr1aΔGEC mice (Fig. 5H) compared with their control counterparts (Fig. 5G). Furthermore, the zymogenic cells were found to be scattered throughout the gastric gland in the mutant stomach in contrast to their normal basal location in controls.
Fig. 5.
Epithelial BMP signaling regulates gastric epithelial cell lineage specification. Adult gastric mucosa of the corpus region of Bmpr1aΔGEC and control mice was stained with specific markers for each major gastric cell type. Immunostaining with Ulex europaeus lectin (UEA-1) (in green) showed expansion in the pit cell population in Bmpr1aΔGEC mice (B) compared with controls (A). Evans Blue served as counterstain for all immunofluorescences (in red). Immunostaining with Griffonia simplicifolica II (GSII) (in green) revealed an increase in the population of mucous neck cells in Bmpr1aΔGEC mice (D) compared with controls (C). H+/K+-ATPase immunostaining (in green) showed a mild decrease in the number of labeled acid-secreting parietal cells in Bmpr1aΔGEC mice (F) compared with control littermates (E). Pepsinogen immunostaining (PgAs) (in green) demonstrated an increase in the zymogenic cell population in Bmpr1aΔGEC (H) compared with control mice (G). qRT-PCR analysis of Muc5ac expression was found to be decreased (I), whereas Muc6 expression was increased in Bmpr1aΔGEC compared with control mice (n = 4) (J). Gastric parietal cells were counted in control and mutant mice (n = 3). Statistical analysis of the number of H+/K+-ATPase-positive cells revealed a significant decrease in parietal cells of the gland units in 90-day-old Bmpr1aΔGEC mice (K). Measurement of basal and stimulated gastric acid secretion, in 16-h-fasted mice, showed that basal and histamine-induced intragastric acidity was significantly lower by 2.18-fold and 3.58-fold, respectively, in Bmpr1aΔGEC mice compared with controls (L) (n = 6). Two-way ANOVA (K) or Student's t-test (I, J, and L); *P < 0.05; **P < 0.01; ***P < 0.0001. Error bars represent SE.
Loss of Bmpr1a in foregut endoderm affects duodenal architecture, proliferation, and cytodifferentiation.
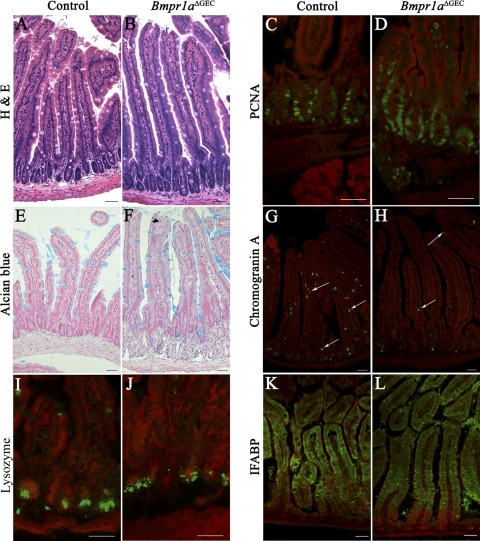
In addition to the stomach, the foregut endoderm also gives rise to the duodenum. We and others have previously reported the phenotypic alteration observed in the intestinal epithelium following loss of BMP signaling (2, 17, 18). Because, in the present model, deletion of Bmpr1a occurred earlier during development (E8.5) than in previous studies (E14.5), we thus investigated the morphological changes occurring with the loss of BMP signaling early in foregut endoderm in the duodenum. Of note, the majority of the general phenotypes found in the duodenum of Bmpr1aΔGEC mice also resembled those previously reported in the jejunum by our group (2). Briefly, histological analysis with H&E staining showed that the duodenum of Bmpr1aΔGEC mice displayed abnormal epithelial morphology with elongated villi, multiplication of crypt units, and absence of de novo crypt phenomenon (Fig. 6B) compared with controls (Fig. 6A). Proliferation assays showed an increase in the number of proliferating cells in Bmpr1aΔGEC mice (Fig. 6D) compared with controls (Fig. 6C). We next investigated possible changes in the cytodifferentiation program of the four intestinal epithelial cell types in Bmpr1a mutant mice. Alcian blue staining, which allows visualization of goblet cells, showed no significant change in the number of these cells in Bmpr1aΔGEC mice (Fig. 6F) compared with controls (Fig. 6E). Immunostaining with the general enteroendocrine cell marker chromogranin A revealed fewer chromogranin A-positive cells in the intestinal epithelium of Bmpr1aΔGEC mice (Fig. 6H) compared with control littermates (Fig. 6G). The status of Paneth cells was also investigated. In contrast with the other intestinal cell types, Paneth cells migrate toward the base of the crypts. To evaluate the presence of Paneth cells in mice with impaired epithelial BMP signaling, immunostaining against lysozyme, a Paneth cell marker, was performed. A more compact staining was observed for Paneth cells in Bmpr1aΔGEC mice (Fig. 6J) compared with controls (Fig. 6I). Location of these cells at the bottom of the crypts was not affected by the loss of BMP signaling. Lastly, absorptive cell differentiation was not affected by loss of BMP signaling as revealed by the absence of modulation in staining intensity and localization of the enterocyte specific marker, IFABP, in both mutant (Fig. 6L) and control mice (Fig. 6K).
Fig. 6.
Loss of epithelial BMP signaling impacts duodenal architecture, proliferation, and cytodifferentiation. H&E staining performed on the duodenum of 90-day-old mutant and control mice showed elongated villi, multiplication of crypt units, and absence of de novo crypt phenomenon in Bmpr1aΔGEC mice (B) compared with controls (A). Proliferation assays were performed with PCNA immunostaining (in green). Mutant mice displayed a slight increase in cell proliferation (D) compared with control duodenum (C). Evans Blue served as counterstain for all immunofluorescences (in red). Alcian blue staining detecting mucin revealed no variation in the number of goblet cells between control (E) and Bmpr1aΔGEC mice (F). Immunostaining with the general enteroendocrine cell marker, chromogranin A (in green), revealed fewer chromogranin A-positive cells (white arrows) in the duodenal epithelium of Bmpr1aΔGEC mice (H) compared with controls (G). Immunostaining with an anti-lysozyme antibody (in green) suggested a reduction in Paneth cell secretory granule content in Bmpr1aΔGEC mice (J) compared with controls (I). No variations in the level of the enterocyte specific intestinal fatty acid-binding protein (IFABP) marker (in green) were observed by immunofluorescence staining between control (K) and Bmpr1aΔGEC mice (L). For all analyses, n = 6.
Loss of epithelial BMP signaling leads to deregulation of gastric endocrine cells.
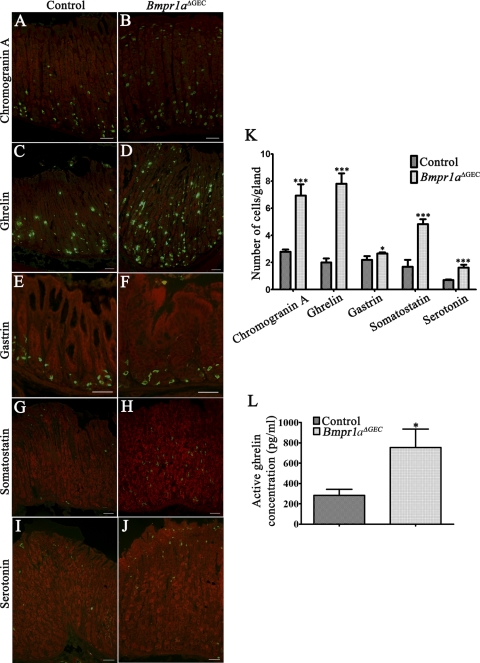
Enteroendocrine cells are found scattered throughout the gastric gland units (45, 48). These cells are the least abundant of the gastric epithelium and are divided into a variety of subpopulations classified according to the hormones they produce. Herein, chromogranin A immunostaining showed a dramatic increase in the number of labeled enteroendocrine cells in Bmpr1aΔGEC mice (Fig. 7B) compared with control littermates (Fig. 7A). Chromogranin A-positive cells counts revealed a significant 2.49-fold increase in enteroendocrine cells in Bmpr1aΔGEC compared with control mice (Fig. 7K). Individual analysis of the various gastric endocrine subpopulations also revealed an increase in all endocrine subpopulations following the loss of epithelial BMP signaling. There was firstly a marked 3.89-fold increase in ghrelin-positive cells in Bmpr1aΔGEC (Fig. 7, D and K) compared with control mice (Fig. 7C), whereas gastrin immunostaining in the antrum was slightly increased by 1.21-fold in the number of gastrin-positive cells in Bmpr1aΔGEC (Fig. 7, F and K) vs. control mice (Fig. 7E). In addition, a 2.88-fold increase in somatostatin-positive cells (Fig. 7, H and K) and a 2.28-fold increase in serotonin-positive cells (Fig. 7, J and K) were also observed in Bmpr1aΔGEC compared with control mice (Fig. 7, G and I, respectively). Circulating ghrelin levels were analyzed and revealed a 2.67-fold increase in Bmpr1aΔGEC mice compared with control littermates (Fig. 7L).
Fig. 7.
Epithelial BMP signaling modulates the gastric endocrine cell population. Chromogranin A immunostaining (in green) showed a dramatic increase in the number of labeled enteroendocrine cells in Bmpr1aΔGEC (B) compared with control littermates (A). Evans Blue served as counterstain for all immunofluorescences (in red). Ghrelin immunostaining (in green) revealed an increase in positive cells in Bmpr1aΔGEC (D) compared with control mice (C). Gastrin immunostaining (in green) in the antrum revealed an increase in positive cells in Bmpr1aΔGEC (F) compared with control mice (E). Immunostaining with somatostatin (in green) showed an increase in positive cells in mutant (H) compared with control mice (G). Serotonin immunostaining (in green) revealed an increase in positive cells in Bmpr1aΔGEC (J) compared with control mice (I). All endocrine-cell subpopulations were counted individually from stomachs of control and mutant mice (n = 3). Statistical analysis confirmed the significant increase in all endocrine subpopulations in Bmpr1aΔGEC mice (K). Circulating active ghrelin levels were analyzed and revealed a 2.67-fold increase in Bmpr1aΔGEC mice compared with control littermates (L). Two-way ANOVA (K), or Student's t-test (L); *P < 0.05; ***P < 0.0001. Error bars represent SE.
Loss of gastric epithelial Bmpr1a leads to a decrease in the metabolism of liquids and solids in mice.
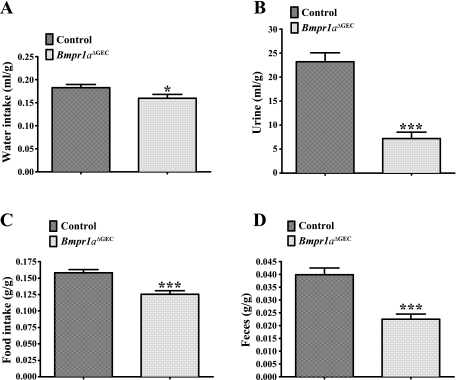
Over the years, it has been shown that the stomach may act as an endocrine organ (5, 10, 19, 62) and possibly alter gastrointestinal (5, 7) and neuroendocrine (19) functions, among others. Using metabolic cages, we therefore investigated the impact of the loss of Bmpr1a in gastric epithelium on both liquid and solid metabolism in mice. Results from male and female mice were combined given that no noticeable variation was observed between the two sexes under normal ad libitum feeding and drinking conditions. Analysis of liquid metabolism (Fig. 8, A and B) revealed that water intake ratios were decreased by 15% in Bmpr1aΔGEC compared with control mice (Fig. 8A). Consistent with this observation, urine ratios indicated 70% less excretion from Bmpr1aΔGEC compared with control mice (Fig. 8B). Analysis of solids metabolism (Fig. 8, C and D) showed that food-intake ratios were decreased by 20% (Fig. 8C), which was consistent with fecal ratios, which were also decreased by 43% (Fig. 8D) in Bmpr1aΔGEC compared with control mice.
Fig. 8.
Impact of loss of gastric epithelial BMP signaling on liquid and solid metabolism. Bmpr1aΔGEC (n = 6) and control mice (n = 6) metabolism was evaluated on a 7-day period to assess the impact of the mutation on diet. Liquid metabolism (A and B) and water ratios (milliliters of water per gram weight) indicate a 15% decrease in consumption for Bmpr1aΔGEC compared with control mice (A), whereas urine ratios (milliliters of urine per gram weight) indicate 70% less excretion for Bmpr1aΔGEC mice compared with controls (B). Solid metabolism (C and D) and food ratios (grams of chow per gram weight) show 20% less consumption for Bmpr1aΔGEC mice compared with controls (C), whereas fecal excretion (grams of feces per gram weight) dropped by 43% for Bmpr1aΔGEC mice compared with controls (D). Water and food intake data were analyzed with the unpaired t-test and urine and feces data with the nonparametric Mann-Whitney test; *P < 0.05, ***P < 0.001. Error bars represent SE.
Loss of gastric epithelial BMP signaling does not promote neoplasia.
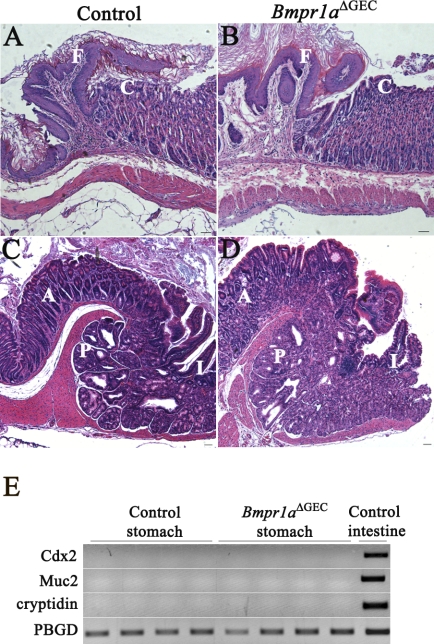
Recent studies have linked gastric tumorigenesis initiation with deregulation in the BMP signaling pathway (3, 4, 41, 49). The formation of tumors at the squamocolumnar and gastrointestinal transition zones has also been reported in another mouse model with impaired Bmpr1a signaling (3). Although very instructive, these latter experiments did not allow the full evaluation of the specific contribution of the epithelium relative to the stroma in this pathology by a selective deregulation of BMP signaling in one compartment over the other. In contrast to the above studies, no polyps were observed herein at the squamocolumnar (Fig. 9, A and B) or gastrointestinal (Fig. 9, C and D) transition zones, as well as in the corpus (Fig. 3F) of Bmpr1aΔGEC mice (Fig. 9, B and D) or controls (Fig. 9, A and C) at 90 days. Furthermore, to further evaluate the possible role of epithelial BMP signaling in gastric tumorigenesis initiation, we investigated for the presence of intestinal markers or spasmolytic polypeptide-expressing metaplasia (SPEM) in Bmpr1aΔGEC gastric mucosa. Routinely, it is considered that the presence of Muc2-expressing goblet cells in the gastric mucosa is a leading marker for intestinal metaplasia (IM) (8, 12). Because goblet cells are not found in the normal stomach, the presence of these cells thereby represents a clear metaplastic process within the intestinal phenotype. In humans, aberrant presence of these cells in the gastric mucosa is defined by Alcian blue staining (12). However, in mice this approach is not as straightforward. Alcian blue-positive cells can be found in deep glandular cells that are also positive for TFF2 and Muc6 (12). In light of this and to avoid false positives, RT-PCR was performed to investigate for the presence of Muc2, a specific goblet cell marker, and other classical markers associated with intestinal metaplasia such as Cdx2 and cryptidin (53, 54, 56). No expression of any of these markers was detected in either Bmpr1aΔGEC or control mice (Fig. 9E).
Fig. 9.
Loss of gastric epithelial BMP signaling does not promote intestinal metaplasia or development of polyposis. H&E staining analysis of both squamocolumnar and gastrointestinal transition zones was performed in Bmpr1aΔGEC and control mice. No polyps or neoplasia were observed at the squamocolumnar (A and B) or gastrointestinal (C and D) transition zones of Bmpr1aΔGEC (B and D) or control mice (A and C) at 90 days (n = 23). RT-PCR analysis for Cdx2, Muc2, and cryptidin revealed no mRNA expression of any of these intestinal markers in gastric mucosa of Bmpr1aΔGEC or control mice (n = 4) (E). I, intestine; C, corpus; A, antrum; P, pylorus; F, forestomach.
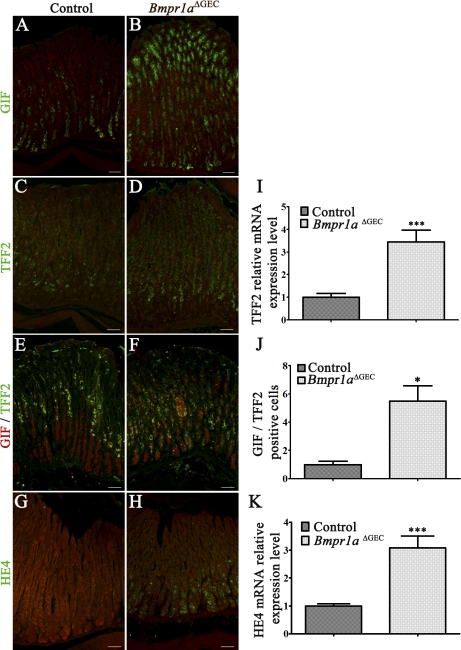
SPEM is observed through transdifferentiation of mature zymogenic cells into SPEM cells (40, 60). These SPEM cells are characterized by the presence of TFF2 at the base of the glands (12). In a first series, the zymogen cell marker, GIF, was used to specifically visualize and localize these cells within the gastric gland of both control (Fig. 10A) and mutant mice (Fig. 10B). An increase in the number of pepsinogen-secreting zymogenic cells was observed in Bmpr1aΔGEC mice similarly to that observed for pepsinogen labeling (Fig. 5H). Immunostaining against TFF2 was next performed to investigate for the presence of SPEM in the gastric mucosa of Bmpr1aΔGEC mice. Presence of TFF2-positive cells was found expressed in the region of the neck and zymogenic-cell populations in the glands of Bmpr1aΔGEC mice (Fig. 10D) compared with the gastric neck region in controls (Fig. 10C). qRT-PCR analysis of TFF2 mRNA expression levels showed a 3.44-fold increase in Bmpr1aΔGEC mice (Fig. 10I). Costaining with GIF and TFF2 confirmed TFF2 labeling in the zymogenic cell population in Bmpr1aΔGEC mice (Fig. 10F) compared with controls (Fig. 10E). Morphometric analysis of the number of cells coexpressing both mucous neck and zymogen markers further support the transdifferentiation of mature zymogenic cells into SPEM cells in Bmpr1aΔGEC mice (Fig. 10J). Immunostaining with HE4, a novel biomarker of SPEM (60), revealed no staining for HE4 in zymogenic cells of control mice (Fig. 10G), whereas staining was observed in Bmpr1aΔGEC mice (Fig. 10H), suggesting the transdifferentiation of zymogenic cells into SPEM following the loss of epithelial BMP signaling. Finally, a 3.08-fold increase in HE4 mRNA expression in Bmpr1aΔGEC mice compared with controls was confirmed by qPCR analysis (Fig. 10K). However, these SPEM cells never developed into neoplasia during the lifespan of these mutant mice.
Fig. 10.
Loss of gastric epithelial BMP signaling results in transdifferentiation of the zymogenic population into spasmolytic polypeptide-expressing metaplasia (SPEM). Immunostaining with gastric intrinsic factor (GIF) (in green) allowed the localization of zymogenic cells in the gastric gland of control (A) and Bmpr1aΔGEC mice (B). Immunostaining with Trefoil factor 2 (TFF2) (in green) revealed positive staining throughout the glands in Bmpr1aΔGEC mice (D) compared with the neck region in controls (C). Evans Blue served as counterstain (in red, A–D). qRT-PCR analysis of TFF2 mRNA expression levels showed a 3.44-fold increase in Bmpr1aΔGEC mice (n = 10) (I). Costaining with gastric intrinsic factor (GIF) (in red) and TFF2 (in green) confirmed that TFF2 labeled the zymogenic cell population (yellow staining) in Bmpr1aΔGEC mice (F) compared with controls (E). The number of cells coexpressing both mucous neck and zymogen cell markers were counted from stomachs of control and mutant mice and revealed a 5.53-fold increase in Bmpr1aΔGEC mice (n = 3) (J). Positive staining with the SPEM biomarker HE4 (in green) was found in zymogenic cells of Bmpr1aΔGEC mice (H) but not in control mice (G) (n = 4). Evans Blue served as counterstain (in red, G and H). qRT-PCR analysis of human epididymis protein 4 (HE4) expression was found to be increased by 3.08-fold in Bmpr1aΔGEC compared with control mice (n = 6) (K). Student's t-test; *P < 0.05; ***P < 0.0001. Error bars represent SE.
DISCUSSION
In recent years, the BMP signaling pathway has been shown to play key crucial roles in gut morphogenesis, cell fate, and adult homeostasis (2, 4, 17, 18). In addition, Bleuming et al. (3) and Oshima et al. (41) revealed a role for BMP signaling in gastric tumorigenesis. However, in these experimental models, they could not exclude a possible role played by mesenchymal BMP signaling in their phenotypes. Our previous work with specific ablation of Bmpr1a in the intestinal epithelium resulted in modest proliferative defects with no significant expansion of stem-cell and progenitor-cell populations and deregulation in terminal cytodifferentiation of secretory cell lineages. Our results showed that specific ablation of BMP signaling only in intestinal epithelial cells was not sufficient to trigger juvenile polyposis syndrome-like phenotype and tumorigenesis in the intestine (2) as previously reported in a model with deletion in all intestinal cells (17, 18). In the present study, we conditionally inactivated Bmpr1a in the mouse early gut endoderm to specify the function of the epithelial BMP signaling cascade in gastric organogenesis, morphogenesis, and maintenance of epithelial cell functions. Again, these mice showed altered specification of gastric epithelial lineage without exhibiting any signs of gastric neoplasia.
It is suggested that intestinal epithelial specification represents a default state for gut endoderm (14). Patterning and acquisition of the distinctive fate of the proximal end of the tube depends on the activation or repression of various factors and pathways such as Wnts (27), BMPs (51), Nkx 2.5, and Gata3 (33, 51). Results herein showed that Bmpr1a was expressed in the epithelium of the forestomach as well as in the pit and isthmus of the gastric gland unit in the corpus and antrum regions. The absence of major abnormalities in corpus or antrum-gland morphogenesis and epithelial proliferation in Bmpr1aΔGEC mice suggests that the BMP signaling pathway is not essential for early development of gastric-unit morphogenesis or for maintaining normal gastric epithelial homeostasis once these units are fully formed. However, the present observations demonstrate that loss of BMP signaling early in foregut endoderm does nonetheless impact on the patterning of the stomach itself. Our results revealed a significant expansion of the forestomach region over the corpus and antrum with an important hyperplasia of squamous epithelium, thereby suggesting that BMP signaling establishes the limits for squamocolumnar transition zones in the stomach.
The formation of tumors at the squamocolumnar and gastrointestinal transition zones has been reported in other mouse models with loss of BMP signaling (4, 41). Bleuming et al. (3) generated a mouse with impaired BMP signaling by using the interferon-inducible promoter Mx1-Cre, which ablated the floxed Bmpr1a from tissues including the gastrointestinal tract, spleen, and liver, as well as the hematopoietic compartment (28). The presence of neoplastic gastric epithelium was observed within 3 wk following the loss of BMP signaling in the stomach. In contrast, in the present study, we found no polyps at either junctions or in the corpus of our Bmpr1aΔGEC mice. In humans, two metaplasias are associated with the precancerous stomach, IM and SPEM (60). Analysis of our Bmpr1aΔGEC mice revealed no expression of intestinal markers such as Cdx2, Muc2, or cryptidin often associated with IM (53, 54, 56). SPEM is observed through transdifferentiation of mature zymogenic cells into SPEM cells following parietal cell loss (40, 60). These SPEM cells are characterized by the presence of TFF2 throughout the glands (12). However, SPEM cells never progress to dysplasia in the absence of inflammation (13). Our study revealed a 30% reduction in parietal cells in Bmpr1aΔGEC adult mice. There was also an upregulation of zymogenic cells positive for TFF2 in Bmpr1aΔGEC mice as well as for the novel biomarker of SPEM, HE4, at the base of the gland, suggesting that loss of epithelial BMP signaling leads to development of SPEM. However, these SPEM cells never developed into neoplasia in mice with impaired gastric epithelial BMP signaling, at least within the short 90-day lifespan of the Bmpr1aΔGEC mice. Such absence of neoplastic lesions in the Bmpr1aΔGEC mice may be the result of two different mechanisms. First, it has become clear that cross talk between all cell compartments is essential for proper gastric morphogenesis as well as maintenance of its homeostatic state in the adult (27, 36, 39, 43, 44, 55). However, previous studies on gastric BMP signaling failed to delineate the specific contribution of the epithelial compartment in the observed phenotypes (4, 41, 49). However, the present results indicate that abrogation of epithelial BMP signaling in the stomach alone is not sufficient to recapitulate the neoplastic features associated with total gastric loss of Bmpr1a (4, 41). These findings support that BMP signaling originating from the epithelium and the mesenchyme differentially regulate some of the gastric epithelial cell behavior and functions. On the other hand, the studies by Bleuming et al. (3) using the interferon-inducible promoter Mx1-Cre to ablate Bmpr1a and Oshima et al. (41) using transgenic mice overexpressing Noggin in the gastric epithelium in combination with elevated levels of prostaglandin E2 could lead to a second interesting perspective. It has been shown that infection of gastric mucosa by Helicobacter pylori leads to inflammation-associated carcinogenesis (12). Both mouse models by Bleuming and Oshima led to the development of neoplasia (3, 41). In contrast, the recent study by Shinohara et al. (49) using transgenic mice overexpressing Noggin in the gastric epithelium as well as our more restrictive model Bmpr1aΔGEC rather presented SPEM. Interestingly, both of these latter models, which are not exposed to any inflammatory trigger, failed to develop neoplasia. One could speculate that induction of the inflammatory response in the context of the loss of BMP signalization could lead to a dramatic cascade of events leading to increased susceptibility to neoplastic initiation.
Despite the fact that BMP signaling does not appear to be essential for gastric epithelial morphogenesis, regulation of glandular proliferation, and general homeostasis, this pathway is nonetheless clearly involved in the regulation of squamous epithelial stem/progenitor cells as well as the regulation of epithelial differentiation programs. The absence of either atrophy or hypertrophy of the gastric gland suggests that glandular stem cells are not influenced by BMP signaling. The analysis of gastric mucosal lineages using a number of histological and immunohistochemical markers indicates that epithelial BMP signaling appears to be involved in the commitment of progenitor cells located in the isthmus of the gastric units. Stem cells give rise to three types of progenitor cells: pre-pit, pre-parietal and pre-neck (22, 23, 25). These progenitor cells ultimately differentiate into their respective lineages. Thus the increase in zymogenic cells could derive from the increase in neck-cell population because these cells give rise to the prezymogenic precursor (22, 23, 25). Then again, in addition to the preparietal cells, a subpopulation of pre-pit and pre-neck cells also contributes to the pool of fully differentiated parietal cells found in gastric units (22, 23, 25). The latter is clearly of interest because parietal cells are the only gastric cell type decreased by the loss of BMP signaling, whereas all other cell types are increased. However, the functionality of these cells, as shown by their ability to produce gastric acid under histamine stimulus, is not impaired by the loss of BMP signaling. Only the acidity level of the gastric content, both basal and stimulated, is lower in Bmpr1aΔGEC mice, which correlates with the decrease in parietal cell number observed in these mice. This decrease in parietal cells is observed at birth and maintained throughout adult life despite the increase in the number of gastrin cells in these mice, which may suggest a hypergastrinemia, which should normally lead to an increase in parietal cells. Such observations were previously reported in the TFF1-null mice model, where pit cell number was increased and parietal cells reduced (26). It was suggested that pre-pit cells could be the source of an unknown molecule that regulates the production of parietal cells (26). In light of the present results, it could be speculated that BMP signaling could be involved in the determination of both pre-pit and pre-neck subpopulations targeted to acquire the parietal phenotype. In absence of BMP signaling, these cells would follow their main path and differentiate into neck and pit cells. Finally, the number of surface mucous cells was found to be increased when gastric epithelial BMP signaling was impaired. The decrease in Muc5ac expression seen in Bmpr1aΔGEC mice, despite the expansion in surface mucous cells, appears somewhat contradictory in regard to the literature (12, 22, 25). We therefore explored the possibility for Muc5ac to be directly transcriptionally regulated by the BR-Smads, which are activated by the BMP signaling cascade. A bioinformatic prediction for the BMP-responsive element (BRE) binding site within the Muc5ac promoter revealed no BRE sequence. Thus it is unlikely that the decrease observed in Muc5ac in mutant mice involves deregulation in its transcription caused by the loss of epithelial BMP signaling. As we have previously shown, BMP signaling can be involved in the terminal differentiation of certain subpopulations of intestinal epithelial cells (2). This particular role for BMP signaling may also occur in the gastric epithelium. Under normal conditions, it could be speculated that epithelial BMP signaling might be involved in the terminal differentiation of the pre-pit precursor into the surface mucous cells, which is characterized by acquisition of Muc5ac expression. Furthermore, one cannot exclude the possibility for the specification of the pre-neck cell population to be impaired by the absence of BMP signaling. Indeed there is a probability where misspecification of these cells might lead to a gain of expression in UEA1 lectin. Under normal conditions, this specific lectin is expressed exclusively by the mucous surface cell in the mouse gastric epithelium (9). Nevertheless, future development of specific markers for the different gastric precursor cells should allow the accurate evaluation of the exact role for BMP signaling on the specification of these cell subpopulations.
Our experiments also revealed that BMP signaling negatively regulates the production of gastric endocrine cells. The fact that all endocrine cells were upregulated in the Bmpr1aΔGEC mice eliminates the possibility of a deregulation stemming from the decrease in parietal cells. Indeed, both somatostatin and gastrin cells are upregulated at the same time in Bmpr1aΔGEC mice, whereas, in a context of acid-secretion regulation, they have an opposite effect (47), thus suggesting that BMP signaling may negatively control the proliferation of endocrine precursor cells in the isthmus of the gland. Results herein further showed that the number of ghrelin cells as well as serum levels were increased in mutant mice. Unexpectedly, metabolism analysis revealed that food intake in Bmpr1aΔGEC mice was decreased despite the upregulation of this hormone, known to normally stimulate appetite (19). Further analysis will be needed to better evaluate the impact and possible networking of gastric endocrine deregulation following the loss of BMP signaling on general metabolism in the mouse.
In summary, the present data indicate that abrogation of epithelial BMP signaling in the stomach alone is not sufficient to recapitulate the phenotypic features associated with total gastric loss of Bmpr1a. Early loss of epithelial BMP signaling in the gut endoderm does not impact on the morphogenesis and epithelial proliferation of the gastric glands although it clearly affects the regional anterior limitation found in the stomach. Our results suggest a possible involvement for epithelial BMP signaling in the commitment and determination of pre-pit and pre-neck subpopulations targeted at acquiring the parietal phenotype as well as a negative regulator for the proliferation and commitment of gastric endocrine precursor cells. Although the loss of epithelial gastric BMP signaling leads to the development of SPEM, neoplasia never developed in these mice during their lifespan, thereby suggesting that the BMP signaling pathway present in the mesenchyme and/or an inflammatory mechanism likely plays a greater role than originally suspected in the control of gastric epithelial cell proliferation, gland morphogenesis, and in the development of gastric cancer.
GRANTS
This research was supported by the Canadian Institutes of Health Research (CIHR) MOP-111104 to N. Perreault, by the Natural Sciences Engineering Research Council of Canada (NSERC) GP6369 to J. Morisset for the pancreatic enzymatic assays, and NIH grant R01-DK053839 to K. Kaestner for the generation of the Foxa3-Cre transgenic line. N. Perreault is a member of the FRSQ-funded Centre de Recherche Clinique Étienne Lebel.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank François Boudreau for critical reading of the manuscript, the QTRN/CIHR Team on Digestive Epithelium intestinal phenotyping platform from the Université de Sherbrooke for histology and phenotyping services, Nathalie Carrier for help with statistical analysis, and Jean Lainé for performing the pancreatic enzymatic assays.
REFERENCES
- 1. Aubin J, Dery U, Lemieux M, Chailler P, Jeannotte L. Stomach regional specification requires Hoxa5-driven mesenchymal-epithelial signaling. Development 129: 4075–4087, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Auclair BA, Benoit YD, Rivard N, Mishina Y, Perreault N. Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology 133: 887–896, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bleuming SA, He XC, Kodach LL, Hardwick JC, Koopman FA, Ten Kate FJ, van Deventer SJ, Hommes DW, Peppelenbosch MP, Offerhaus GJ, Li L, van den Brink GR. Bone morphogenetic protein signaling suppresses tumorigenesis at gastric epithelial transition zones in mice. Cancer Res 67: 8149–8155, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bleuming SA, Kodach LL, Garcia Leon MJ, Richel DJ, Peppelenbosch MP, Reitsma PH, Hardwick JC, van den Brink GR. Altered bone morphogenetic protein signalling in the Helicobacter pylori-infected stomach. J Pathol 209: 190–197, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Chen D, Zhao CM, Hakanson R, Samuelson LC, Rehfeld JF, Friis-Hansen L. Altered control of gastric acid secretion in gastrin-cholecystokinin double mutant mice. Gastroenterology 126: 476–487, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors 22: 233–241, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Cobb S, Wood T, Tessarollo L, Velasco M, Given R, Varro A, Tarasova N, Singh P. Deletion of functional gastrin gene markedly increases colon carcinogenesis in response to azoxymethane in mice. Gastroenterology 123: 516–530, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Correa P. A human model of gastric carcinogenesis. Cancer Res 48: 3554–3560, 1988 [PubMed] [Google Scholar]
- 9. Falk P, Lorenz RG, Sharon N, Gordon JI. Moluccella laevis lectin, a marker for cellular differentiation programs in mouse gut epithelium. Am J Physiol Gastrointest Liver Physiol 268: G553–G567, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 274: G561–G568, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Gao N, White P, Kaestner KH. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell 16: 588–599, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldenring JR, Nomura S. Differentiation of the gastric mucosa III. Animal models of oxyntic atrophy and metaplasia. Am J Physiol Gastrointest Liver Physiol 291: G999–G1004, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Goldenring JR, Ray GS, Coffey RJ, Meunier PC, Haley PJ, Barnes TB, Car BD. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology 118: 1080–1093, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Grapin-Botton A, Melton DA. Endoderm development: from patterning to organogenesis. Trends Genet 16: 124–130, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev 19: 877–890, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Hansson M, Olesen DR, Peterslund JM, Engberg N, Kahn M, Winzi M, Klein T, Maddox-Hyttel P, Serup P. A late requirement for Wnt and FGF signaling during activin-induced formation of foregut endoderm from mouse embryonic stem cells. Dev Biol 330: 286–304, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJ, Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 303: 1684–1686, 2004 [DOI] [PubMed] [Google Scholar]
- 18. He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet 36: 1117–1121, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Inui A, Asakawa A, Bowers CY, Mantovani G, Laviano A, Meguid MM, Fujimiya M. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J 18: 439–456, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Johannesson M, Stahlberg A, Ameri J, Sand FW, Norrman K, Semb H. FGF4 and retinoic acid direct differentiation of hESCs into PDX1-expressing foregut endoderm in a time- and concentration-dependent manner. PLos One 4: e4794, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec 236: 259–279, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec 236: 280–296, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec 236: 297–313, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: general conclusions on cell kinetics in the oxyntic epithelium. Anat Rec 236: 333–340, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Karam SM, Straiton T, Hassan WM, Leblond CP. Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells 21: 322–336, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Karam SM, Tomasetto C, Rio MC. Trefoil factor 1 is required for the commitment programme of mouse oxyntic epithelial progenitors. Gut 53: 1408–1415, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim BM, Buchner G, Miletich I, Sharpe PT, Shivdasani RA. The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev Cell 8: 611–622, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science 269: 1427–1429, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Laine J, Beattie M, LeBel D. Simultaneous kinetic determinations of lipase, chymotrypsin, trypsin, elastase, and amylase on the same microtiter plate. Pancreas 8: 383–386, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Langlois MJ, Roy SA, Auclair BA, Jones C, Boudreau F, Carrier JC, Rivard N, Perreault N. Epithelial phosphatase and tensin homolog regulates intestinal architecture and secretory cell commitment and acts as a modifier gene in neoplasia. FASEB J 23: 1835–1844, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Lee CS, Sund NJ, Behr R, Herrera PL, Kaestner KH. Foxa2 is required for the differentiation of pancreatic alpha-cells. Dev Biol 278: 484–495, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Lee ER, Trasler J, Dwivedi S, Leblond CP. Division of the mouse gastric mucosa into zymogenic and mucous regions on the basis of gland features. Am J Anat 164: 187–207, 1982 [DOI] [PubMed] [Google Scholar]
- 33. Li X, Udager AM, Hu C, Qiao XT, Richards N, Gumucio DL. Dynamic patterning at the pylorus: formation of an epithelial intestine-stomach boundary in late fetal life. Dev Dyn 238: 3205–3217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development 132: 279–289, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Massague J. TGF-beta signal transduction. Annu Rev Biochem 67: 753–791, 1998 [DOI] [PubMed] [Google Scholar]
- 36. McLin VA, Henning SJ, Jamrich M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology 136: 2074–2091, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Mishina Y. Function of bone morphogenetic protein signaling during mouse development. Front Biosci 8: d855–d869, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis 32: 69–72, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Moniot B, Biau S, Faure S, Nielsen CM, Berta P, Roberts DJ, de Santa Barbara P. SOX9 specifies the pyloric sphincter epithelium through mesenchymal-epithelial signals. Development 131: 3795–3804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nomura S, Yamaguchi H, Ogawa M, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 288: G362–G375, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Oshima H, Itadani H, Kotani H, Taketo MM, Oshima M. Induction of prostaglandin E2 pathway promotes gastric hamartoma development with suppression of bone morphogenetic protein signaling. Cancer Res 69: 2729–2733, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Perreault N, Beaulieu JF. Primary cultures of fully differentiated and pure human intestinal epithelial cells. Exp Cell Res 245: 34–42, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Perreault N, Katz JP, Sackett SD, Kaestner KH. Foxl1 controls the Wnt/beta-catenin pathway by modulating the expression of proteoglycans in the gut. J Biol Chem 276: 43328–43333, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Perreault N, Sackett SD, Katz JP, Furth EE, Kaestner KH. Foxl1 is a mesenchymal Modifier of Min in carcinogenesis of stomach and colon. Genes Dev 19: 311–315, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann NY Acad Sci 1014: 1–12, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Roberts DJ. Molecular mechanisms of development of the gastrointestinal tract. Dev Dyn 219: 109–120, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Schubert ML. Gastric exocrine and endocrine secretion. Curr Opin Gastroenterol 25: 529–536, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology 134: 849–864, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Shinohara M, Mao M, Keeley TM, El-Zaatari M, Lee HJ, Eaton KA, Samuelson LC, Merchant JL, Goldenring JR, Todisco A. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology 139: 2050–2060, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shroyer NF, Wong MH. BMP signaling in the intestine: cross-talk is key. Gastroenterology 133: 1035–1038, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Smith DM, Nielsen C, Tabin CJ, Roberts DJ. Roles of BMP signaling and Nkx2.5 in patterning at the chick midgut-foregut boundary. Development 127: 3671–3681, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Sweetser DA, Hauft SM, Hoppe PC, Birkenmeier EH, Gordon JI. Transgenic mice containing intestinal fatty acid-binding protein-human growth hormone fusion genes exhibit correct regional and cell-specific expression of the reporter gene in their small intestine. Proc Natl Acad Sci USA 85: 9611–9615, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tanabe H, Sato T, Watari J, Maemoto A, Fijiya M, Kono T, Ashida T, Ayabe T, Kohgo Y. Functional role of metaplastic Paneth cell defensins in Helicobacter pylori-infected stomach. Helicobacter 13: 370–379, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Tatematsu M, Tsukamoto T, Inada K. Stem cells and gastric cancer: role of gastric and intestinal mixed intestinal metaplasia. Cancer Sci 94: 135–141, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Theodosiou NA, Tabin CJ. Sox9 and Nkx2.5 determine the pyloric sphincter epithelium under the control of BMP signaling. Dev Biol 279: 481–490, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Tsukamoto T, Mizoshita T, Tatematsu M. Gastric-and-intestinal mixed-type intestinal metaplasia: aberrant expression of transcription factors and stem cell intestinalization. Gastric Cancer 9: 156–166, 2006 [DOI] [PubMed] [Google Scholar]
- 57. van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev 87: 1343–1375, 2007 [DOI] [PubMed] [Google Scholar]
- 58. van den Brink GR, Hardwick JC, Nielsen C, Xu C, ten Kate FJ, Glickman J, van Deventer SJ, Roberts DJ, Peppelenbosch MP. Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut 51: 628–633, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435: 959–963, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Weis VG, Goldenring JR. Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer 12: 189–197, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xiao C, Ogle SA, Schumacher MA, Orr-Asman MA, Miller ML, Lertkowit N, Varro A, Hollande F, Zavros Y. Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology 138: 550–561, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zavros Y, Rieder G, Ferguson A, Samuelson LC, Merchant JL. Genetic or chemical hypochlorhydria is associated with inflammation that modulates parietal and G-cell populations in mice. Gastroenterology 122: 119–133, 2002 [DOI] [PubMed] [Google Scholar]
- 63. Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol 25: 221–251, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.