Abstract
Hydrogen sulfide (H2S) is produced endogenously by l-cysteine metabolism. H2S modulates several ion channels with an unclear mechanism of action. A possible mechanism is through reduction-oxidation reactions attributable to the redox potential of the sulfur moiety. The aims of this study were to determine the effects of the H2S donor NaHS on NaV1.5, a voltage-dependent sodium channel expressed in the gastrointestinal tract in human jejunum smooth muscle cells and interstitial cells of Cajal, and to elucidate whether H2S acts on NaV1.5 by redox reactions. Whole cell Na+ currents were recorded in freshly dissociated human jejunum circular myocytes and NaV1.5-transfected human embryonic kidney-293 cells. RT-PCR amplified mRNA for H2S enzymes cystathionine β-synthase and cystathionine γ-lyase from the human jejunum. NaHS increased native Na+ peak currents and shifted the half-point (V1/2) of steady-state activation and inactivation by +21 ± 2 mV and +15 ± 3 mV, respectively. Similar effects were seen on the heterologously expressed NaV1.5 α subunit with EC50s in the 10−4 to 10−3 M range. The reducing agent dithiothreitol (DTT) mimicked in part the effects of NaHS by increasing peak current and positively shifting steady-state activation. DTT together with NaHS had an additive effect on steady-state activation but not on peak current, suggesting that the latter may be altered via reduction. Pretreatment with the Hg2+-conjugated oxidizer thimerosal or the alkylating agent N-ethylmaleimide inhibited or decreased NaHS induction of NaV1.5 peak current. These studies show that H2S activates the gastrointestinal Na+ channel, and the mechanism of action of H2S is partially redox independent.
Keywords: patch clamp, intestine, smooth muscle, dithiothreitol, thimerosal
hydrogen sulfide (h2s) is a gas produced endogenously through l-cysteine metabolism predominantly by two enzymes, cystathione γ-lyase (CSE) and cystathione β-synthase (CBS) (reviewed in Refs. 4, 14, 19). H2S is detected in mammalian tissues and in circulation. Moreover, H2S and/or HS− have been shown to be an active molecule, with clear pharmacological effects on smooth muscle tissues, including relaxation of vascular, corpus cavernosum, uterine, and gastrointestinal smooth muscle.
Ion channels regulate contractility of gastrointestinal smooth muscle. H2S is known to target various ion channels. Evidence for this involvement of channel activity is strongest for the KATP channel with some evidence for modulation of BK, Cl−, and l-type and T-type Ca2+ channels (15, 19, 42). In human intestinal smooth muscle cells, channels that allow entry of ions into the cell include NaV1.5 [Na+ current: (10, 27, 38, 48), CaV1.2 L-type Ca2+ current: (6, 7, 22, 47), CaV3.2 T-type Ca2+ current: (8, 47)], and nonselective cation channels (16). The mechanism by which H2S modulates ion-channel activity is still largely unknown, and, although the chemistry of H2S favors redox modification of proteins, there has been little study of this effect. H2S and alternative byproducts of cysteine metabolism appear to have potency as redox reagents (46, 50) and can affect both TTX-resistant and TTX-sensitive Na+ channels. For example, l-cysteine (0.1 mM) prevents inhibition of peak NaV1.5 current by the oxidizing ion Hg2+ but not Cd2+ in hH1-transfected COS7 cells (49). Cysteine oxidized as cysteic acid can be decarboxylated into taurine. In turn, taurine (5–20 mM) can positively shift the voltage dependence of activation of TTX-sensitive Na+ channels, negatively shift the voltage dependence of inactivation of TTX-sensitive and TTX-resistant Na+ channels in rat dorsal root ganglia neurons, and inhibit peak sodium current by 25% (50).
As a blocker of oxidation-mediated inhibition of Na+ current, l-cysteine shares an effect similar to the effects of common reducing agents [β-mercaptoethanol, dithiothreitol (DTT)] and opposite of the effects of taurine or oxidizing molecules (thimerosal, Hg2+, Zn2+, Cd2+, and H2O2). In general, reducing agents either can shift the voltage dependence of steady-state activation and inactivation to more positive potentials (5) or increase peak current (5, 18). In contrast, oxidizing reagents shift steady-state inactivation to more negative potentials or reversibly inhibit peak current (5, 18, 21, 37) (Table 1). The mechanisms of these effects are not clear. Potentially, redox reagents can directly influence formation of disulfide bonds or can indirectly affect PKC-mediated activation of channels through the redox state of pyridine nucleotides NADH/NAD+ (1, 2, 9, 18, 20, 45). The aims of our study were first to determine the effect of the H2S donor NaHS on sodium current carried by NaV1.5 in myocytes from the human jejunum circular smooth muscle layer and second to test the interdependence of redox molecules on NaHS-induced changes of the NaV1.5 pore (α) subunit.
Table 1.
Effects of reducing, oxidizing, or alkylating reagents on peak current and steady-state kinetics of sodium channels
| Effect | Peak Current | Activation V1/2 | Inactivation V1/2 |
|---|---|---|---|
| Reducing Agents | |||
| βME | →(50 | →(5) | |
| DTT | ↑(1, 5, 9, 18, 21, 37) | ←(21) | =(21)→(37) |
| l-cysteine | ↓(49) | ||
| Oxidizing Agents | |||
| Cd2+ | ↓(18, 49) | ||
| H2O2 | ↓(21) | =(21) | ←(21) |
| Hg2+ | ↓(9, 18, 49) | ←(18) | |
| Taurine | ↓(46, 50) | →(50) | ←(50) |
| Thimerosal | ↓(5, 30, 37) =(9, 18) | ←(30) =(5) →(37) | ←(5, 30, 37) |
| Zn2+ | ↓(18) | ||
| Alkylating Agent | |||
| NEM | ↓(34, 36) | ← (36) | ← (36) |
Values in parentheses are references.
βME, β-mercaptoethanol; DTT, dithiothreitol; NEM, N-ethylmaleimide;
↑, increase in current; ↓, decrease in current; →, rightward (positive) shift in V1/2; ←, leftward (negative) shift in V1/2; =, no change.
MATERIALS AND METHODS
Preparation of smooth muscle cells from the circular layer of human jejunum.
Human jejunum was obtained as surgical waste from gastric bypass procedures performed for morbid obesity. Use was approved by the Mayo Clinic Institutional Review Board. Tissues were harvested into F-12 solution (F-12; Gibco, Invitrogen, Grand Island, NY; 10 ml/l antibiotic antimicotic A5955; Sigma, St. Louis, MO; 14 mM NaHCO3; pH 7.35) and delivered to the laboratory within 20 min. The circular muscle layer of human jejunum was dissected, and myocytes were dissociated from tissue according to our protocol described previously (39), using papain (24 U/mg, 1.5 mg/ml) and DL-DTT (0.3 mg/ml or ∼2 mmol/l) as reagents before two rinses with Ca2+-free Hanks solution (Cellgro, Herndon, VA). Cells were resuspended in 7 ml of chilled Ca2+-free Hanks solution plus 3 ml of modified minimal essential medium (10.4 g/l MEM, Sigma; containing in mM, 4.17 Na+, 0.5 Ca2+, 0.5 Mg2+, 2 Cl−, 4.17 HCO3−, 10 HEPES, pH 7.2, 276 mOSM). Cells adhered to the recording chamber glass coverslip by gravity within 5–20 min at 22°C before recording.
RT-PCR.
Total RNA from human jejunum was isolated using RNAbee (Tel-Test, Friendswood, TX) in accordance with the user's manual. RT-PCR amplifications were performed using GeneAmp Gold PCR Core Kit (Applied Biosystem, Foster City, CA) following the manufacturer's instructions, and the quality and quantity of the extracted RNA was determined spectrophotometrically by checking the 260/280 absorbance ratios. RT was performed using a mixture of random hexamers and oligo(dT) primers; the reaction protocol consisted of annealing at 25°C for 10 min, followed by one cycle at 42°C for 20 min. The product of the RT reaction was then amplified using the following gene-specific primers designed for human CSE and CBS: CSE forward, 5′-ACATGTCCGAATGGAAAAGC-3′; CSE reverse, 5′-ACTTTCACTTGGAGGGTGTG-3′; CBS forward, 5′-AGGGCTTTCTGAAGGAGGAG-3′; CBS reverse, 5′-CCACGAAGTTCAGCAAGTCA-3′. The primers were designed on the basis of published sequence of human CSE and CBS using Primer3 software (Ref. 31; http://frodo.wi.mit.edu/primer3/). The amplification protocol consisted of 95°C for 10 min, followed by 35 cycles at 94°C, for 20 s; 60°C, for 30 s; and 72°C for 30 s, ending with 72°C for 7 min. The PCR products were separated on 1% agarose gel with their identity confirmed by sequencing (Mayo Molecular Core).
Immunohistochemistry.
Full-thickness human jejunum was immunostained for both CBS and CSE. Cryostat sections 12 μm in thickness were cut from paraformaldehyde (PFA; 4%)-fixed or fresh-frozen tissue embedded in optimal cutting temperature compound. Whereas the antibody raised against CSE (CTH; Abnova, Taipei, Taiwan; 1:1,000) required the use of fresh-frozen sections fixed in PFA for 10 min, the antibody raised against CBS (Abnova; 1:300) was used on tissue fixed before sectioning. Tissue sections were rinsed in 1× PBS followed by a 2-h incubation in 1× PBS containing 0.3% Triton X-100 (Pierce, Rockford, IL) and 1% bovine albumin serum (Sigma-Aldrich). Antibodies were diluted in 1× PBS, 0.3% Triton X-100, and 1% BSA and incubated overnight at 4°C. Tissue sections were rinsed in 1× PBS three times, followed by a 1-h incubation with a Cy-3-conjugated donkey anti-mouse secondary antibody (Millipore, Billerica, MA) diluted at 1:500 in 1× PBS, 0.3% Triton X-100, and 1% BSA. Slides were rinsed three times in 1× PBS and mounted in SlowFade Gold with DAPI (Invitrogen, Carlsbad, CA).
Transfection of HEK cells.
A construct containing SCN5A (H558/Q1077) encoding the Na+ channel α-subunit, NaV1.5, was cotransfected with pEGFP-C1 (Clontech, Palo Alto, CA) into human embryonic kidney (HEK)293 cells (American Type Culture Collection, Manassas, VA) using LIPOFECTAMINE 2000 Reagent (Invitrogen) as described previously (23, 28, 32). Transfected HEK293 cells were cultured for 24 h and selected for patch clamp by green fluorescent protein fluorescence.
Electrophysiology.
Patch-clamp electrodes were pulled from Kimble KG-12 glass on a P-97 puller (Sutter Instrument, Novato, CA) and coated with heat-cured R6101 compound (Dow Corning, Midland, MI). Whole cell currents were recorded from amphotericin-perforated patches on jejunum myocytes or standard dialyzed patches on transfected HEK293 cells with an Axopatch 200B amplifier, Digidata 1322A, and pCLAMP 9 software (Molecular Devices, Union City, CA) as described previously (17, 32, 39). To measure peak current, steady-state activation, and current-voltage (I-V) relationships of Na+ currents in myocytes from the human jejunum circular muscle layer (Figs. 2–3), cells were held at −100 mV and stepped for 50 ms to test voltages from −80 through +35 mV in 5-mV increments. Na+ currents from SCN5A-transfected HEK293 cells (Figs. 4–9) were recorded by stepping for 50 ms to test voltages from −80 through 35 mV from a holding potential of −120 mV. Start-to-start time between sweeps was 1 s. Nifedipine (1 μM) was added to the control Ringer's solution to block L-type Ca2+ current and reveal Na+ I-V curves and steady-state kinetics (Figs. 2–3).
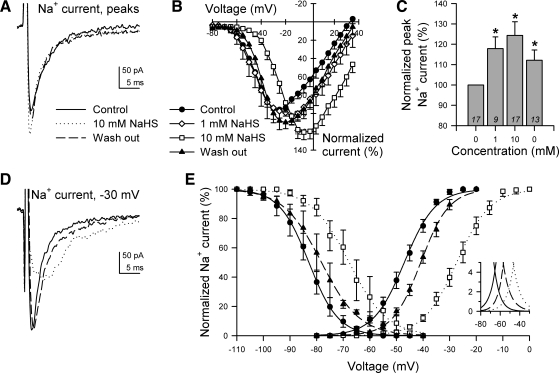
Fig. 2.
NaHS increased peaks and resulted in positive shifts in the steady-state activation and inactivation of Na+ current in myocytes from human jejunum circular smooth muscle. A: representative whole cell voltage-clamp traces displaying maximum peak inward Na+ current, which were elicited by stepping from −100 mV to −25 mV (control), +10 mV (10 mM NaHS), or −20 mV (wash out). These traces were recorded between sequential rinses of NaCl Ringer's solution with 0 (control), 10, or 0 mM NaHS (wash out). B: normalized current-voltage relationships (n = 12) in the presence of nifedipine to block L-type Ca2+ current. C: normalized peak Na+ currents in the presence of 0 (control), 1, 10, or 0 mM (wash out) extracellular NaHS (*P < 0.05 to control). N values are shown in italics. D: representative whole cell Na+ currents produced by stepping from −100 to −30 mV with 0 (control, solid line), 10 (dotted line), or 0 (wash out, dashed line) mM extracellular NaHS. E: steady-state activation and inactivation curves of Na+ currents with 0 (control), 10, or 0 (wash out) mM extracellular NaHS. Inset: Na+ channel window currents. V1/2 of inactivation (mV): control, −83 ± 2; 10 mM NaHS, −68 ± 4*; wash out, −79 ± 2* (n = 5, *P < 0.05 to control). V1/2 of activation (mV): control, −47 ± 1; 10 mM NaHS, −26 ± 2*; wash out, −40 ± 1* (n = 13, *P < 0.05 to control).
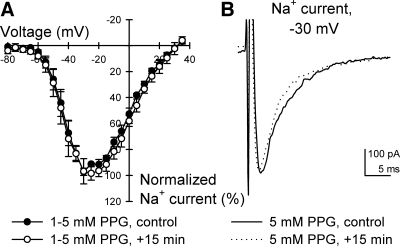
Fig. 3.
CSE inhibitor propargylglycine (PPG) had no effect on Na+ current after 15-min exposure in human jejunum smooth muscle cells. A: normalized current-voltage relationships showing peak Na+ currents after immediate (control) or 15-min exposure to 5 mM extracellular PPG (n = 4, P > 0.05 to controls at all step voltages). B: representative peak whole cell Na+ currents elicited by stepping from −100 to −30 mV at 0 or 15 min exposure to PPG.
Fig. 4.
NaHS increased peaks and shifted positive the steady-state activation and inactivation of Na+ current in NaV1.5-transfected human embryonic kidney (HEK)293 cells. A: normalized current-voltage relationships showing peak Na+ currents at all step voltages tested (n = 12). B: representative whole cell Na+ currents elicited by stepping from −100 to −40 mV in the presence of 0 mM (control), 3 mM, or 0 mM NaHS (wash out). C: steady-state activation and inactivation curves of NaV1.5 exposed to no drug control wash (●), 3 mM NaHS (□), or no drug wash out (▴). Inset: NaV1.5 window currents. D: normalized maximum peak Na+ currents in the presence of no drug control wash (CTRL), 1 × 10−5 to 3 × 10−3 M NaHS, or no drug wash out (WASH). N values are shown in italics. E: V1/2 of activation (●) and inactivation (○) plotted vs. concentration of NaHS (CTRL, no drug control wash; 1 × 10−5 to 3 × 10−3 M NaHS; WASH, no drug wash out). *P < 0.05 vs. control.
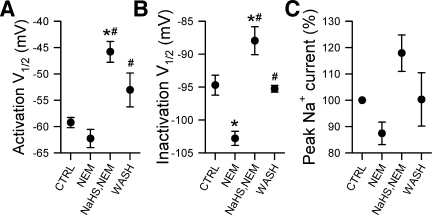
Fig. 9.
N-ethylmaleimide (NEM) inhibited the NaHS-induced increase in peak current of NaV1.5 but did not inhibit NaHS-induced changes in steady state kinetics. A–C: V1/2 of activation (A), V1/2 of inactivation (B), and normalized maximum peaks (C) of Na+ currents following no drug control wash (CTRL), 5 min of exposure to 1 mM NEM, 3 mM NaHS after pretreatment with 1 mM NEM, or no drug wash out (WASH) (n = 4–6, *P < 0.05 vs. control, and #P < 0.05 vs. only NEM by a one-way ANOVA with Tukey's posttest).
Inactivation of Na+ current in jejunum myocytes (Fig. 2) was measured by stepping from a holding potential of −100 mV, to prepulse voltages between −110 mV and −40 mV in 5-mV increments for 3 s each, to −110 mV for 0.2 ms to normalize capacitance transients, and then to a test voltage of −40 mV for 100 ms to preferentially open Na+ channels. Start-to-start time was 4 s between sweeps. In SCN5A-transfected HEK293 cells (Figs. 4, 6–9), inactivated Na+ currents were measured by stepping from a holding potential of −100 mV to a prepulse potential between −130 and −60 mV in 5-mV increments for 3 s each, to −110 mV for 0.2 ms, and then to a test potential of −40 mV for 100 ms.
Fig. 6.
DTT augmented the NaHS-induced positive shift in V1/2 of steady-state activation of NaV1.5 heterologously expressed in HEK293 cells. A–C: V1/2 of activation (A), V1/2 of inactivation (B), and normalized maximum peaks (C) of Na+ currents in the presence of no drug control wash, 3 mM NaHS, 3 mM DTT + 3 mM NaHS, or no drug wash out (n = 6, *P < 0.01 vs. control or wash out, and #P < 0.01 vs. NaHS alone by repeated measures ANOVA with Tukey's posttest).
Drugs and solutions.
The pipette solution contained (in mM): 145 Cs+, 125 methanesulfonate, 35 Cl−, 5 Na+, 5 Mg2+, 2 EGTA, buffered with 5 HEPES; pH was adjusted to 7.0 with CsOH. Amphotericin B was dissolved in DMSO (∼3 mg/50 μl) and suspended in the pipette solution (10 μl/ml) for use as backfill. The control extracellular solution for native cell experiments (Figs. 2–3) was modified Ringer's solution (in mM): 149.2 Na+, 159 Cl−, 4.74 K+, 2.54 Ca2+, 0.001 nifedipine, 5.5 dextrose, buffered with 10 HEPES. For experiments with HEK293 cells transfected with SCN5A, Cs+ (Figs. 4, 6–9) or N-methyl-d-glucamine (NMDG+, Fig. 5) partially replaced Na+ to reduce the current size to levels that could be recorded accurately. Ionic concentrations after pH adjustment were (in mM): 15 Na+, 139.2 NMDG+ or Cs+, 159 Cl−, 4.74 K+, 2.54 Ca2+, 5.5 dextrose, buffered with 10 HEPES; pH was adjusted to 7.4 with HCl or NaOH. Osmolality of solutions measured 300 mmol/kg. Liquid junction potentials were corrected during analysis.
Fig. 5.
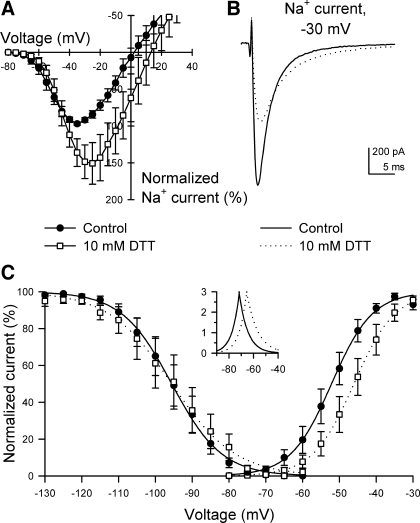
Reducing agent dithiothreitol (DTT) increased peak Na+ current and shifted steady-state activation positive in HEK293 cells heterologously expressing NaV1.5. A: normalized current-voltage relationships showing peak Na+ currents at all step voltages tested (n = 7). B: representative whole cell Na+ currents elicited by stepping from −100 to −30 mV with 0 (control) or 10 mM extracellular DTT. C: steady-state activation and inactivation curves of NaV1.5 exposed to 0 mM (control, ●) or 10 mM DTT (□). Inset, NaV1.5 window currents. V1/2 of inactivation (mV): control, −96 ± 3; 10 mM DTT, −95 ± 4 (n = 4, P > 0.05 to control). V1/2 of activation (mV): control, −52 ± 2; 10 mM DTT, −46 ± 2* (n = 7, *P < 0.05 to control).
H2S donor NaHS (10−5 to 10−2 M) was dissolved freshly in control extracellular solutions. The predicted equilibrium potential for Na+ would shift by up to +2 or +5 mV with the addition of 3 or 10 mM Na+ from NaHS to the 15 or 149.2 mM Na+ extracellular solutions, respectively. At physiological pH and 25°C, 40% of the sulfide anion (HS−) exists as H2S (11, 13). DL-DTT (10−4 to 10−2 M, Figs. 5–6), thimerosal (10−6 to 10−2 M, Figs. 7–8), and N-ethylmaleimide (NEM, 10−3 M, Fig. 9) were added to the extracellular solution as reducing, oxidizing, and alkylating agents, respectively. All chemicals were purchased from Sigma Aldrich. Working concentrations of reagents matched pH 7.5 by alkacid paper strip test (Fisher Scientific) before exposure to cells. When a stable baseline was reached after run up of inward current, cells were rinsed with control Ringer's solution (150 or 15 mM Na+). Because Nav1.5 can be mechanoactivated by perfusion (32, 38), mechanically activated currents recorded immediately after the first rinse were assigned as controls. Previous observations in our laboratory showed that steady-state current-voltage dependency of inward Na+ currents may shift left (negative) up to −1 mV per consecutive rinse.
Fig. 7.
Thimerosal inhibited peak Na+ current and shifted steady-state kinetics of NaV1.5 heterologously expressed in HEK293 cells. A: normalized current-voltage relationships showing peak Na+ currents at all step voltages tested (n = 6). B: representative whole cell Na+ currents elicited by stepping from −100 to −50 mV before, during, or after washes of 3 mM thimerosal. C: steady-state activation and inactivation curves of NaV1.5 exposed to no drug control wash (●), 3 mM thimerosal (□), or no drug wash out (▴). Inset: NaV1.5 window currents. D: normalized maximum peak Na+ currents in the presence of no drug control wash, 1 × 10−5 to 3 × 10−3 M thimerosal, or no drug wash out. N values are shown in italics. E: V1/2 of activation (●) and inactivation (○) plotted vs. concentration of thimerosal (no drug control wash; 1 × 10−5 to 3 × 10−3 M thimerosal). *P < 0.05 vs. control.
Fig. 8.
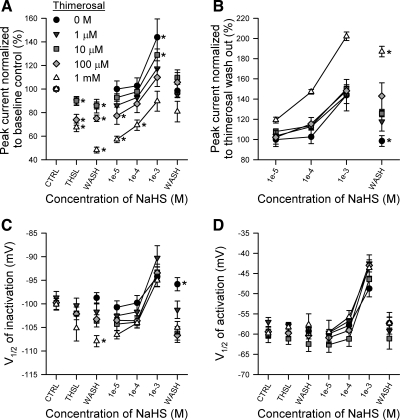
Thimerosal (THSL) is a noncompetitive inhibitor of the NaHS-induced increase in peak current of NaV1.5 and does not inhibit NaHS-induced changes in kinetics. A: normalized maximum peak Na+ currents in the presence of (from left to right): no drug control wash (CTRL), 0 or 10−6 to 10−3 M thimerosal (THSL), thimerosal washout (WASH), 10−5 to 10−3 M NaHS, or no drug wash out (WASH). Data were normalized to raw peak currents of CTRL (100%). *P < 0.05 vs. control by a paired two-tailed Student's t-test of current densities. B: normalized maximum peak Na+ currents in the presence of 10−5 to 10−3 M NaHS or no drug wash out. Data are the same as shown in A but were normalized to raw peak currents of THSL. *P < 0.05 comparing data within each column by a one-way ANOVA with Tukey's posttest. C and D: V1/2 of steady-state inactivation (C) or activation (D) of NaV1.5 exposed to the same series of rinses described in A. N = 36 total cells, 3–12 records per symbol, *P < 0.05 comparing data within each column by a one-way ANOVA with Tukey's posttest. ●, data from Fig. 4, D and E.
Data analysis.
Data were analyzed with Clampfit 9 software (Molecular Devices, Sunnyvale, CA), Microsoft Excel 2003 (Microsoft, Redmond, WA), SigmaPlot 8 (Systat Software, San Jose, CA), and InStat (GraphPad Software, La Jolla, CA). I-V relationships plot each step's peak inward current vs. the step voltage. For I-V or steady-state activation curves (Figs. 2–9), maximum inward peak current was normalized to 100% by the equation: |INORM| = 100*(IV/IMAX), where INORM is normalized peak current, IV is the negative peak of each trace, and IMAX is the maximum peak inward current of a designated control (such as the peak inward current at −30 mV from the no-drug control). Therefore, each trace per record was expressed as a percentage of peak. Steady-state inactivation curves were normalized with the same equation, except that peak currents were measured at the test pulse and plotted vs. the pretest pulse potential. Steady-state kinetics (Figs. 2, 4–9) were fitted with a sigmoid three-parameter curve (Boltzmann equation): y = a/[1 + e−(x−x0)/b], such that a is the y-intercept (∼100%), b is the slope, and x0 is the voltage of half-maximal activation, V1/2. We used data from cells surviving more than five sequential rinses of reagent to calculate EC50 values by fitting a logistic four-parameter curve: y = y0 + a/(1 + x/x0)b, such that x0 is EC50, the half-maximal effective concentration. Statistical comparisons were performed on raw data using a paired two-tailed Student's t-test (Figs. 2–5, 7, 8A), a repeated-measures ANOVA with Tukey's posttest (Fig. 6), or a one-way ANOVA with Tukey's posttest (Figs. 8, B–D, and 9). Statistical significance was accepted when P < 0.05. Data are expressed as mean values ± SE.
RESULTS
Hydrogen sulfide-producing enzymes are present in the circular layers of human jejunum.
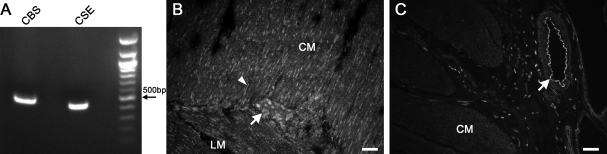
To determine whether H2S could be produced endogenously in the human gastrointestinal tract, we tested for the presence of mRNA for H2S-producing enzymes CBS and CSE in a human jejunum circular smooth muscle cDNA library. mRNA for both enzymes was found to be present (Fig. 1A), consistent with previous reports (24, 29). To determine cellular expression of CSE and CBS, we carried out immunohistochemistry on human jejunum fresh-frozen sections. CSE appeared to be predominantly in neurons in the myenteric plexus as well as in fibers in the muscle layers (Fig. 1B). CBS appeared to be expressed predominantly in blood vessels with minimal expression in the muscle layers (Fig. 1C).
Fig. 1.
Cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) mRNAs are expressed in human jejunum. A: transcripts encoding CBS and CSE were amplified by RT-PCR from RNA isolated from the circular smooth muscle layer of human jejunum. B: immunostaining for CSE in a section of human jejunum. Arrow, neuron. Arrowhead, fiber. CM, circular muscle. LM, longitudinal muscle. Scale bar = 50 μm. C: immunostaining for CBS in a section of human jejunum. Arrow, blood vessel. Scale bar = 50 μm.
Hydrogen sulfide affects NaV1.5 function by increasing maximum peak inward currents and shifting the voltage dependence of activation and inactivation.
The H2S donor NaHS was applied at various concentrations to myocytes from human jejunum circular smooth muscle. Both 1 mM and 10 mM NaHS increased peak Na+ current by 18 ± 6% and 24 ± 7%, respectively (−122 ± 39 pA control to −141 ± 44 pA 1 mM NaHS, n = 9, P < 0.01; −147 ± 23 pA control to −177 ± 27 pA 10 mM NaHS, n = 17, P < 0.01) (Fig. 2, A–C). Additionally, NaHS (10 mM) shifted the voltage of maximum peak Na+ currents by +19 ± 2 mV (n = 17, Fig. 2B), the V1/2 of activation by +21 ± 2 mV (−47 ± 1 mV control to −26 ± 2 mV with NaHS, n = 13, P < 0.01, Fig. 2, D and E), and the V1/2 of inactivation by +15 ± 3 mV (−83 ± 2 mV control to −68 ± 4 mV with NaHS, n = 5, P < 0.05, Fig. 2E). Although wash out completely reversed the effects on the peak current, neither shift in voltage dependence was completely reversible (after wash out, V1/2 of activation: −40 ± 1 mV, n = 13, P < 0.01 to control or NaHS; V1/2 of inactivation: −79 ± 2 mV, n = 5, P < 0.05 to control or NaHS) (Fig. 2E).
To test whether addition of NaHS changed Na+ concentration sufficiently to increase peak current, we increased the bath Na+ concentration to 160 mM Na+, equal to that predicted from the greatest dose of NaHS used. Peak currents of NaV1.5-transfected HEK293 cells in control solution were −749 ± 58 pA, and with 10 mM additional Na+, peak currents increased to −834 ± 80 pA (n = 6, P = 0.08 to control). Unlike with NaHS, this trend did not reach significance, and the increase was 11% compared with 24% with 10 mM NaHS. Additionally, the rinse with the greater extracellular NaCl concentration caused a reversible shift in the V1/2 of activation of NaV1.5 of only −1.0 ± 0.2 mV (−49.1 ± 0.5 mV control, to −50.1 ± 0.4 mV with +10 mM Na+, to −49.3 ± 0.4 mV wash out; n = 6, P < 0.01 control vs. +10 mM Na+, P > 0.05 control vs. wash out), indicating no major change in voltage dependence of activation.
We tested whether Na+ current in myocytes dissociated from human jejunal circular smooth muscle was modulated by endogenous H2S production from the muscle cells themselves. The CSE inhibitor propargylglycine (PPG) was added to the extracellular solution. PPG (1–5 mM) had no effect on peak Na+ current (−263 ± 80 pA control to −264 ± 78 pA PPG, n = 4, P > 0.05) after 15-min application to human jejunum smooth muscle cells (Fig. 3). The V1/2 of steady-state activation also did not shift (−44 ± 2 mV control to −44 ± 3 mV PPG, n = 4, P > 0.05, Fig. 3A).
The data are consistent with activation of the Na+ channel in human jejunum smooth muscle cells by extracellular NaHS (1–10 mM). Previous studies showed that the Na+ current in these cells is carried by NaV1.5. Thus we tested whether the effects of NaHS on Na+ current could be reproduced by the α-subunit of NaV1.5 when expressed in HEK293 cells (Fig. 4). In response to NaHS, peak Na+ current increased over drug-free control by 44 ± 16% at the 1 mM dose and by 46 ± 14% at the 3 mM dose (−100 ± 22 pA/pF control to −134 ± 24 pA/pF with 1 mM NaHS to −163 ± 31 pA/pF with 3 mM NaHS, n = 6–9, P < 0.05 to control; Fig. 4D) and was washed out effectively (1 ± 5% decrease, −94 ± 37 pA/pF control to −89 ± 31 pA/pF wash out, n = 3, P > 0.05). The EC50 for NaHS on NaV1.5 peak current was 1.5 ± 0.5 mM (n = 7, Table 2). The voltage dependence of activation and inactivation also shifted positive at concentrations greater than 0.1 and 1.0 mM, respectively, and returned to control levels after wash out (V1/2 of activation: −59 ± 1 mV control, to −58 ± 2 mV with 0.1 mM NaHS, to −49 ± 2 mV with 1 mM NaHS, to −57 ± 2 mV wash out; V1/2 of inactivation: −97 ± 2 mV control, to −94 ± 2 mV with 1 mM NaHS, to −96 ± 1 mV wash out; n = 3–12, P < 0.01 NaHS to control or P > 0.05 wash out to control). EC50 values for NaHS on NaV1.5 are summarized in Table 2.
Table 2.
Effects and EC50 values of NaHS and redox reagents on NaV1.5
| Peak Current |
Activation V1/2 |
Inactivation V1/2 |
||||
|---|---|---|---|---|---|---|
| Effect | EC50, mM | Effect | EC50, mM | Effect | EC50, μM | |
| NaHS | ↑ | 1.5 ± 0.5 | → | 0.6 ± 0.1 | → | 840 ± 90 |
| DTT | ↑ | 1.7 ± 0.5 | → | 1.3 ± 0.2 | = | n/a |
| Thimerosal | ↓ | 1.4 ± 0.5 | → | 4.6 ± 1.3 | ← | 180 ± 60 |
Applicable values are means ± SE. EC50 values are expressed as averages of fits from n = 4–7 cells exposed to ≥5 concentrations. n/a, not applicable.
The reducing agent DTT produces an additive effect with NaHS on the positive shift in steady-state activation.
Reducing agents such as β-mercaptoethanol and DTT reverse the oxidative block by Hg2+ of maximum peak Na+ current in guinea pig ventricular myocytes, neuroblastoma, and μ1-transfected COS7 cells (5, 9, 18). If NaHS acts directly on NaV1.5 by sulfhydryl modification, then the effects of DTT on NaV1.5 expressed heterologously in HEK293 cells should be similar to the effects of NaHS observed in the native Na+ current experiments (Fig. 2). Similar to NaHS, DTT (10 mM) increased maximum peak Na+ current by 59 ± 28% (−55 ± 16 pA/pF control to −70 ± 16 pA/pF with DTT, n = 6, P < 0.05; Fig. 5A) and shifted the voltage dependence of steady-state activation by +6 ± 1 mV (−52 ± 2 mV control to −46 ± 2 mV with DTT, n = 7, P < 0.05; Fig. 5C). However, DTT had no effect on the V1/2 of steady-state inactivation (−85 ± 2 mV control to −85 ± 4 mV with DTT, n = 6, P > 0.05; Fig. 5C). The EC50 values for DTT on the Na+ current increase and steady-state kinetic shift were 1.7 ± 0.5 mM and 1.3 ± 0.2 mM, respectively (n = 5, Table 2).
These data illustrate that DTT alone had effects on NaV1.5 expressed in HEK cells that were similar but not identical to the effects of NaHS on the native channel in jejunum myocytes. We then used the HEK expression system to determine whether the effects of DTT are additive to the effects of NaHS (Fig. 6). After NaHS was added and steady-state achieved, the bath solution was changed subsequently to 3 mM DTT with 3 mM NaHS. This change produced an additional +5 ± 2 mV shift in steady-state activation beyond a +16 ± 5 mV shift from NaHS alone (−60 ± 1 mV control to −44 ± 2 mV with NaHS to −38 ± 1 mV with both DTT and NaHS, n = 6, P < 0.05; Fig. 6A). However, the NaHS + DTT mixture (3 mM each) did not significantly shift inactivation (−99 ± 1 mV control to −89 ± 2 mV with NaHS to −86 ± 3 mV with both DTT and NaHS, P < 0.05 DTT + NaHS to control, P = 0.13 DTT + NaHS to 3 mM NaHS alone; Fig. 6B), or increase peak current over 3 mM NaHS alone (6 ± 2% increase, −163 ± 31 pA/pF with NaHS to −176 ± 37 with both NaHS and 3 mM DTT, n = 6, P = 0.09 to NaHS alone, Fig. 6C). These observations demonstrate a partial overlap between the effects of NaHS and DTT and suggest more than one mechanism of action. The increase in maximum peak current induced by NaHS may be through a redox mechanism shared by DTT, whereas the shifts in steady-state inactivation and steady-state activation are not through a shared mechanism.
The oxidizing agent thimerosal does not competitively inhibit hydrogen sulfide-induced increases in maximum peak Na+ current in HEK293 cells expressing NaV1.5.
To further test the possibility that NaHS was acting on NaV1.5 peak current through a redox mechanism, we used thimerosal, an organic Hg2+-conjugated oxidizing reagent (reviewed in Ref. 3). Thimerosal was shown previously to be an inhibitor of NaV1.4 or NaV1.5 with reduction in maximum peak Na+ current, a left shift in steady-state inactivation and a right shift in steady-state activation (5, 37). First, we confirmed previous findings for the effects of thimerosal on TTX-resistant Na+ channels (37) and in particular NaV1.5 (Fig. 7). Thimerosal at 3 mM irreversibly decreased maximum peak current of NaV1.5 by up to 54 ± 5% (−109 ± 30 pA/pF control to −47 ± 14 pA/pF with thimerosal to −19 ± 6 pA/pF wash out, n = 4–6, P < 0.05 thimerosal or wash out to control and P < 0.05 wash out to thimerosal), left-shifted steady-state inactivation (−98 ± 1 mV control to −105 ± 2 mV with thimerosal to −106 ± 3 mV wash out, n = 6, P < 0.05 to control) and right-shifted steady-state activation (−58 ± 1 mV control to −49 ± 2 mV with thimerosal to −48 ± 2 mV wash out, n = 4–6, P < 0.05 to control). EC50 values for thimerosal on NaV1.5 are shown in Table 2.
Inhibiting maximum peak current of NaV1.5 would allow use of thimerosal as an antagonist to NaHS if the latter's effect were attributable to redox biochemistry of NaV1.5. On the basis of this hypothesis, thimerosal would favor formation of stable disulfide bridges and decrease Na+ channel permeability (18); however, we found that thimerosal reacts with aqueous NaHS, yielding a white precipitate. Fortunately, irreversibility of NaV1.5 inhibition by the oxidant meant that we could test the effects of NaHS after wash out of thimerosal from the bath. Thus we added a single dose of thimerosal to HEK293 cells expressing NaV1.5, washed with drug-free control solution, then rinsed through a series of increasing extracellular concentrations of NaHS before final wash out (Fig. 8). Single doses of thimerosal (10−6 to 10−3 M) inhibited Na+ maximum peak current in a concentration-dependent manner, and wash out did not reverse the block (n = 5–8, P < 0.05 to control; Fig. 8A, second and third columns). Subsequent addition of NaHS (10−5 to 10−3 M) reversed thimerosal-induced inhibition by returning peak Na+ current back to (10−6, 10−4, 10−3 M thimerosal) or above (0, 10−5 M thimerosal) baseline. However, peak currents in the presence of NaHS were proportional to thimerosal blockade; the currents were not significantly different when normalized to take into account the level of inhibition of Na+ current by thimerosal (n = 3–5, P > 0.05 by one-way ANOVA per NaHS concentration; Fig. 8B), and the percent increase in Na+ current induced by NaHS was not different to that observed without thimerosal. Likewise, thimerosal did not inhibit NaHS-induced shifts in steady-state inactivation or activation of NaV1.5 at any concentration tested (n = 3–12, P > 0.05 by one-way ANOVA per NaHS concentration, Fig. 8, C and D). Thus, although NaHS had the opposite effect to thimerosal on peak NaV1.5 current, the interaction was not competitive with respect to the degree of thimerosal inhibition.
Exposure to the alkylating agent NEM inhibits a subsequent NaHS-mediated increase in maximum peak Na+ current.
To clarify whether the increase in peak Na+ current by NaHS was due to the reduction of available cysteine residues on NaV1.5, we modified those cysteines with the alkylating agent NEM (36) before addition of NaHS (Fig. 9). After 5 min of exposure to NEM (1 mM), maximum peak Na+ current was −80 ± 28 pA/pF compared with −98 ± 38 pA/pF in the control (n = 6, P = 0.12 by a paired two-tailed Student's t-test). NaHS (3 mM) applied after NEM resulted in a peak current of −107 ± 39 pA/pF; this value was not statistically different from control (n = 6, P > 0.05 by one-way ANOVA with Tukey posttest). Positive shifts in steady-state activation and inactivation mediated by NaHS were unaffected by pretreatment with NEM (Fig. 9, A and B), again suggesting that, of the three parameters examined, only peak Na+ current may be modulated by NaHS via a redox-dependent mechanism.
DISCUSSION
The main findings of this study are that the H2S donor NaHS increased peak sodium currents in myocytes from the human jejunum circular smooth muscle layer and in HEK293 cells heterologously expressing NaV1.5. NaHS also right shifted the voltage dependence of Na+ current inactivation and activation, thereby increasing the number of Na+ channels available at more depolarized potentials in myocytes from the circular layer in human jejunum. This shift in window current may further contribute to depolarization of the membrane potential. Therefore, the TTX-resistant Na channel NaV1.5 can be added to the known ion channels that are modulated by H2S. Most data heretofore have shown that the effect of H2S involves the KATP channel. H2S has been shown to open KATP channels in cells from cardiovascular, endocrine, and nervous systems, thereby regulating smooth muscle contractility, inflammation, nociception, pain, and cell death (reviewed in Ref. 42). The KATP channel antagonist glibenclamide can inhibit H2S-mediated vasorelaxation (52), and the CSE inhibitor PPG can decrease KATP whole cell currents. H2S or NaHS is considered to directly interact with KATP channels by increasing the open probability, but not by changing the single-channel conductance, of KATP channels (41, 51). One mechanism proposed for direct interaction between H2S and KATP channels is S-sulfhydration, a process by which a donor transfers a sulfhydryl (−SH) group to form a covalent persulfide (−SSH) in the target protein (25, 26). H2S has also been proposed to modulate large-conductance Ca2+-sensitive K+ (BKCa) channels, but the described effects on this channel are less congruent. NaHS decreased open probability and right shifted steady-state activation of BKCa channels expressed in HEK293 cells, whereas it increased whole cell BKCa current in rat pituitary tumor (GH3) cells (35, 43). This discrepancy may reflect differences in the subunit composition of the channels under study in the two different cell types.
H2S also affects Cl− secretion. In guinea pig and human colon, Cl− secretion stimulated by NaHS and l-cysteine was blocked by TTX, but NaHS-induced Cl− secretion was not found in T84 human colon epithelial cells, suggesting that TTX-sensitive Na+ channels may be necessary for H2S-stimulated Cl− secretion (33, 42). TTX-sensitive and TTX-resistant Na+ channels have different sensitivities but generally not opposite effects in response to l-cysteine, taurine, or redox reagents (Table 1; 37, 49, 50). Because H2S was shown to be an activator of TTX-resistant NaV1.5 in our study, the interrelationship between H2S, NaV1.5, and Cl− channels in the gastrointestinal tract deserves study.
The H2S donor NaHS also reduces L-type Ca2+ current density and inhibits recovery from depolarization-induced inactivation in rat cardiomyocytes, but this inhibition was not accompanied by a change in kinetics (40). For T-type Ca2+ channels, the H2S precursor l-cysteine and redox reducer DTT selectively increased CaV3.2 but not CaV3.1 or CaV3.3 T-type Ca2+ currents expressed in HEK cells (12). In undifferentiated NG108–15 cells, NaHS increased T-type Ca2+ currents, an effect that was inhibited by oxidizing reagent DTNB or subsequently reversed by subeffective concentrations of NaHS or DTT (15). These data suggested that H2S may act as a nociceptive messenger via a redox pathway.
Our data on Nav1.5 showed that the oxidizing agent thimerosal (10−6 to 10−3 M) did not competitively inhibit the NaHS-induced increase in peak Na+ current. In contrast to the work on the T-type Ca2+ channel that used single concentrations of each drug, we examined the interrelationship of these two drugs by utilizing a 1,000-fold concentration range of the oxidizer against a 100-fold range of NaHS. Although there was an inhibition of NaHS-induced changes in peak current by thimerosal, suggesting similar sites of action of these drugs, thimerosal altered the efficacy of NaHS (Fig. 8A) but had no effect on the potency of NaHS (Fig. 8B), indicating noncompetitive antagonism via distinct sites of action. In addition, thimerosal had little effect on NaHS-induced changes in kinetics, further suggesting independent mechanisms of action. The data obtained with NEM, a drug that alkylates cysteine residues, further support the redox-independent actions of NaHS on activation and inactivation kinetics of the NaV1.5 channel. The data also support the possibility that NaHS increases peak Na+ current by acting on available cysteine residues or disulfide bonds via a redox-dependent mechanism.
Although the mechanism of the H2S-generating NaHS on the TTX-resistant Na+ channel NaV1.5 remains to be fully elucidated, one possibility lies in the potential action of HS−. At pH 7.4 and 22°C, HS− supplied by NaHS will exist in equilibrium with dissolved H2S. The effects reported in this study could be attributed to either or both species because both are active biological molecules (11). Increased extracellular Na+ from NaHS is not likely to account for the observed changes because Na+ dissociated from NaCl in control experiments did not produce a shift in NaV1.5 steady-state voltage dependence, nor did an increase in sodium current reach significance. HS− can produce a strong alkaline solution in absence of a buffer, and a high pH solution can increase Na+ current and shift its voltage dependence to more negative voltages (44); however, the opposite (positive) shift was observed here, and our solutions were strongly buffered with HEPES (10 mM), meaning no change in pH was detected after addition of NaHS to the extracellular solutions. Although smooth muscle cells express message for both CSE and CBS, the lack of detectable protein in smooth muscle cells and the lack of effect of CSE inhibitor PPG on myocytes suggest that any potential source of H2S in the gastrointestinal tract in vivo would not likely be smooth muscle cells.
In summary, the studies described in this manuscript determined that H2S activates the TTX-resistant NaV1.5 expressed in the human gastrointestinal tract. H2S also causes a parallel shift in activation and inactivation, resulting in the same sized window current but shifted to more positive potentials, thereby allowing Na+ entry into smooth muscle cells at more depolarized voltages. The NaHS-induced shifts in steady-state kinetics of NaV1.5 were augmented by a reducing agent but not affected by thimerosal or NEM. Therefore, the mechanisms of action of H2S on NaV1.5 appear to be, at least in part, redox independent.
GRANTS
This work was supported by NIH grants DK52766, DK57061, and DK17238.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Gary Stoltz for tissue dissection and cell dissociation and Kristy Zodrow for secretarial assistance.
REFERENCES
- 1. Benitah JP, Ranjan R, Yamagishi T, Janecki M, Tomaselli GF, Marban E. Molecular motions within the pore of voltage-dependent sodium channels. Biophys J 73: 603–613, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chahine M. Cardiac metabolic state and Brugada syndrome: a link revealed. Circ Res 105: 721–723, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Elferink JG. Thimerosal: a versatile sulfhydryl reagent, calcium mobilizer, and cell function-modulating agent. Gen Pharmacol 33: 1–6, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Elsey DJ, Fowkes RC, Baxter GF. Regulation of cardiovascular cell function by hydrogen sulfide (H(2)S). Cell Biochem Funct 28: 95–106, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Evans JR, Bielefeldt K. Regulation of sodium currents through oxidation and reduction of thiol residues. Neuroscience 101: 229–236, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Farrugia G, Holm AN, Rich A, Sarr MG, Szurszewski JH, Rae JL. A mechanosensitive calcium channel in human intestinal smooth muscle cells. Gastroenterology 117: 900–905, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Farrugia G, Rich A, Rae JL, Sarr MG, Szurszewski JH. Calcium currents in human and canine jejunal circular smooth muscle cells. Gastroenterology 109: 707–717, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Gibbons SJ, Strege PR, Lei S, Roeder JL, Mazzone A, Ou Y, Rich A, Farrugia G. The alpha1H Ca2+ channel subunit is expressed in mouse jejunal interstitial cells of Cajal and myocytes. J Cell Mol Med 13: 4422–4431, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hisatome I, Kurata Y, Sasaki N, Morisaki T, Morisaki H, Tanaka Y, Urashima T, Yatsuhashi T, Tsuboi M, Kitamura F, Miake J, Takeda S, Taniguchi S, Ogino K, Igawa O, Yoshida A, Sato R, Makita N, Shigemasa C. Block of sodium channels by divalent mercury: role of specific cysteinyl residues in the P-loop region. Biophys J 79: 1336–1345, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holm AN, Rich A, Miller SM, Strege P, Ou Y, Gibbons S, Sarr MG, Szurszewski JH, Rae JL, Farrugia G. Sodium current in human jejunal circular smooth muscle cells. Gastroenterology 122: 178–187, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med 47: 1346–1353, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Joksovic PM, Nelson MT, Jevtovic-Todorovic V, Patel MK, Perez-Reyes E, Campbell KP, Chen CC, Todorovic SM. Cav3.2 is the major molecular substrate for redox regulation of T-type Ca2+ channels in the rat and mouse thalamus. J Physiol 574: 415–430, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem 285: 21903–21907, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kasparek MS, Linden DR, Kreis ME, Sarr MG. Gasotransmitters in the gastrointestinal tract. Surgery 143: 455–459, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawabata A, Ishiki T, Nagasawa K, Yoshida S, Maeda Y, Takahashi T, Sekiguchi F, Wada T, Ichida S, Nishikawa H. Hydrogen sulfide as a novel nociceptive messenger. Pain 132: 74–81, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Koh SD, Monaghan K, Ro S, Mason HS, Kenyon JL, Sanders KM. Novel voltage-dependent non-selective cation conductance in murine colonic myocytes. J Physiol 533: 341–355, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kraichely RE, Strege PR, Sarr MG, Kendrick ML, Farrugia G. Lysophosphatidyl choline modulates mechanosensitive L-type Ca2+ current in circular smooth muscle cells from human jejunum. Am J Physiol Gastrointest Liver Physiol 296: G833–G839, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurata Y, Hisatome I, Tsuboi M, Uenishi H, Zhang G, Oyaizu M, Sato R, Imanishi S. Effect of sulfhydryl oxidoreduction on permeability of cardiac tetrodotoxin-insensitive sodium channel. Life Sci 63: 1023–1035, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Linden DR, Levitt MD, Farrugia G, Szurszewski JH. Endogenous production of H2S in the gastrointestinal tract: still in search of a physiologic function. Antioxid Redox Signal 12: 1135–1146, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu M, Sanyal S, Gao G, Gurung IS, Zhu X, Gaconnet G, Kerchner LJ, Shang LL, Huang CL, Grace A, London B, Dudley SC., Jr Cardiac Na+ current regulation by pyridine nucleotides. Circ Res 105: 737–745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo A, Ma J, Zhang P, Zhou H, Wang W. Sodium channel gating modes during redox reaction. Cell Physiol Biochem 19: 9–20, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Lyford GL, Strege PR, Shepard A, Ou Y, Ermilov L, Miller SM, Gibbons SJ, Rae JL, Szurszewski JH, Farrugia G. alpha(1C) (Ca(V)1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am J Physiol Cell Physiol 283: C1001–C1008, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Makielski JC, Ye B, Valdivia CR, Pagel MD, Pu J, Tester DJ, Ackerman MJ. A ubiquitous splice variant and a common polymorphism affect heterologous expression of recombinant human SCN5A heart sodium channels. Circ Res 93: 821–828, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Martin GR, McKnight GW, Dicay MS, Coffin CS, Ferraz JG, Wallace JL. Hydrogen sulphide synthesis in the rat and mouse gastrointestinal tract. Dig Liver Dis 42: 103–109, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration (Abstract). Sci Signal 2: ra72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mustafa AK, Gadalla MM, Snyder SH. Signaling by gasotransmitters (Abstract). Sci Signal 2: re2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ou Y, Gibbons SJ, Miller SM, Strege PR, Rich A, Distad MA, Ackerman MJ, Rae JL, Szurszewski JH, Farrugia G. SCN5A is expressed in human jejunal circular smooth muscle cells. Neurogastroenterol Motil 14: 477–486, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Ou Y, Strege P, Miller SM, Makielski J, Ackerman M, Gibbons SJ, Farrugia G. Syntrophin gamma 2 regulates SCN5A gating by a PDZ domain-mediated interaction. J Biol Chem 278: 1915–1923, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Parajuli SP, Choi S, Lee J, Kim YD, Park CG, Kim MY, Kim HI, Yeum CH, Jun JY. The inhibitory effects of hydrogen sulfide on pacemaker activity of interstitial cells of Cajal from mouse small intestine. Korean J Physiol Pharmacol 14: 83–89, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ribeiro MA, Cabral HO, Costa PF. Modulatory effect of NO on sodium currents in a neuroblastoma cell line: aspects of cell specificity. Neurosci Res 58: 361–370, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Bioinformatics Methods and Protocols, edited by Krawetz S, Misener S. Totowa, NJ: Humana Press, 2000, p. 365–386 [DOI] [PubMed] [Google Scholar]
- 32. Saito YA, Strege PR, Tester DJ, Locke GR, 3rd, Talley NJ, Bernard CE, Rae JL, Makielski JC, Ackerman MJ, Farrugia G. Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. Am J Physiol Gastrointest Liver Physiol 296: G211–G218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, Kimura H, Ishii I, De Giorgio R, Campi B, Schemann M. Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon. Gastroenterology 131: 1542–1552, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Shrager P. Slow sodium inactivation in nerve after exposure to sulhydryl blocking reagents. J Gen Physiol 69: 183–202, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sitdikova GF, Weiger TM, Hermann A. Hydrogen sulfide increases calcium-activated potassium (BK) channel activity of rat pituitary tumor cells. Pflügers Arch 459: 389–397, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Song J, Jang YY, Shin YK, Lee C, Chung S. N-Ethylmaleimide modulation of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. Brain Res 855: 267–273, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Song J, Jang YY, Shin YK, Lee MY, Lee C. Inhibitory action of thimerosal, a sulfhydryl oxidant, on sodium channels in rat sensory neurons. Brain Res 864: 105–113, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Strege PR, Holm AN, Rich A, Miller SM, Ou Y, Sarr MG, Farrugia G. Cytoskeletal modulation of sodium current in human jejunal circular smooth muscle cells. Am J Physiol Cell Physiol 284: C60–C66, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Strege PR, Mazzone A, Kraichely RE, Sha L, Holm AN, Ou Y, Lim I, Gibbons SJ, Sarr MG, Farrugia G. Species dependent expression of intestinal smooth muscle mechanosensitive sodium channels. Neurogastroenterol Motil 19: 135–143, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res 79: 632–641, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Tang G, Wu L, Liang W, Wang R. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol Pharmacol 68: 1757–1764, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Tang G, Wu L, Wang R. Interaction of hydrogen sulfide with ion channels. Clin Exp Pharmacol Physiol 37: 753–763, 2010. [Erratum. J Neurophysiol 1997 (May): 2586, 2010.] [DOI] [PubMed] [Google Scholar]
- 43. Telezhkin V, Brazier SP, Cayzac S, Muller CT, Riccardi D, Kemp PJ. Hydrogen sulfide inhibits human BK(Ca) channels. Adv Exp Med Biol 648: 65–72, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Tombaugh GC, Somjen GG. Differential sensitivity to intracellular pH among high- and low-threshold Ca2+ currents in isolated rat CA1 neurons. J Neurophysiol 77: 639–653, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Valdivia CR, Ueda K, Ackerman MJ, Makielski JC. GPD1L links redox state to cardiac excitability by PKC-dependent phosphorylation of the sodium channel SCN5A. Am J Physiol Heart Circ Physiol 297: H1446–H1452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Warenycia MW, Steele JA, Karpinski E, Reiffenstein RJ. Hydrogen sulfide in combination with taurine or cysteic acid reversibly abolishes sodium currents in neuroblastoma cells. Neurotoxicology 10: 191–199, 1989 [PubMed] [Google Scholar]
- 47. Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Ca2+ currents in human colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 269: G378–G385, 1995 [DOI] [PubMed] [Google Scholar]
- 48. Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Fast Na+ current in circular smooth muscle cells of the large intestine. Pflügers Arch 423: 485–491, 1993 [DOI] [PubMed] [Google Scholar]
- 49. Yatsuhashi T, Hisatome I, Kurata Y, Sasaki N, Ogura K, Kato M, Kinugasa R, Matsubara K, Yamawaki M, Yamamoto Y, Tanaka Y, Ogino K, Igawa O, Makita N, Shigemasa C. L-cysteine prevents oxidation-induced block of the cardiac Na+ channel via interaction with heart-specific cysteinyl residues in the P-loop region. Circ J 66: 846–850, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Yu SS, Yu K, Gu Y, Ruan DY. Taurine-induced modulation of voltage-sensitive Na+ channels in rat dorsal root ganglion neurons. Brain Res Bull 66: 259–267, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Zhang Z, Huang H, Liu P, Tang C, Wang J. Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening K ATP channels. Can J Physiol Pharmacol 85: 1248–1253, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008–6016, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]