Abstract
Telomerase mutations and significantly shortened chromosomal telomeres have recently been implicated in human lung pathologies. Natural telomere shortening is an inevitable consequence of aging, which is also a risk factor for development of lung disease. However, the impact of shortened telomeres and telomerase dysfunction on the ability of lung cells to respond to significant challenge is still largely unknown. We have previously shown that lungs of late generation, telomerase null B6.Cg-Terctm1Rdp mice feature alveolar simplification and chronic stress signaling at baseline, a phenocopy of aged lung. To determine the role telomerase plays when the lung is challenged, B6.Cg-Terctm1Rdp mice carrying shortened telomeres and wild-type controls were subjected to partial pneumonectomy. We found that telomerase activity was strongly induced in alveolar epithelial type 2 cells (AEC2) of the remaining lung immediately following surgery. Eighty-six percent of wild-type animals survived the procedure and exhibited a burst of early compensatory growth marked by upregulation of proliferation, stress response, and DNA repair pathways in AEC2. In B6.Cg-Terctm1Rdp mice carrying shortened telomeres, response to pneumonectomy was characterized by decreased survival, diminished compensatory lung growth, attenuated distal lung progenitor cell response, persistent DNA damage, and cell growth arrest. Overall, survival correlated strongly with telomere length. We conclude that functional telomerase and properly maintained telomeres play key roles in both long-term survival and the early phase of compensatory lung growth following partial pneumonectomy.
Keywords: lung, alveolar epithelial type 2 cells, bronchoalveolar epithelial stem cells, proliferation
alveolar epithelial type 2 cells (AEC2) are thought to maintain lung alveolar epithelium by producing surfactant and by serving as the progenitor for alveolar epithelial type 1 cells, which mediate lung gas exchange (1, 17). Putative epithelial progenitor populations have been noted in other compartments of the lung, and although each population expresses specific markers, the main method of identification is by response to injury (30, 48, 54). In distal lung, AEC2 and surfactant protein-C (SP-C)/Clara cell secretory protein (CCSP) double-positive bronchoalveolar epithelial stem cells (BASC) share the capacity to survive damage and proliferate and/or increase in number in response to regeneration signals (25, 44, 48, 49).
Our laboratory and others have demonstrated AEC2 participation in recovery from hyperoxic injury (11, 14, 45). Upregulation of proliferative and repair pathways, as well as activity of the telomere-maintaining shelterin complex and telomerase, the enzyme that catalyzes telomere repair, characterize injury-responsive AEC2 (16, 33, 49). Telomerase contributes to cell integrity by preventing excess telomere degradation following cellular division and blocking and/or facilitating repair of DNA lesions. Telomerase is active in proliferation-competent progenitors within a variety of organs, including lung, and correlates with self-renewal capacity (16, 20, 24, 33, 39, 59). In humans, mutations of the shelterin complex, including telomerase, have been found to underlie dyskeratosis congenita (DKC) and certain hematological disorders (3, 13, 59). In lung, telomerase mutations have been linked to idiopathic pulmonary fibrosis of the lung (IPF) (2, 4, 22, 57), a condition hypothesized to be initiated in some cases by epithelial hypoplasia (35, 53). We recently showed that the number and integrity of AEC2 is reduced in mice lacking functional telomerase (35). In this experimental model, significant defects are not noted in early generations. However, sequential inbreeding results in progressively shorter telomeres and significant deficiencies in the progenitor populations in many organs, including lung (7, 35).
Due to evidence of a role for telomerase in preservation and repair of distal lung epithelium, we chose partial pneumonectomy (PNX), the surgical resection of the left lung in mice as described by Sukurai et al. (55), as a model to study compensatory response to injury in the presence and absence of functional telomerase. Following PNX, remaining lung tissue exhibits “recruitment of alveolar capillary reserves, remodeling of existing tissue, and regenerative growth of acinar tissue” (27), resulting in increased numbers of AEC2 (26, 47). In wild-type (WT) mice, the early burst of growth over postoperative days (POD) 1 to 7, which includes upregulation of lung adaptive and developmental factors (21, 30, 56, 62), is followed by restoration of lung mass by POD10 (9, 19, 46) and restoration of histological and functional features by POD21 (58). In our experiments, WT C57Bl/6J and telomerase null (terc−/−) B6.Cg-Terctm1Rdp mice were subjected to PNX. We observed that telomerase activity in WT AEC2 peaked at POD3 and that survival to POD7 strongly correlated with both telomere length and baseline numbers of progenitors prior to surgery. AEC2 and BASC, which robustly respond to PNX in WT mice, fail to do so in terc−/− cells because of the initiation of growth arrest during the initial phase of recovery.
MATERIALS AND METHODS
Animals.
Breeding pairs of C57BL/6J WT mice and homozygous telomerase null B6.Cg-Terctm1Rdp (terc−/−) mice, purchased from Jackson Laboratories (Bar Harbor, ME), were the founders for WT and terc−/− second (F2), third (F3), and fourth (F4) generation cohorts. For all experiments, randomized numbers of male and female mice were used at 10 ± 2 wk. Animals were handled according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the Saban Research Institute/Childrens Hospital Los Angeles (CHLA) and were maintained under standard conditions in the pathogen-free facility at CHLA.
Pneumonectomy procedure.
Surgery was performed by aseptic technique as outlined by the CHLA IACUC standard operating procedure for survival surgery, which is based on the National Research Council's Guide. The approach we used was a modification of Sukurai et al.'s method (55), presented in a recent publication (29). Briefly, prior to the PNX surgery, animals were mechanically ventilated via tracheotomy by use of a volume-controlled, small animal mechanical ventilator (Harvard Model 687, Holliston, MA). A thoracotomy (THX) was performed to access the left lung. The left lung was elevated into the THX and a single 5-mm neuroclip (Fine Science Tools, Foster City, CA) was applied at the hilum by using a curved hemostat. The left lung was then resected and the ribs, soft tissue, and overlying skin were closed in two layers, by use of a running 5-0 monofilament suture. Experimental control mice underwent sham operations of only tracheotomy and THX, with identical preoperative and postoperative care as described. Postsurgery, mice exhibiting signs of distress were compassionately terminated.
Telomerase activity assay.
Quantitative analysis of telomerase activity in isolated AEC2 was performed by using the Telo TAGGGG Telomerase PCR ELISAPLUS system from Roche (Indianapolis, IN) according to manufacturer's instructions. For each n, samples were run in duplicate along with positive and negative telomerase controls, heat-treated duplicates of each sample, and controls for the PCR step.
Lung weight/body weight measurement.
Following THX or PNX, animals were euthanized by use of Nembutal at 200 mg/kg ip and weighed. Lung tissue was harvested according to our standard procedure (34), with the exception that perfusion to complete exsanguination utilized only 3 ml of sterile, normal saline. Right lung was sharply separated from the bronchus, heart, thymic tissue, trachea, surgical clip if present, and any other adherent clot or fibrinous exudate. Isolated right lung tissue was weighed on a calibrated scale and the ratio of lung weight to body weight (lung weight/body weight) was calculated.
Immunohistochemistry.
Lungs were fixed and paraffin embedded according to protocols previously described (34). SP-C expression in situ was determined by using a specific rabbit antibody from Seven Hills Bioreagents (Cincinnati, OH). CC10 and Ki-67 were detected by using a goat polyclonal antibody and a mouse monoclonal antibody, respectively, from Santa Cruz Biotechnologies (Santa Cruz, CA). Cy-3- and FITC-labeled secondary antibodies were from Sigma, as were purified rabbit and goat IgGs used as nonspecific primary antibody controls. Expression of 8-oxoguanine (8-OHdG) was detected using the OxyDNA Assay kit from Calbiochem (EMD Bioscience, La Jolla, CA), also according to manufacturer's instructions. After immunohistochemical processing and washing, sections were counterstained and mounted with Vecta-Shield mounting medium containing 4,6-diamidino-2-phenylindole (DAPI) from Vector Laboratories (Burlingame, CA). Sections were viewed via a Leica DM IV fluorescent microscope and images acquired by use of OpenLab software (Improvision/Perkin Elmer, Waltham, MA). Cell counts used to generate AEC2 percentages were performed on micrographs by using the Cell Counter (for SP-C-positive cells) and Automatic Nuclei Counter (ITCN) plug-ins for ImageJ software (http://rsb.info.nih.gov/ij/).
AEC2 isolation and Western blotting.
AEC2 isolation and Western blotting followed our standard protocols (34). C-PARP, P-SAPK/JNK, P-ERK1/2, and p21 antibodies were from Cell Signaling. HSP-27 antibody was from Stressgen (Enzo Life Sciences, Plymouth Meeting, PA). Anti-PCNA, anti-early growth response protein-1 (Egr-1), and anti-actin antibodies were purchased from Santa Cruz Biotechnologies. Horseradish peroxidase-labeled secondary antibodies were from Sigma.
Statistics.
One-way analysis of data for which more than two samples were compared was performed by the nonparametric Kruskal-Wallis test or Spearman nonparametric rank correlation. Within these groups, pairwise tests were done with nonparametric rank tests. Where only two sets of data were compared, Student's t-test was used. Data were deemed significant at P < 0.05.
RESULTS
Telomerase activity is upregulated in WT AEC2 following PNX.
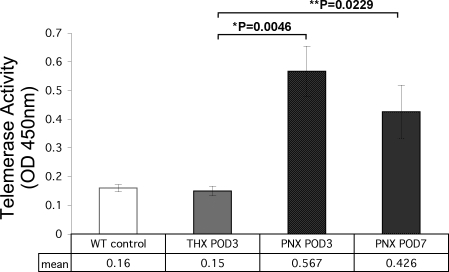
Using our standard protocols, we isolated AEC2 from the right lung tissue of WT mice at PNX POD3 and 7, as well as from sham-operated (THX) animals at POD3. This protocol produces a 90–95% pure AEC2 population by staining for SP-C expression (34). Cell extracts were analyzed for the presence of telomerase activity via an ELISA-based detection system. We have previously shown that telomerase activity in WT AEC2 is low at baseline but can be induced by hyperoxic lung injury. In the case of PNX, telomerase activity peaked at 3 days post-PNX to a level significantly above those observed in AEC2 isolated from nonoperated baseline control lung (Fig. 1). AEC2 isolated from sham-operated WT mice at this time point showed essentially baseline levels of activity. Telomerase activity in AEC2 from partially pneumonectomized and recovering WT mice moderately decreased over time but was still significantly above baseline levels by POD7. These data show that, in the right lung remaining following removal of the left lung, a 3.5-fold increase in telomerase activity occurs during the compensatory growth phase. Because terc−/− mice are null for telomerase, AEC2 from these animals, across all generations and at all sham and postoperative time points, exhibited no telomerase activity (not shown).
Fig. 1.
Telomerase activity in wild-type (WT) alveolar epithelial type 2 cells (AEC2) at 3 and 7 days following partial pneumonectomy (PNX) or sham thoracotomy (THX). WT mice were subjected to PNX or THX as described. Right lungs were harvested on days 3 and 7 of recovery and AEC2 were isolated. Telomerase activity was assayed in fresh isolates. For both PNX postoperative day (POD)3 and POD7 time points, telomerase activity in isolated AEC2 was significantly higher than the level present in AEC2 isolated from sham-operated lung (*P = 0.0046 and **P = 0.0229 by Student's t-test; n = 3 for all groups).
PNX survival is compromised by telomerase knockdown and correlates strongly and proportionately with telomere length.
Following partial pneumonectomy on WT, terc−/−F2, terc−/−F3, and terc−/−F4 mice age 10 ± 2 wk, animals were monitored for adverse reactions to surgery, up to and including natural deaths. Survival at POD7 was recorded as the number of animals operated on that survived and appeared fit by day 7 following surgery, as shown in Table 1. One hundred percent of WT, terc−/−F2, and terc−/−F3 sham-operated mice monitored through POD7 survived. Although 12 of 14 WT mice that underwent PNX survived through day 7, lower long-term survival rates (7/10) were observed for terc−/−F2 mice, which we have previously shown carry telomeres approximately one-half the length of WT (35). Even lower rates of survival (10/17) were encountered for terc−/−F3 mice, carrying telomeres one-quarter the length of WT. We found that the next generation of telomerase null mice, terc−/−F4, which carry severely shortened telomeres and, as previously reported, exhibit a notable baseline lung pathology of alveolar simplification and chronic stress signaling, were incapable of tolerating the surgery. All terc−/−F4 mice died intraoperatively or by POD1 (POD7 survival rate 0/5). Of note, whereas all WT, terc−/−F2, and terc−/−F3 sham-operated mice survived, none of the sham-operated terc−/−F4 mice survived, indicating a severe intolerance to anesthesia, ventilation, and/or even minimal surgery. For this reason, our ongoing studies focused only on WT, terc−/−F2, and terc−/−F3 cohorts. We also limited the study to the first week following surgery, i.e., the time frame when telomerase activity in WT survivors peaks. Our observations suggested a relationship between terc−/− generation survival to POD7 and telomere length. For statistical analysis, POD7 survival numbers for WT, terc−/−F2, and terc−/−F3 were converted to percentage values (animals surviving to POD7/total number of animals undergoing PNX). For WT mice, the rate of survival was 85.71%. For terc−/−F2, POD7 survival was 70.00%, whereas for terc−/−F3 it was 58.82%. Mean telomere lengths for these cohorts, as determined by our previous study, are 41,788 ± 4,897 bp (mean ± SE) for WT, 22,211 ± 4,769 bp for terc−/−F2, and 14,920 ± 4,499 bp for terc−/−F3 (34). When these previously obtained mean telomere lengths were correlated to POD 7 survival expressed as a percentage, the relationship was found to be very strong, with an R2 value of 0.9743. These data indicate that telomere shortening has a significant impact on the ability of the whole animal to survive PNX. Telomerase is dysfunctional in all three terc−/− cohorts undergoing PNX. Thus this genetic deletion may also have influenced long-term survival. However, given that telomerase was absent in all terc−/− generations examined, it appears that telomere length has a significant impact on survival.
Table 1.
Postsurgery survival to POD 7
| WT | terc−/−F2 | terc−/−F3 | terc−/−F4 | |
|---|---|---|---|---|
| THX | 7/7 (100%) | 4/4 (100%) | 4/4 (100%) | 0/2 (0%) |
| PNX | 12/14 (85.71%) | 7/10 (70.00%) | 10/17 (58.82) | 0/5 (0%) |
Survival to postoperative day 7 (POD7) by partially pneumonectomized (PNX) or sham-operated (THX) wild-type (WT) and terc−/− 2nd, 3rd, and 4th generation (F2, F3, and F4, respectively) mice.
The compensatory increase in lung mass in post-PNX terc−/− lung is significantly diminished compared with the response observed in WT lung.
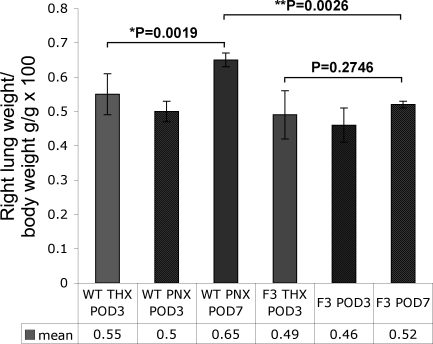
To determine the gross differences in the recovery of lung mass post-PNX, body weights and wet lung weights of the right lung tissue from THX control mice at POD3 (THX POD3), as well as equivalent measurements taken from PNX animals at POD3 and 7 (PNX POD3 and PNX POD7) were analyzed. Though mean body weight for mature telomerase null mice is slightly smaller than that of age-matched WT animals, the ratio of lung weight/body weight prior to surgery did not differ significantly between the two strains, indicating no specific impact on lung development at a gross level because of telomerase knockdown (not shown). However, following surgery, the early compensatory response of the remaining lung tissue that initiates the process of restoring lung mass differed significantly when WT and terc−/−F3 cohorts were compared (Fig. 2). At POD3 there was no significant difference in the ratio of right lung weight/body weight in post-PNX mice compared with post-THX mice in either cohort. In addition, the lung to body weight ratio of WT PNX POD3 samples did not differ significantly from samples harvested from terc−/−F3 mice at the same, early time point. However, at POD 7, notable differences between WT and terc−/−F3 samples were observed. In WT animals, right lung weight increased such that the lung weight/body weight ratio was significantly greater than the ratio observed at POD3 (P = 0.0019), indicating a notable increase in right lung mass. In contrast, no significant change in the lung weight/body weight ratio was observed in PNX POD7 terc−/−F3 samples compared with PNX POD3 samples (P = 0.2746). In addition, the lung weight/body weight ratios in WT animals at POD7 was significantly greater than the ratios calculated for terc−/−F3 POD7 mice (P = 0.0026). (For all cohorts, n = 3–5.) These data indicate that a significant increase in lung mass at this early time point, which in WT mice is notably just past the peak of telomerase activity initiated by PNX, does not occur in terc−/−F3 mice.
Fig. 2.
Lung weight-to-body weight ratios (lung weight/body weight) at 3 and 7 days post-PNX or THX. The ratio of right lung weight to whole body weight (lung weight/body weight) was calculated for pneumonectomized or sham-operated mice at POD3 (THX and PNX groups) or POD7 (PNX only), and the resulting values were each multiplied by 100. By Student's t-test, the difference in lung weight/body weight for WT mice at PNX POD7 was significant compared with the ratio for THX samples (*P = 0.0019) and also significant compared with the ratio for terc−/− 3rd generation (F3) PNX at the same time point (**P = 0.0026). No significant difference was noted when ratios for terc−/−F3 THX and PNX POD7 were compared (P = 0.2746; n = 3–8 for all groups).
Telomerase null mouse lung exhibits diminished numbers of progenitor AEC2 and BASC at baseline.
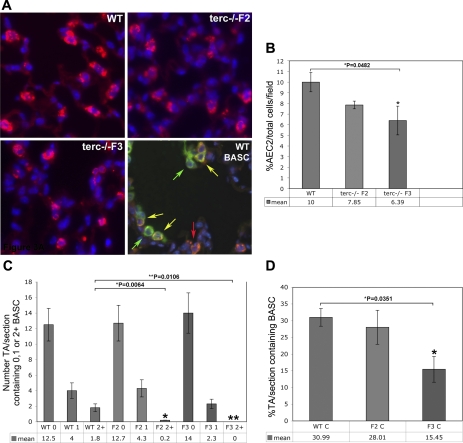
Our previous studies showed that functional telomerase activity and adequate telomere length are essential for the maintenance of AEC2 integrity at baseline. We also reported diminished numbers of AEC2 in terc−/−F4 lung under normal conditions (35). Given the observation that F4 mice failed to survive PNX surgery and that although F2 and F3 animals survived, albeit at a lower rate than WT, we wished to determine whether one underlying factor was the baseline number of key progenitor cell populations in these cohorts. In the present study, we examined the baseline status of AEC2 in terc−/−F2 and F3 lung, as well as the number of BASC within terminal airways (TA). Examples of lung tissue from WT, terc−/−F2, and terc−/−F3 mice are presented in Fig. 3A, where immunohistochemistry using an antibody to AEC2 marker surfactant protein C (SP-C) stains AEC2 cytoplasm red. In these experiments, sections were counterstained with DAPI to visualize nuclei and allow assessment of the total number of cells present in each microscopic field by using automated cell and nuclei counting software from ImageJ. The bottom right of this same figure shows immunohistochemical detection of BASC in WT lung, which double stain positive for SP-C and the airway Clara cell marker CC10. In this micrograph, CC10-positive cells stain green and the double-positive BASC are yellow.
Fig. 3.
WT and terc−/− AEC2 and bronchoalveolar epithelial stem cells (BASC) in situ at baseline. Sections from nonoperated mouse lung were fixed and subjected to immunohistochemistry using rabbit and goat primary antibodies, a Cy3-labeled anti-rabbit IgG secondary antibody, and a FITC-labeled anti-goat IgG secondary antibody. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). To control for nonspecific antibody staining, purified rabbit or goat IgG, at the same concentrations as specific primary antibodies, were used to probe adjacent sections (not shown). A: representative sections used for AEC2 and BASC quantitation in situ. Expression of AEC2 marker surfactant protein-C (SP-C; red) was analyzed for each sample. Total cells per field were detected by nuclear DAPI staining (blue). Top and bottom left: AEC2 distribution in WT, terc−/−F2, and terc−/−F3 lung. Bottom right: a representative WT section probed by using an additional antibody for Clara cell marker CC10 (green) visualized with FITC-labeled secondary antibody. Overlay of images of SP-C and CC10 expression patterns reveals the presence of SP-C/CC10 double-positive BASC (yellow). Section presented shows a bronchoalveolar duct junction located in a transitional airway of distal lung tissue. B: baseline percentage of AEC2 per total cells per field. SP-C-labeled sections from nonoperated WT, terc−/−F2, and terc−/−F3 lungs were observed microscopically and the number of total cells as represented by DAPI staining, as well as the number of SP-C-positive cells, were counted within the same microscopic field at ×20. For each sample, n = 6–12 (fields chosen from slides containing sections from 2–3 animals with 3–6 sections/slide). The difference in AEC2 percentage for terc−/−F2 was not significant compared with WT (P = 0.1471). The difference between WT and terc−/−F3 AEC2 percentage was significant (*P = 0.0482). C: baseline distribution of BASC per transitional airway (TA). SP-C/CC10 double-labeled sections from nonoperated WT, terc−/−F2, and terc−/−F3 lungs were observed microscopically and the number of BASC contained in every transitional airway in each section were recorded. For each sample, n = 4–8, representing complete sections from 2 animals analyzed per group. Values were grouped so that each cohort could be compared for BASC density per TA and the mean percentage of TAs per section containing 0, 1, or 2+ BASC (0, 1, or 2+, respectively) was calculated. No significant difference was noted in the percentage of TA/section that contained 0 or 1 BASC when WT, terc−/−F2, and terc−/−F3 samples were compared. Both terc−/−F2 and terc−/−F3 sections exhibited a lower percentage of TAs that contained 2 or more BASC compared with WT (*P = 0.0064 for terc−/−F2 vs. WT and **P = 0.0106 for terc−/−F3 vs. WT). D: baseline percentage TA/section that contain BASC. To consolidate distribution data, values from the analyses shown in C were grouped to determine the mean percentage of TAs per section that contained 1 or more BASC. The mean percentage of TAs per section containing BASC in terc−/−F2 samples was not significantly different from the percentage found in WT lung. The percentage of BASC-containing TAs per section in terc−/−F3 lung was significantly lower than that of WT (*P = 0.0351). C, control.
We assumed that prior to the initiation of injury the presence of a healthy population of progenitor cells fully capable of an adequate response to insult is a basic requirement for survival, both during and following PNX. The quantitation of the data acquired from these micrographs, shown in Fig. 3B, showed that the number of AEC2, detected as a percentage of SP-C-positive cells per total cells per field, decreased in proportion to shortening telomere lengths [values from our previous study (35)]. For terc−/−F3 lung, the decrease significant (terc−/−F3: mean 6.39% AEC2·total cells−1·field−1 vs. WT: mean 10.00% AEC2·total cells−1·field−1; P = 0.0482). Using previously acquired telomere measurements we found a very strong correlation between mean telomere length and the mean percentage of AEC2 per total cells per field (R2 = 0.9625). In addition, the number of AEC2 present in lung prior to surgery strongly correlated with a decreased ability of terc−/−F2 and terc−/−F3 cohorts to survive PNX out to POD7 (R2 = 0.9505).
To determine whether telomere shortening and telomerase dysfunction had an impact on the numbers of BASC at baseline, lung sections from WT, terc−/−F2, and terc−/−F3 were double stained for SP-C and CC10 expression and multiple sections were analyzed for the presence of double staining BASC at the bronchoalveolar duct junctions, the transitional airway-to-alveolus portion of terminal airways. Using the method devised by Kim et al. (30) and Nolen-Walston et al. (44), we scored each terminal airway per section for the number of BASC present. These data are presented in Fig. 3C. Although the majority of TAs at baseline for all three cohorts contained no visible BASC, differences were observed for those rare TAs that contained two or more of these progenitor cells. These differences were significant, since only 0.2 TAs/section in terc−/−F2 lung contained multiple BASC, vs. 1.8 TAs/section for WT (P = 0.0064). The difference was also significant when TAs in terc−/−F3 lung were examined, where no TAs containing multiple BASC were observed (P = 0.0106). When the percentage of TAs in each section that contained 1, 2, or more BASC at baseline was calculated, differences between WT and terc−/−F3 lung were also apparent (Fig. 3D). In this case, only 15.45% of TAs per section in terc−/−F3 lung contained BASC, whereas that percentage was 30.99% in WT lung. This difference was significant (P = 0.0351). In contrast, no significant difference in BASC-positive TA percentage was observed when WT and terc−/−F2 lung sections were compared. By using this measurement for the presence of BASC at baseline, a moderate correlation between the percentage of BASC-positive TAs and telomere length was found (R2 = 0.6811). However, a stronger correlation was found between the percentage of BASC-positive TAs in lung prior to surgery and long-term survival of PNX to POD7. In this case, the higher baseline percentage of BASC-positive TAs in WT lung correlated positively with better rates of survival, whereas the significantly lower baseline levels of BASC-positive TAs in terc−/−F3 correlated with the much poorer outcome of this cohort following PNX (R2 = 0.8654). Decreased baseline levels of AEC2 and BASC, particularly in terc−/−F3 lung, indicated a compromised ability of telomerase null cells carrying the most severely shortened telomeres to respond to even minor requirements of normal lung maintenance. These data therefore led to the hypothesis that challenge of terc−/−F3 mice with partial pneumonectomy would result in an attenuated response by progenitor AEC2 and BASC populations.
The robust increases in AEC2 and BASC during the early compensatory growth phase following PNX in WT lung are not observed in terc−/−F3 lung.
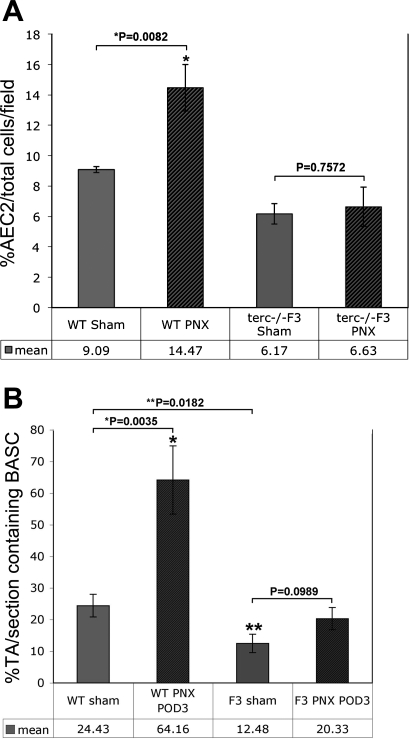
The compensatory growth that characterizes the lung following partial pneumonectomy is because of a complex, coordinated response that includes cellular proliferation, angiogenesis, and alveolar hypertrophy. We wished to determine the specific response of distal lung progenitor AEC2 and BASC populations to this challenge. Experimental subjects were sham operated and partially pneumonectomized WT and terc−/−F3 animals, from which lungs were harvested at POD3. This early time point was chosen to represent the most likely timing of the burst of compensatory growth activity by progenitor populations. To analyze the response of AEC2, the percentage of SP-C-positive cells per total numbers of lung cells was counted in multiple microscopic fields (n = 6–9; 2 fields per 4–6 sections prepared from at least 2 individual animals). Data in Fig. 4A show that WT AEC2 increase significantly in number in POD3 lung remaining following PNX compared with numbers observed in lung from sham-operated WT animals (P = 0.0082). In contrast, the AEC2 response in terc−/−F3 lung post-PNX was negligible and the difference compared with sham samples was not statistically significant (P = 0.7572). We also noted a slight, though not significant, overall decrease in AEC2 percentage in sham-operated samples from both cohorts compared with control samples, as presented in Fig. 3B, and postulated that barotrauma from ventilation may have had some impact on AEC2 viability.
Fig. 4.
AEC2 and BASC in situ post-PNX. A: AEC2 per total cells per field at THX and PNX POD3. SP-C-labeled sections from postsurgery POD3 PNX and THX WT and terc−/−F3 lungs were observed microscopically and the number of total cells as represented by DAPI staining as well as the number of SP-C-positive cells were counted within the same microscopic field at ×20. For each sample, n = 6–10 (fields chosen from slides containing sections from 2 animals with 3–5 sections/slide). The mean percentage of AEC2/total cells in PNX POD3 WT lung was significantly higher than the percentage in THX WT controls (*P = 0.0082). The difference in AEC2 percentage in PNX vs. THX terc−/−F3 lung was not significant (P = 0.7572). B: percentage TA/section containing BASC at THX and PNX POD3. The mean percentage of TAs per section that contained 1 or more BASC was calculated for postsurgery POD3 PNX and THX WT and terc−/−F3 samples. For each sample, n = 8–12 (complete sections from 2 animals with 4–6 sections/slide). The difference in the mean percentage of TAs containing BASC per section in PNX vs. THX POD3 WT lung was highly significant (*P = 0.0035). The percentage of TAs containing BASC in POD3 sham-operated WT lung was significantly greater than the percentage in terc−/−F3 THX POD3 lung (**P = 0.0182). The percentages of TAs per section containing BASC when PNX vs. THX POD3 terc−/−F3 sections were compared and the difference by Student's t-test was not significant (P = 0.0989).
To determine whether the same attenuated response to PNX by terc−/−F3 lung occurred in the BASC population, the number of transitional airways containing one, two, or more BASC per section were counted (n = 8–12 sections prepared from 2–3 individual animals). The resulting data, shown in Fig. 4B, show a significant elevation in the number of WT TAs that contain BASC at POD3 following surgery. In right lungs of sham-operated mice, just under a quarter of TAs contain BASC, whereas in lung tissue remaining following PNX, well over half of TAs contain one or more BASC (24.43 vs. 64.16%). The increase in the number of TAs harboring this progenitor population in WT lung is highly significant (P = 0.0035). The number of TAs that contain BASC in POD3 PNX lung also increases in terc−/−F3 mice. However, the overall increase in the number of these cells following PNX must be considered modest, since the levels in sham-operated terc−/−F3 show that this lung is already at a deficit even in controls, since right lungs from mice that undergo THX alone exhibit essentially half the number of BASC containing TAs vs. what is observed in WT lung following a sham operation (12.48 vs. 24.43%; P = 0.0182). A rise in the number of terc−/−F3 TAs that contain BASC following PNX is detectable, but not quite significant, with an increase to 20.33% (P = 0.0989). Although there is some response on the part of BASC in terc−/−F3 lung remaining following PNX, the response at the critical, early POD3 time point never approaches the level of activation seen in WT lungs, which are mounting a robust compensatory growth response at this time.
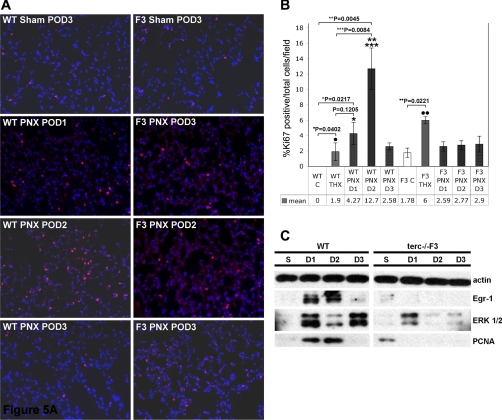
The compensatory growth response in WT lung and AEC2 is characterized by elevation of proliferation and repair markers not observed in terc−/− lung cells.
To determine the mechanism underlying the poor terc−/−F3 progenitor cell compensatory growth response, the expression of markers for proliferation and repair were analyzed in lung tissue by immunohistochemistry and in isolated AEC2 by Western blotting. These data showed a burst of Ki-67 expression in WT lung at POD1 and 2, which precedes the rise in AEC2 and BASC numbers at POD3 previously described. Ki-67-positive cells exhibit red nuclear staining in the photomicrographs presented in Fig. 5A. The elevated expression of Ki-67, which participates in both proliferation and repair pathways, resolves by POD3, indicating an early, rapid, but transitory response to PNX stimulation (Fig. 5A, left). In contrast, expression of Ki-67 in terc−/−F3 lung is slightly elevated even at baseline, but not significantly so, whereas only a modest increase over control was observed during the critical POD1 and POD2 time points (Fig. 5A, right). These immunohistochemical data were quantified by counting cells exhibiting Ki-67 nuclear staining and calculating the percentage positive per total number of cells, as determined by DAPI staining, per microscopic field (n = 6, 3 fields per 2 sections) (Fig. 5B). The increase in expression over baseline at POD1 and POD2 was significant in WT samples (P = 0.0217 and P = 0.0045, respectively). We also noted increased Ki-67 expression over respective baselines in sham-operated WT and terc−/−F3 lung (P = 0.0402 and P = 0.0221). We speculate this may be a transient response to barotrauma during mechanical ventilation at the time of surgery. Compared with the increased Ki-67 expression noted at POD3 post-THX, the increase in Ki-67 in WT samples was significant at POD2 (P = 0.0084), but not POD1 (P = 0.1205). No significant change in the level of Ki-67 expression over baseline was noted post-PNX in any terc−/−F3 samples (P = 0.3057 at POD1 and P = 0.206 at POD2), indicating these cells do not respond to PNX by inducing at least one of the proliferation/repair pathways that is activated in WT. In addition, the smaller number of Ki-67-positive cells in post-PNX terc−/−F3 samples, which presumably were exposed to the same level of barotrauma as terc−/−F3 sham-operated animals, indicated an additional trauma imposed by the response to partial pneumonectomy.
Fig. 5.
Proliferation marker expression in whole lung and AEC2 WT and terc−/−F3 post-PNX. A: Ki-67 expression in whole lung. Representative sections from sham-operated samples at POD3 and postpneumonectomy samples harvested at POD1, POD2, and POD3 (D1, D2, and D3, respectively) were fixed and subjected to immunohistochemistry using Ki-67 primary antibody and Cy-3 secondary antibody (red). Nuclei were stained with DAPI. Nonoperated control samples were also prepared and analyzed for Ki-67 expression (not shown). Negative controls for Ki-67 antibody binding were performed on adjacent sections using mouse IgG in place of specific primary antibody (not shown). B: percentage of Ki-67-positive cells per total cells per field in WT and terc−/−F3 lung post-PNX. For each group, n = 6 (fields chosen from slides containing sections from 2 animals with 3 sections/slide). Ki-67-positive cells per total number of cells per microscopic field were quantitated by calculating the percentage of marker positive (red) nuclei per total nuclei as stained by DAPI in each ×20 field analyzed. The percentage of Ki-67-positive cells in WT POD2 lung was significantly higher than the percentages in WT nonoperated control (**P = 0.0045) and WT THX POD3 control (***P = 0.0084). WT POD1 and POD3 samples exhibited significantly more Ki-67-positive cells than nonoperated control (*P = 0.0217 for PNX POD1), but not sham-operated control (P = 0.1205 for PNX POD1). Both WT and terc−/−F3 THX control samples showed elevated Ki-67 expression compared with nonoperated controls and in both cohorts and these differences were significant by Student's t-test (·P = 0.0217; ··P = 0.0221). No significant change in the percentage of Ki-67-positive cells was observed in post-PNX terc−/−F3 lung samples compared with terc−/−F3 nonoperated control at any post-PNX time point. All post-PNX terc−/−F3 samples exhibited significantly fewer Ki-67-positive cells than sham-operated control samples. C: expression of proliferative markers early growth response protein-1 (Egr-1), MAP kinase ERK1/2, and PCNA in AEC2 harvested post-PNX from WT and terc−/−F3 mice. AEC2 were isolated from right lungs harvested from sham-operated mice at POD3 (S) and from pneumonectomized mice at POD1, POD2, and POD3. Cell lysates were probed for expression of Egr-1, PCNA, and MAP kinase ERK1/2, with expression of actin used as a loading control. Blot shown is representative of multiple blots (3–5) using samples from 3–4 different animals/time point.
To focus more specifically on proliferation, the levels of expression of Egr-1, activated, phosphorylated proliferative response MAP kinase ERK1/2, and proliferating cell nuclear antigen (PCNA) were assessed in AEC2 isolated from sham-operated and PNX postoperative lung at POD1 through POD3 (Fig. 5C). We noted that, as in the Ki-67 assay, terc−/−F3 AEC2 exhibit modest but measurable levels of proliferation marker expression at baseline. We hypothesize that constant, low level attrition of vulnerable AEC2 and other lung cells because of telomere shortening (reflected by the lower baseline numbers of AEC2 and BASC) requires moderate upregulation of proliferation rates and that this compensation is not required in quiescent, homeostatic normal lung. However, when severely challenged by partial pneumonectomy, the proliferative response of terc−/−F3 AEC2 differs starkly from that of WT. Western blotting data show a rapid induction of early response markers in WT AEC2, including upregulation of strong Egr-1 expression at POD1 and POD2 and activation of ERK1/2, as signified by the appearance of its activated, phosphorylated form in samples from POD1–3. In contrast, Egr-1 is not upregulated in terc−/−F3 AEC2 at any time point, and phosphorylation of ERK1/2 occurs modestly and transiently, at POD1. In this same cell population, PCNA appears to be mildly induced by ventilation in terc−/−F3 samples, but not in WT. Paralleling Egr-1 expression, PCNA is elevated in WT AEC2 at PNX POD1 and POD2. This induction tails off by POD3. In contrast, AEC2 in post-PNX terc−/−F3 lung fail to upregulate PCNA, further indicating a nonproliferative response by these cells. Taken together, these data show that one possible underlying cause of the poor outcome in terc−/− animals following PNX is a “compensatory growth” response that is significantly attenuated. In contrast to what is observed in WT progenitors, terc−/−F3 AEC2 fail to upregulate proliferative pathways following PNX, raising the possibility that these cells are growth arrested. Alternatively, given the lower numbers of Ki-67-positive cells in terc−/−F3 PNX samples compared with the numbers observed in post-THX tissue (Fig. 5, A and B), we speculated that PNX in the context of telomerase downregulation and telomere shortening might also trigger an apoptotic response.
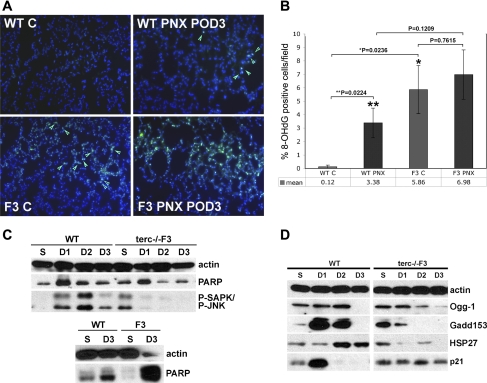
The inefficient post-PNX proliferation response of terc−/− lung and AEC2 is accompanied by enhancement of baseline DNA damage.
To determine whether alterations in other pathways occur in addition to the atypical post-PNX proliferation response of terc−/− lung and progenitor populations, which might also contribute to the poor survival rate of these cohorts, we examined markers for DNA damage and apoptosis in whole lung, as well as AEC2 isolated from WT and terc−/−F3 animals. We previously reported that lung tissue from terc−/−F4 mice contains low, but significant, levels of 8-OHdG, an oxidative adduct of guanine that signals the presence of DNA damage. A similar low level of 8-OHdG was observed in control terc−/−F3 lung, which appears as nuclear and/or mitochondrial green fluorescent staining (green arrows) in the example shown in Fig. 6A. (Note that some fluorescence in this sample is due to red blood cell autofluorescence, which can be distinguished from nuclear and mitochondrial lesions by shape.) As previously reported, this figure also shows that control WT tissue rarely exhibited any 8-OHdG-positive cells. The number of 8-OHdG-positive cells in WT lung increased following PNX at POD3, though an even greater abundance of this damage marker was observed in terc−/−F3 PNX lung at the same time point. These data were quantified by counting the number of 8-OHdG-positive cells per total number of cells per microscopic field (n = 12 for each sample; 3 microscopic fields counted per 2 sections from 2 different animals) (Fig. 6B). As expected, the percentage of 8-OHdG-positive cells in terc−/−F3 lung at baseline was significantly greater than the percentage in WT control (P = 0.0236). The mean percentage of lung cells exhibiting DNA damage in terc−/−F3 lung at PNX POD3 is double that observed in WT lung, though because of variations in the number of positive cells across each section in both cohorts, these values were not statistically different (P = 0.1209). Although the increase in the percentage of damaged cells in WT PNX POD3 samples compared with baseline was significant (P = 0.0224), the increase in terc−/−F3 PNX POD3 samples was not significantly greater than the elevated basal level of damaged cells in terc−/−F3 control (P = 0.7615).
Fig. 6.
DNA damage/repair and cell cycle marker expression in AEC2 and whole lung post-PNX. A: representative sections used for 8-oxoguanine (8-OHdG) quantitation in situ. Representative sections from nonoperated and post-PNX POD3 lungs were fixed and subjected to immunohistochemistry using 8-OHdG primary antibody and FITC-labeled secondary antibody (green). Nuclei were stained with DAPI (blue). Negative controls for 8-OHdG antibody binding were performed on adjacent sections using rabbit IgG in place of specific primary antibody (not shown). B: percentage of 8-OHdG-positive cells per total cells per field post-PNX. Multiple tissue samples from nonoperated WT or terc−/−F3 right lung or corresponding samples from tissue remaining post-PNX at POD3 were fixed and probed for the presence of 8-OHdG adducts. For each group, n = 6 (fields chosen from slides containing sections from 2 animals with 3 sections/slide). At baseline, the percentage of 8-OHdG-positive cells in terc−/−F3 nonoperated lung was significantly higher than the level observed in WT nonoperated lung (*P = 0.0236). Conversely, the percentage of 8-OHdG-positive cells was not significantly greater post-PNX in terc−/−F3 POD3 lung than in WT at the same time point (P = 0.1209). The percentage of 8-OHdG-positive cells in WT PNX POD3 lung was higher than the percentage in nonoperated control (**P = 0.0224), but the percentage of 8-OHdG-positive cells in terc−/−F3 PNX POD3 lung was not significant compared with percentages in nonoperated terc−/−F3 lung (P = 0.7615). C: expression of stress activated MAP-kinase phospho-SAPK/phospho-JNK and apoptotic marker C-PARP. AEC2 and whole lung tissue were isolated from right lungs harvested from sham-operated mice at POD3 and from pneumonectomized mice at POD1, POD2, and POD3. Cell lysates were probed for expression of chaperone HSP27, MAP kinase P-SAPK/P-JNK and apoptotic marker cleaved PARP. Whole lung tissue lysates from sham-operated (S) and PNX POD3 (D3) mice were probed for expression of apoptotic marker PARP in both its cleaved (bottom band) and uncleaved (top band) forms. Expression of actin was used as a loading control. Blot shown is representative of multiple blots (3–5) using samples from 3–4 different animals/time point. D: expression of DNA damage markers and repair enzymes. AEC2 were isolated from right lungs harvested from sham-operated mice at POD3 and from pneumonectomized mice at POD1, POD2, and POD3. Cell lysates were probed for expression of DNA repair enzymes Ogg-1 and Gadd153, chaperone HSP27, and cell cycle control protein p21. Expression of actin was used as a loading control. Blot shown is representative of multiple blots (2–3) using samples from 2–3 different animals/time point.
The expression of apoptotic and protective markers were then examined in AEC2 isolated from WT vs. terc−/−F3 sham-operated and post-PNX lung at early postoperative time points and in whole lung tissue collected from the same animals at the same time. We previously reported that expression of the stress activated kinase phospho-SAPK/JNK (P-SAPK/P-JNK) is nonexistent in WT AEC2 at baseline but is present in terc−/−F4 cells, indicating chronic stress. As shown in Fig. 6C, expression of P-SAPK/P-JNK is likewise negligible in AEC2 from sham-operated WT lung but is present in AEC2 isolated from sham-operated terc−/−F3 lung. P-SAPK/P-JNK expression is induced in post-PNX WT AEC2, with peak expression occurring at POD2. However, P-SAPK/P-JNK expression is negligible in post-PNX terc−/−F3 AEC2, which we postulate may indicate an inability of this population to mount an elevated protective response. We also analyzed the expression of the apoptotic marker PARP, particularly its cleaved form, the appearance of which indicates activation of caspases and the presence of apoptotic cells within a given population. These analyses show that for both WT and terc−/−F3 samples, low levels of cleaved PARP are present in AEC2 isolated from both sham-operated and post-PNX lung, with peak expression for both cohorts at POD1. However, in the whole tissue samples shown in Fig. 6D, negligible levels of either uncleaved-PARP (top band) or C-PARP in sham-operated terc−/−F3 lung give way to highly elevated levels of both species in terc−/−F3 PNX POD3 lung. In contrast, whole lung from WT animals, whether post-sham operation or PNX, showed a low level of cleaved PARP and essentially no uncleaved PARP at the POD3 time point (though the level of uncleaved PARP did increase at that time). Thus it appears that a similar level of apoptosis can be detected in AEC2 from both WT and terc−/−F3 lung post-PNX, perhaps because of barotrauma. However, the overall level of apoptosis in terc−/−F3 lung is elevated by contributions from other lung cell populations, which may account for the higher mean number of 8-OHdG-positive cells observed in terc−/−F3 lung sections as seen in Fig. 6A.
terc−/− AEC2 mount an ineffective response to enhanced DNA damage and appear to growth arrest following PNX.
Because WT and terc−/−F3 AEC2 exhibit similar levels of cleaved PARP expression but WT AEC2 appear to overcome the effects of mild DNA damage and not only survive but proliferate, we wished to determine whether these differing responses were due to a failure not only in stress management but also, specifically, in DNA damage repair. As shown in Fig. 6D, the chaperone/heat shock protein HSP27, which functions as a stress and apoptosis inhibitor, is elevated in both WT and terc−/−F3 AEC2 in response to ventilation when samples from sham-operated mice are analyzed. This low level of expression was also observed at POD1 in WT AEC2, but expression markedly increased at the critical POD2 and POD3 time points. In contrast, HSP27 expression decreased in terc−/−F3 post-PNX AEC2, indicating that these cells are no longer mounting a protective response. A similar pattern was observed when post-PNX terc−/−F3 AEC2 were probed for expression of the DNA repair enzymes Ogg-1 and GADD153. Unlike the expression observed in WT samples, where Ogg-1 and especially GADD153 peaked at high levels at the POD1 and POD2 time points and then decreased, expression of these enzymes in terc−/−F3 AEC2 was induced moderately or not at all.
The expression of the cell cycle arrest protein p21 reflected the sharp differences in response to pneumonectomy by WT and terc−/−F3 lung. In WT AEC2, p21, which normally induces a brief period of growth arrest to allow DNA repair to occur, is present at a moderate level in WT sham samples and is then strongly induced at POD1 but cannot be detected following this time point. In contrast, p21 expression is consistently present in all terc−/−F3 sham samples and at all post-PNX time points analyzed at moderate levels, reflecting continuous growth arrest in response to the stress of ventilation and the stimulation imposed by PNX. Thus, although WT and terc−/−F3 AEC2 show DNA damage and similar levels of cleaved PARP at the critical PNX POD1–3 time points, only WT AEC2 are capable of simultaneously inducing both protective and repair pathways (Fig. 6D), as well as being able to respond to proliferative stimuli (Fig. 5). These positive responses to PNX are all lacking in terc−/−F3 AEC2 and whole lung.
DISCUSSION
To the best of our knowledge, we are the first to examine the role of telomerase and telomere length in the adaptation by normal murine lung tissue that occurs following partial pneumonectomy. Pneumonectomy offers a model of lung compensatory growth that is reproducible and quantifiable (52). The examination of lung responses to partial pneumonectomy is also considered a useful method for determining the mechanisms underlying lung cell potential for proliferation (9, 25–27, 44, 58).
A number of studies have shown that the induction of postpneumonectomy compensatory growth provides an environment conducive to tumor cell proliferation, reflecting the power of the growth signals that are unleashed by lung volume reduction (8–10, 31, 37, 39). It is therefore not surprising that upregulation of telomerase activity occurs during the initial phase of this process, which was confirmed by our study. These data indicated that there may be a normal role for telomerase upregulation in response to strong stimulatory signals.
The reduced ability of terc−/− mice to respond to pneumonectomy is in sharp contrast to the WT response and to previous studies in WT animals that showed that alveolar epithelial expansion, alveolar thickening, and neoalveolarization are all critical components of the compensatory lung growth that follows pneumonectomy (18, 58). Our data further agree with the previously published finding, that in WT mice, BASC increase in numbers following PNX (25, 44). Although the confirmation of BASC as a functional epithelial progenitor population requires further analysis beyond the scope of the present study, this characteristic response to challenge makes them a useful marker for the ability of distal lung tissue to respond positively to growth signals. Our present study demonstrates that although WT AEC2 and BASC increase in number following PNX, these populations are significantly impaired during the initial compensatory growth phase in terc−/− lung. Our earlier findings showed that AEC2 from terc−/− mice are chronically stressed at baseline (35). We now show that this chronically stressed status, plus reduced baseline numbers of progenitors prior to PNX, both presumably caused by telomere shortening, affect the initial ability of terc−/− lung to respond to the biomechanical signals sent out by lung volume reduction. This observation is consistent with studies showing that acutely shortened or impaired telomeres diminish cellular ability to respond to environmental stimuli (23, 33, 50, 51) and that telomere shortening accompanies reduced capacity for proliferation. In addition to the role of telomere shortening of stem/progenitor cells in aging (20), the consequences of shortened telomeres are manifested in diseases such as DKC, bone marrow failure resulting in aplastic anemia, and IPF (4, 5, 57). Parallel to the increasing severity of respiratory symptoms in progressive generations of terc−/− mice, human families afflicted with DKC and IPF because of mutations in TERC or TERT show earlier onset and greater severity of symptoms with successive generations (4, 5).
To determine the basis of this observation on a molecular level, further experiments showed that AEC2 from terc−/− mice carrying shortened telomeres exhibit strongly attenuated expression of proliferation markers. ERK1/2 is a major transducer of extracellular proliferation signals and is responsible for transferring these signals to downstream nuclear effectors of cellular proliferation. ERK1/2 responds upstream to epidermal growth factor receptor signaling and targets PCNA downstream, both of which proliferative markers have been shown to be strongly upregulated following PNX (21, 41). Other early response genes are upregulated immediately following PNX, including Egr-1 and TTF-1 (Nkx2.1), the latter of which is a critical factor in lung development (31, 43, 56). We found that another proliferation marker, Ki-67, which has been used as a prognostic marker for aggressive lung tumors (42), is also upregulated in normal WT tissue following PNX, but is more modestly induced in terc−/−F3 lung. The fact that proliferation of terc−/− AEC2 and other lung cells is so compromised immediately following PNX indicates one potential mechanism that may underlie their deficient compensatory response.
Complementary data were produced by probing AEC2 for protective markers and expression of DNA repair enzymes, which were strongly induced in WT, but not terc−/−F3 cells. Although phospho-JNK has been implicated in transducing stress signaling that results in cell death, it can also induce the upregulation of protective molecules, such as the chaperone HSP27. These molecules are both upregulated in WT AEC2, presumably by compensatory growth-triggered stimulation. The lack of P-JNK and HSP27 in post-PNX terc−/−F3 AEC2, along with elevated levels of apoptotic markers and a diminished proliferation response, presents a cellular milieu following PNX where biomechanical stimulation occurs but is not efficiently addressed.
A more direct indicator of the altered telomerase null response to PNX is the persistent expression of p21 in AEC2 during the initial compensatory growth phase. p21 is a direct target of the cell cycle regulatory protein p53. Activation of p53 in cells that carry a normal gene (true for both WT and terc−/− AEC2), but are not proliferation competent, triggers upregulation of p21, which eventually results in senescence (61). Since most lung epithelial cells are highly quiescent, this pathway may be the source of the low level of terminal deoxynucleotidyl-mediated dUTP nick end labeling (TUNEL)-positive cells observed in PNX-stimulated lung in both cohorts. However, in proliferation-competent cells, p21 is counteracted by strong proliferation signals, such as those induced by compensatory growth, and although cells may arrest briefly to perform any required DNA repair, they will then proceed through the cell cycle. Shortened telomeres can have a significant impact on this normal response, such that a chronic, low level of DNA damage is observed and cells are much more prone to senescence (15). We observed that p21 is strongly expressed 1 day following PNX in WT AEC2. However, it is strongly downregulated thereafter, during a time frame when WT AEC2 proliferation increases. Conversely, a moderate level of p21 expression persists in terc−/−F3 AEC2 following PNX, indicating that a significant number of these progenitors may be undergoing growth arrest at a time when proliferation should be highly stimulated.
Although a direct molecular connection between telomerase dysfunction, telomere shortening, and a poor response to PNX remains elusive, we speculate that significant alterations in critical molecular pathways that control immediate proliferation responses following PNX underlie the negligible increase in remaining lung tissue volume in terc−/−F3 animals. It is also apparent that the inability to mount this early drive to restore lung volume is tied to long-term survival. Thus our studies demonstrate that insufficient telomerase activity in distal lung epithelial progenitor cells, especially when coupled with acutely shortened telomeres, has a profound impact on the ability of the lung to initiate compensatory growth.
GRANTS
This research was funded by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) Grants R01 HL065352 (B. Driscoll), NIH/NHLBI P01 HL60231, R01s HL44060, HL44977, GM096195 (D. Warburton), NIH Minority Supplement to P01 HL60231 (G. N. Williams), California Institute of Regenerative Medicine (CIRM) Training Grant TG2-01168 (D. Warburton, B. Driscoll), and CIRM Fellowship Award via TG2-01168 (S. Jackson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Sue Buckley for invaluable technical advice, Dr. Tracey Grikscheit for consultation on the PNX technique, and Dr. Henri Ford for support and input on the construction of this manuscript. The authors are indebted to Dr. Connie Hsia for inspiration and early technical discussions and to Dr. Hsia and Dr. Wei Shi for providing critiques of the manuscript.
REFERENCES
- 1.Adamson YR, Bowden DH. The Type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest 30: 35–42, 1974 [PubMed] [Google Scholar]
- 2.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, Phillips JA, 3rd, Lansdorp PM, Loyd JE, Armanios MY. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 105: 13051–13056, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armanios M, Chen JL, Chang YPC, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, Hamosh A, Greider CW. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci USA 102: 15960–15964, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 356: 1317–1326, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Armanios M, Alder JK, Parry EM, Karim B, Strong MA, Greider CW. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Genet 85: 823–832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet 10: 45–61, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91: 25–34, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Brachner A, Sasgary S, Pirker C, Rodgarkia C, Mikula M, Mikulits W, Bergmeister H, Setinek U, Wieser M, Chin SF, Caldas C, Micksche M, Cerni C, Berger W. Telomerase- and alternative telomere lengthening-independent telomere stabilization in a metastasis-derived human non-small cell lung cancer cell line: effect of ectopic hTERT. Cancer Res 66: 3584–3592, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Brown LM, Rannels SR, Rannels DE. Implications of post-pneumonectomy compensatory lung growth in pulmonary physiology and disease. Respir Res 2: 340–347, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown LM, Welch DR, Rannels SR. B16F10 melanoma cell colonization of mouse lung is enhanced by partial pneumonectomy. Clin Exp Metastasis 19: 369–376, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Buckley S, Driscoll B, Anderson KD, Warburton D. Apoptosis and DNA damage in AEC2 cultured from hyperoxic rats. Am J Physiol Lung Cell Mol Physiol 274: L714–L720, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Cagle PT, Thurlbeck WM. Postpneumonectomy compensatory lung growth. Am Rev Respir Dis 138: 1314–1326, 1988 [DOI] [PubMed] [Google Scholar]
- 13.Calado RT, Young NS. Telomere diseases. N Engl J Med 361: 2353–2565, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Chintagari NR, Guo Y, Bhaskaran M, Chen J, Gao L, Jin N, Weng T, Liu L. Gene expression of rat alveolar type II cells during hyperoxia exposure and early recovery. Free Radic Biol Med 43: 628–642, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng Y, Chan SS, Chang S. Telomere dysfunction and tumour suppression: the senescence connection. Nat Rev Cancer 8: 450–458, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driscoll B, Buckley S, Kim CB, Anderson KD, Warburton D. Telomerase in alveolar epithelial development and repair. Am J Physiol Lung Cell Mol Physiol 279: L1191–L1198, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Evans MJ, Cabral LJ, Stevens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 22: 142–150, 1975 [DOI] [PubMed] [Google Scholar]
- 18.Fehrenbach H, Voswinckel R, Michl V, Mehling T, Fehrenbach A, Seeger W, Nyengaard JR. Neoalveolarization contributes to compensatory lung growth following pneumonectomy in mice. Eur Respir J 31: 515–522, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Fernandez L, Le Cras T, Ruiz M, Glover D, Kron I, Laubach V. Differential vascular growth in postpneumonectomy compensatory lung growth. J Thorac Cardiovasc Surg 133: 309–316, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behaviour. Science 309: 1253–1256, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Foster DJ, Yan X, Bellotto DJ, Moe OW, Hagler HK, Estrera AS, Hsia CC. Expression of epidermal growth factor and surfactant proteins during postnatal and compensatory lung growth. Am J Physiol Lung Cell Mol Physiol 283: L981–L990, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Garcia CK, Wright WE, Shay JW. Human diseases of telomerase dysfunction: insights into tissue aging. Nucleic Acids Res 35: 7406–7416, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemann MT, Rudolph KL, Strong MA, DePinho RA, Chin L, Greider CW. Telomere dysfunction triggers developmentally regulated germ cell apoptosis. Mol Biol Cell 12: 2023–2030, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br J Cancer 96: 1020–1024, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman AM, Shifren A, Mazan MR, Gruntman AM, Lascola KM, Nolen-Walston RD, Kim CF, Tsai L, Pierce RA, Mecham RP, Ingenito EP. Matrix modulation of compensatory lung regrowth and progenitor cell proliferation in mice. Am J Physiol Lung Cell Mol Physiol 298: L158–L168, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsia CCW, Herazo LF, Fryder-Doffey F, Weibel ER. Compensatory lung growth occurs in adult dogs after right pneumonectomy. J Clin Invest 94: 405–412, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsia C. Signals and mechanisms of compensatory lung growth. J Appl Physiol 97: 1992–1998, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Hsia CC, Johnson RL., Jr Further examination of alveolar septal adaptation to left pneumonectomy in the adult lung. Respir Physiol Neurobiol 151: 167–177, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Jackson SR, Williams GN, Lee J, Baer JF, Warburton D, Driscoll B. A modified technique for partial pneumonectomy in the mouse. J Invest Surg 24: 81–86, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchoalveolar stem cells in normal lung and lung cancer. Cell 121: 823–835, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Landesberg LJ, Ramalingam R, Lee K, Rosengart TK, Crystal RG. Upregulation of transcription factors in lung in the early phase of postpneumonectomy lung growth. Am J Physiol Lung Cell Mol Physiol 281: L1138–L1149, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Lantuejoul S, Salon C, Soria JC, Brambilla E. Telomerase expression in lung preneoplasia and neoplasia. Int J Cancer 120: 1835–1841, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Lechel A, Manns MP, Rudolph KL. Telomeres and telomerase: new targets for the treatment of liver cirrhosis and hepatocellular carcinoma. J Hepatol 41: 491–497, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Reddy R, Barsky L, Weinberg K, Driscoll B. Contribution of proliferation and DNA damage repair to alveolar epithelial type 2 cell recovery from hyperoxia. Am J Physiol Lung Cell Mol Physiol 290: L685–L694, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Reddy R, Barksy L, Scholes J, Chen H, Shi W, Driscoll B. Lung alveolar integrity is compromised by telomere shortening in telomerase-null mice. Am J Physiol Lung Cell Mol Physiol 296: L57–L70, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J 30: 835–839, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Maniwa Y, Kanki M, Okita Y. Importance of the control of lung recurrence soon after surgery of pulmonary metastases. Am J Surg 179: 122–125 [DOI] [PubMed] [Google Scholar]
- 38.Marchetti A, Bertacca G, Buttitta F, Chella A, Quattrocolo G, Angeletti CA, Bevilacqua G. Telomerase activity as a prognostic indicator in stage I non-small cell lung cancer. Clin Cancer Res 5: 2077–2081, 1999 [PubMed] [Google Scholar]
- 39.Marchetti A, Pellegrini C, Buttitta F, Falleni M, Romagnoli S, Felicioni L, Barassi F, Salvatore S, Chella A, Angeletti CA, Roncalli M, Coggi G, Bosari S. Prediction of survival in stage I lung carcinoma patients by telomerase function evaluation. Lab Invest 82: 729–736, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Mason RJ, Williams MC, Moses HL, Mohla S, Berberich MA. Stem cell in lung development, disease, and therapy. Am J Respir Cell Mol Biol 16: 355–363, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto K, Nagayasu T, Hishikawa Y, Tagawa T, Yamayoshi T, Abo T, Tobinaga S, Furukawa K, Koji T. Keratinocyte growth factor accelerates compensatory growth in the remaining lung after trilobectomy in rats. J Thorac Cardiovasc Surg 137: 1499–1507, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Meert AP, Martin B, Verdebout JM, Paesmans M, Berghmans T, Ninane V, Sculier JP. Correlation of different markers (p53, EGF-R, c-erbB-2, Ki-67) expression in the diagnostic biopsies and the corresponding resected tumors in non-small cell lung cancer. Lung Cancer 44: 295–301, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Minoo P, Li C, Liu HB, Hamdan H, deLemos R. TTF-1 is an epithelial morphoregulatory transcriptional factor. Chest 111: 135S–137S, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Nolen-Walston RD, Kim CF, Mazan MR, Ingenito EP, Gruntman AM, Tsai L, Boston R, Woolfenden AE, Jacks T, Hoffman AM. Cellular kinetics and modeling of bronchoalveolar stem cell response during lung regeneration. Am J Physiol Lung Cell Mol Physiol 294: L1158–L1165, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Reilly MA. DNA damage and cell cycle checkpoints in hyperoxic lung injury: braking to facilitate repair. Am J Physiol Lung Cell Mol Physiol 281: L291–L305, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Rannels DE, Rannels SR. Compensatory growth of the lung following partial pneumonectomy. Exp Lung Res 14: 157–182, 1988 [DOI] [PubMed] [Google Scholar]
- 47.Rannels S, Rannels E. Alterations in type II pneumocytes cultured after partial pneumonectomy. Am J Physiol Cell Physiol 254: C684–C690, 1988 [DOI] [PubMed] [Google Scholar]
- 48.Rawlins EL, Hogan BLM. Epithelial stem cells of the lung: privileged few or opportunities for many? Development 133: 2455–2465, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Reddy R, Buckley S, Doerken M, Barksy L, Weinberg K, Anderson K, Warburton D, Driscoll B. Isolation of a putative progenitor subpopulation of alveolar epithelial type 2 cells. Am J Physiol Lung Cell Mol Physiol 286: L658–L667, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447: 725–729, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Rubio MA, Davalos AR, Campisi J. Telomere length mediates the effects of telomerase on the cellular response to genotoxic stress. Exp Cell Res 298: 17–27, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Sakurai MK, Lee S, Arsenault DA, Nose V, Wilson JM, Heymach JV, Puder M. Vascular endothelial growth factor (VEGF) accelerates compensatory lung growth after unilateral pneumonectomy. Am J Physiol Lung Cell Mol Physiol 292: L742–L747, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, Thannickal VJ, Moore BB, Christensen PJ, Simon RH. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med 181: 254–263, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc 5: 328–333, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sukurai MK, Greene AK, Wilson J, Fauza D, Pruder M. Pneumonectomy in the mouse: technique and perioperative management. J Invest Surg 18: 201–205, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Takahashi Y, Izumi Y, Kohno M, Kimura T, Kawamura M, Okada Y, Nomori H, Ikeda E. Thyroid transcription factor-1 influences the early phase of compensatory lung growth in adult mice. Am J Respir Crit Care Med 181: 1397–1406, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA 104: 7552–7557, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voswinckel R, Motejl Fehrenbach A, Wegmann M, Mehlig T, Fehrenbach H, Seeger W. Characterisation of post pneumonectomy lung growth in adult mice. Eur Respir J 24: 524–532, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat Genet 365: 447–449, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Westhoff JH, Schildhorn C, Jacobi C, Homme M, Hartner A, Braun H, Kryzer C, Wang C, von Zglincki T, Kranzlin B, Gretz N, Melk A. Telomere shortening reduces regenerative capacity after acute kidney injury. J Am Soc Nephrol 21: 327–336, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang H. Molecular signaling and genetic pathways of senescence: Its role in tumorigenesis and aging. J Cell Physiol 210: 567–574, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Zhang Q, Bellotto DJ, Ravikumar P, Moe OW, Hogg RT, Hogg DC, Estrera AS, Johnson RL, Jr, Hsia CC. Postpneumonectomy lung expansion elicits hypoxia-inducible factor-1α signaling. Am J Physiol Lung Cell Mol Physiol 293: L497–L504, 2007 [DOI] [PubMed] [Google Scholar]