Abstract
Apoptosis of lung epithelial and endothelial cells by exposure to cigarette smoke (CS) severely damages the lung tissue, leading to the pathogenesis of emphysema, but the underlying mechanisms are poorly understood. We have recently established a direct correlation between decreased lipid raft CFTR expression and emphysema progression through increased ceramide accumulation. In the present work, we investigated the role of membrane CFTR in regulating apoptosis and autophagy responses to CS exposure. We report a constitutive and CS-induced increase in the number of TUNEL-positive apoptotic cells in Cftr−/− murine lungs compared with Cftr+/+ murine lungs that also correlated with a concurrent increase in the expression of ceramide, NF-κB, CD95/Fas, lipid raft proteins, and zonula occludens (ZO)-1/2 (P < 0.001). We also verified that stable wild-type CFTR expression in CFBE41o− cells controls constitutively elevated caspase-3/7 activity (−1.6-fold, P < 0.001). Our data suggest that membrane CFTR regulates ceramide-enriched lipid raft signaling platforms required for the induction of Fas-mediated apoptotic signaling. In addition, lack of membrane CFTR also modulates autophagy, as demonstrated by the significant increase in constitutive (P < 0.001) and CSE-induced (P < 0.005) perinuclear accumulation of green fluorescent protein-microtubule-associated protein 1 light chain-3 (LC3) in the absence of membrane CFTR (CFBE41o− cells). The significant constitutive and CS-induced increase (P < 0.05) in p62 and LC3β expression in CFTR-deficient cells and mice corroborates these findings and suggest a defective autophagy response in the absence of membrane CFTR. Our data demonstrate the critical role of membrane-localized CFTR in regulating apoptotic and autophagic responses in CS-induced lung injury that may be involved in the pathogenesis of severe emphysema.
Keywords: cystic fibrobsis transmembrane conductance regulator, epithelium, emphysema, ceramide, autophagy, apoptosis
exposure to cigarette smoke (CS) and/or other environmental factors contribute to the pathogenesis of chronic obstructive pulmonary disease (COPD) and severe emphysema (48), which are major causes of morbidity and mortality in the United States and worldwide (2). In the United States, CS exposure is the primary factor for the pathogenesis of emphysema and COPD (12), whereas in developing countries, other environmental factors, such as smoke from burning biomass fuels or air pollutants, are more critical factors (1, 13). Although the physiological relevance of CS-induced pulmonary cell apoptosis in the progress of chronic lung injury, emphysema, and COPD has been extensively investigated (8), the underlying mechanisms still remain elusive. A significant increase in apoptosis of alveolar epithelial and endothelial cells in the lungs of COPD subjects has been reported, and since this is not compensated for by increased proliferation of these structural cells with important pathophysiological functions, the final outcome is the destruction of lung tissue and the development of emphysema (8). The accumulation and/or upregulation of ceramide and other sphingolipids in the lungs of COPD subjects and CS-exposed mice is considered to trigger inflammatory and apoptotic signaling (4, 12, 32, 40), but the precise mechanism of increased ceramide levels is not apparent. We and others are currently investigating the therapeutic efficacy of selectively targeting ceramide accumulation pathways as a strategy to reverse the debilitating effects of apoptosis in lung injury (4, 12) and emphysema (4).
The critical role of CFTR in regulating NF-κB-mediated inflammatory signaling is well documented (3, 16, 46). We (4, 46) recently demonstrated that lipid raft CFTR is a critical modulator of the innate and adaptive immune response. Our recent study (4) also suggested that lipid raft CFTR expression inversely correlates with the severity of emphysema and ceramide accumulation. We and others (4, 6) have found that CS or CS extract (CSE) exposure decreases membrane CFTR expression in murine lungs and human cells, respectively. Concurrently, ceramide-enriched membrane platforms are critical for CD95/Fas (TNF receptor superfamily, member 6) clustering through the aggregation of lipid rafts that lead to Fas-mediated apoptosis (15, 24), one of the critical factors mediating the pathophysiology of emphysema. Therefore, one can postulate that changes in lipid raft CFTR expression may be crucial for the induction of apoptosis during chronic lung injury and severe emphysema. Indeed, it has been shown that knockdown of CFTR expression in alveolar macrophages or CFTR deficiency in epithelial cells confers to them a proinflammatory phenotype and induces apoptosis (18, 50). CFTR may also regulate lung injury and inflammation by controlling autophagy, a key survival pathway of the cell (26). A recent study by Luciani et al. (19) demonstrated a critical role of CFTR in regulating autophagy. Defective or lack of membrane CFTR leads to the inhibition of autophagy, resulting in the accumulation of protein aggregates that is proposed to cause lung inflammation in cystic fibrosis (CF) (19). Moreover, activation of Fas may also induce autophagy (31, 52). Although CF cells demonstrate exaggerated apoptosis (36) and aberrant autophagy (19), the role of membrane or lipid raft-localized CFTR in regulating CS-induced apoptotic and autophagy responses is not apparent.
In the present study, we identified the critical role of membrane CFTR in regulating CS-induced apoptosis and autophagy during lung injury. Our data suggest that CFTR-dependent lipid rafts regulate apoptotic and autophagic responses by modulating Fas expression. Lack of membrane CFTR leads to defective autophagy and enhanced apoptosis in acute CS-induced lung injury.
MATERIALS AND METHODS
Reagents and treatments.
CFBE41o-wt-CFTR and CFBE41o− (from Dr. Dieter Gruenert), 16HBEo−, and human embryonic kidney (HEK)-293 cells were cultured at 37°C with 5% CO2 in MEM or DMEM-F-12 media supplemented with 10% FBS and 1% penicillin, streptomycin, and amphotericin B (PSA) (Invitrogen). We used normal 16HBEo− and CFBE41o− cells for the green fluorescent protein (GFP)-microtubule-associated protein 1 light chain-3 (LC3) reporter assay, and CFBE41o-wt-CFTR and CFBE41o− cells were used to evaluate whether the effects of CFTR deficiency on apoptosis induction could be reversed by stable wild-type (WT) CFTR expression in CFBE41o− cells. We verified the role of WT CFTR in regulating apoptotic and autophagy responses using HEK-293 cells transiently transfected with WT CFTR-enhanced GFP (EGFP) and/or pEGFP. For in vitro experiments, cells were treated with 200 μg/ml CS extract (CSE; Murty Pharmaceuticals) or DMSO vehicle for the indicated time points. Cells were transfected with EGFP-LC3B plasmid using Lipofectamine 2000 reagent (Invitrogen) as previously described (46) followed by CSE treatment and analyzed by immunofluorescence microscopy to detect and count GFP-LC3-positive cells. HEK-293 cells were similarly transiently transfected with pEGFP or WT CFTR-EGFP plasmids and treated with CSE (200 μg/ml) or DMSO vehicle for 24 h. Mice were exposed to CS (acute, 5 days; or subchronic, 4 wk) or Pseudomonas aeruginosa LPS (Sigma) as previously described (4).
Murine experiments.
All animal experiments were carried out in accordance with The Johns Hopkins University Animal Care and Use Committee-approved protocols. We used age-, weight-, and sex-matched (8 wk old) B6–129S6-Cftr−/− [Cftrtm1Kthc-TgN(FABPCFTR)] (41, 42) and Cftr+/+ inbred mouse strains (procured from Case Western Reserve University Animal Resource Center; representative data are shown for at least n = 3 mice in all experiments). All mice were housed in a controlled environment under pathogen-free conditions. Mice (3 mice/group, 8 wk old) were exposed to CS using the TE-2 cigarette smoking machine (Teague Enterprises, Davis, CA). CS was generated by burning research grade cigarettes (3R4F, 0.73 mg nicotine/cigarette) purchased from the Tobacco Research Institute (University of Kentucky, Lexington, KY) for 5 h/day for 5 days (acute exposure) or 4 wk (subchronic exposure). An average total particulate matter of 150 mg/m3 was recorded in real time during the smoking protocols. The control group of mice was exposed to filtered room air, and all mice were killed 2 h after the last CS exposure. To evaluate the effect of lipid raft CFTR, we used our previously described method (4) using cyclodextrin (CD) treatment under conditions known to disrupt lipid rafts and deplete CFTR. Subchronic CS-exposed mice were treated intratracheally with CD (2 × 50 μg in PBS as vehicle; see scale in Fig. 3D) at the indicated time points and killed 2 h after the last CS exposure. To verify the findings from CS exposure, we also used the P. aeruginosa LPS-induced lung injury model as previously described (4). Briefly, Cftr+/+ and Cftr−/− mice were treated with 20 μg/mouse P. aeruginosa LPS by intratracheal instillation for 24 h. Lungs from CS-exposed or P. aeruginosa LPS-treated mice were harvested, fixed in 10% buffered formalin phosphate (Fisher Scientific), paraffin embedded, and cut into longitudinal sections (5 μm thick) on glass slides for immunostainings or to detect the number of apoptotic cells by TUNEL assay.
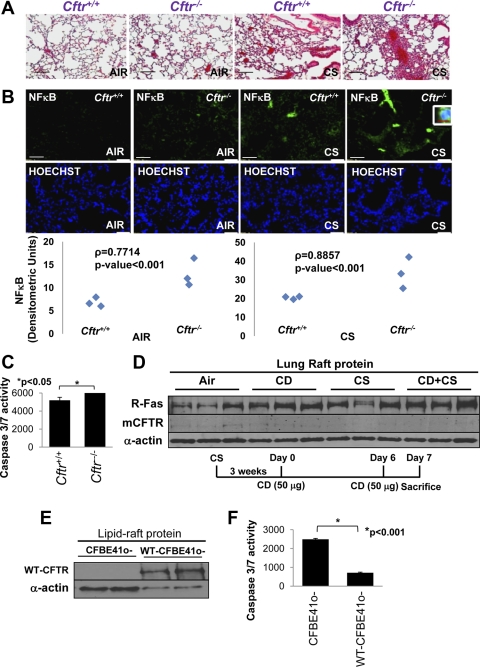
Fig. 3.
Absence of CFTR worsens CS-induced inflammation and apoptotic cell death. A: hematoxylin and eosin (H&E) staining of longitudinal lung sections from Cftr+/+ or Cftr−/− (n = 3) mice exposed to room air or CS was used to detect the inflammatory milieu in the lungs. The data indicate that the absence of CFTR triggers higher constitutive as well as CS-induced inflammation in murine lungs. Black bar = 50 μm. B: NF-κB immunostaining of these murine lung sections (top) demonstrated that the presence of CFTR (Cftr+/+ mice) significantly inhibited NF-κB activation (bottom) and nuclear translocation (inset, top right) in acute CS-induced lung injury (P < 0.001, bottom). White scale bars = 50 μm; red scale bar = 10 μm. Middle: costaining with Hoechst dye was used to localize the nucleus (blue). C: total lung lysates of Cftr+/+ and Cftr−/− mice showed a significant increase (P < 0.05, mean ± SE) in caspase-3/7 activity in the absence of CFTR. D: immunoblot analysis of the lipid raft protein fraction of murine (Cftr+/+, n = 3) lungs from cyclodextrin (CD)-treated (at the indicated time points) and/or subchronic CS-exposed mice showed an increase in Fas levels, whereas membrane CFTR (m-CFTR) expression decreased. α-Actin was used as a loading control. E and F: immunoblot analyis of lipid raft fractions of CFBE41o− and CFBE41o-wt-CFTR cells showed the correlation of stable lipid raft wild-type (WT) CFTR expression (E) with a significant (P < 0.001, mean ± SE) decrease in caspase-3/7 activity (F). The data imply that CFTR controls CS-induced lung injury and apoptosis.
Immunohistology and TUNEL assay.
Longitudinal tissue sections from murine lungs were immunostained with primary antibodies (1–2 μg/ml) for ceramide (mouse monoclonal, Alexis Biochemical), Fas [rabbit polyclonal, Santa Cruz Biotechnology (SCBT)], NF-κB (rabbit polyclonal, SCBT), p62 (mouse monoclonal, BD Biosciences), zonula occludens (ZO)-1 (rabbit polyclonal, SCBT)/ZO-2 (goat polyclonal, SCBT), and LC3β (goat polyclonal, SCBT) followed by secondary antibodies (1:200 dilution) using our previously described protocol (47). The secondary antibodies used were goat anti-rabbit IgG FITC (1 μg/ml, SCBT), donkey anti-mouse IgG Alexa fluor 594 (10 μg/ml, Invitrogen), and donkey anti-goat Dylight 594 (1.25 μg/ml, Jackson ImmunoResearch). Nuclei were detected by Hoechst (2 μg/ml, Invitrogen) staining, whereas hematoxylin and eosin (H&E) was used to evaluate lung morphology and the inflammatory state. Images were captured by Axiovert 200 Carl Zeiss fluorescence microscope using the Zeiss Axiocam HRC camera and Axiovision software. The numbers of apoptotic cells in longitudinal lung sections from Cftr+/+ and Cftr−/− mice exposed to CS or P. aeruginosa LPS were quantified by a DeadEnd Fluorometric TUNEL kit (Promega).
Autophagy reporter assay.
16HBEo− and CFBE41o− cells were transiently transfected with EGFP-LC3B plasmid (vector backbone: pEGFP-C3, Addgene) for a total of 48 h. Cells were treated with 200 μg/ml CSE for the last 24 h and analyzed by immunofluorescence microscopy (4) using an Axiovert 200 Carl Zeiss fluorescence microscope, Zeiss Axiocam HRC camera, and Axiovision software as described above. The perinuclear localization of GFP-LC3 was determined at ×100 magnification. The number of GFP-LC3-positive perinuclear aggregates was counted in each well, and representative data of triplicate samples are shown.
Caspase-3/7 assay.
CFBE41o− and CFBE41o-wt-CFTR cells were cultured as previously described (4), and 104 cells/well were plated in an opaque 96-well flat-bottom tissue culture plate (100 μl volume/well). Cells were treated with 200 μg/ml CSE for 24 h or an equal volume of DMSO vehicle as a control. Caspase-3/7 activity was quantified using a Caspase-Glo 3/7 assay (Promega) as previously described (47). Briefly, equal volumes of the caspase-3/7 reagent were added to each well, and the plate was incubated for 30–60 min in the dark. The luminescence was measured, and the fold change in caspase activity was calculated.
Flow cytometry.
HEK-293 cells were transiently transfected with pEGFP or WT CFTR-EGFP plasmid and treated with DMSO vehicle or CSE (200 μg/ml) for 24 h. Cells were either analyzed for WT CFTR-EGFP expression or the number of p62-positive cells using the BD FACS Caliber flow cytometer as previously described (4). Next, we quantified the percentage of apoptotic cells (in the M1 phase) using propidium iodide (Sigma) staining as previously described (33). Briefly, cells were treated as above and fixed with ice-cold 70% ethanol followed by propidium iodide (1 mg/ml) staining and flow cytometry (33). Cells in the M1 phase were gated to quantify the statistical change in the percentage of apoptotic cells.
Immunoblot analysis of total and lipid raft proteins.
Total lung lysates were isolated as previously described (4, 5, 23) from Cftr+/+ and Cftr−/− mice (air or CS exposed), subjected to 10% SDS-PAGE, and analyzed for changes in the expression of the apoptotic marker Fas (SCBT), aggresome/defective autophagy marker p62 (BD Bioscience), the lipid raft marker ZO-1 (SCBT), and CFTR (rabbit polyclonal, SCBT) by immunoblot analysis. Lysates from CFBE41o− and CFBE41o-wt-CFTR (DMSO or CSE treated) or HEK-293 cells (pEGFP or WT CFTR-EGFP) were similarly analyzed for p62 (BD Bioscience), Fas (SCBT), or CFTR [SCBT; 596 (CF Foundation and University of North Carolina under a Material Transfer Agreement)] expression. Blots were reprobed for α- or β-actin (Sigma) as the loading control. The lipid raft protein fraction was isolated from cells or murine lungs (air, subchronic CS, and/or CD) as we have previously described (4) and immunoblotted for Fas (SCBT), CFTR (SCBT, mice; and 596-ab, cells), and α-actin (Sigma). We also isolated lipid raft proteins from Cftr+/+ and Cftr−/− mice lungs and spotted (20 μg, dot blot) them on a 0.45-μm nitrocellulose membrane followed by immunoblot analysis for ceramide (Alexis) and α-actin.
Statistical analysis.
Data are expressed as means ± SE (SD for flow cytometry analysis) of at least three experiments. Student t-test and ANOVA were used to determine the statistical significance. Microscopy data were analyzed by densitometry (MATLAB R2009b, Mathworks, Natick, MA) followed by Spearman's correlation coefficient analysis to calculate the significance among the indicated groups.
RESULTS
CFTR controls alveolar cell apoptosis in CS-induced lung injury.
Alveolar cell apoptosis is the primary cause of CS-induced emphysema development (8). We (4) have previously shown that lipid raft-localized CFTR regulates inflammatory signaling via ceramide in COPD subjects with emphysema and CS-exposed mice. To elucidate the role of CFTR in lung cell apoptosis, we quantified the number of apoptotic cells in the lungs of Cftr+/+ and Cftr−/− mice exposed to either room air or acute CS by TUNEL assay. We found a significant constitutive increase in TUNEL-positive apoptotic cells (predominantly alveolar type II) in Cftr−/− murine lungs (P < 0.01) compared with Cftr+/+ murine lungs that was further aggravated by acute CS exposure (Fig. 1A and Supplemental Material, Supplemental Fig. S1, A and B).1 The expression and lipid raft clustering of the CD95/Fas receptor is essential for the activation of apoptotic signaling (15). We found a significant increase in Fas expression in Cftr−/− murine lungs compared with Cftr+/+ (P < 0.001; Fig. 1B) that also correlated with a substantial increase in lung ceramide accumulation (P < 0.001; Fig. 1C). Expression of Fas (apoptosis) and ceramide (apoptosis and inflammation) was further elevated in CS-exposed Cftr−/− murine lungs compared with Cftr+/+ (P < 0.001; Fig. 1, B and C, top and bottom), indicating that CFTR deficiency mediates CS-induced lung injury by inducing apoptotic cell death. We were able to verify Fas accumulation in lipid rafts (discussed below for Fig. 3D). We also confirmed this finding in the P. aeruginosa LPS-induced lung injury model and found that Cftr−/− mice have higher number of TUNEL-positive apoptotic cells compared with Cftr+/+ mice (P < 0.05; Supplemental Fig. S1, A and B). These results demonstrate that CFTR controls ceramide accumulation and alveolar cell apoptosis in CS-induced lung injury.
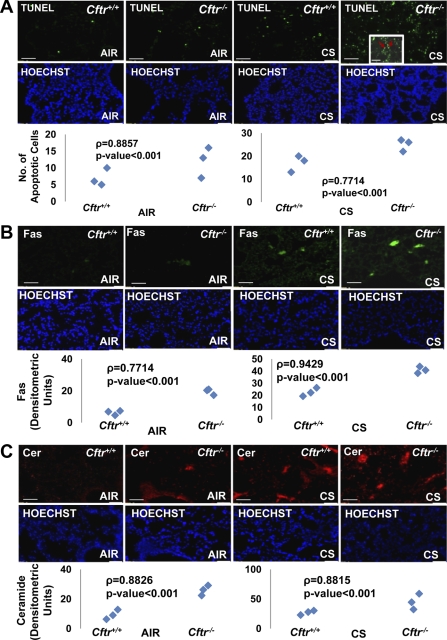
Fig. 1.
CFTR controls cigarette smoke (CS)-induced apoptosis in murine lungs. A, top: paraffin-embedded longitudinal lung sections from Cftr+/+ or Cftr−/− mice (n = 3) exposed to room air or CS were used to detect the number of apoptotic cells by TUNEL assay. The inset shows a higher-magnification image of a CS-exposed Cftr−/− murine lung section showing alveolar type II TUNEL-positive cells (red arrows). Middle: costaining with Hoechst dye was used to localize the nucleus (blue). Bottom: the data show a significant increase in constitutive and CS-induced TUNEL-positive apoptotic cells (P < 0.001) in Cftr−/− mice compared with Cftr+/+ mice. B and C, top: longitudinal lung sections from the same groups of mice were immunostained for Fas (B) and ceramide (Cer; C) expression. Middle: costaining with Hoechst dye was used to localize the nucleus (blue). Bottom: the representative sections (B and C) show that the absence of CFTR triggers a significant increase in Fas and ceramide expression in murine lungs, which was further upregulated by CS exposure (P < 0.001). White scale bars = 50 μm. The data indicate that CFTR regulates CS-induced apoptosis in murine lungs.
CFTR regulates the expression of lipid raft markers in murine lungs.
We (4) have previously shown that CFTR negatively regulates lipid raft clustering and that the absence of CFTR leads to ceramide accumulation in lipid rafts. Here, we evaluated the correlation of increased expression of the lipid raft markers ZO-1 and ZO-2 with the changes in Fas and ceramide expression in Cftr−/− murine lungs. Since Fas receptor clustering to ceramide-enriched raft platforms is essential to trigger the apoptotic cascade (14, 15), we hypothesized that CFTR controls the clustering of Fas in lipid rafts to regulate apoptotic signaling. To confirm this, we analyzed the protein expression of ZO-1 and ZO-2 in longitudinal lung sections of Cftr+/+ and Cftr−/− mice exposed to acute CS by immunostaining. The data indicated a significant upregulation of both constitutive and CS-induced ZO-1/2 expression in Cftr−/− murine lungs (P < 0.001; Fig. 2, A and B). We verified this by immunoblotting total lung protein extracts of air- or CS-exposed Cftr+/+and Cftr−/− mice to clearly show constitutive differences in ZO-1 expression (Fig. 2C). We did not see a significant change in CS-induced ZO-1 expression in Cftr+/+ and Cftr−/− murine lungs. It may be possible that ZO-1 localizes/clusters in the lipid raft (insoluble protein fraction) upon CS exposure. Since we used soluble total protein extracts, we may not have detected the upregulation of ZO-1 upon CS exposure (as shown in Fig. 2A by immunostaining). Further studies are required using purified lipid raft fractions from the lungs of these mice to verify the data. Moreover, we also found a significant increase in ZO-1/ceramide costaining in CS-exposed Cftr−/− mice compared with Cftr+/+ mice (Supplemental Fig. S2A). These data suggest that the absence of lipid raft CFTR leads to ceramide accumulation and raft clustering, which may induce the formation of signaling platforms involved in Fas receptor clustering and activation of the apoptosis pathway.
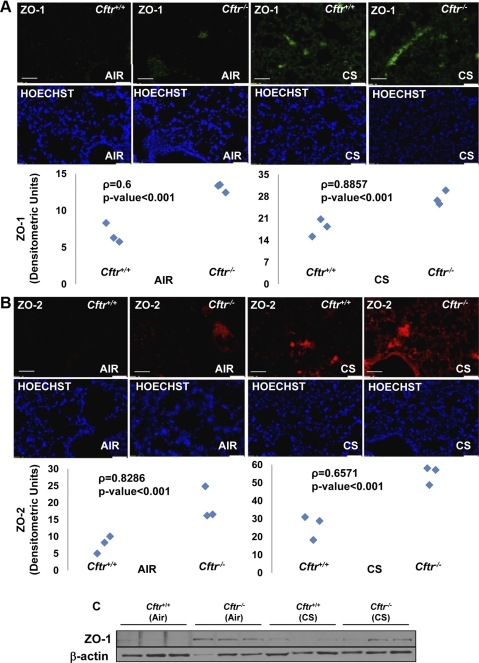
Fig. 2.
Defective CFTR increases the expression of lipid raft proteins. A and B, top: longitudinal lung sections from Cftr+/+ or Cftr−/− mice (n = 3) exposed to room air or CS were immunostained for zonula occludens (ZO)-1 (A) or ZO-2 (B). Middle: costaining with Hoechst dye was used to localize the nucleus (blue). Bottom: expression of these lipid raft proteins (ZO-1/2) was significantly elevated in the absence of CFTR, which was further increased by CS exposure (P < 0.001). White scale bars = 50 μm. C: Western blot analysis of total lung lysates from Cftr+/+ or Cftr−/− mice exposed to room air or CS showed upregulation of ZO-1 expression in the absence of CFTR. β-Actin was used as a loading control. The data imply that CFTR regulates the expression of lipid raft proteins, thereby modulating the clustering of signaling receptors.
CFTR controls CS-induced inflammation and apoptosis in murine lungs.
Acute CS exposure triggers NF-κB-mediated inflammatory signaling in murine lungs (51). To verify if the absence of CFTR aggravates CS-mediated NF-κB activation and inflammation, we used the Cftr−/− murine model and stained longitudinal lung sections from CS-exposed Cftr+/+ and Cftr−/− mice with H&E and NF-κB (using an antibody). We found that Cftr−/− mice showed a significant constitutive increase in inflammation, NF-κB activation, and nuclear localization compared with Cftr+/+ mice (P < 0.001; Fig. 3, A and B). Acute CS exposure enhanced the inflammation, NF-κB levels, and nuclear localization (Fig. 3B, inset, top right) in Cftr−/− murine lungs compared with Cftr+/+ murine lungs, indicating that CFTR regulates inflammatory signaling in response to CS exposure through NF-κB activation. We also verified that Cftr−/− murine lungs have constitutively higher caspase-3/7 activity compared with Cftr+/+ (Fig. 3C). Next, to evaluate if lipid raft CFTR regulates Fas-mediated apoptosis, we treated Cftr+/+ mice with methyl-β-cyclodextrin (CD) (4, 46) and/or subchronic CS. We observed that both CD and CS treatment increased lipid raft Fas expression, whereas CFTR levels decreased (Fig. 3D). Cotreatment with CD and CS had a synergistic effect. We also verified this using cell lines and found that stable WT CFTR expression in CFBE41o− and CFBE41o-wt-CFTR cells controls constitutively elevated caspase-3/7 activity (−1.6-fold, P < 0.001) in CFBE41o− cells (Fig. 3, E and F). Our data indicate that lipid raft CFTR controls alveolar cell apoptosis (Figs. 1 and 2) by regulating membrane ceramide accumulation (Fig. 1 and Supplemental Figs. S1C and S2, A and B) as a mechanism to induce Fas receptor clustering and caspase-3/7 activity (Fig. 3). Since intratracheal instillation of active caspase-3 or ceramide induces an emphysema like-phenotype in a murine emphysema model (49), the observed decrease in CS-induced membrane CFTR and subsequent ceramide accumulation contributes to the pathogenesis of severe emphysema, as recently demonstrated by our group (4).
WT CFTR controls CSE-induced apoptosis.
To confirm our hypothesis that WT CFTR controls Fas-mediated apoptotic signaling, we overexpressed WT CFTR in HEK-293 cells and found a significant decrease in constitutive Fas expression (P < 0.05; Fig. 4A). We also found that WT CFTR expression in HEK-293 cells was significantly downregulated by CSE treatment (P < 0.001; Fig. 4B, bottom) supporting our recent data (4). Next, we verified that WT CFTR expression controls CSE-induced apoptosis, as demonstrated by a decrease in the number of cells in the M1 phase (Fig. 4C). The data indicate that WT CFTR regulates Fas expression and CSE-induced apoptosis.
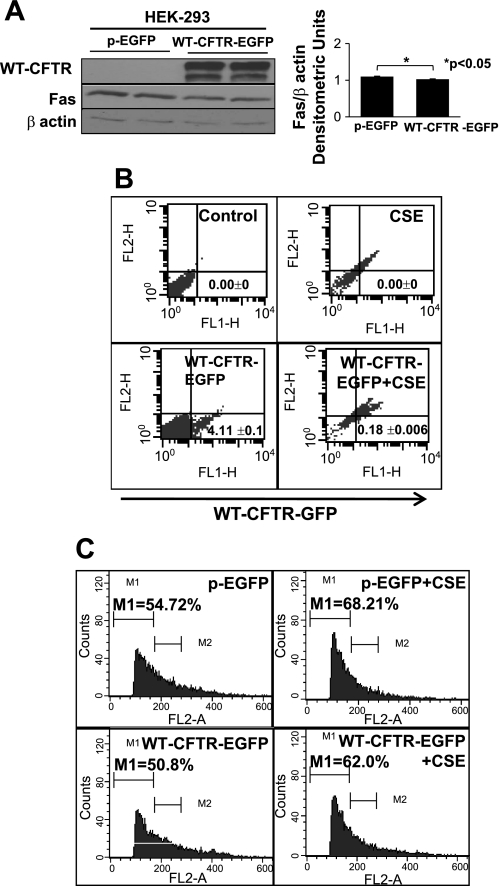
Fig. 4.
WT CFTR controls Fas expression and apoptosis. A: Western blot analysis of total protein lysates from human embryonic kidney (HEK)-293 cells transiently transfected with control [enhanced green fluorescent protein (EGFP) plasmid (pEGFP)] or WT CFTR-green fluorescent protein (GFP) plasmids (left) showed a significant decrease (P < 0.05, mean ± SE; right) in Fas expression in the presence of WT CFTR. β-Actin was used as a loading control. B: flow cytometry results of HEK-293 cells transiently transfected with WT-CFTR-GFP plasmid with or without CS extract (CSE; 200 μg/ml) showed that CSE treatment significantly decreased (P < 0.001, mean ± SD) WT CFTR expression (bottom right). C: propidium iodide (PI) staining of HEK-293 cells transiently transfected with control (pEGFP) or WT-CFTR-GFP plasmids with or without CSE showed that WT CFTR controls CSE-induced apoptosis, as demonstrated by the decrease in the number of cells in the M1 phase (bottom right). The data demonstrate that overexpression of WT CFTR controls Fas expression and CSE-induced apoptosis.
CFTR augments the CS-induced autophagy response.
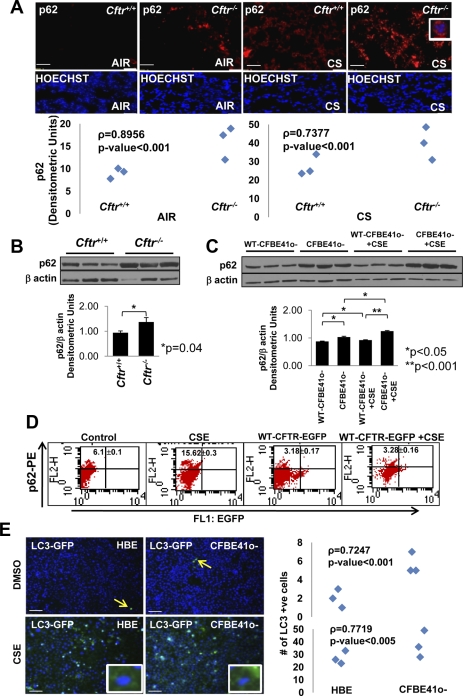
Autophagy is a critical cellular homeostatic process that disposes the damaged protein aggregates (aggresomes) that are associated with several chronic inflammatory diseases and cancer (9, 34). A recent study by Luciani et al. (19) clearly demonstrated a critical role of CFTR in maintaining the robust autophagic machinery, and defective CFTR results in the inhibition of autophagy, leading to an inflammatory outcome. We analyzed the accumulation of p62 (a defective autophagy marker) in longitudinal lung sections of room air- and acute CS-exposed Cftr+/+ and Cftr−/− mice. We demonstrated a significant upregulation (P < 0.05) of p62 expression in Cftr−/− murine lungs compared with Cftr+/+ murine lungs (Fig. 5, A and B). Acute CS exposure induced p62 expression and perinuclear accumulation (Fig. 5A, inset, top right) in murine lungs that was significantly higher (P < 0.001) in Cftr−/− mice compared with Cftr+/+ mice. We further evaluated this hypothesis in two CSE-exposed human cell lines: CFBE41o-wt-CFTR and CFBE41o− cells. We demonstrated that intrinsically higher p62 expression in CFBE41o− cells can be corrected by stable WT CFTR expression in CFBE41o-wt-CFTR cells (P < 0.05; Fig. 5C). Moreover, treatment of these cells with CSE further induced p62 expression, but it was significantly lower in the presence of WT CFTR (P < 0.001; Fig. 5C). We verified this observation in CSE-treated HEK-293 cells overexpressing WT CFTR and observed a significant decrease in CSE-induced p62-positive cells with CFTR expression (Fig. 5D). The accumulation of LC3 punctate (perinuclear aggregates) is considered a reliable marker of autophagy (19). We found a significant increase in the perinuclear accumulation of GFP-LC3 in CFBE41o− cells compared with 16HBEo− cells (P < 0.001; Fig. 5E, top), which was markedly induced by CSE treatment (P < 0.005; Fig. 5E, bottom). Moreover, we also found an increase in constitutive as well as acute CS-induced LC3 expression in Cftr−/− murine lungs compared with Cftr+/+ murine lungs (Supplemental Fig. S2C). We not only confirmed here the findings of Luciani et al. but also demonstrated the critical role of CFTR in regulating CS-induced autophagy responses. The absence of CFTR resulted in a significant increase in p62-positive perinuclear aggregates (aggresomes) that may explain unrelenting inflammation and apoptosis. Our data demonstrate a novel mechanism by which CFTR protects alveolar epithelial cells from CS-induced aggresome formation and the resulting inflammatory/apoptotic phenotype.
Fig. 5.
Absence of CFTR triggers CS-induced aberrant autophagy responses. A, top: longitudinal lung sections of Cftr+/+ and Cftr−/− mice exposed to room air or CS were immunostained for p62. Middle: costaining with Hoechst dye was used to localize the nucleus (blue). Bottom: the data indicate a significant increase in p62 expression (P < 0.001) and perinuclear localization (inset, top right) in the absence of CFTR (both constitutive and after CS exposure). B: Western blot analysis of total protein lysates from Cftr+/+ and Cftr−/− mice (top) demonstrated a significant constitutive increase in p62 protein levels in Cftr−/− murine lungs compared with Cftr+/+ murine lungs (P = 0.04, mean ± SE; bottom). C: the Western blot analysis (top) data showed that the intrinsically higher p62 protein expression in CFBE41o− cells could be significantly downregulated by stable WT CFTR expression (CFBE41o-wt-CFTR cells, P < 0.05, mean ± SE; bottom). Treatment with CSE further induced p62 levels in CFBE41o− cells, although the increase was much lower in the presence of WT CFTR (P < 0.05; bottom). D: flow cytometry analysis of HEK-293 cells showed that WT CFTR expression significantly controls CSE induced in p62-positve cells (P < 0.001, mean ± SD). The percentage of p62-positive cells is shown as the sum of the top right and upper left quadrants. E: CFBE41o− cells showed a significant (P < 0.001; right) increase in the perinuclear accumulation of the autophagy marker GFP-microtubule-associated protein 1 light chain-3 (LC3) (inset, left) compared with 16HBEo− cells (n = 3), which have normal membrane CFTR levels. The data also show a further significant (P < 0.005; right) increase in CSE-induced GFP-LC3 perinuclear aggregates (inset) in the absence of membrane CFTR (CFBE41o− cells). White scale bars = 50 μm; red scale bar = 10 μm. The data imply that WT CFTR is required for a protective autophagy response in acute CS-mediated lung injury.
DISCUSSION
We selected the acute CS exposure model to detect early events involved in CS-induced lung injury. Based on our recent data (4), we investigated the role of CFTR in regulating apoptosis and autophagy in CS-induced lung injury. We demonstrate that CFTR-dependent lipid rafts inhibit the expression of TNF receptor superfamily member 6 (Fas), which may prevent alveolar-epithelial cell apoptosis in response to CS exposure. Our data confirm that membrane CFTR controls Fas-mediated apoptosis by regulating the expression of ceramide and the lipid raft proteins ZO-1 and ZO-2. Moreover, CFTR also regulates alveolar homeostasis by maintaining a robust autophagy response to CS-induced lung injury. The absence of CFTR results in alveolar apoptosis and defective autophagy (increased perinuclear p62 accumulation), which may exacerbate the CS-induced lung injury and lead to the pathogenesis of severe emphysema.
Recent data from our laboratory (4) have demonstrated the critical role of CFTR in regulating ceramide signaling during lung injury and emphysema. CFTR is localized in the lipid rafts and regulates the pathophysiology of chronic lung diseases such as CF, COPD, and emphysema (4, 10, 50). Although the development of chronic lung disease and emphysema clearly involves apoptosis and inflammation of alveolar epithelial and endothelial cells (28) that results in nonreversible damage to the lung tissue (12, 30), the factors governing apoptotic cell death and chronic inflammation are not apparent. It is becoming clear that CF subjects have a significant accumulation of alveolar apoptotic and inflammatory cells as well as defective autophagy (19, 38, 43, 44). Moreover, lack of CFTR triggers ceramide accumulation by regulating membrane acidic sphingomyelinase activity and/or intracellular pH (4, 29, 39), which assists in the formation of signaling platforms that are required for clustering of the Fas receptor and the consequent activation of the apoptotic cascade (24). We hypothesized that since CFTR expression is significantly downregulated by CS exposure (4) and in COPD subjects (6) with severe emphysema (4), it may be one of the possible regulatory components of the apoptotic signaling complex that responds to CS-induced lung injury. Our present work confirms this hypothesis and shows that lack of CFTR triggers a higher number of apoptotic cells in the lungs of CS-exposed mice. We also observed a similar increase in Fas, ceramide, and ZO-1/2 expression, suggesting that membrane CFTR controls apoptosis by controlling the lipid raft clustering of Fas (Fig. 6). We verified that the expression of a common lipid raft loading control (α-actin) did not differ from β-actin under conditions of CFTR deficiency or CS exposure (Supplemental Fig. S3). This rules out a global effect of CFTR deficiency or CS exposure on lipid raft proteins.
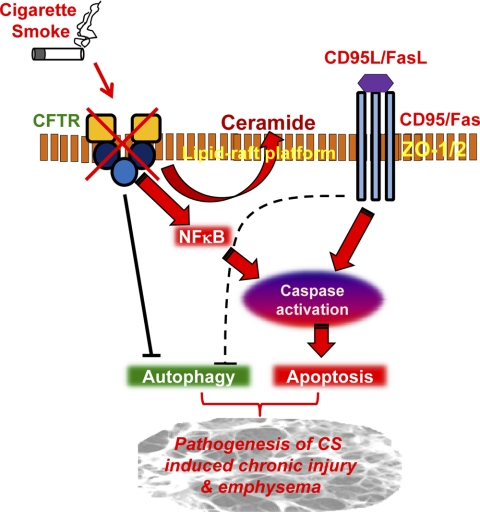
Fig. 6.
Schematic showing the regulation of CS-induced lung injury by lipid raft CFTR. The schematic illustrates the critical role of lipid raft CFTR in controlling apoptotic (CD95/Fas) and autophagy responses to CS-induced lung injury. Our model predicts that the CS-mediated decrease in lipid raft CFTR expression modulates these responses via NF-κB/Fas, resulting in increased apoptosis and aberrant autophagy. Our data suggest that environmental factors such as CS exposure modulate the lipid raft clustering by controlling lipid raft CFTR expression, which regulates membrane ceramide accumulation. We anticipate that the CS-mediated decrease in lipid raft CFTR expression induces membrane ceramide accumulation, which results in lipid raft fusion and large-scale clustering of membrane receptors (like Fas) as a mechanism for the pathogenesis of chronic lung injury and emphysema. FasL, Fas ligand.
Previous reports on the role of CFTR in regulating apoptosis are not conclusive (21, 22, 27, 29, 36, 50) . It has been reported that CF epithelial cells have increased expression and secretion of Fas and Fas ligand, respectively (11). Moreover, the increased apoptosis of tracheal and pancreatic CF cells correlated with the increase in inflammatory cytokines and NF-κB activation (36). There is evidence for a direct role of CFTR in apoptosis, where small interfering (si)RNA-mediated knockdown of CFTR conferred a proinflammatory phenotype and apoptosis induction in alveolar macrophages (50). Furthermore, defective CFTR is also known to hamper the mechanism of efferocytosis, or phagocytosis of apoptotic cells, resulting in the accumulation of apoptotic cells with proinflammatory consequences (17, 45). It is known that ceramide accumulation mediates inflammation, cell death, and infection susceptibility in CF mice (39), indicating that CFTR deficiency promotes apoptosis in both CF cell lines and Cftr−/− mice. A plethora of evidence suggests that CFTR deficiency triggers NF-κB-mediated inflammation in CF cell lines and Cftr−/− murine lungs (35, 36, 46) that, by itself, induces apoptotic cell death. Moreover, the role of CFTR in apoptosis is complex, cell type specific, and requires further evaluation. It may be plausible that defective CFTR also modulates other cellular homeostatic mechanisms that lead to a prolonged proinflammatory lung phenotype in CF subjects (19).
It has been recently reported that defective CFTR induces aggresome formation, inflammation, and aberrant autophagy in CF airway epithelial cell lines and human CF subjects (19, 20). We investigated, based on our recent findings (4) in emphysematous lung tissue, that decreased CFTR expression may correlate with the deregulation of autophagy in CS-induced lung injury. Our present data suggest that the absence of membrane CFTR alters the autophagy response to CS. The increase in perinuclear p62 and LC3β accumulation in Cftr−/− murine lungs compared with Cftr+/+ murine lungs implies that CFTR deficiency leads to an accumulation of damaged proteins triggered by CS exposure. In support of our observations, Monick et al. (25) identified an autophagy defect in alveolar macrophages from smokers, suggesting that altered autophagy may play a crucial role in the pathogenesis of CS-induced lung injury and emphysema. We also observed increased perinuclear accumulation of GFP-LC3 in the CSE-treated CFBE41o− cells compared with 16HBEo− cells, indicating an accumulation of autophagosomes in membrane CFTR-deficient epithelial cells. Recent reports by Choi and colleagues (7, 37) have demonstrated that LC3β is a positive regulator of CS-induced lung epithelial cell death. Our data corroborate their findings, as we observed a positive correlation between CSE-induced perinuclear GFP-LC3 accumulation and increased epithelial cell death that was aggravated in CFTR-deficient cells. Moreover, we also observed a concurrent increase in p62 and LC3β accumulation (Fig. 5A and Supplemental Fig. S2C) and expression suggesting a defective clearance of autophagosomes in the lungs of Cftr−/− mice exposed to CS.
In conclusion, our present work correlates lack of membrane CFTR expression with an increase in CS-induced apoptosis and defective autophagy. Moreover, we also found that CFTR-dependent lipid rafts regulate ceramide release and Fas expression and clustering as a mechanism to control CS-induced alveolar cell apoptosis and autophagy (Fig. 6). We propose that rescue or restoration of optimal functional CFTR in the lipid rafts may be a promising therapeutic strategy to control CS-induced apoptosis, lung injury, and emphysema.
GRANTS
This work was supported by grants from Cystic Fibrosis Foundation, the Flight Attendant Medical Research Institute, and National Institutes of Health (RR-025005 and RHL-096931) to N. Vij.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Dieter Gruenert for providing the CFBE41o− and CFBE41o-wt-CFTR cell lines.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Lung Cellular and MolecularPhysiology website.
REFERENCES
- 1. Balmes JR. When smoke gets in your lungs. Proc Am Thorac Soc 7: 98–101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barnes PJ. New therapies for chronic obstructive pulmonary disease. Med Princ Pract 19: 330–338, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Becker KA, Riethmuller J, Luth A, Doring G, Kleuser B, Gulbins E. Acid sphingomyelinase inhibitors normalize pulmonary ceramide and inflammation in cystic fibrosis. Am J Respir Cell Mol Biol 42: 716–724, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Bodas M, Min T, Mazur S, Vij N. Critical modifier role of membrane-cystic fibrosis transmembrane conductance regulator-dependent ceramide signaling in lung injury and emphysema. J Immunol 186: 602–613, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bodas M, Min T, Vij N. Early-age-related changes in proteostasis augment immunopathogenesis of sepsis and acute lung injury. PLoS One 5: e15480, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med 173: 1139–1144, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, Ifedigbo E, Parameswaran H, Ryter SW, Choi AM. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci USA 107: 18880–18885, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res 7: 53, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dohm CP, Kermer P, Bahr M. Aggregopathy in neurodegenerative diseases: mechanisms and therapeutic implication. Neurodegener Dis 5: 321–338, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Dudez T, Borot F, Huang S, Kwak BR, Bacchetta M, Ollero M, Stanton BA, Chanson M. CFTR in a lipid raft-TNFR1 complex modulates gap junctional intercellular communication and IL-8 secretion. Biochim Biophys Acta 1783: 779–788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Durieu I, Amsellem C, Paulin C, Chambe MT, Bienvenu J, Bellon G, Pacheco Y. Fas and Fas ligand expression in cystic fibrosis airway epithelium. Thorax 54: 1093–1098, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Filosto S, Castillo S, Danielson A, Franzi L, Khan E, Kenyon N, Last J, Pinkerton K, Tuder R, Goldkorn T. Neutral sphingomyelinase 2: a novel target in cigarette smoke-induced apoptosis and lung injury. Am J Respir Cell Mol Biol 44: 350–360, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fullerton DG, Bruce N, Gordon SB. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg 102: 843–851, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grassme H, Cremesti A, Kolesnick R, Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene 22: 5457–5470, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem 276: 20589–20596, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Hamai H, Keyserman F, Quittell LM, Worgall TS. Defective CFTR increases synthesis and mass of sphingolipids that modulate membrane composition and lipid signaling. J Lipid Res 50: 1101–1108, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol 11: R795–R805, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Jacquot J, Tabary O, Le Rouzic P, Clement A. Airway epithelial cell inflammatory signalling in cystic fibrosis. Int J Biochem Cell Biol 40: 1703–1715, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, Gavina M, Pulze L, Giardino I, Pettoello-Mantovani M, D'Apolito M, Guido S, Masliah E, Spencer B, Quaratino S, Raia V, Ballabio A, Maiuri L. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol 12: 863–875, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina DL, Settembre C, Gavina M, Raia V, Ballabio A, Maiuri L. Cystic fibrosis: a disorder with defective autophagy. Autophagy 7: 92–94, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Maiuri L, Raia V, De Marco G, Coletta S, de Ritis G, Londei M, Auricchio S. DNA fragmentation is a feature of cystic fibrosis epithelial cells: a disease with inappropriate apoptosis? FEBS Lett 408: 225–231, 1997 [DOI] [PubMed] [Google Scholar]
- 22. McKeon DJ, Condliffe AM, Cowburn AS, Cadwallader KC, Farahi N, Bilton D, Chilvers ER. Prolonged survival of neutrophils from patients with Delta F508 CFTR mutations. Thorax 63: 660–661, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Min T, Bodas M, Mazur S, Vij N. Critical role of proteostasis-imbalance in pathogenesis of COPD and severe emphysema. J Mol Med; http://www.springerlink.com/content/h3077753lw797wk1/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miyaji M, Jin ZX, Yamaoka S, Amakawa R, Fukuhara S, Sato SB, Kobayashi T, Domae N, Mimori T, Bloom ET, Okazaki T, Umehara H. Role of membrane sphingomyelin and ceramide in platform formation for Fas-mediated apoptosis. J Exp Med 202: 249–259, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Monick MM, Powers LS, Walters K, Lovan N, Zhang M, Gerke A, Hansdottir S, Hunninghake GW. Identification of an autophagy defect in smokers' alveolar macrophages. J Immunol 185: 5425–5435, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moreau K, Luo S, Rubinsztein DC. Cytoprotective roles for autophagy. Curr Opin Cell Biol 22: 206–211, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moriceau S, Lenoir G, Witko-Sarsat V. In cystic fibrosis homozygotes and heterozygotes, neutrophil apoptosis is delayed and modulated by diamide or roscovitine: evidence for an innate neutrophil disturbance. J Innate Immun 2: 260–266, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Morissette MC, Parent J, Milot J. Alveolar epithelial and endothelial cell apoptosis in emphysema: what we know and what we need to know. Int J Chron Obstruct Pulmon Dis 4: 19–31, 2009 [PMC free article] [PubMed] [Google Scholar]
- 29. Noe J, Petrusca D, Rush N, Deng P, VanDemark M, Berdyshev E, Gu Y, Smith P, Schweitzer K, Pilewsky J, Natarajan V, Xu Z, Obukhov AG, Petrache I. CFTR regulation of intracellular pH and ceramides is required for lung endothelial cell apoptosis. Am J Respir Cell Mol Biol 41: 314–323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park JW, Ryter SW, Choi AM. Functional significance of apoptosis in chronic obstructive pulmonary disease. Copd 4: 347–353, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Park MA, Zhang G, Norris J, Hylemon PB, Fisher PB, Grant S, Dent P. Regulation of autophagy by ceramide-CD95-PERK signaling. Autophagy 4: 929–931, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 11: 491–498, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc 1: 1458–1461, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Rodriguez-Gonzalez A, Lin T, Ikeda AK, Simms-Waldrip T, Fu C, Sakamoto KM. Role of the aggresome pathway in cancer: targeting histone deacetylase 6-dependent protein degradation. Cancer Res 68: 2557–2560, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Rottner M, Freyssinet JM, Martinez MC. Mechanisms of the noxious inflammatory cycle in cystic fibrosis. Respir Res 10: 23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rottner M, Kunzelmann C, Mergey M, Freyssinet JM, Martinez MC. Exaggerated apoptosis and NF-κB activation in pancreatic and tracheal cystic fibrosis cells. FASEB J 21: 2939–2948, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Ryter SW, Lee SJ, Choi AM. Autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. Expert Rev Respir Med 4: 573–584, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tabary O, Corvol H, Boncoeur E, Chadelat K, Fitting C, Cavaillon JM, Clement A, Jacquot J. Adherence of airway neutrophils and inflammatory response are increased in CF airway epithelial cell-neutrophil interactions. Am J Physiol Lung Cell Mol Physiol 290: L588–L596, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, Weller M, Tummler B, Lang F, Grassme H, Doring G, Gulbins E. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med 14: 382–391, 2008. 18376404 [Google Scholar]
- 40. Uhlig S, Gulbins E. Sphingolipids in the lungs. Am J Respir Crit Care Med 178: 1100–1114, 2008 [DOI] [PubMed] [Google Scholar]
- 41. van Heeckeren AM, Schluchter MD, Drumm ML, Davis PB. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol 287: L944–L952, 2004 [DOI] [PubMed] [Google Scholar]
- 42. van Heeckeren AM, Schluchter MD, Xue W, Davis PB. Response to acute lung infection with mucoid Pseudomonas aeruginosa in cystic fibrosis mice. Am J Respir Crit Care Med 173: 288–296, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vandivier RW, Fadok VA, Hoffmann PR, Bratton DL, Penvari C, Brown KK, Brain JD, Accurso FJ, Henson PM. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest 109: 661–670, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vandivier RW, Fadok VA, Ogden CA, Hoffmann PR, Brain JD, Accurso FJ, Fisher JH, Greene KE, Henson PM. Impaired clearance of apoptotic cells from cystic fibrosis airways. Chest 121: 89S, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Vandivier RW, Richens TR, Horstmann SA, deCathelineau AM, Ghosh M, Reynolds SD, Xiao YQ, Riches DW, Plumb J, Vachon E, Downey GP, Henson PM. Dysfunctional cystic fibrosis transmembrane conductance regulator inhibits phagocytosis of apoptotic cells with proinflammatory consequences. Am J Physiol Lung Cell Mol Physiol 297: L677–L686, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vij N, Mazur S, Zeitlin PL. CFTR is a negative regulator of NFκB mediated innate immune response. PLoS One 4: e4664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vij N, Roberts L, Joyce S, Chakravarti S. Lumican regulates corneal inflammatory responses by modulating Fas-Fas ligand signaling. Invest Ophthalmol Vis Sci 46: 88–95, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respir Care 48: 1204–1213, 2003 [PubMed] [Google Scholar]
- 49. Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 295: L1–L15, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu Y, Krause A, Hamai H, Harvey BG, Worgall TS, Worgall S. Proinflammatory phenotype and increased caveolin-1 in alveolar macrophages with silenced CFTR mRNA. PLoS One 5: e11004, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-κB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol 291: L46–L57, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Zhang Y, Wu Y, Cheng Y, Zhao Z, Tashiro S, Onodera S, Ikejima T. Fas-mediated autophagy requires JNK activation in HeLa cells. Biochem Biophys Res Commun 377: 1205–1210, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.