Abstract
We previously reported that zinc thiolate signaling contributes to hypoxic contraction of small, nonmuscularized arteries of the lung. The present studies were designed to investigate mechanisms by which hypoxia-released zinc induces contraction in isolated pulmonary endothelial cells and to delineate the signaling pathways involved in zinc-mediated changes in the actin cytoskeleton. We used fluorescence-based imaging to show that hypoxia induced time-dependent increases in actin stress fibers that were reversed by the zinc chelator, N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN). We further showed that hypoxia-induced phosphorylation of the contractile protein myosin light chain (MLC) and assembly of actin stress fibers were each TPEN sensitive. Hypoxia and zinc-induced inhibition of MLC phosphatase (MLCP) were independent of the regulatory subunit (MYPT1) of MLCP, and therefore hypoxia-released zinc likely inhibits MLCP at its catalytic (PP1) subunit. Inhibition of PKC by Ro-31–8220 and a dominant-negative construct of PKC-ε attenuated hypoxia-induced contraction of isolated pulmonary endothelial cells. Furthermore, zinc-induced phosphorylation of MLC (secondary to inhibition of MLCP) was PKC dependent, and hypoxia-released zinc promoted the phosphorylation of the PKC substrate, CPI-17. Collectively, these data suggest a link between hypoxia, elevations in labile zinc, and activation of PKC, which in turn acts through CPI-17 to inhibit MLCP activity and promote MLC phosphorylation, ultimately inducing stress fiber formation and endothelial cell contraction.
Keywords: pulmonary circulation, endothelial cells, actin stress fibers
our recent data showed that acute hypoxia-induced increases in nitric oxide (NO) biosynthesis result in increases in intracellular free zinc that in turn contribute to vasoconstriction of small, intra-acinar arteries of lung (8). Because this anatomic site is composed primarily of endothelial cells with only solitary or discontinuous smooth muscle-like cells (e.g., pericytes) in their wall (13), we investigated the potential for hypoxia-zinc-mediated contraction in pulmonary endothelium and confirmed that isolated pulmonary (but not systemic) endothelial cells constricted in hypoxia (8) and that these contractile events were associated with hypoxia-induced increases in labile zinc. The mechanism by which zinc can induce vasoconstriction in the pulmonary vasculature is not known. However, zinc-associated proteins account for roughly 10% of the human proteome (26), and many of these putative targets for hypoxia-released zinc are involved in signaling pathways regulating cellular contractility.
Endothelial cells contain all the molecular machinery required to generate contractile force via the actomyosin motor. Contraction is initiated by phosphorylation of the 20-kDa regulatory myosin light chain (MLC) at S19/T18 (17). The phosphorylation of MLC is dependent on a balance between the activities of calcium/calmodulin-dependent MLC kinase (MLCK) and MLC phosphatase (MLCP). Remodeling of the endothelial actin cytoskeletal in response to either hypoxia or the procoagulant protein, thrombin, involves MLC and actin-related proteins (16). One potential connection between these signaling pathways and zinc is the relationship between divalent metal ions and sulfhydryl residues in the activation of type 1 and type 2A serine/threonine phosphoprotein phosphatases (PPases), with zinc having been shown to be a potent inhibitor of λ-PPase (43). A further link is provided by the indirect evidence supporting a role for zinc in strengthening of focal adhesions (3) although the mechanism(s) underlying this phenomenon are not clear. Likely participants in endothelial contractile pathways include Rho/Rho kinase (ROCK) and PKC isoforms, the latter of which are known to be tightly regulated by zinc (27) and participate in hypoxic pulmonary vasoconstriction (HPV) (24) (i.e., PKC-ε).
The present studies were designed to investigate the mechanisms by which hypoxia-released zinc induces contraction in pulmonary endothelium and to delineate the signaling pathways involved in zinc-mediated changes in the actin cytoskeleton. We hypothesized that hypoxia-induced alterations in zinc homeostasis promote endothelial cell contraction via changes in the phosphorylation status of MLC and increased formation/stabilization of actin stress fibers.
MATERIALS AND METHODS
Chemicals and materials.
All reagents were purchased from Sigma-Aldrich (www.sigmaaldrich.com, St. Louis, MO) unless otherwise noted. Details of the dominant-negative PKC-ε herpes simplex virus were provided elsewhere (34). The enhanced green fluorescent protein (EGFP) actin construct was a gift from Michael Davidson at The Florida State University.
Cell culture.
Cells were grown at 37°C with 5% CO2. Details regarding the culture of sheep pulmonary artery endothelial cells (SPAEC) are described elsewhere (36). Rat pulmonary microvascular endothelial cells (RPMVEC) were purchased from VEC Technologies (VEC Technologies, Rensselaer, NY) and grown in complete MCDB-131 media (Lonza, Allendale, NJ). Rat pulmonary artery endothelial cells (RPAEC) were grown in DMEM (Fisher Scientific, Waltham, MA) with 10% fetal bovine serum, 2 mM glutamine, and 100 U/ml penicillin/streptomycin.
Immunofluorescence.
Cells were seeded on glass coverslips coated with laminin (11.34 μg/ml, Fisher Scientific). Hypoxic exposures were performed inside a hypoxic chamber (Coy Laboratory Products, Grass Lake, MI). Following treatment, cells were fixed, and permeabilized in 2% paraformaldehyde with 0.1% Triton X-100. Alexa Fluor488 Phalloidin was used for F-actin staining of stress fibers. Sequential XYZ-sections (1024X1024 pixels, at Nyquist axial frequency) were obtained using an Olympus Fluoview 1000 confocal microscope (Bethlehem, PA) equipped with a ×60 oil-immersion optic (NA, 1.43). Three-dimensional images were reconstructed using Metamorph (Molecular Devices, Downingtown, PA), and quantification of actin stress filaments was determined by volume rendering in Imaris (Bitplane, Saint Paul, MN).
Live cell imaging.
Cells were seeded on 35-mm laminin-coated glass-bottom dishes (MatTek, Ashland, MA) and imaged in a closed, thermocontrolled (37°C) stage-top incubator (Tokai-Hit, Tokyo, Japan). Images were obtained using a Nikon TE2000E (Melville, NY) microscope equipped with a ×40 oil-immersion objective (Nikon, CFI PlanFluor, NA 1.3) and Q-Imaging Retiga EXI camera (Burnaby, BC, Canada). MetaMetamorph (Molecular Devices) was used to collect and analyze data and to drive the microscope. Hypoxic conditions were obtained by bubbling the media with hypoxic gas (90% N2-5% CO2-1.5% O2), which reduced oxygen tension to 15 ± 2 mmHg, as measured using a Clarke electrode.
Total internal reflectance fluorescence imaging.
Cells were imaged on a Nikon TiE inverted microscope with a 1.49 NA oil-immersion objective capable of both epifluorescence and total internal reflectance fluorescence (TIRF) microscopy illumination through the objective. EGFP was excited with a 488-nm Coherent Sapphire laser (Coherent, Santa Clara, CA). Laser intensities were controlled using a Neos AOTF mounted on a Prairie Technologies laser bench (Madison, WI). To ensure correct image registration, a triple-pass filter cube (488 nm, 561 nm, 638 nm) and matched emitter filters were used (Chroma, Brattleboro, VT). Images were collected using a Photometrics HQ2 Coolsnap camera (Photometrics, Tucson, AZ) at full resolution. Data were collected and analyzed using NIS-Elements (Nikon).
Western blotting.
Mouse monoclonal PKC-ε antibody (E-5) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and used at a 1:500 dilution. MLC2, phospho-MLC (Thr18/Ser19), MYPT1, and phospho-MYPT1 (Thr853) antibodies were purchased from Cell Signaling Technology (Beverly, MA) and used at 1:1,000 dilutions. CPI-17 and phospho-CPI-17 antibodies were purchased from Santa Cruz Biotechnology and used at 1:100 dilutions. Total protein levels were normalized to the level of β-actin for each sample, and phosphorylated protein expressed relative to total protein levels.
Membrane fractionation.
Cells were lysed (100 mM Tris·HCl, pH 7.4, 1%, vol/vol Nonidet P-40, 10 mM NaF, 1 mM vanadate, 10 μg/ml of aprotinin, 10 μg/ml of leupeptin) and centrifuged for 10 min at 14,000 revolution/min. The supernatant was further centrifuged at 43,000 g for 30 min (21) to separate cytoplasmic and membranes fraction.
PKC-ε immunoprecipitation and enzyme activity assay.
Cells were lysed in modified RIPA buffer (100 mM Tris·HCl, pH 7.4, 1%, vol/vol, Nonidet-P40 10 mM NaF, 1 mM vanadate, 10 μg/ml of aprotinin, 10 μg/ml of leupeptin). Insoluble material was removed by centrifugation, and protein concentrations were determined using the Bio-Rad DC protein assay (Bio-Rad, Hercules. CA). Equal amounts of protein were precleared with protein A-Sepharose and incubated with antibody for 2 h at 4°C. The immune complexes were isolated with Protein A-Sepharose, washed, and eluted. Equal amounts of immunocomplex were then subjected to PKC-ε kinase assay, as described previously (6).
Statistical analysis.
Data are presented as means ± SD. Comparisons between more than two groups were done using ANOVA followed by Dunnett's posttest. A value of P < 0.05 was considered statistically significant.
RESULTS
Hypoxia induces zinc-dependent changes in the actin cytoskeleton of isolated pulmonary microvascular endothelial cells.
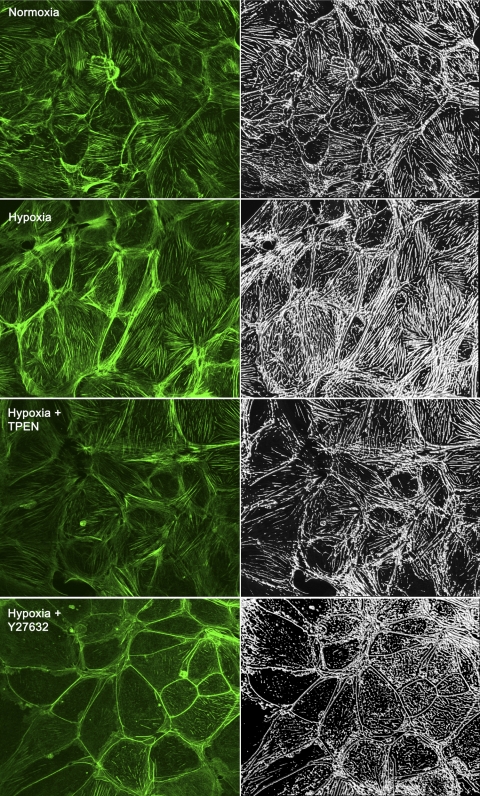
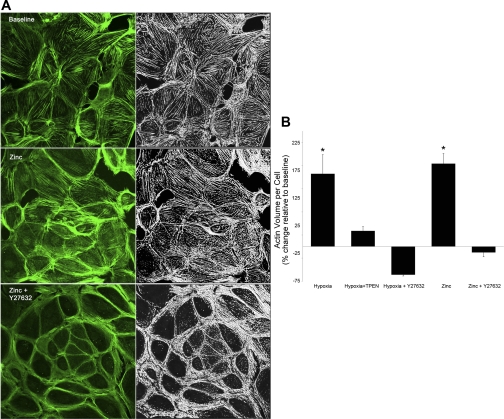
We previously reported that hypoxia induced increases in labile zinc in small intra-acinar arteries of the isolated perfused mouse lung (8). The observation that hypoxic vasoconstriction was blunted in the lungs of mice in which the major zinc binding protein (metallothionein; MT) was knocked out (MT−/− mice), or in wild-type mice perfused with the zinc chelator, N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN), led us to hypothesize that observed increases in intracellular zinc contribute to constriction in the pulmonary microvasculature. The anatomic site in question was shown to be composed primarily of endothelial cells (8), and these initial investigations confirmed the potential for hypoxia-zinc-mediated contraction in isolated primary cultures of pulmonary endothelium. In the present report, we first assessed the zinc dependency of hypoxia-induced changes in the actin cytoskeleton in isolated rat pulmonary microvascular endothelial cells (RPMVEC). Hypoxic exposure increased the abundance or total volume of actin per cell, as well as the alignment of actin stress fibers (Fig. 1, mean data Fig. 2B) compared with normoxia. Treatment with TPEN reduced the effects of hypoxia on the actin cytoskeleton (Figs. 1 and 2B), suggesting that hypoxia-induced changes in intracellular zinc contribute to the formation or stabilization of actin stress filaments in RPMVEC. Although early reports suggest that zinc can alter skeletal muscle contractility (19), little is known about the effects of zinc on the intracellular contractile apparatus in either muscle or nonmuscle cells. Our data in fixed pulmonary endothelial cells showed that exogenous zinc (in the presence of the zinc ionophore, pyrithione) also increased the abundance and altered the distribution of actin stress fibers (Fig. 2, A and B), consistent with a contractile phenotype.
Fig. 1.
Hypoxia induces zinc-dependent changes in the actin cytoskeleton of isolated rat pulmonary microvascular endothelial cells (RPMVEC). Each image is a projected 3D reconstruction with Alexa 647-phalloidin staining of filamentous actin (left, green) and volume-rendered images of actin abundance (right, white). Hypoxia (30 min) increased both the total volume of actin per cell (mean data shown in Fig. 2B) and the alignment of actin stress fibers compared with normoxia. Treatment with the zinc chelator, N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN) (25 μM), or the Rho kinase inhibitor, Y27632 (10 μM), reduced the effects of hypoxia on the actin cytoskeleton.
Fig. 2.
Exogenous zinc increases actin stress fibers in isolated pulmonary microvascular endothelial cells. A: each image is a projected 3D reconstruction with Alexa 647-phalloidin staining of filamentous actin (left, green) and the volume-rendered image of actin abundance (right, white). Exogenous zinc (30 min, 10 μM + 2 μM pyrithione) increased both the total volume of actin per cell, as well as the alignment of actin stress fibers compared with baseline. The Rho kinase inhibitor, Y27632 (10 μM), reduced the effects of exogenous zinc on the actin cytoskeleton. B: mean data for an average of 125–200 cells per condition. *Significantly different from baseline (P < 0.01).
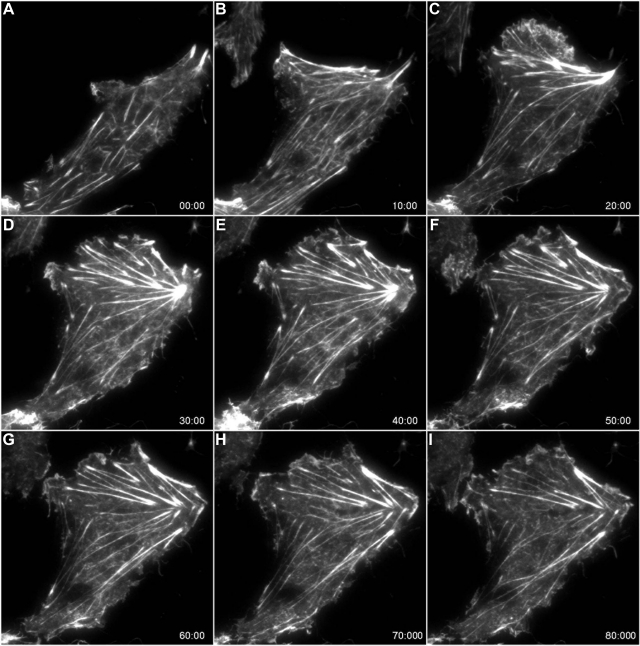
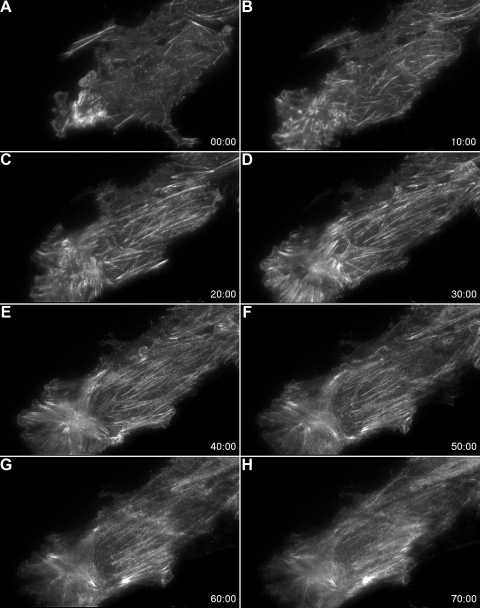
We next used TIRF microscopy of EGFP-tagged actin to examine hypoxia- and zinc-induced contractile events in live cultures of RPMVEC. TIRF relies on an evanescent wave generated perpendicular to the optical axis when light reflects off of a surface at an incident angle. The intensity of the evanescent wave decays exponentially and effectively penetrates only 100–150 nm beyond the coverslip into the cell, thereby confining the excitation of fluorophores to an extremely thin axial slice (20). As a result the signal-to-noise ratio in TIRF is extremely high. Although the approach will only image events at the basal surface of the cell, one can be certain that emitted signal is only derived from this region of the cell, as opposed to other methods that are not specifically constrained in the z-axis to a single plane (e.g., confocal). Using TIRF, we observed that hypoxia caused time-dependent and reversible (Fig. 3) increases in the abundance and alignment of basal stress fibers (Figs. 3 and 4, Supplemental movies S1 and S2; supplemental material for this article is available online at the American Journal of Physiology Lung Cellular and Molecular Physiology website). Consistent with the data in fixed cells (Fig. 1), the addition of the zinc chelator, TPEN, during hypoxia, resulted in the rapid disassembly of actin stress filaments (Fig. 4, Supplemental movie S2). We previously showed that isolated RPMVEC that were embedded in a flexible collagen matrix actively contracted in response to hypoxic stimuli (8). The resultant thickness of this collagen gel exceeded the working distance of the high numerical aperture objective (NA, 1.49; WD 120 μm) required for TIRF imaging. Thus for the TIRF studies, the cells were plated directly on laminin coated glass resulting in a stiff matrix, which permitted tension generation (stress fiber formation and stabilization) but precluded cellular contraction because of the rigidity of the underlying substrate (22).
Fig. 3.
Total internal reflectance fluorescence (TIRF) microscopy of enhanced green fluorescent protein (EGFP)-actin reveals hypoxia-induced time-dependent changes in the actin cytoskeleton. A–E: changes in basal stress fibers that occur in response to hypoxia (exposure was initiated after the baseline image taken in A). The cells were returned to normoxia at 40 min (following collection of the image shown in E). F–I: disassembly of stress fibers during the recovery period.
Fig. 4.
TIRF microscopy of EGFP-actin reveals that hypoxia-induced changes in the actin cytoskeleton are reversed by zinc chelation. A–E: response to a 40-min period of hypoxia (instituted after the collection of the baseline image shown in A). The addition of the zinc chelator, TPEN, during hypoxia (following collection of the image shown in E) resulted in a time-dependent disassembly of hypoxia-induced basal stress fibers (F–H).
Hypoxia and zinc induce MLCK-independent phosphorylation of MLC.
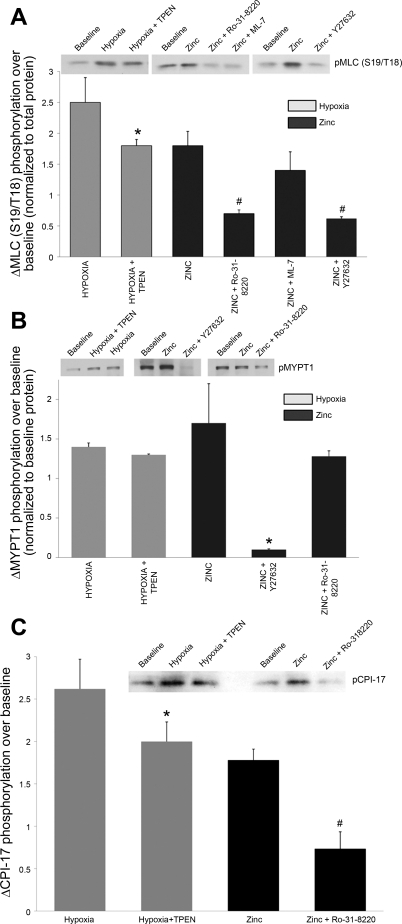
Similar to the contraction of smooth muscle, changes in contractile force in endothelial cells are accomplished via regulation of the level of phosphorylation of the regulatory MLC (33). In conjunction with the observed hypoxia-induced augmentation of actin stress fibers (Figs. 1–4), Western blotting revealed increases in diphosphorylated (2.5 ± 0.4-fold) MLC (Fig. 5A), which were reduced in the presence of TPEN (to a 1.8 ± 0.1-fold change). TPEN alone had no effect on phosphorylation of MLC (1.0 ± 0.1-fold change over control). Addition of exogenous zinc (10 μM + 2 μM pyrithione) also resulted in small but significant increases in MLC phosphorylation (1.6 ± 0.5-fold increase), supporting a role for zinc in modulating endothelial contractility. The MLCK-specific inhibitor ML-7 (4), which competes with ATP binding to the active sites on MLCK, had no effect on zinc-induced changes in stress fiber formation or MLC phosphorylation (1.4 ± 0.3-fold change with zinc in the presence of Ro-31–8220, Fig. 5A), suggesting that MLCK is not a downstream target for zinc in promoting increased actomyosin interactions. ML-7 alone had no effect on the phosphorylation of MLC (0.8 ± 0.2-fold change over baseline).
Fig. 5.
Hypoxia-induced changes in zinc homeostasis modulate myosin light chain (MLC) phosphorylation in isolated RPMVEC. A: representative Western blots and mean data for diphosphorylated MLC (S19/T18). Phosphorylated protein is expressed relative to total protein level (n = 3 to 5 separate experiments; *significantly different from hypoxia; #significantly different from zinc). B: representative Western blots and mean data for phosphorylated MYPT1 (T853, n = 3; *P <0.05). C: hypoxia- and zinc- induced phosphorylation of CPI-17 (T38) was suppressed by TPEN (n = 4, *P < 0.05) or PKC inhibition, respectively (Ro-31–8220, n = 3, #P < 0.05).
The Rho family of small GTPases plays a key role in regulating the endothelial contractile apparatus through inhibition of MLCP activity (25). ROCK signaling in smooth muscle has been shown to be critical to sustaining chronic HPV (40), and hypoxia-induced Rho/ROCK activation is associated with increased cell stiffness in pulmonary microvascular endothelial cells (2). Consistent with these reports, we found that hypoxia-mediated changes in the actin cytoskeleton (Figs. 1 and 2B), as well as hypoxia-induced increases in phosphorylated MLC (Fig. 5A), were dramatically reduced in pulmonary endothelial cells in the presence of the Rho kinase inhibitor, Y-27632 (10 μM). Furthermore, Y-27632 virtually abolished zinc-mediated increases in basal stress fiber formation (Fig. 2, A and B) and significantly decreased the zinc-induced phosphorylation of MLC (Fig. 5A).
Hypoxia-induced changes in MLCP activity are modulated by altered zinc homeostasis.
MLCP activity requires binding of the regulatory domain, MYPT1, to the catalytic domain (PP1c) as well as the substrate (myosin). Phosphorylation of MYPT1 reduces its binding affinity for myosin, inhibiting the activity of the holoenzyme (15, 39). Western blot analysis showed increases in phospho-MYPT1 (Thr853) in response to either hypoxia or exogenous zinc (1.4 ± 0.2-fold and 1.7 ± 0.1-fold, respectively; Fig. 5B). Exposure of RPMVECs to hypoxia or zinc in the presence of the ROCK inhibitor, Y-27632, resulted in a decrease in MYPT1 phosphorylation at Thr853 (Fig. 5B), further supporting the involvement of Rho/ROCK signaling in promoting stress fiber formation in response to zinc. Whereas hypoxia-induced MLC phosphorylation was shown to be sensitive to zinc chelation, TPEN had no effect on the phosphorylation of MYPT1 in response to hypoxia, suggesting that the inhibitory effects of hypoxia-released zinc on phosphatase function (and thus the phosphorylation status of MLC) could be mediated by zinc-related signaling that targets the catalytic subunit (PP1c) of the enzyme.
Independently of Rho/ROCK-mediated changes in cell contractility, PKC signaling pathways also regulate the organization of cytoskeletal proteins through the inhibition of MLCP and resulting increases in MLC phosphorylation (33). Of relevance to our data showing zinc-induced changes in endothelial cell contractility, intracellular zinc concentrations have been reported to influence PKC activity and processing (11, 12, 27). Accordingly, we found that zinc-induced phosphorylation of MLC was suppressed by PKC inhibition with Ro-31–8220 (Fig. 5A), suggesting that PKC-related signaling can transduce the effects of zinc to pulmonary endothelial cell contraction. Unlike the inhibition of MLCP by Rho/ROCK, PKC-mediated phosphatase inhibition acts through phosphorylation of the PKC substrate, CPI-17 (PKC-potentiated inhibitory protein of 17 kDa) rather than phosphorylation of MYPT1 (14), explaining why Ro-31–8220 had no effect on MYPT1 phosphorylation (Fig. 5B). Phosphorylation of CPI-17 at T38 promotes inhibition of the catalytic subunit of PP1 and the activity of the MLCP holoenzyme (14). We observed that hypoxia-induced phosphorylation of CPI-17 (T38) was suppressed by TPEN and that PKC inhibition (Ro-31–8220, Fig. 5C) reduced the zinc-mediated phosphorylation of the protein. Collectively these data indicate that hypoxia-released zinc contributes to changes in the actin cytoskeleton and contraction of pulmonary endothelium through activation of the PKC-CPI-17 signaling pathway and inhibition of MLCP.
Zinc-induced activation of PKC-ε modulates hypoxia-induced contraction of pulmonary endothelium.
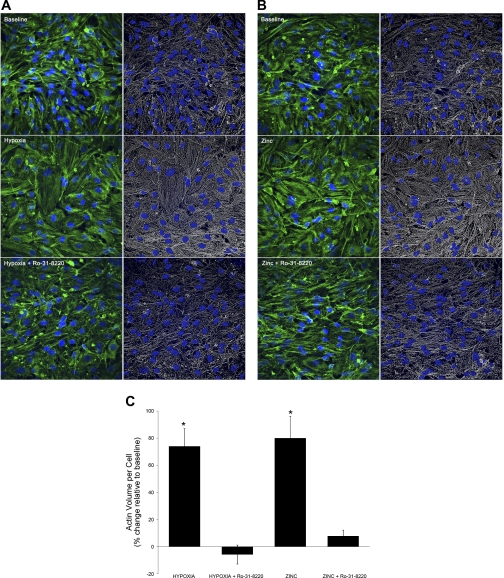
PKC activators, including mimetics of the natural ligand diacyglycerol (i.e., phorbol myristate acetate), stimulate contraction and potentiate HPV, whereas PKC inhibitors decrease HPV (29). Although the roles of individual isoforms of PKC in the control of pulmonary vascular reactivity have not been clearly defined, wide-body deletion of PKC-ε was shown to blunt murine HPV, leaving ANGII and KCl responses preserved (24). We postulated that PKC-ε, a zinc-sensitive signaling molecule in HPV (24), transduces hypoxia-mediated changes in zinc homeostasis. A role for PKC in mediating hypoxia-induced endothelial cell contraction was initially investigated in RPAEC using Ro-31–8220, which inhibits PKC-ε with an IC50 of 0.024 μM (42). As in RPMVEC (Fig. 1), either hypoxia (Fig. 6, A and C) or zinc (Fig. 6, B and C) increased the abundance or total volume of actin per cell, as well as the alignment of actin stress fibers in RPAEC compared with baseline. Treatment with Ro-31–8220 reduced the effects of both hypoxia and zinc on the actin cytoskeleton, further supporting a role for PKC signaling in mediating the effects of hypoxia-induced changes in intracellular zinc on the formation or stabilization of actin stress filaments in RPMVEC.
Fig. 6.
PKC-ε contributes to hypoxia- and zinc-induced stress fiber formation and contraction of pulmonary endothelium. Each image is a projected 3D reconstruction with Alexa 647-phalloidin staining of filamentous actin (phalloidin, green; nuclei, blue) and volume-rendered images of actin abundance (actin volume, white; nuclei, blue). Hypoxia (30 min) (A) or zinc (10 μM) (B) increased both alignment of stress fiber and the total volume of actin per cell compared with normoxia (C). Treatment with the PKC inhibitor, Ro-31–8220 (2 μM), attenuated the effects of hypoxia on the actin cytoskeleton (P < 0.01, C).
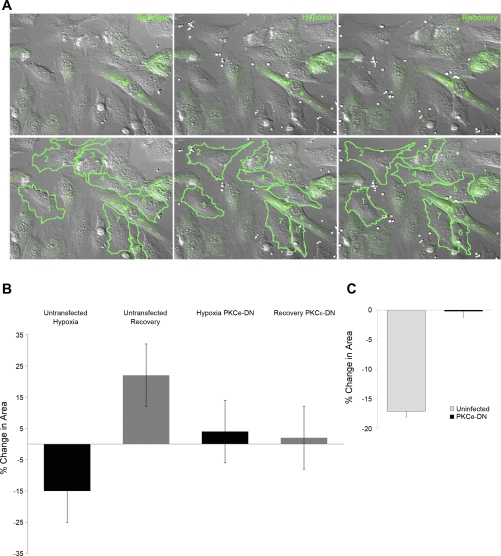
We adopted a dominant-negative approach to study the role of PKC-ε, specifically, in modulating hypoxia-induced endothelial cell contraction. The infection efficiency was extremely low in RPAEC, and thus, for these studies, they were replaced with SPAEC, which have been shown to 1) increase NO production when exposed to acute hypoxia (8), 2) increase intracellular labile zinc in response to NO (35, 36), and 3) contract in response to hypoxia (Fig. 7). Hypoxia-dependent contractile behavior was studied at the level of the single cell using high-resolution differential interference contract imaging of changes in cell surface area. We compared hypoxia-induced contractile responses between cells expressing the dominant-negative PKC-ε construct with uninfected cells within the same experiment (example shown in Fig. 7, A and B). Mean data from four separate experiments (Fig. 7C, with 2–6 cells per group, per experiment) suggested that PKC-ε serves as an effector molecule mediating hypoxia-induced constriction in the pulmonary vasculature in that the 17.0 ± 2.5% decrease in area in the uninfected cells was significantly greater than the contraction observed in cells expressing PKC-ε dominant negative (0.2 ± 1.5%, P = 0.012, Fig. 7C).
Fig. 7.
Dominant-negative (DN) PKC-ε attenuates hypoxia-induced contraction of sheep pulmonary artery endothelial cells (SPAEC). A: top: representative field of cells at baseline, during hypoxia (at 30 min) and following the normoxic recovery period (30 min). Bottom: an example of the analysis with regions of interest drawn around the uninfected cells in this field. The contractile responses of cells expressing the GFP-tagged PKC-εDN construct (green) were compared with cells that were not infected. In this example, the 7 uninfected cells were compared with 8 infected (green) cells. B: mean data for this example show a reversible (−15 ± 9%) decrease in cell surface area in response to hypoxia, whereas the infected cells in this field showed a variable response (mean change of +4 ± 11%). C: mean data for 4 separate experiments (with 2–6 cells per group, per experiment, P < 0.05).
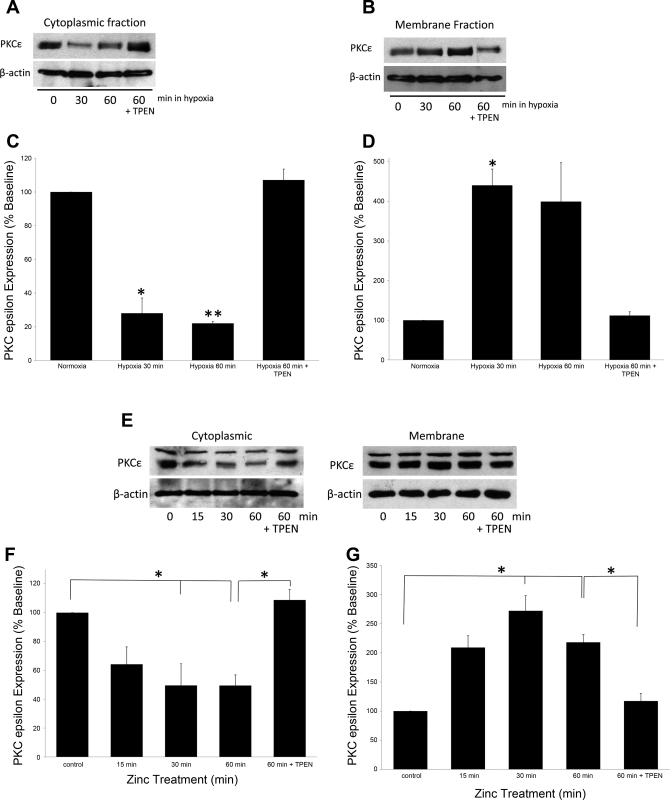
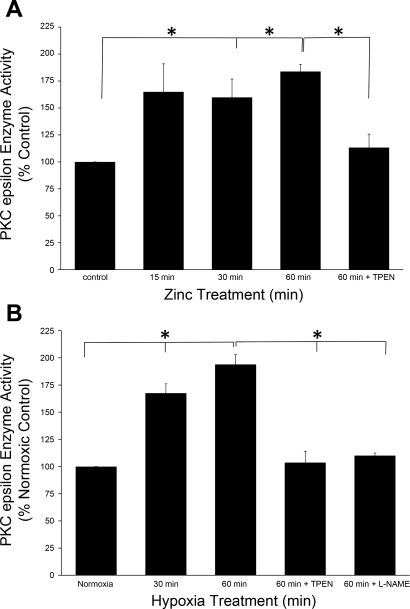
PKC enzyme activity is associated with the physical translocation of the enzyme from the cytosol to the cell membrane (30, 37). Using cell fractionation followed by Western blot analysis, we observed a time-dependent decrease in PKC-ε protein levels in the cytosolic fraction (P < 0.05, Fig. 8, A and C) and a corresponding increase in the appearance of the protein in the membrane fraction (P < 0.05, Fig. 8, B and D) when primary cultures of pulmonary endothelial cells were exposed to acute hypoxia. These hypoxia-induced effects were reversed by addition of the zinc-specific chelator, TPEN (10 μM, P < 0.001). Similar changes in PKC-ε localization were achieved by addition of exogenous zinc to the media during normoxia (Fig. 8, E–G, P < 0.05). Furthermore, exogenous zinc (10 μM)-induced increases (P < 0.05) in PKC-ε enzyme activity were prevented by TPEN (10 μM, Fig. 9A). Lastly, hypoxia induced time-dependent increases in PKC-ε enzyme activity (Fig. 9B) were reversed by the addition of either the NO synthase (NOS) inhibitor, nitro-l-arginine methyl ester (l-NAME) (1 mM, P < 0.05), or the zinc-specific chelator TPEN (10 μM, P < 0.001), confirming that the effects of hypoxic exposure on PKC-ε enzyme function in isolated pulmonary endothelial cells were regulated by NO-mediated changes in zinc homeostasis.
Fig. 8.
Hypoxia and zinc induce translocation of PKC-ε in isolated pulmonary endothelial cells. Western blot analysis of PKC-ε revealed a translocation of protein from the cytoplasm to the membrane fraction following hypoxia (A–D). These changes were reversed by the addition of 10 μM TPEN. Treatment with exogenous zinc also resulted in a significant loss of PKC-ε in the cytoplasm and accumulation in the membrane fraction (E–G) that was reversed by TPEN (10 μM). Protein levels were normalized to β-actin (n = 3, *P < 0.05, **P < 0.001).
Fig. 9.
Hypoxia-induced increases in PKC-ε activity are dependent on nitric oxide synthase (NOS) and zinc. A: zinc-induced increases in PKC-ε activity were reversed by TPEN (10 μM, n = 3, P < 0.05). B: hypoxia-induced increases in enzyme activity were reversed by the competitive NOS inhibitor nitro-l-arginine methyl ester (l-NAME) (1 mM) or the zinc-specific chelator, TPEN (10 μM, n = 3, *P < 0.05).
DISCUSSION
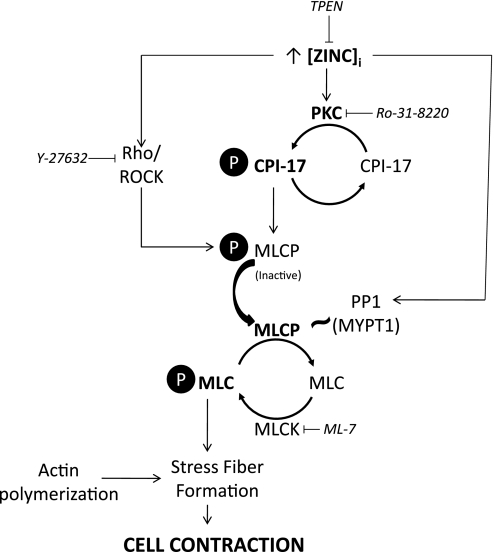
The process of endothelial cell contraction requires the formation of contractile actin stress fibers containing bipolar arrays of myosin II between consecutive α-actinin foci (28). Interactions between actin and myosin are dependent on the phosphorylation state of MLC, which in turn is determined by the balance between MLCK and MLCP activities. Although in vitro evidence supports activation of MLCK by zinc, it is not known whether this is a direct effect on the protein (31), nor is it known whether this activation contributes to force generation in vivo. Hypoxia has been shown to induce cytoskeletal changes in isolated pulmonary microvascular endothelial cells independent of MLCK activation (2). In accordance with these findings, we show that zinc is also capable of promoting MLC phosphorylation and stress fiber formation when MLCK is inhibited by ML-7, suggesting that zinc acts through inhibition of MLCP in promoting cell contractility (Fig. 10). In vitro data show that zinc can interact directly with the catalytic core of MLCP (PP1c) and cause conformational changes that promote destabilization of the holoenzyme (43). In our experience, zinc chelation (TPEN) decreased hypoxia-induced MLC phosphorylation (Fig. 5A) and caused disassembly of actin stress fibers during hypoxia (Figs. 1 and 3) but had no impact on phosphorylation of the regulatory subunit, MYPT1, of MLCP (Fig. 5B). These data imply that hypoxia released zinc signals through the PP1 subunit of MLCP to affect changes in enzyme activity and endothelial cell contraction (Fig. 10). In contrast, inhibition of ROCK had a marked effect on zinc-mediated phosphorylation of MYPT1. Although the Rho-associated binding protein kinase 1 (ROCK1) contains a conserved, cysteine-rich zinc-binding region in the C1 domain (23), the relationship between zinc and the ROCK pathway has not been investigated in the context of cellular contractility. Further studies are needed to determine what effects changes in intracellular zinc have on the Rho-binding properties and enzyme function of ROCK1.
Fig. 10.
Putative signaling pathways mediating zinc-induced endothelial cell contraction. Interactions between actin and myosin and, ultimately, endothelial cell contraction, are dependent on the phosphorylation state of MLC. This in turn is determined by the balance between MLC kinase (MLCK) and MLC phosphatase (MLCP). Our data showed that zinc was capable of promoting MLC phosphorylation and stress fiber formation when MLCK was inhibited by ML-7, suggesting that zinc acts through inhibition of MLCP in promoting cell contractility. Zinc chelation (TPEN) decreased hypoxia-induced MLC phosphorylation and caused disassembly of actin stress fibers during hypoxia but had no impact on phosphorylation of the regulatory subunit, MYPT1, of MLCP implying that hypoxia released zinc signals through the PP1 subunit of MLCP to affect changes in enzyme activity and endothelial cell contraction. Zinc-induced phosphorylation of MLC (secondary to inhibition of MLCP) was also shown to be PKC dependent (Ro-31–8220), with hypoxia-released zinc promoting the phosphorylation of the PKC substrate, CPI-17. Collectively, these data suggest a link between hypoxia, elevations in labile zinc, and activation of PKC, which in turn acts through CPI-17 to inhibit MLCP activity and promote MLC phosphorylation, ultimately inducing stress fiber formation and endothelial cell contraction.
Intracellular zinc concentrations influence PKC activity and processing in a number of cell types (11, 12, 27). We recently observed that zinc contributed to tert-butyl hydroperoxide-induced necrosis, in part, via a PKC-dependent pathway, providing one potential link between zinc and PKC in pulmonary endothelium (5, 38). Whereas oxidative stress is thought to activate PKC by modifying zinc thiolate clusters of the regulatory domain of the NH2 terminus, relieving autoinhibition and facilitating cofactor independent PKC activation (18), it has been suggested that changes in intracellular zinc affect PKC activity by targeting the C1 domain of the enzyme, which contains cysteine-rich finger-like motifs that bind zinc (11). We showed that inhibition of PKC by Ro-31–8220 attenuated hypoxia-induced contraction of isolated pulmonary endothelial cells (Fig. 6) and that zinc-induced phosphorylation of the contractile regulatory protein, MLC, was PKC dependent (Fig. 5A). We further showed that hypoxia-released zinc promoted the phosphorylation of the PKC substrate, CPI-17 (Fig. 5C). PKC acts through CPI-17 to stabilize the phosphorylation of MLC by inactivating MLC phosphatase, ultimately inducing stress fiber formation and cell contraction (Fig. 10). Such signaling has been shown to regulate the reorganization of cytoskeletal proteins and affect changes in barrier function in pulmonary endothelium (21). Although there is compelling evidence supporting a critical role for zinc in maintaining epithelial barrier function in the context of inflammatory stress (9) and it is not unreasonable to propose that zinc may also play a role in regulating endothelial barrier function, further studies are required to determine the downstream effects of the hypoxia/zinc pathway on endothelial permeability in the lung.
Investigating a role for individual PKC isoforms in the described zinc signaling pathways regulating endothelial contractility is complicated by the lack of specificity of the pharmacological inhibitors and activators of the enzyme. Ro-31–8220 has an IC50 of 0.024 μM for PKC-ε (42); however, at high concentrations it can also inhibit PKC-α, PKC-βII, and PKC-γ. The difficulties in using such inhibitors are further demonstrated by observations that Ro-31–8220 can activate c-Jun expression and inhibit MAPK phosphatase-1 expression (1, 7). Although we cannot eliminate the possibility that multiple isoforms of PKC play a role in modulating the response to hypoxia observed in pulmonary endothelium, the observation that the dominant-negative approach to specifically inhibit PKC-ε also significantly attenuated the hypoxia-induced contraction of isolated cells confirmed the importance of the PKC-ε isoform in mediating pulmonary endothelial cell contraction. Similarly, although zinc-induced changes in MLC phosphorylation were abrogated by pharmacological inhibition of Rho kinase, it has been reported that Y-27632 can exert nonspecific inhibitory effects on PKC-mediated vascular responses at concentrations in excess of 10 μM (10). Therefore, future studies incorporating specific knockdown of the genes in question are required to delineate the precise pathways regulating these events.
The specificity of the proposed zinc pathways in mediating hypoxia-induced contraction in the pulmonary vasculature is not known. We have previously shown that both hypoxia-induced changes in labile zinc and hypoxia-induced endothelial cell contraction (8) were unique to cells derived from lung in that aortic endothelium did not exhibit these responses to low PO2. However, we do not anticipate that the tissue specificity of HPV is conferred by activation of PKC-ε in that PKC has been shown to be an important signaling molecule mediating contractile responses in both the systemic and pulmonary circulation (32, 41). In our hands, the hypoxia-induced changes in PKC-ε activity were both l-NAME and TPEN sensitive, arguing a role for NO-induced changes in labile zinc in mediating enzyme function. NO donors have also been shown to induce activation, translocation, and nitration of PKC-ε in cardiac myocytes (5). In fact, both endothelial NOS (eNOS) and inducible NOS proteins are associated with PKC-ε in the heart (30) although data obtained in PKC-ε−/− mice suggested a role for PKC-ε in the regulation of eNOS expression during chronic hypoxic exposure rather than the direct regulation of enzymatic activity (24). Our data suggest a role for PKC in mediating the downstream effects of hypoxia-induced changes in NO synthesis and intracellular zinc homeostasis to produce contraction of pulmonary endothelium. Although data obtained using dominant-negative approaches, and isoform-specific antibodies, directly implicated the involvement of PKC-ε, we cannot eliminate the possibility that other pathways, or other isoforms of PKC, also contribute to HPV and/or the generation of contractile force in isolated pulmonary endothelial cells.
GRANTS
This work was funded in part by NIH HL081421 (C. St Croix), NIH R37 HL65697 (B. Pitt), R01CA142580-01 and R01CA129127-01 (Q. Wang), and 1 U54 RR022241-01 (S. Watkins).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the technical expertise of Christina Goldbach and Greg Gibson.
REFERENCES
- 1.Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett 399: 333–338, 1996 [DOI] [PubMed] [Google Scholar]
- 2.An SS, Pennella CM, Gonnabathula A, Chen J, Wang N, Gaestel M, Hassoun PM, Fredberg JJ, Kayyali US. Hypoxia alters biophysical properties of endothelial cells via p38 MAPK- and Rho kinase-dependent pathways. Am J Physiol Cell Physiol 289: C521–C530, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Avnur Z, Geiger B. Substrate-attached membranes of cultured cells isolation and characterization of ventral cell membranes and the associated cytoskeleton. J Mol Biol 153: 361–379, 1981 [DOI] [PubMed] [Google Scholar]
- 4.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J 371: 199–204, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balafanova Z, Bolli R, Zhang J, Zheng Y, Pass JM, Bhatnagar A, Tang XL, Wang O, Cardwell E, Ping P. Nitric oxide (NO) induces nitration of protein kinase Cepsilon (PKCepsilon ), facilitating PKCepsilon translocation via enhanced PKCepsilon -RACK2 interactions: a novel mechanism of no-triggered activation of PKCepsilon. J Biol Chem 277: 15021–15027, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Bandyopadhyay G, Standaert ML, Zhao L, Yu B, Avignon A, Galloway L, Karnam P, Moscat J, Farese RV. Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J Biol Chem 272: 2551–2558, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Beltman J, McCormick F, Cook SJ. The selective protein kinase C inhibitor, Ro-31–8220, inhibits mitogen-activated protein kinase phosphatase-1 (MKP-1) expression, induces c-Jun expression, and activates Jun N-terminal kinase. J Biol Chem 271: 27018–27024, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Bernal PJ, Leelavanichkul K, Bauer E, Cao R, Wilson A, Wasserloos KJ, Watkins SC, Pitt BRand, St, Croix CM. Nitric-oxide-mediated zinc release contributes to hypoxic regulation of pulmonary vascular tone. Circ Res 102: 1575–1583, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besecker B, Bao S, Bohacova B, Papp A, Sadee W, Knoell DL. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cytoprotection in lung epithelia. Am J Physiol Lung Cell Mol Physiol 294: L1127–L1136, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Budzyn K, Paull M, Marley PD, Sobey CG. Segmental differences in the roles of rho-kinase and protein kinase C in mediating vasoconstriction. J Pharmacol Exp Ther 317: 791–796, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Chou SS, Clegg MS, Momma TY, Niles BJ, Duffy JY, Daston GP, Keen CL. Alterations in protein kinase C activity and processing during zinc-deficiency-induced cell death. Biochem J 383: 63–71, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Csermely P, Szamel M, Resch K, Somogyi J. Zinc can increase the activity of protein kinase C and contributes to its binding to plasma membranes in T lymphocytes. J Biol Chem 263: 6487–6490, 1988 [PubMed] [Google Scholar]
- 13.Donoghue L, Tyburski JG, Steffes CP, Wilson RF. Vascular endothelial growth factor modulates contractile response in microvascular lung pericytes. Am J Surg 191: 349–352, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J Biochem (Tokyo) 118: 1104–1107, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci 22: 32–39, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Gavard J, Gutkind JS. Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from Galpha12/13 and Galpha11/q. J Biol Chem 283: 29888–29896, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goeckeler ZM, Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol 130: 613–627, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med 28: 1349–1361, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Isaacson A, Sandow A. Effects of zinc on responses of skeletal muscle. J Gen Physiol 46: 655–677, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane LP, Watkins SC. Dynamic regulation of Tec kinase localization in membrane-proximal vesicles of a T cell clone revealed by total internal reflection fluorescence and confocal microscopy. J Biol Chem 280: 21949–21954, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Kolosova IA, Ma SF, Adyshev DM, Wang P, Ohba M, Natarajan V, Garcia JG, Verin AD. Role of CPI-17 in the regulation of endothelial cytoskeleton. Am J Physiol Lung Cell Mol Physiol 287: L970–L980, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Maxwell IZ, Heisterkamp A, Polte TR, Lele TP, Salanga M, Mazur E, Ingber DE. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys J 90: 3762–3773, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol 16: 5313–5327, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Littler CM, Morris KG, Jr, Fagan KA, McMurtry IF, Messing RO, Dempsey EC. Protein kinase C-epsilon-null mice have decreased hypoxic pulmonary vasoconstriction. Am J Physiol Heart Circ Physiol 284: H1321–H1331, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285: 895–898, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Maret W, Li Y. Coordination dynamics of zinc in proteins. Chem Rev 109: 4682–4707, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Murakami K, Whiteley MK, Routtenberg A. Regulation of protein kinase C activity by cooperative interaction of Zn2+ and Ca2+. J Biol Chem 262: 13902–13906, 1987 [PubMed] [Google Scholar]
- 28.Naumanen P, Lappalainen P, Hotulainen P. Mechanisms of actin stress fibre assembly. J Microsc 231: 446–454, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Orton EC, Raffestin B, McMurtry IF. Protein kinase C influences rat pulmonary vascular reactivity. Am Rev Respir Dis 141: 654–658, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Ping P, Takano H, Zhang J, Tang XL, Qiu Y, Li RC, Banerjee S, Dawn B, Balafonova Z, Bolli R. Isoform-selective activation of protein kinase C by nitric oxide in the heart of conscious rabbits: a signaling mechanism for both nitric oxide-induced and ischemia-induced preconditioning. Circ Res 84: 587–604, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Rainteau D, Wolf C, Lavialle F. Effects of calcium and calcium analogs on calmodulin: a Fourier transform infrared and electron spin resonance investigation. Biochim Biophys Acta 1011: 81–87, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen H, Takuwa Y, Park S. Protein kinase C in the regulation of smooth muscle contraction. FASEB J 1: 177–185, 1987 [PubMed] [Google Scholar]
- 33.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Srinivasan R, Wolfe D, Goss J, Watkins S, de Groat WC, Sculptoreanu A, Glorioso JC. Protein kinase C epsilon contributes to basal and sensitizing responses of TRPV1 to capsaicin in rat dorsal root ganglion neurons. Eur J Neurosci 28: 1241–1254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.St Croix CM, Stitt MS, Leelavanichkul K, Wasserloos KJ, Pitt BR, Watkins SC. Nitric oxide-induced modification of protein thiolate clusters as determined by spectral fluorescence resonance energy transfer in live endothelial cells. Free Radic Biol Med 37: 785–792, 2004 [DOI] [PubMed] [Google Scholar]
- 36.St Croix CM, Wasserloos KJ, Dineley KE, Reynolds IJ, Levitan ES, Pitt BR. Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. Am J Physiol Lung Cell Mol Physiol 282: L185–L192, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Takayama M, Ebihara Y, Tani M. Differences in the expression of protein kinase C isoforms and its translocation after stimulation with phorbol ester between young-adult and middle-aged ventricular cardiomyocytes isolated from Fischer 344 rats. Jpn Circ J 65: 1071–1076, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Tang ZL, Wasserloos K, St Croix CM, Pitt BR. Role of zinc in pulmonary endothelial cell response to oxidative stress. Am J Physiol Lung Cell Mol Physiol 281: L243–L249, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Velasco G, Armstrong C, Morrice N, Frame S, Cohen P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr850 induces its dissociation from myosin. FEBS Lett 527: 101–104, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Ward JP, McMurtry IF. Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol 9: 287–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weissmann N, Voswinckel R, Hardebusch T, Rosseau S, Ghofrani HA, Schermuly R, Seeger W, Grimminger F. Evidence for a role of protein kinase C in hypoxic pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol 276: L90–L95, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J 294: 335–337, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhuo S, Dixon JE. Effects of sulfhydryl regents on the activity of lambda Ser/Thr phosphoprotein phosphatase and inhibition of the enzyme by zinc ion. Protein Eng 10: 1445–1452, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.