Abstract
Cigarette smoking is the major cause of chronic obstructive pulmonary disease (COPD) and predisposes subjects to severe respiratory tract infections. Epidemiological studies have shown that cigarette smokers are seven times more likely to contract influenza infection than nonsmokers. The mechanisms underlying this increased susceptibility are poorly characterized. Retinoic acid-inducible gene (RIG)-I is believed to play an important role in the recognition of, and response to, influenza virus and other RNA viruses. Our study focused on how cigarette smoke extract (CSE) alters the influenza-induced proinflammatory response and suppresses host antiviral activity in the human lung using a unique lung organ culture model. We first determined that treatment with 2–20% CSE did not induce cytotoxicity as assessed by LDH release. However, CSE treatment inhibited influenza-induced IFN-inducible protein 10 protein and mRNA expression. Induction of the major antiviral cytokine IFN-β mRNA was also decreased by CSE. CSE also blunted viral-mediated RIG-I mRNA and protein expression. Inhibition of viral-mediated RIG-I induction by CSE was prevented by the antioxidants N-acetyl-cysteine and glutathione. These findings show that CSE suppresses antiviral and innate immune responses in influenza virus-infected human lungs through oxidative inhibition of viral-mediated induction of the pattern recognition receptor RIG-I. This immunosuppressive effect of CSE may play a role in the enhanced susceptibility of smokers to serious influenza infection in the lung.
Keywords: cytokine, smoking, retinoic acid-inducible gene I, interferon-inducible protein-10
chronic obstructive pulmonary disease (COPD) is the fifth leading cause of morbidity and mortality in the developed world and represents a substantial economic and social burden (39). Cigarette smoking is a well-known contributing factor for COPD and respiratory tract infections. Oxidants present in cigarette smoke (CS) may play a role in the pathogenesis of COPD (5).
Influenza virus is a highly contagious agent that causes upper and lower respiratory tract infection resulting in 200,000 hospitalizations and 36,000 deaths in the United States per year (15, 36). The devastating Spanish influenza A virus (IAV) infected about one-third of the world's population and killed 40 million people during the pandemic of 1918. In 2009, a new pandemic of H1N1 influenza has emerged and spread globally, the first influenza pandemic in >40 yr. Epidemiological studies have shown that cigarette smokers are seven times more likely to contract influenza than nonsmokers (3). Influenza infection in healthy smokers is associated with a higher incidence of lower respiratory tract illness as well as a longer duration of symptoms than in nonsmokers. This may be due to impairment of the host antiviral response by CS (4). However, the mechanisms of the immunosuppressive effect of CS and the enhanced susceptibility to influenza infection are unknown.
The innate immune system responds to influenza through three classes of microbial pathogen sensors, called pattern recognition receptors (PRRs). First, most cells use the cytosolic sensor retinoic acid-inducible gene (RIG)-I to detect influenza virus (18). Second, endosomal-based Toll-like receptors (TLRs) are also involved. TLR3, a double-stranded (ds)RNA sensor, may be used by some epithelial cells to detect the viral replicative intermediate dsRNA (12). Plasmacytoid dendritic cells (pDCs) use TLR7 to recognize influenza genomic RNA upon release in late endosomes (24). Finally, the nucleotide-binding domain and leucine-rich-repeat-containing proteins (NLRP), including NLRP3 and nucleotide-binding oligomerization domain 2 (NOD2), may serve as intracellular mediators of influenza virus-initiated host-cell signaling via the regulation of caspase-1 (2, 32, 35). Recognition of influenza virus subsequently initiates signaling cascades through both RIG-I and TLR7, resulting in the production of proinflammatory cytokines and type-I IFNs, which limit viral replication and increase resistance to infection. NLRPs are mainly involved in the regulation of IL-1β maturation through the formation of a biochemical complex called the inflammasome (1). The inflammasome regulates the activation of caspase-1 and subsequent cleavage of IL-1β and IL-18 precursors into their functional forms, which are then released from the cell (35).
We (6, 9) have developed a human lung organ culture model to study the local lung response to human pathogens. Precision-cut lung slices have been used in toxicology studies and have advantages over the use of isolated, cultured epithelial cells for the study of infectious disease. The structural integrity of lung tissue is maintained (31), and this allows for cell-cell interactions in a more complex and native three-dimensional system. We (42) adapted this model to study cytokine responses to influenza virus in the human lung. Detailed mechanistic studies of intracellular processes such as signal pathway activation can be examined in human tissue without risk to the host.
The aim of this study was to evaluate whether CS extract (CSE) inhibits influenza virus-induced cytokine responses in the human lung. This study also evaluated whether modulation of the antiviral cytokine response by CSE is due to inhibition of viral induction of RIG-I. CS is known to contain abundant free radical species and reactive oxidants. There is ample evidence that these reactive oxidants play a role in CS-induced adverse health consequences. Thus, the role of oxidants in this phenomenon was also examined.
MATERIALS AND METHODS
Preparation of influenza virus stock.
H1N1 influenza virus A/PR/34/8 was passaged in Madin-Darby canine kidney (MDCK) cells. Viruses were grown in MDCK cells in DMEM-F-12 with ITS+ (BD Biosciences, Franklin Lakes, NJ) and trypsin (22), harvested at 72 h postinfection, and titered by plaque assay in MDCK cells. There was no detectable endotoxin in the final viral preparations used in the experiments as determined by limulus amebocyte lysate assay (Cambrex, Walkersville, MD). The lower limit of detection of this assay is 0.1 EU/ml or ∼20 pg/ml LPS.
Lung explant culture and CSE preparation.
Human lung tissue was obtained from patients undergoing resection for lung cancer in accordance with protocols approved by the Institutional Review Boards of the University of Oklahoma, Veterans Administration Hospital, Baptist-Integris Hospital, St. Anthony's Hospital, and Mercy Health Center (all in Oklahoma City, OK). Only tissue that did not contain tumor was used for experiments. The tumor-free lung tissue was transported in sterile PBS (pH 7.2) containing 200 μg gentamicin/ml, 100 U penicillin/ml, 100 μg streptomycin/ml, and 2.5 μg amphotericin B/ml (PBS + antibiotics), and the tissue was subsequently stored at 4°C in PBS + antibiotics for no longer than 4 h. Lung segments were inflated with lung slice medium (LSM) containing 1.5% agarose, 1-cm cores were prepared, and cores were sliced into 500-μm-thick sections as previously described (9). LSM consisted of minimal essential medium (Sigma, St. Louis, MO) supplemented with 1.0 μg bovine insulin/ml, 0.1 μg hydrocortisone/ml, 0.1 μg retinyl acetate/ml, 200 μg gentamicin/ml, 100 U penicillin/ml, 100 μg streptomycin/ml, and 1.25 μg amphotericin B/ml. Each slice was placed in 0.5 ml LSM in a single well of a 24-well plate and then incubated at 37°C in 5% CO2. LSM was replaced before the slices were subjected to the experimental treatments.
CSE was prepared by a modification of the method developed by Carp and Janoff (8). Briefly, one (100 mm) cigarette without filter was combusted with a pump. The smoke was bubbled through 25 ml LSM at a speed of 50 ml/min. The resulting suspension was filtered through a 0.22-μm pore filter (Lida Manufacturing, Kenosha, WI) to remove bacteria and large particles. This solution, considered to be 100% CSE, was diluted and applied to lung slice cultures within 30 min of preparation. The nicotine concentration of 100% CSE was 73.48 ± 1.08 μg/ml. The cytotoxicity of CSE for the human lung was assessed by a lactate dehydrogenase (LDH) assay and cell viability assay.
LDH assay and intracellular dehydrogenase assay.
Lung slices cultured in 24-well plates in 0.5 ml of fresh LSM were incubated with 0%, 2%, 10%, 15%, 20%, 50%, or 100% CSE or 10 μM staurosporine as a positive control (Enzo Biochem, Farmingdale, NY) for 24 h at 37°C in 5% CO2. For the LDH assay, the supernatant and lung slices were collected in 0.1% Triton X-100 (Fisher, Waltham, MA) and stored at −20°C. Slices were homogenized by three freeze-thaw cycles along with mechanical disruption. Lung slice homogenates were clarified by centrifugation at 12,000 g for 10 min at 4°C, and triplicate 100-μl aliquots of the clarified lysates and supernatants were added to separate wells of a 96-well plate. LDH activity was measured by detection using a coupled enzymatic reaction using a commercially available kit (BioVision Research Products, Mountain View, CA) according to the manufacturer's directions. The amount of LDH activity was assessed by detection of the reaction product, formazan, at 500 nm using a spectrophotometer (Vmax Microplate reader, Molecular Devices, Sunnyvale, CA). Cytotoxicity, expressed as the percentage of LDH released, was calculated as follows: [supernatant LDH activity/(supernatant LDH activity + cell lysate LDH activity)] × 100. The viability of the cells in the slices was assessed by measuring intracellular dehydrogenase activity with slices treated with CSE as described above. Cell Counting Kit-8 (Dojindo Molecular Technologies, Rockville, MD) reagent (50 μl) was added to the slice medium, and slices were incubated for an additional 24 h. The absorbance at 450 nm of the reduced reagent indicated the activity of dehydrogenases in live cells.
Infection of human lung slices with IAV and determination of cytokine release by ELISA.
After an overnight incubation of the lung slices, the culture medium was replaced with fresh LSM. For each data point, three lung slices with or without CSE treatment were each exposed to 6 × 106 plaque-forming units (PFU)/ml of influenza virus PR8 and allowed to incubate at 37°C with 5% CO2 for the indicated periods. The amount of virus was derived from our previous publication (42) and represents ∼6 multiplicity of infection/cell. Virus diluent was used as a negative control, and PMA (100 ng/ml)-LPS (1 μg/ml) was used as a positive control. After stimulation, media supernatants were harvested and stored at −20°C before ELISA.
Cytokine monocyte chemoattractant protein (MCP)-1 ELISAs were performed using anti-cytokine monoclonal primary antibodies and biotinylated anti-cytokine polyclonal secondary antibodies (R&D Systems, Minneapolis, MN). Interferon-inducible protein-10 (IP-10) and IFN-β were measured using commercially available ELISA kits (BD Biosciences, San Jose, CA, and PBL InterferonSource, Piscataway, NJ, respectively). Plates were developed using TMB reagent (BD Biosciences).
RIG-I protein determination by immunoblot analysis.
Lung slices with or without CSE treatment were stimulated with 6 × 106 PFU/ml of influenza virus PR8 strain. Mock-infected, negative control slices were exposed to an equivalent volume of virus-free diluent. After 24 h of incubation, slices were harvested, homogenized, and then lysed in 500 μl of cold lysis buffer [150 mM NaCl, 50 mM Tris (pH 8.0), 10 mM EDTA, 10 mM NaF, 10 mM sodium pyrophosphate, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and 10 μg leupeptin/ml]. Lung slice homogenates were clarified by centrifugation at 10,000 g at 4°C for 10 min, and the clarified lysates were mixed with SDS-PAGE sample buffer [60 mM Tris (pH 6.8), 10% glycerol, and 2.3% SDS] and heated to 95°C for 5 min. Samples were separated by 4–15% gradient gel and electrophoretically transferred to polyvinylidene difluoride membranes. For the detection of proteins, membranes were immunoblotted with rabbit polyclonal antibody specific for RIG-I (Abcam, Cambridge, MA) and GAPDH (R&D Systems). Membranes were developed with horseradish peroxidase-labeled goat anti-rabbit IgG (Cell Signaling Technology) and chemilluminescent reagents (Pierce Biotechnology, Rockford, IL). Blots were developed using the Syngene G:box Bioimaging System and GeneTools software (Syngene, Frederick, MD) and quantified using ImageQuant software (BD/Molecular Dynamics, Bedford, MA).
Measurement of mRNA expression by relative end-point RT-PCR.
Total RNA from lung slices was extracted using a modified TRIzol (Invitrogen, Carlsbad, CA) protocol and spectrophometrically quantitated, and the integrity was verified by formaldehyde agarose gel electrophoresis.
Equal amounts (1 μg) of RNA for each sample were used with oligo(dT) as primers for the production of cDNA (SuperScript II First-Strand Synthesis System for RT-PCR, Invitrogen) to produce cDNA. Gene-specific primers for the receptors and GAPDH housekeeping genes were used in standard PCR on a MJ Research DNA Engine thermal cycler with the following program: 1 cycle of 94°C for 2 min followed by 32 cycles of 94°C for 30 s, 56°C for 30 s, and 68°C for 2 min and ending with 68°C for 7 min of extension. Primer sequences were as follow: RIG-I, forward 5′-TCCTTTATGAGTATGTGGGCA-3′ and reverse 5′-TCGGGCACAGAATATCTTTG-3′; IFN-β, forward 5′-GCTCTCCTGTTGTGCTTCTCCAC-3′ and reverse 5′-CAATAGTCTCATTCCAGCCAGTGC-3′; GAPDH, forward 5′-GGAAGGTGAAGGTCGGAGT-3′ and reverse 5′-GAAGATGGTGATGGGATTTC-3′; TLR3, forward 5′-GTCTGGGAACATTTCTCTTC-3′ and reverse 5′-GATTTAAACATTCCTCTTCGC-3′; NLRP3, forward 5′-CTGTGTGTGGGACTGAAGCAC-3′ and reverse 5′-GCAGCTCTGCTGTTTCAGCAC-3′; NOD2, forward 5′-GAAGTACATCCGCACCGAG-3′ and reverse 5′-GACACCATCCATGAGAAGACAG-3′; IL-6, forward 5′-AGGAGCCCAGCTATGAACT-3′ and reverse 5′-TGAGATGCCGTCGAGGATG-3′; IL-8, forward 5′-GACTTCCAAGCTGGCCGTG-3′ and reverse 5′-CCACAACCCTCTGCACCC-3′; MCP-1, forward 5′-GTGATCTTCAAGACCATTGTG-3′ and reverse 5′-ATTCTTGCAAAGACCCTC-3′; IP-10, forward 5′-TCTAGAACCGTACGCTGTACCTGC-3′ and reverse 5′-CTGGTTTTAAGGAGATCT-3′; and IFN-γ, forward 5′-GGTCATTCAGATGTAGCGG-3′ and reverse 5′-CACTCTCCTCTTTCCAATTC-3′. After PCR, samples were separated on a 1.5% agarose gel and then stained with ethidium bromide (Invitrogen) for imaging, and band volumes were calculated using ImageQuant 5.0 software (Molecular Dynamics). Amplified DNA band densities were normalized to the corresponding GAPDH densities to correct for potential differences in input cDNA.
Immunohistochemistry on lung tissue explants.
To examine which cell types were affected by CSE in the lung tissue, we performed immunohistochemical staining for IP-10 and IFN-β after influenza infection. Lung slices were exposed to 6 × 106 PFU/ml of influenza virus or virus diluents with or without 2% CSE treatment. Brefeldin A (LC Laboratories, Wofford, MA) was added at a concentration of 5 μg/ml to block protein export to enhance cytokine detection (9). After the incubation, lung slices were fixed with 4% paraformaldehyde in PBS at room temperature for 30 min and then imbedded in paraffin. Sections (3–5 μm) were mounted on glass slides and immunoprobed with a goat anti-human polyclonal antibody for IP-10 (R&D Systems) or IFN-β (Abcam), an anti-viral nucleoprotein polyclonal antibody (43), and an anti-CD68 monoclonal antibody (Dakocytomation, Carpinteria, CA) for macrophages or an anti-cytokeratin monoclonal antibody (Dakocytomation) for epithelial cells. After being washed, sections were probed with a donkey anti-goat secondary antibody conjugated to Alexa fluor 350, a donkey anti-rabbit secondary antibody conjugated to Alexa fluor 546, and a donkey anti-mouse secondary antibody conjugated to Alexa fluor 647, and the cell nuclei were stained with SYTOX green (all from Molecular Probes). Transmitted light and fluorescent microscopy images were obtained using a Zeiss LSM-510META laser scanning confocal microscope.
Statistical analysis.
Where applicable, data are expressed as means ± SE. Statistical significance was determined by one-way ANOVA with a Student-Newman-Keuls post hoc correction for multiple comparisons. Significance was considered at P < 0.05.
RESULTS
CSE attenuates influenza-mediated induction of innate immune cytokine responses and RIG-I in the human lung.
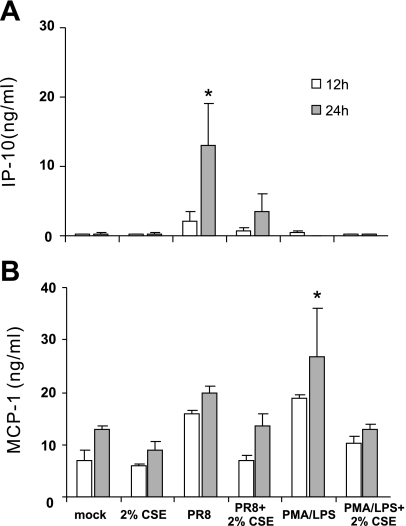
The effect of CSE on the lung innate immune cytokine response to IAV was examined. Cultured lung slices were exposed to 2% CSE or no CSE for 24 h before stimulation with 6 × 106 PFU/ml of IAV PR8. Virus diluents (mock) were used as negative controls, and PMA (100 ng/ml)-LPS (1 μg/ml) was used as a positive control for cytokine induction. The effects of these stimuli and CSE on cytokine release were measured with ELISA of lung slice media supernatants (Fig. 1). IAV PR8 induced a 79-fold IP-10 (P < 0.05) increase but only a 2-fold MCP-1 (no significant difference) increase in cytokine release from the human lung after 24 h of exposure. Consistent with our previous findings, PMA-LPS did not induce IP-10, which suggests that the human lung IP-10 response is relatively specific to virus (42). Pretreatment of the lung slices with 2% CSE decreased IAV-stimulated IP-10 release by 73% and appeared to decrease MCP-1 by 32%, although the latter effect did not reach statistical significance. This suppression of the IP-10 innate immune cytokine response to IAV by CSE is consistent with effects previously demonstrated by others when LPS was used as the stimulus (38, 41). The fact that differential IP-10 and MCP-1 induction occurred may be due to direct regulation of IP-10 by RIG-I while MCP-1 was controlled by other pathways, as has been shown by others (14, 34). This differential induction of the cytokines suggested that the IP-10 response is a specific cytokine response to influenza virus.
Fig. 1.
Cigarette smoke extract (CSE) suppresses influenze A virus (IAV)-stimulated innate immune cytokine release in the human lung. Human lung slices were exposed for the times indicated to 6 × 106 plaque-forming units (PFU)/ml of IAV PR8 in the presence or absence of 2% CSE. IFN-inducible protein-10 (IP-10; A) and monocyte chemoattractant protein-1 (MCP-1; B) cytokine protein levels were determined by ELISA in lung slice supernatants. Data are expressed as means ± SE from 3 separate lung slice donor experiments. Statistical significance was determined by ANOVA. Means were compared with data from the negative control group. *P < 0.05.
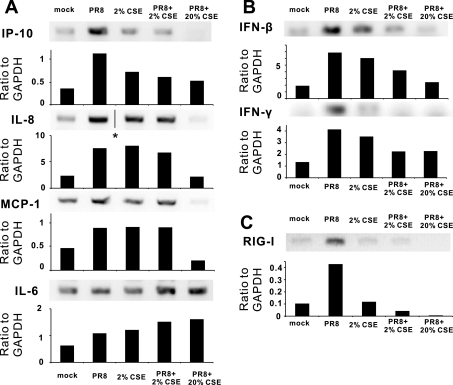
To confirm that inhibition of cytokine protein induction was reflected at the level of transcription, we measured cytokine mRNA levels in lung slices exposed to IAV with or without CSE using relative end-point RT-PCR. Cultured lung slices were exposed to 0%, 2%, and 20% CSE for 24 h before stimulation with 6 × 106 PFU/ml of IAV PR8. Additional human lung slices were mock treated with virus diluent with or without 2% CSE or 2% CSE alone, all as additional negative controls. Consistent with the ELISA results, 2% CSE decreased viral-mediated IP-10 mRNA induction by 65% (Fig. 2A). This concentration of CSE caused minimal inhibition of IAV-mediated IL-8 and MCP-1 mRNA induction. Furthermore, 20% CSE completely inhibited the cytokine induction of IP-10, IL-8, and MCP-1 by IAV. CSE (2%) alone also modestly induced mRNA expression of IP-10 (1.8-fold over mock), IL-8 (3-fold), MCP-1 (1.7-fold), and IL-6 (1.7-fold). Neither concentration of CSE inhibited the induction of IL-6 mRNA, and, in fact, appeared to enhance viral-mediated induction of this cytokine. The enhancement of IL-6 mRNA induction versus the inhibition the antiviral cytokines IP-10, IL-8, and MCP-1 by 20% CSE suggests that viral-mediated induction of this proinflammatory cytokine is controlled by a different pathway. The increase of IL-6 mRNA also suggests that the mRNA decrease of the other cytokines was not likely due to CSE-induced nonspecific cytotoxic effects.
Fig. 2.
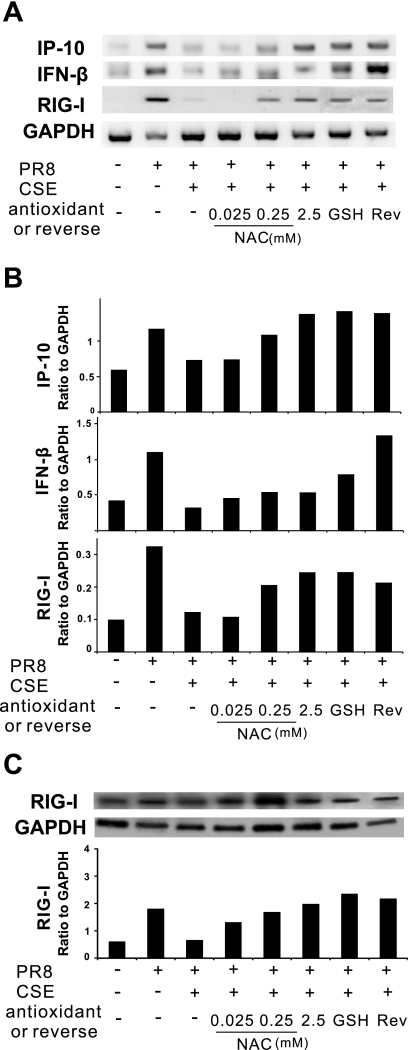
CSE suppresses IAV-stimulated cytokine and retanoic acid-inducible gene (RIG)-I mRNA expression in the human lung. Human lung slices were exposed to 6 × 106 PFU/ml of IAV PR8 in the presence or absence of CSE for 24 h. Total RNA was then isolated from lung slices. Relative end-point RT-PCR products were separated by agarose gel electrophoresis, and mRNA expression was determined by densitometry of the appropriate bands on ethidium bromide-stained gels. Transcript levels of cytokines (A), IFNs (B), and RIG-I (C) were normalized relative to the constitutively expressed GAPDH gene. Data are representative of 3 separate experiments. *Lanes were not adjacent but came from the same original gel.
As there may be differential effects of CSE on proinflammatory versus antiviral cytokine induction, we examined mRNA induction of the two antiviral cytokines IFN-β and IFN-γ (Fig. 2B). IAV increased mRNA levels of both cytokines by threefold. Viral-mediated induction of both cytokine mRNAs was suppressed 60% by 2% CSE. When CSE was increased to 20%, inhibition increased to 85% for IFN-β but stayed at 60% for IFN-γ. It should be noted that, although we saw a significant induction of IFN-β and IFN-γ mRNA by PR8 virus, cytokine protein levels were below the limit of detection (<100 pg/ml) by ELISA.
RIG-I is critical for triggering antiviral immune responses to IAV (29–30). Overexpression of RIG-I enhances the induction of IP-10 mRNA and protein by immunostimulants (14, 34). We next sought to determine the effect of CSE on RIG-I mRNA expression in the human lung (Fig. 2C). After 24 h of exposure to 2% CSE, RIG-I expression induced by IAV was decreased to background levels. RIG-I expression in the presence of IAV was completely inhibited by 20% CSE. These findings suggest that CSE-mediated immunosuppression of antiviral responses is due to the prevention of RIG-I induction by IAV.
Immunosuppression by CSE is not due to nonspecific cytotoxicity.
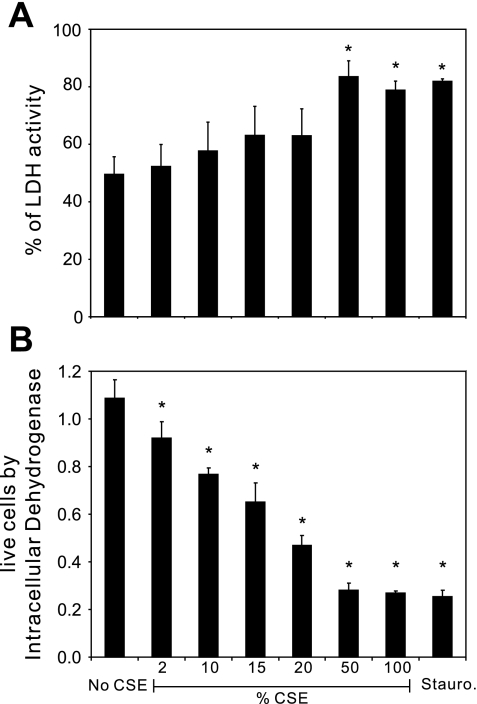
To determine whether immunosuppression by CSE could be due to nonspecfic cytotoxicity, we performed two sets of experiments. First, we measured LDH release from lung slices during exposure to CSE. Lung slices were exposed to increasing concentrations of CSE from 2%, 10%, 15%, 20%, 50%, and 100% for 24 h followed by measurements of LDH in the media and homogenized lung slices. Staurosporine was used as a positive control for cytotoxicity (Fig. 3). Basal LDH release was fairly high (49%), likely due to the large cut surface of the lung after it had been sliced. High percentages of CSE (50% and 100%) stimulated significant cytotoxicity, comparable to that seen with staurosporine. In contrast, concentrations of CSE at or below 20% did not cause a statistically significant increase in cytotoxicity. We also performed cell viability assays to detect the activity of dehydrogenases in cells. The activity is directly proportional to the number of living cells. We found that CSE induced cell death in lung slices in a dose-dependent manner and that 2% CSE induced ∼16% of total cell death after 24 h.
Fig. 3.
Effect of CSE on cell viability in human lung slices. Lung slices were incubated with 0%, 2%, 10%, 15%, 20%, 50%, and 100% CSE for 24 h. Staurosporine (10 μM)-treated lung slices were used as a positive control for cytotoxicity. CSE-free medium was used as a negative control. A: LDH activity of the supernatant and lung slices was measured using an LDH-Cytotoxicity Assay Kit (BioVision Research Products) according to the manufacturer's instructions. Cytotoxicity is expressed as the percentage of supernatant-released LDH to total (supernatant + tissue) LDH. B: the relative number of live cells was determined using Cell Counting Kit-8, which detects the activity of dehydrogenases in live cells. Results are shown as means ± SE from 4 separate experiments. Means were compared with data from the negative control group. *P < 0.05.
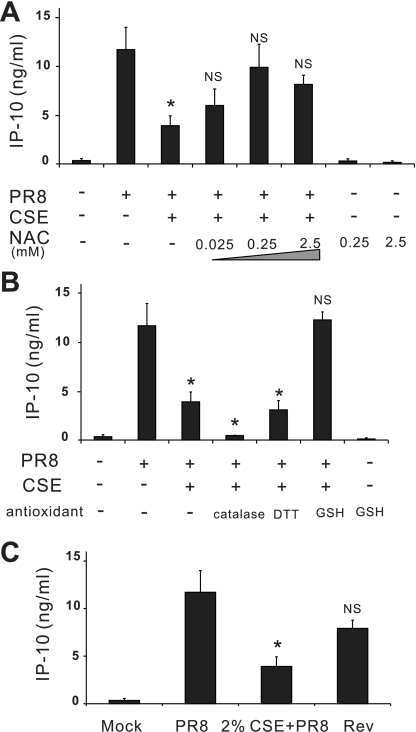
We also performed CSE washout experiments to determine whether the effects seen could be due to nonspecific cytotoxicity. Lung slices were first treated with 2% CSE in medium. After 24 h, 2% CSE medium was replaced with normal LSM without CSE for another 4 h. Lung slices were then challenged with 6 × 106 PFU/ml of IAV PR8. Washout of CSE completely restored the IP-10 antiviral cytokine response to that of CSE-unexposed slices (Fig. 4C). These data suggest that immunsuppression by 2% CSE was not due to nonspecific cytotoxicity.
Fig. 4.
Antioxidants prevent CSE-mediated suppression of IAV induction of IP-10 in the human lung. For antioxidant treatment, lung slices were treated with increasing amounts of N-acetyl-cysteine (NAC; A) or 1,000 mU/ml catalase, 1 mM DTT, or 1 mM GSH (B) for 3 h before 2% CSE was added. All antioxidants were maintained throughout the experiment. In additional wells [C; reverse group (Rev)], there was no antioxidant treatment, and CSE was removed after 24 h of treatment and incubated for another 4 h in normal lung slice medium before treatment with virus. All indicated slices were exposed to 6 × 106 PFU/ml of IAV PR8 for 24 h. IP-10 protein levels were determined by ELISA of lung slice supernatants. Data are expressed as means ± SE from 3 separate lung slice donor experiments. Statistical significance was determined by ANOVA. Means were compared with data from the PR8-treated group. NS, no significant difference. *P < 0.05.
Antioxidants prevent the immune cytokine responses from being suppressed by CSE.
Smoke induces oxidant-mediated cell damage. To investigate whether the CSE-mediated innate immune suppression was related to oxidants, we studied whether antioxidants prevented CSE-mediated effects on cytokine responses in our human model. Lung slices were preincubated with increasing amounts of N-acetyl-cysteine (NAC) for 3 h before 2% CSE was added. NAC was maintained throughout the experiment. IP-10 induction by influenza was measured under these conditions. As shown in Fig. 4A, exogenous NAC restored the lung IP-10 response to virus in lungs treated with CSE. We also examined the effect of pretreatment with several other reducing reagents: glutathione (GSH), catalase, and DTT. As shown in Fig. 4B, GSH at 1 mM completely restored IP-10 induction by IAV. Catalase (1,000 U/ml) and DTT (1 mM) at the indicated concentrations failed to protect the human lung from CSE-mediated immunosuppresion. In fact, catalase-treated lung slices did not produce IP-10 in response to influenza. NAC and GSH alone did not induce IP-10.
We next examined the protective effects of NAC and GSH on IAV induction of IP-10 and IFN-β mRNA in the presence of CSE using relative end-point RT-PCR (Fig. 5A). Consistent with the findings above at the level of translation, NAC prevented CSE-mediated suppression of IP-10 and IFN-β mRNA induction by IAV in the human lung. GSH at 1 mM completely prevented the suppression of viral induction of IP-10 and partially diminished the suppression of viral induction of IFN-β mRNA by 2% CSE.
Fig. 5.
Antioxidants prevent CSE-mediated suppression of IAV-induced cytokine and RIG-I expression in the human lung. Lung slices were treated with increasing amounts of NAC or GSH (1 mM) for 3 h before 2% CSE was added. For the reverse group, CSE was removed after 24 h of treatment and incubated for another 4 h in normal lung slice medium before treatment with virus. All indicated slices were exposed to 6 × 106 PFU/ml of IAV PR8 for 24 h. A and B: relative end-point RT-PCR was used to determine mRNA expression. Transcript levels of cytokines and RIG-I were normalized relative to the constitutively expressed GAPDH gene. C: Western blot analysis was used to determine RIG-I protein expression in lung slices. Membranes were probed with anti-RIG-I or anti-GAPDH antibodies. Protein expression of RIG-I was normalized relative to GAPDH. Data are representative of 3 separate experiments.
These findings suggest that the CSE-mediated innate immunosuppression of cytokine responses to IAV occurs via oxidants.
Antioxidants prevent CSE-mediated suppression of RIG-I induction by IAV.
To determine if the antioxidant protection against CSE-mediated immunosuppression was due to the preservation of antiviral RIG-I responses, mRNA and protein levels of RIG-I were assessed by relative end-point RT-PCR and Western blot analysis. RIG-I mRNA induction by IAV was protected from 2% CSE-mediated inhibition by NAC in a dose-dependent manner (Fig. 5A). GSH also prevented the CSE-mediated suppression of antiviral RIG-I responses in the human lung. These results were confirmed at the level of translation by Western blot analysis as both antioxidants prevented the inhibition of IAV induction of RIG-I protein by CSE (Fig. 5B).
The recovery of the cytokine response to IAV in the reverse group shown in Fig. 4C can also be traced back to RIG-I recovery. By relative end-point RT-PCR, we found that the mRNA induction of IP-10 and IFN-β was restored after removing 2% CSE. The restoration of the cytokine responses was accompanied with and preceded by the restoration of RIG-I mRNA and protein induction by IAV (Fig. 5, A and B). Taken together, these findings suggest that both prevention by antioxidants of CSE-induced blunting of IAV immune responses and cellular recovery of these responses after CSE washout is due to the restoration of RIG-I induction by IAV.
Other PRRs in IAV infection in the human lung model.
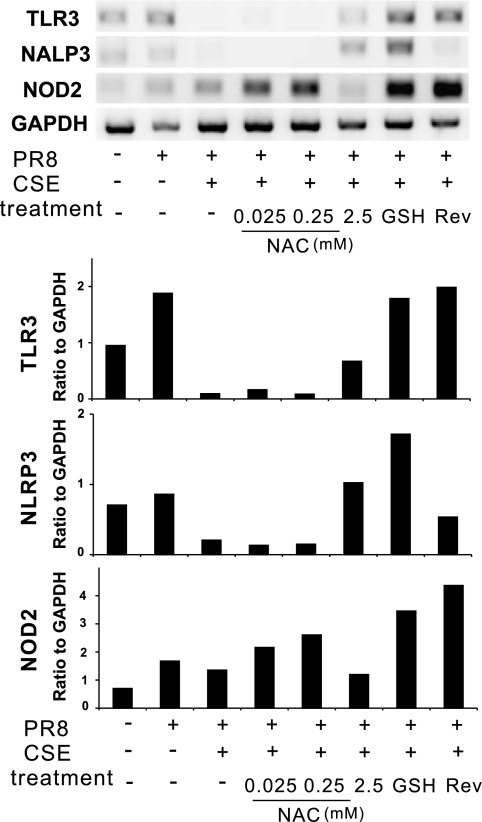
We next examined whether other important PRRs (i.e., TLR3, NLRP3, and NOD2) are involved in the innate immune cytokine responses to virus in the human lung and whether these responses were suppressed by CSE. We thus examined the induction of these PRRs during IAV infection in the presence or absence of CSE in our human lung model (Fig. 6). NLRP3 was not induced by virus. There was 1.8- and 2.4-fold induction of TLR3 and NOD2 by IAV, respectively. This was less than that seen with the induction of RIG-I by virus (4.2-fold; Fig. 2C). CSE (2%) completely suppressed TLR3 induction by virus but did not affect NOD2 induction. NAC did not consistently, or in a dose-response fashion, prevent suppression by CSE of IAV-mediated induction of TLR3. GSH treatment in the presence of CSE caused a twofold increase of NOD2 mRNA induction by IAV, whereas GSH alone did not affect NOD2 levels (not shown). Removing CSE and allowing the cells to recover restored the induction of TLR3 and NOD2 by IAV. As IAV induction of these PRRs in the presence of CSE and/or antioxidants does not parallel cytokine responses to virus, this suggests that TLR3, NLRP3, and NOD2 play a lesser role in IAV-mediated cytokine responses in the human lung than RIG-I.
Fig. 6.
Effects of CSE and antioxidants on induction of mRNA expression of other pattern recognition receptors by IAV in the human lung. Lung slices were treated with increasing amounts of NAC or GSH (1 mM) for 3 h before 2% CSE was added. For the reverse group, CSE was removed after 24 h of treatment and incubated for another 24 h in normal lung slice medium. Lung slices were exposed as indicated to 6 × 106 PFU/ml of IAV PR8 for 24 h. Relative end-point RT-PCR was used to determine mRNA expression. Transcript levels of Toll-like receptor 3 (TLR3), nucleopeptide-binding domain and leucine-rich repeat-containing protein 3 (NLRP3), and nucleotide-binding oligomerization domain 2 (NOD2) were normalized relative to GAPDH mRNA expression. Data are representative of 3 separate experiments.
CSE suppresses IP-10 and IFN-β induction by influenza virus in macrophages and epithelial cells in the human lung.
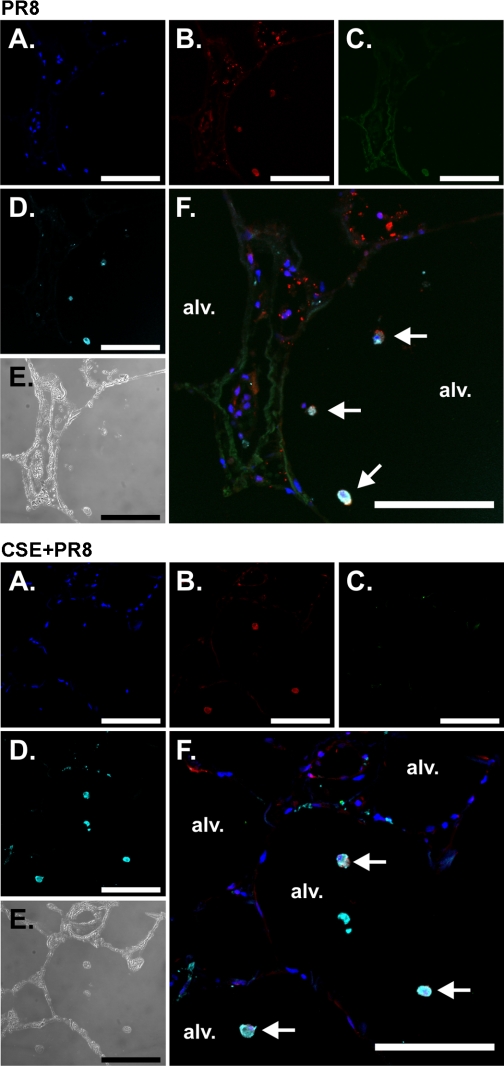
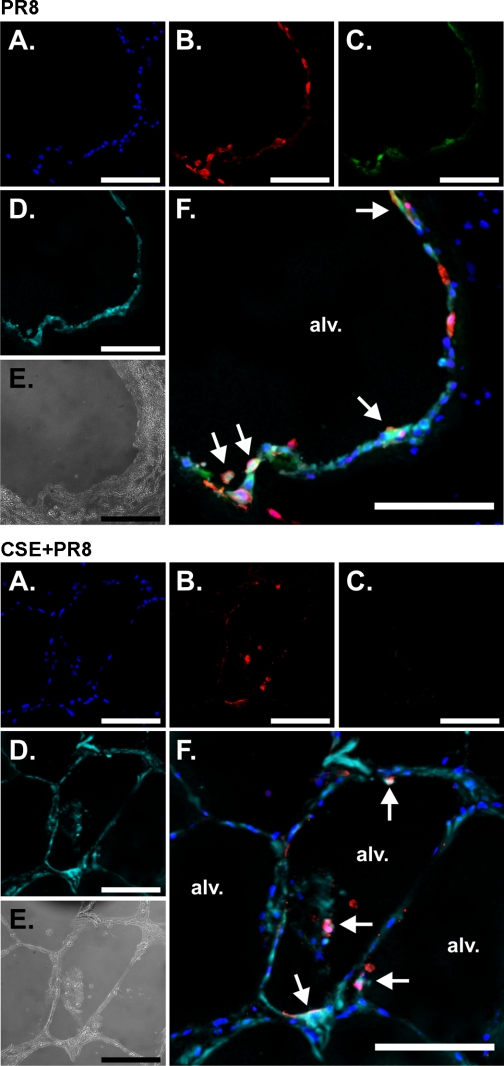
To determine which cell types in the lungs were affected by CSE exposure, we performed immunohistochemistry on virus-exposed lung slices. Lung slices were first treated with or without 2% CSE and then exposed to virus at 6 × 106 PFU/ml or virus-free buffer for 24 h in the presence of brefeldin A to enhance the detection of cytokines. Slices were then processed for immunohistochemistry for the detection of influenza virus nucleoprotein and the cytokines IP-10 and IFN-β. Macrophages and epithelial cells were detected using anti-CD68 monoclonal and anti-cytokeratin monoclonal antibodies, respectively. Tissues exposed to virus diluents were used to demonstrate basal cytokine detection. An additional negative control was performed for IP-10 and IFN-β detection using the same staining protocol but with IP-10 and IFN-β primary antibodies omitted. There was minimal background immunofluorescence in the absence of IP-10 and IFN-β primary antibodies (not show). Detection of IP-10 and IFN-β was significantly enhanced by influenza virus infection [Figs. 7 (top, C) and 8 (top, C), PR8 only]. CSE-treated lung slices did not produce either IP-10 from macrophages or IFN-β from epithelial cells [Figs. 7 (bottom, C) and 8 (bottom, C), CSE + PR8]. Thus, the results demonstrated that CSE suppressed the induction of both cytokines by PR8.
Fig. 7.
CSE suppresses IP-10 induction by influenza virus in macrophages in the human lung. Lung slices were exposed to 6 × 106 PFU/ml of influenza virus PR8 or virus diluents for 24 h in the presence of brefeldin A (BFA) to enhance the detection of cytokines. Slices were then processed for immunohistochemistry for the detection of the chemokine IP-10 using goat polyclonal antibodies, viral nucleoprotein (NP) using rabbit polyclonal antibody, and macrophages using anti-CD68 monoclonal antibody. Nuclei were stained with SYTOX green. Top: PR8 alone. Bottom: CSE + PR8. A–D: fluorescent images that demonstrate nuclei (A; blue), NP (B; red), IP-10 (C; green), and macrophages (D; cyan). E: bright-field images that demonstrate that lung architecture is preserved during the experiment. F: overlays of the fluorescent images that demonstrate that the primary cellular source of IP-10 is alveolar macrophages (arrows). Bars = 100 μm.
Fig. 8.
CSE suppresses IFN-β induction by influenza virus in epithelial cells in the human lung. Lung slices were exposed to 6 × 106 PFU/ml of influenza virus PR8 or virus diluents for 24 h in the presence of BFA to enhance the detection of cytokines. Slices were then processed for immunohistochemistry for the detection of IFN-β using goat polyclonal antibodies, viral NP using rabbit polyclonal antibody, and epithelial cells using anti-cytokeratin monoclonal antibody. Top: PR8 alone. Bottom: CSE + PR8. A–D: fluorescent images that demonstrate nuclei (A; blue), NP (B; red), IFN-β (C; green), and epithelial cells (D; cyan). E: bright-field images. F: overlays of the fluorescent images that demonstrate that the primary cellular sources of the cytokines are epithelial cells (arrows). Bars = 100 μm.
DISCUSSION
The present study demonstrates that CSE inhibits IAV-induced antiviral cytokine responses in the human lung. This is preceded by inhibition of viral-mediated induction of RIG-I by CSE. RIG-I and cytokine induction by influenza in the presence of CSE was restored in a dose-dependent manner by the addition of the antioxidants NAC and GSH. Also, the suppression of RIG-I and cytokine induction induced by 2% CSE was reversible. These findings suggest that CSE suppresses the antiviral and innate immune responses of influenza-infected human lungs through oxidative inhibition of viral-mediated induction of RIG-I and that this is not likely due to direct cytotoxicity. The depression of PRR induction and antiviral cytokine responses by CSE is likely important in the enhanced susceptibility of smokers to influenza infection in the lung.
CS contains a high concentration of free radicals and other oxidants, such as nitric oxide, superoxide anions, and hydrogen peroxide, and induces oxidative stress and inflammation in vitro and in vivo. The imbalance between oxidants and antioxidants is involved in the development of pulmonary emphysema (7, 33). Here, NAC, a GSH precursor and free radical scavenger, was able to block the suppressive effect of CSE in a dose-dependent manner. We did not observe this effect with the other antioxidants, catalase and DTT, at the tested doses. It is interesting to note that, at the lowest concentration of NAC restoration, IP-10 mRNA induction did not completely correlate with IP-10 protein induction for unknown reasons. The results support the hypothesis that oxidative stress induced by CS is one of the main elements that cause significant damage to immune responses in smokers or secondhand smokers.
It has been shown that CSE induces reversible DNA damage and that oxidants play a role in this damage in human lung fibroblasts and epithelial cells (20, 23). The reduced mRNA induction of RIG-I could be caused by direct damage to the DNA segment of this important pathogen sensor. However, the enhanced IL-6 mRNA induction in CSE-treated tissue by IAV makes this possibility less likely, as the damage appears to be specific for certain cytokines and sensors (Fig. 2A). CSE-caused oxidative stress may thus specifically inhibit the transcription of RIG-I.
The enhancement of IL-6 and IL-8 mRNA induction by 2% CSE suggests that viral-mediated induction of these proinflammatory cytokines is controlled by different pathways, at least in human lungs. In mice, IL-6 and KC (a mouse homolog of IL-8) induction upon smoke exposure was both reduced in TLR4-deficient mice, suggesting that both are regulated through the TLR4/MyD88 pathway in the mouse lung (11). Thus, the TLR4/MyD88 pathway is also possibly involved in human IL-6 and IL-8 mRNA induction. CSE induced IL-8 production in both primary human bronchial epithelial cells and the BEAS-2B epithelial cell line (13). The increased IL-8 production by CSE was not due to transcriptional regulation but was associated with IL-8 mRNA stabilization. It is possible that IL-6 mRNA induction in our model does not rely on message stabilization, and this could be responsible for the differential IL-6 and IL-8 induction by 20% CSE.
Local inflammation and elevated cytokine production are the hallmarks of host innate immune responses that act to protect against infection by viruses and are essential in the local control of invading microbes. The expression of PRRs in uninfected, unstimulated cells or animals is negligible, which might be critical in preventing uncontrolled inflammation (28, 40). Unrestrained or excessive stimulation of the innate immune response has been postulated to cause a cytokine “storm” that contributes to the lethality of highly pathogenic viruses in humans, mice, and macaques (10, 17, 21). Therefore, controlled activation of various PRRs by different viruses is important for the generation of optimal antiviral responses. As different classes of PRRs are characterized in mouse models, elucidating the role of each specific PRR and its function in human innate responses will be required. As it maintains the normal architecture present in native tissue and contains the diversity of cell types found in the normal lung, our human lung organ culture model, unlike artificial models using differentiated cell lines, provides a relevant platform in which to perform this work.
In the present study, we identified RIG-I as an important PRR that senses IAV infection and activates cytokine production and antiviral cytokine defenses in the human lung. RIG-I induction by IAV was the greatest of the PRRs we tested. Also, suppression of RIG-I by CSE was most consistently correlated with suppression of antiviral cytokine responses, as was restoration of RIG-I and cytokine responses by antioxidants. Induction of the other PRRs by IAV and restoration of these responses by our treatments did not correlate with cytokine responses to the virus. This suggests that these PRRs do not play a major role in lung cytokine responses to IAV. Although we demonstrated that RIG-I is critical for the initiation of the early antiviral cytokine response in the human lung, other PRRs may also be indispensable for other responses to IAV. For example, NLRP3 triggers activation of the inflammasome in mice, which further induces caspase-1 activation and the release of IL-1β and IL-18. These caspase-1-dependent cytokines are broadly protective and function to contain the extent of lung damage and associated epithelial necrosis (35). Thus, NLRP3-initiated innate immune responses may modulate the severity of influenza pneumonia in mice. We should note that the role of NLRP3 in human influenza pneumonia has not been established, and we did not see induction of NLRP3 in our human model.
Although we found that CSE attenuates the influenza-induced innate antiviral response in our human model, as other observations from human cells (25–26), there are some data suggesting that CS exposure selectively enhances viral-induced pulmonary innate responses in mice (16, 27). Influenza virus is not a natural pathogen of mice and requires several passages through them to become mouse adapted and cause disease. The clinical symptoms of the disease in these animals are different from those seen in humans, the natural host. For example, unlike in humans, most mouse strains become hypothermic during infection, and infection is usually lethal (37). These contrasting results may be due to the differences in the innate immune systems of mice and humans. The other possibility is that poly(I-C) is used in the mouse studies as the immunostimulant. However, Mda5, instead of RIG-I, is responsible for the recognition of poly(I-C), and Mda5 is not involved in influenza virus recognition (19).
Together, these findings suggest that oxidant-mediated immunosuppression of the antiviral host response, specifically that due to RIG-I, may be responsible for the increased severity of infection with IAV in smokers. As the effect appears to be modulated through oxidants and can be negated by the addition of antioxidants, this suggests that therapy directed at restoring antioxidant tone in the lung may be helpful as adjunctive therapy in susceptible patients. As demonstrated when CSE was washed out of our human lung organ cultures, the other method for restoring this RIG-I antiviral response is, of course, smoking cessation.
GRANTS
This work was partially supported by a Clinical Innovator Award from the Flight Attendant Medical Research Institute and by the Oklahoma Health Research Program from Oklahoma Center for the Advancement of Science and Technology (to W. Wu) and by National Institute of Allergy and Infectious Diseases Grant 1-U19-AI-62629 (to J. P. Metcalf).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Elizabeth Duggan for excellent technical support and Dr. Gillian Air for providing the influenza virus strain. The authors also acknowledge assistance from the Oklahoma Medical Research Foundation Imaging Analysis core facility.
In addition, the authors acknowledge the kind assistance of the Departments of Pathology of the Veterans Administration Hospital, the University of Oklahoma Medical Center, Baptist-Integris Hospital, St. Anthony's Hospital, and Mercy Health Center (all in Oklahoma City, OK).
REFERENCES
- 1. Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20: 319–325, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30: 556–565, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med 164: 2206–2216, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Aronson MD, Weiss ST, Ben RL, Komaroff AL. Association between cigarette smoking and acute respiratory tract illness in young adults. JAMA 248: 181–183, 1982 [PubMed] [Google Scholar]
- 5. Baughman RP, Corser BC, Strohofer S, Hendricks D. Spontaneous hydrogen peroxide release from alveolar macrophages of some cigarette smokers. J Lab Clin Med 107: 233–237, 1986 [PubMed] [Google Scholar]
- 6. Booth JL, Coggeshall KM, Gordon BE, Metcalf JP. Adenovirus type 7 induces interleukin-8 in a lung slice model and requires activation of Erk. J Virol 78: 4156–4164, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carnevali S, Petruzzelli S, Longoni B, Vanacore R, Barale R, Cipollini M, Scatena F, Paggiaro P, Celi A, Giuntini C. Cigarette smoke extract induces oxidative stress and apoptosis in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 284: L955–L963, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Carp H, Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis 118: 617–621, 1978 [DOI] [PubMed] [Google Scholar]
- 9. Chakrabarty K, Wu W, Booth JL, Duggan ES, Nagle NN, Coggeshall KM, Metcalf JP. Human lung innate immune response to Bacillus anthracis spore infection. Infect Immun 75: 3729–3738, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 12: 1203–1207, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doz E, Noulin N, Boichot E, Guenon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF, Couillin I. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol 180: 1169–1178, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem 280: 5571–5580, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Hudy MH, Traves SL, Wiehler S, Proud D. Cigarette smoke modulates rhinovirus-induced airway epithelial cell chemokine production. Eur Respir J 35: 1256–1263, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Imaizumi T, Kumagai M, Taima K, Fujita T, Yoshida H, Satoh K. Involvement of retinoic acid-inducible gene-I in the IFN-γ/STAT1 signalling pathway in BEAS-2B cells. Eur Respir J 25: 1077–1083, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, Black S, Shinefield H, Fukuda K. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 342: 232–239, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, Elias JA. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest 118: 2771–2784, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kash JC, Tumpey TM, Proll SC, Carter V, Perwitasari O, Thomas MJ, Basler CF, Palese P, Taubenberger JK, Garcia-Sastre A, Swayne DE, Katze MG. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature 443: 578–581, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23: 19–28, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441: 101–105, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Kim H, Liu X, Kobayashi T, Conner H, Kohyama T, Wen FQ, Fang Q, Abe S, Bitterman P, Rennard SI. Reversible cigarette smoke extract-induced DNA damage in human lung fibroblasts. Am J Respir Cell Mol Biol 31: 483–490, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, Feldmann F, Alimonti JB, Fernando L, Li Y, Katze MG, Feldmann H, Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445: 319–323, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Liu C, Air GM. Selection and characterization of a neuraminidase-minus mutant of influenza virus and its rescue by cloned neuraminidase genes. Virology 194: 403–407, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Liu X, Conner H, Kobayashi T, Kim H, Wen F, Abe S, Fang Q, Wang X, Hashimoto M, Bitterman P, Rennard SI. Cigarette smoke extract induces DNA damage but not apoptosis in human bronchial epithelial cells. Am J Respir Cell Mol Biol 33: 121–129, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA 101: 5598–5603, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mian MF, Lauzon NM, Stampfli MR, Mossman KL, Ashkar AA. Impairment of human NK cell cytotoxic activity and cytokine release by cigarette smoke. J Leukoc Biol 83: 774–784, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Mian MF, Stampfli MR, Mossman KL, Ashkar AA. Cigarette smoke attenuation of poly I:C-induced innate antiviral responses in human PBMC is mainly due to inhibition of IFN-β production. Mol Immunol 46: 821–829, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Motz GT, Eppert BL, Wortham BW, Amos-Kroohs RM, Flury JL, Wesselkamper SC, Borchers MT. Chronic cigarette smoke exposure primes NK cell activation in a mouse model of chronic obstructive pulmonary disease. J Immunol 184: 4460–4469, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Opitz B, Puschel A, Schmeck B, Hocke AC, Rosseau S, Hammerschmidt S, Schumann RR, Suttorp N, Hippenstiel S. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem 279: 36426–36432, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Opitz B, Rejaibi A, Dauber B, Eckhard J, Vinzing M, Schmeck B, Hippenstiel S, Suttorp N, Wolff T. IFNβ induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cell Microbiol 9: 930–938, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314: 997–1001, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Placke ME, Fisher GL. Adult peripheral lung organ culture–a model for respiratory tract toxicology. Toxicol Appl Pharmacol 90: 284–298, 1987 [DOI] [PubMed] [Google Scholar]
- 32. Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. Activation of innate immune antiviral responses by Nod2. Nat Immunol 10: 1073–1080, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Snider GL. Emphysema: the first two centuries–and beyond. A historical overview, with suggestions for future research: part 1. Am Rev Respir Dis 146: 1334–1344, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Taima K, Imaizumi T, Yamashita K, Ishikawa A, Fujita T, Yoshida H, Takanashi S, Okumura K, Satoh K. Expression of IP-10/CXCL10 is upregulated by double-stranded RNA in BEAS-2B bronchial epithelial cells. Respiration 73: 360–364, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30: 566–575, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA 292: 1333–1340, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Van Reeth K. Cytokines in the pathogenesis of influenza. Vet Microbiol 74: 109–116, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L. Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J Immunol 175: 2684–2691, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. Eur Respir J 30: 993–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Wang J, Wu S, Jin X, Li M, Chen S, Teeling JL, Perry VH, Gu J. Retinoic acid-inducible gene-I mediates late phase induction of TNF-α by lipopolysaccharide. J Immunol 180: 8011–8019, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Witherden IR, Vanden Bon EJ, Goldstraw P, Ratcliffe C, Pastorino U, Tetley TD. Primary human alveolar type II epithelial cell chemokine release: effects of cigarette smoke and neutrophil elastase. Am J Respir Cell Mol Biol 30: 500–509, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Wu W, Booth JL, Duggan ES, Wu S, Patel KB, Coggeshall KM, Metcalf JP. Innate immune response to H3N2 and H1N1 influenza virus infection in a human lung organ culture model. Virology 396: 178–188, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang H, Air GM. Expression of functional influenza virus A polymerase proteins and template from cloned cDNAS in recombinant vaccinia virus infected cells. Biochem Biophys Res Commun 200: 95–101, 1994 [DOI] [PubMed] [Google Scholar]