Abstract
Remodeling of right coronary artery (RCA) occurs during right ventricular hypertrophy (RVH) induced by banding of the pulmonary artery (PA). The effect of RVH on RCA endothelial function and reactive oxygen species (ROS) in vessel wall remains unclear. A swine RVH model (n = 12 pigs) induced by PA banding was used to study RCA endothelial function and ROS level. To obtain longitudinal coronary hemodynamic and geometric data, digital subtraction angiography was used during the progression of RVH. Blood flow in the RCA increased by 82% and lumen diameter of RCA increased by 22% over a 4-wk period of RVH. The increase in blood flow and the commensurate increase in diameter resulted in a constant wall shear stress in RCA throughout the RVH period. ROS was elevated by ∼100% in RCA after 4 wk of PA banding. The expressions of p47phox, NADPH oxidase (NOX1, NOX2, and NOX4) were upregulated in the range of 20–300% in RCA of RVH. The endothelial function was compromised in RCA of RVH as attributed to insufficient endothelial nitric oxide synthase cofactor tetrahydrobiopterin. In vivo angiographic analysis suggests an increased basal tone in the RCA during RVH. In conclusion, stretch due to outward remodeling of RCA during RVH (at constant wall shear stress), similar to vessel stretch in hypertension, appears to induce ROS elevation, endothelial dysfunction, and an increase in basal tone.
Keywords: remodeling, endothelial nitric oxide synthase uncoupling, reactive oxygen species
right ventricular hypertrophy (RVH) results in significant remodeling of right coronary artery (RCA) (3–7, 10, 12). The morphometric data of RCA main trunk, arterioles, and capillaries in RVH suggest outward remodeling in main trunk and increase in numbers of resistance and capillary vessels (12). A hemodynamic analysis showed RCA compensatory adaption during RVH to restore the perfusion at the arteriolar and capillary levels and increase blood flow in the main trunk (10), in proportion to increase in right ventricle (RV) mass (12). The effect of RVH on RCA endothelial function, however, remains unclear.
Endothelial function plays an important role in vascular pathophysiology and is a biomarker/mediator of cardiovascular risk factors (2, 4, 5, 12, 16, 21, 24). Endothelial dysfunction has also been shown to be a predictor of adverse outcomes in patients with coronary artery disease (11, 12). Nitric oxide (NO) is well known as endothelium-dependent vasodilator and is believed to play an atheroprotective role. Reactive oxygen species (ROS) are free radicals found in all vascular cells that are involved in remodeling in both physiological and pathological conditions (1, 4–8). ROS can inactivate NO and decrease NO bioavailability in blood vessels that may compromise endothelium-dependent vasorelaxation (4, 14, 15, 36, 40). Recent studies suggest that an imbalance between superoxide and NO levels, rather than the individual levels, may have harmful consequences on the endothelium (4, 15, 24). Numerous observations suggest that ROS are involved in vascular remodeling in response to mechanical stimulations, including stretch (14, 15, 24, 32). The RCA in RVH experiences outward remodeling (increase in diameter) and axial elongation (12). It is unknown whether ROS, endothelial function, and vascular tone change during RCA remodeling in RVH.
Our hypothesis is that elevated ROS, endothelial dysfunction, and increased tone accompany RCA remodeling in RVH. Uncoupled endothelial NO synthase (eNOS) due to insufficient tetrahydrobiopterin (BH4) may also contribute to endothelial dysfunction. We used digital subtraction angiography (DSA) to quantify RCA remodeling longitudinally based on quantitative angiographic images in swine. The ex vivo endothelium-dependent vasorelaxation in response to vasodilator was measured to evaluate endothelial function. ROS production was confirmed by electron paramagnetic resonance (EPR) spectroscopy, chemiluminescence analysis, and ethidium fluorescence analysis. The expression of NADPH oxidase was also measured to underscore the role of ROS production in a circumferentially stretched RCA at constant wall shear stress (WSS).
MATERIALS AND METHODS
Animal preparation.
The experiments were conducted on seven 3- to 4-mo-old Yorkshire pigs, and five age- and weight-matched animals served as a sham group. All experiments were performed in accordance with national and local ethical guidelines, including the Institute of Laboratory Animal Research Guide, Public Health Service policy, and Animal Welfare Act, and approved University of California at Irvine and Indiana University School of Medicine IACUC protocols.
A thoracotomy was performed along the fourth intercostal space. The chest cavity was exposed to provide access to the pulmonary artery (PA), as well as the RCA. A glycerin-filled silicone occluder was fitted around the PA, and the filling tube was exteriorized to allow for cuff occlusion at a later time. A transonic flow probe (TD420, Transonic) was acutely placed on the proximal RCA, and flow rate was recorded. Once the probe was removed at the conclusion of coronary flow measurements, the chest was closed, and the animal was allowed to recover for 1 wk.
The degree of RVH, and the related increase in coronary blood flow, was imposed by the pressure gradient across the PA. A 7-Fr Swan-Ganz catheter was inserted through the jugular sheath and guided into the RV. The PA was banded upon inflation of the silicone occluder. The banding was set, and the occluder was locked when the desired systolic RV pressure was reached. The pressure increase ranged from 35 to 50% of baseline. The pressure gradient across the PA was monitored throughout the duration of the study. The sham group was treated identically, except the occluder was not inflated.
The RCA was imaged before and immediately after banding and again on scheduled days for the duration of the study. A period of 4 wk after onset of occlusion was deemed sufficient to observe most of the remodeling in RVH (3, 12, 22, 31, 39). At the end of 4 wk, the animal was anesthetized, and the heart was exposed similar to prior surgery. The transonic flow probe was placed in the same previous RCA region to record coronary flow rate, and the animal was euthanized. The heart was excised and immediately stored in 4°C HEPES physiological saline solution (HEPES-PSS) (in mmol/l: 142 NaCl, 4.7 KCl, 2.7 sodium HEPES, 3 HEPES acid, 1.17 MgSO4, 2.79 CaCl, 5.5 glucose). The RCA was excised carefully and used for various measurements. The degree of hypertrophy was assessed by measuring RV-to-left ventricle (LV) mass ratio (RV/LV).
Longitudinal measurements of blood flow and volume.
Blood flow, lumen diameter, and volume were measured using DSA techniques (27–29) based on video densitometry. Briefly, a 7-Fr catheter was inserted into the carotid sheath and placed at the inlet of the RCA. A radiopaque contrast material (iohexol, 350 mg iodine/ml) was injected into the RCA while biplane digital images were obtained. Measurements were made when the contrast material displaced blood in the RCA. Volume was obtained by measuring the integrated intensity within a region of interest that circumscribed the vessel. The integrated intensity of the artery was directly converted to a volume measurement by comparison with integrated intensities of calibration phantoms of known volumes (29). The volume ratio was the ratio of the volume at a given day to the volume at day 0. Blood flow was computed using the time difference between volume images (30). The normalized WSS was calculated by the equation N WSS = Q̇i/Q̇f(Df/Di)3, where Q̇ and D represent flow and diameter of vessel, respectively, and i and f represent initial (day 0) and scheduled (days 4, 7, 14, 28, 30) states, respectively.
To determine vascular tone, nitroglycerin was administered as a bolus (0.3 mg) intracoronary at the inlet of the RCA. The radiopaque contrast material was injected into the RCA after nitroglycerin administration, and biplane digital images were obtained (28). The volume ratio with administering nitroglycerin was the ratio of the maximal volume at a particular time point to maximal volume at day 0 (baseline). The comparison of the percentages with/without administering nitroglycerin was used to indicate the in vivo tone of RCA (28).
EPR spectroscopy.
The vascular segment for EPR was videotaped from the side (∼4 mm in length) and cross-sectional views under stereo microscope, and the volume of the segment was calculated based on the product of cross-sectional area and axial length. The ROS generation was expressed as moles per unit of volume. A measure of the ROS concentration in the tissue samples was determined from EPR spectra obtained by incubating the tissue samples with the spin trapping agent N-tert-butyl-α-phenylnitrone 190 mM in HEPES-PSS for 30 min at 37°C in the dark. A Bruker ESP X-band spectrometer equipped with a TE102 cavity was utilized to detect signals (frequency: 9.4 GHz, power: 25.2 mW). All experiments were done at liquid nitrogen temperature. ROS concentrations were determined with 2,2,6,6-tetramethylpiperidine 1-oxyl solution (0.1 μmol/l) used as a concentration standard. All EPR parameters and conditions applied to both standard and experimental samples.
Chemiluminescence.
A highly sensitive chemiluminescence probe, luminol derivative L-012 (Wako Chemicals) was used to detect ROS in the vascular tissue. Three arterial segments, 2–3 mm in length, were incubated in 96-well plate with HEPES-PSS at 37°C for 1 h. NADPH (0.3 mol/l) and L-012 (500 μmol/l) were administered in wells (40). Light emission was detected with an ultra-sensitive photon counter (Wallac EnVision, 2104 Multilabel Reader, PerkinElmer). Counts were obtained at 1-min interval for 40 min. ROS levels were reported as relative light units after subtracting background luminescence and were normalized to dry tissue weight (mg).
Ethidium fluorescence analysis.
A ring of RCA was sampled from the same region of the RV in both control and PA banded animals. The RCA tissue was incubated with HEPES-PSS containing dihydroethidium (DHE; 0.2 μmol/l) at 35°C for 30 min, where HEPEPS-PSS was previously aerated by nitrogen to remove possible ROS in the solution. The RCA tissue was rinsed three times with PSS and then sectioned into 20-μm slides by a cryomicrotom (CM1850 Leica, Germany). Confocal microscopy (LSM 510 META, Zeiss) was used to visualize the fluorescence (excitation wavelength/emission wavelength: 518/605 nm). A gray-scale analysis was carried out to determine the fluorescence area fraction, and the autofluorescence of vascular tissue was subtracted.
NOXs and eNOS.
Briefly, the protein extracts (∼25 μg) from arterial tissues were fractionated on 10% SDS-PAGE gel, transferred onto polyvinylidene difluoride membrane, and probed with the following primary antibodies: anti-NOX1 (1:250, Santa Cruz Biotechnology), anti-NOX2 (1:250, Santa Cruz Biotechnology), anti-NOX4 (1:250, Santa Cruz Biotechnology), anti-p47phox (1:500, Santa Cruz Biotechnology), or anti-eNOS (1:1,000 dilution in blocking buffer, BD Transduction Laboratory). Blots were incubated with horseradish peroxidase-conjugated secondary antibody. The signal was detected by enhanced chemiluminescence (Amersham) and evaluated by densitometry (Sigma Scan). β-Actin was used for normalization.
Ex vivo endothelium-dependent relaxation.
An isovolumic myograph recently developed by our group was employed to evaluate the endothelial function of RCA (18–20), which maintains physiological loading similar to a pressure myograph, but measures tension with the high sensitivity of a wire myograph. Briefly, the RCA was cannulated on both ends in a physiological bath with HEPES-PSS and stretched to in situ length. The pressure and external diameter were measured with pressure transducer (Mikro-Tip SPR-524, Millar Instruments) and digital diameter tracking (DiamTrak v3+, Australia), respectively. The internal diameter was computed using the incompressibility assumption of vessel wall (18–20). The circumferential tension (product of pressure and internal radius) of the vessel was calculated. The vessel segment was precontracted to an approximate pressure by acetylcholine at submaximal concentration (10−7-10−5 mol/l), which resulted in somewhat different concentration of acetylcholine for each segment. In general, the concentration was ∼80% higher in sham than in RVH vessels. The precontracted RCA was relaxed by bradykinin at a series of doses from 10−12 to 10−7 mol/l. The endothelium-dependent vasodilatation was expressed as percent decrease in tension, which is calculated by the equation: %Tension = (Td − Ti)/(Tmax − Ti) × 100. The tension at every dose (Td), physiological level (Ti), and submaximum tension (Tmax) by vasocontrictor (acetylcholine) are shown. In additional experiments, the vessel segments were incubated with either BH4 (10−7 mol/l) and l-arginine (10−7 mol/l) or apocynin (10−6 mol/l) for 40 min, and the endothelium-dependent relaxation of RCA was measured. The endothelium-independent vasorelaxation to sodium nitroprusside (dose-response relaxation: 10−10-10−5 mol/l) served as reference.
Statistical analysis.
The data are expressed as means ± SD, unless otherwise stated. The correlation of ROS production, NOX, endothelium-dependent relaxation, and RCA blood flow to RV/LV mass (degree of RVH) were analyzed using a linear least squares fit. Variance analysis (ANOVA) was used with time- and dose-dependent comparisons (Bonferroni). Student's t-test was used to detect differences between pairwise groups. For all analyses, P < 0.05 level was used to indicate statistical significance.
RESULTS
The body weight and systemic blood pressure of PA banded animals were not significantly changed compared with sham group (Table 1). RV systolic pressure in PA banding group was significantly higher than that in sham group (Table 1). The RV/LV was defined as the ratio of the RV free wall mass to LV plus septal wall mass. In PA banding group, RV/LV was over three times that of sham group, which indicated significant RV hypertrophy during PA banding (Table 1).
Table 1.
Hemodynamic and physiological parameters
| PA Banding |
Sham |
|||
|---|---|---|---|---|
| Day 0 | Day 28 | Day 0 | Day 28 | |
| Body weight, kg | 41.2 ± 3.3 | 49.8 ± 4.7 | 40.7 ± 3.8 | 53.6 ± 6.2 |
| RV/LV, g/g | N/A | 0.73 ± 0.11* | N/A | 0.21 ± 0.06 |
| Systemic pressure, mmHg | 68 ± 8 | 64 ± 6 | 69 ± 8 | 70 ± 11 |
| RV systolic pressure, mmHg | 37 ± 7 | 52 ± 11* | 36 ± 7 | 38 ± 9 |
| RCA blood flow, ml/min | 23.8 ± 4.3 | 44.3 ± 7.3* | 22.7 ± 4.6 | 25.9 ± 5.1 |
Values are means ± SD. PA, pulmonary artery; RV/LV, weight ratio of right to left ventricles; RV, right ventricle; RCA, right coronary artery; N/A, not applicable.
P < 0.05, significant difference vs. sham group.
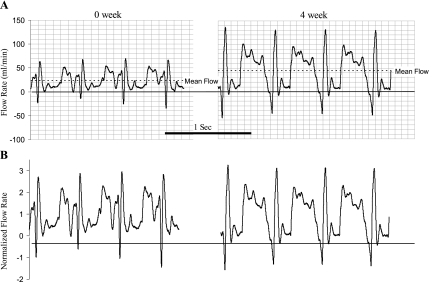
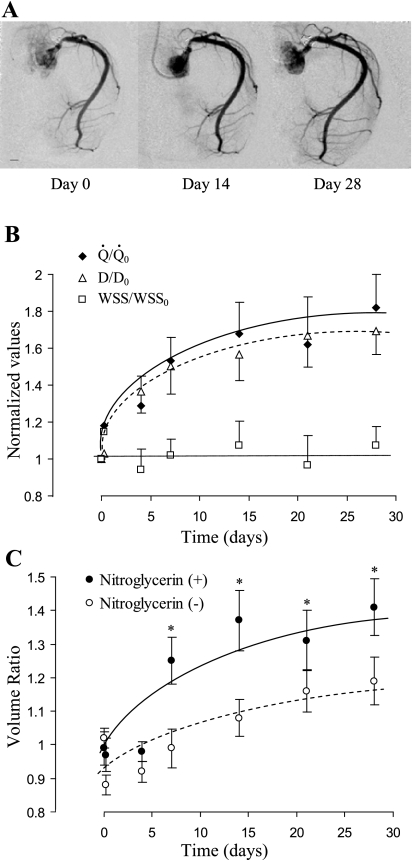
The blood flow in RCA based on measurement of Transonic probe significantly increased in the PA banding group compared with the sham group (Table 1). The flow rate measured by Transonic Doppler (Fig. 1) shows similar waveform pattern with mean value of 23.8 ± 4.3 ml/min in sham control and 44.3 ± 7.3 ml/min after 4 wk of PA banding (Fig. 1A). When the phasic flow curves were normalized relative to the respective mean values of flow rate, the waveform appears similar, which suggests that only the mean value changed and not the oscillatory component (Fig. 1B). Angiographic DSA was used weekly to longitudinally quantify RCA blood flow and luminal diameter, and the typical images are shown in Fig. 2A. The RCA experienced a small step increase in blood flow with the onset of PA banding and a gradual increase in blood flow over time (Fig. 2B). The gradual chronic increase in blood flow was accompanied by an increase in RCA diameter. The RCA diameter cubed in Fig. 2B shows a close proportion to blood flow. Since WSS is proportional to the ratio of blood flow to diameter cubed, the WSS was essentially unchanged throughout the experimental duration. The total increase in blood flow after 4 wk was 1.82 times the sham control using DSA measurement, which was not statistically different from the Transonic measurement (1.87 times). Furthermore, we determined the WSS at onset of banding and after 4-wk banding with Transonic measurement to be 11.0 ± 0.9 and 10.4 ± 0.8 dyn/cm2, respectively (P = 0.37).
Fig. 1.
The Transonic flow tracing curves of blood flow in right coronary artery (RCA) at day 0 and day 28 (4 wk) of right ventricular (RV) hypertrophy (RVH). A: real-time recordings at day 0 and 4 wk of RVH. B: flow curves were normalized by the mean flow rate.
Fig. 2.
A: video-densitometric images of RCA were from the same pig to show the progress (day 0, day 7, and day 28) of RCA remodeling after pulmonary artery (PA) banding. All images are taken at the same magnification. Scale length is 5 mm. B: flow and inner diameter were measurements based on digital subtraction angiography (DSA), and wall shear stress (WSS) was calculated based on the ratio of flow to diameter cubed. Normalized flow (Q̇/Q̇0), diameter cubed [(D/D0)3], and WSS (WSS/WSS0) were defined as the ratio at a given day relative to day 0. Both Q̇/Q̇0 and (D/D0)3 increased gradually with time (one-way ANOVA, P < 0.05). The increase is exponential thereafter, as shown through the best fit line. WSS/WSS0 showed no significant change with time (one-way ANOVA, P > 0.05). C: volumetric growth of RCA from day 0 to day 28. In vivo lumen volume was measured using DSA techniques based on video densitometry. The volume ratio was calculated as the ratio of volume at a particular time point to day 0 (before banding). At the particular time point, lumen volume was first measured without administration of nitroglycerin (basal tone) and then measured again with administration of nitroglycerin as a bolus (0.3 mg, intracoronary) at the inlet of the RCA. The volume ratio of RCA at basal tone significantly increased during RVH (one-way ANOVA, P < 0.05). With administration of nitroglycerin, the volume ratio was significantly increased (two-way ANOVA, *P < 0.05). Data are expressed as means ± SE.
The lumen volume ratio at basal tone of the RCA significantly increased (P < 0.05) during the progression of RVH (Fig. 2C) as a result of circumferential expansion and axial elongation of RCA. With administration of nitroglycerin, the increase in volume ratio at vasodilation was greater than that at basal tone (P < 0.05) (Fig. 2C). The lumen volume at vasodilation reflects the maximal diameter of a blood vessel with relaxed vascular smooth muscle. The increase in the differences of volume ratios between basal tone and vasodilation implies greater vascular tone during the progression of RVH.
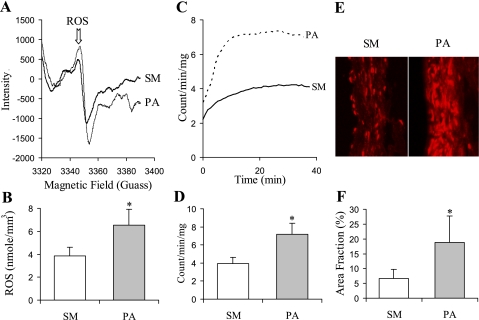
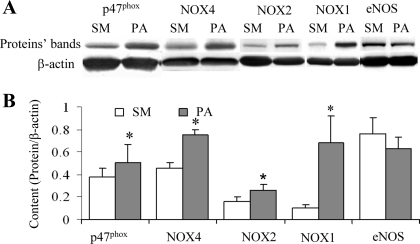
The oxidative stress in arterial tissue was measured with spin trap, chemiluminescence analysis, and fluorescence assay. The typical tracing curves of spin trap are presented in Fig. 3A. ROS species concentration measured using spin trap indicates an increase in ROS after 4 wk of PA banding (Fig. 3B). Luminol derivative L-012 is highly sensitive in the physiological range of pH range (7.5) and reacts with ROS generated from various biological tissues. The typical tracing curves of L-012 enhanced chemiluminescence in sham and PA banding are presented in Fig. 3C, and the increase in ROS levels is summarized in Fig. 3D. The fluorescent area of ethidium combined nucli in the section of RCA in PA banding revealed a significantly higher level compared with sham (Fig. 3, E and F). We further evaluated the expression of subunits of NOX: NOX1, NOX2, NOX4, and p47phox (Fig. 4) to identify the sources of ROS. NOX oxidase is the major source of superoxide, and p47phox is essential to NOX oxidase function. The upregulation of enzyme expression increased the generation of superoxide and elevated oxidative stress in tissue. Although the expression of eNOS was attenuated after 4 wk of PA banding, the difference was not statistically significant (Fig. 4).
Fig. 3.
A: The typical electron paramagnetic resonance (EPR) spectrum for a RCA segment exposed to N-tert-butyl-α-phenylnitrone (PBN). B: reactive oxygen species (ROS) production measured with EPR normalized by volume (mm3) of vessel tissue. C: typical time course of ROS formation in arterial tissue with L-012 enhanced chemiluminescence. D: ROS concentration averaged by time and normalized by dry weight of vessel tissue. E: ethidium fluorescence images. F: ROS production represented as area fraction of fluorescence. Values are means ± SD. SM, sham group. PA, PA banding group. *Statistically difference compared with control (P < 0.05).
Fig. 4.
Expression of the proteins of p47phox, NADPH oxidase (NOX4, NOX2, NOX1), and endothelial nitric oxide synthase (eNOS). A: Western blotting bands of the proteins. B: the semiquantification of the proteins content normalized by β-actin content. Values are means ± SD. *Statistically difference (P < 0.05).
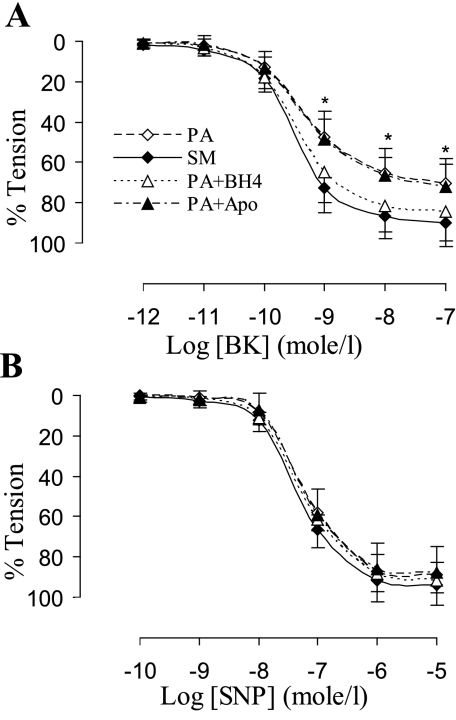
Endothelial function was evaluated in this study by ex vivo acetylcholine precontractile endothelium-dependent vasorelaxation in response to bradykinin. The endothelium-dependent relaxation was compromised after 4 wk of PA banding (Fig. 5A) compared with sham group (two-way ANOVA, P < 0.05). The supplement of BH4 and l-arginine restored the endothelial function in PA banding group (Fig. 5A), which suggests insufficient eNOS cofactors in the PA banding model. Administration of apocynin did not restore the endothelial dysfunction (Fig. 5), which implies that ROS may affect endothelial function through oxidization of eNOS cofactor (BH4). NO donor (sodium nitroprusside) induced endothelium-independent vasorelaxation showed that vascular smooth muscle relaxation in response to NO was unchanged (Fig. 5B).
Fig. 5.
A: endothelial function represented by endothelium-dependent vasorelaxation in response to bradykinin (BK; precontracted with acetylcholine 10−7-10−6 mol/l). The endothelial function of RCA in PA banding (PA) was significantly dysfunctional compared with SM (two-way ANOVA, *P < 0.05). Vessel segments in PA banding incubated with tetrahydrobiopterin (PA+BH4) for 40 min showed restoration of endothelium-dependent vasorelaxation. Apocynin (PA+Apo) did not improve endothelium-dependent vasorelaxation of vessel segment in PA banding. B: endothelium-independent vasorelaxation in response to sodium nitroprusside (SNP; precontracted with acetylcholine 10−7-10−6 mol/l) (two-way ANOVA, P > 0.05). Values are means ± SD.
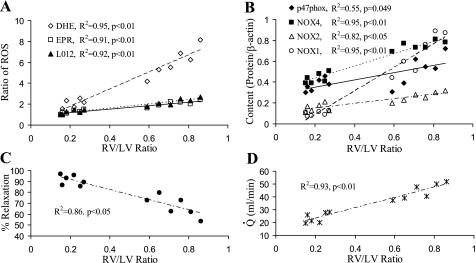
The ROS production measured using semiquantitative methods shows a positive correlation with RV/LV (Fig. 6A). Although the slopes are different, it is likely that ethidium fluorescence assay is less accurate than EPR and chemiluminescence analysis, which have similar slopes. The subunits of NOX shows a linear correlation with RV/LV as well (Fig. 6A). The endothelium-dependent relaxation shows an inverse correlation with increase in RV/LV (Fig. 6C). The blood flow in RCA increases linearly during ventricular hypertrophy (Fig. 6D). This implies that RCA blood flow correlates in the same way to the various parameters that correlate with RV/LV.
Fig. 6.
Correlation between the degree of oxidative stress/endothelial dysfunction and the degree of RVH [RV-to-left ventricle ratio (RV/LV)]. A: ROS productions normalized by their baseline values in various measurements [dihydroethidium (DHE), EPR, L012-enhanced chemiluminescence] correlates to RV/LV. B: expressions of p47phox, NOX1, NOX2, and NOX4 correlates to RV/LV. C: maximal endothelium-dependent relaxation correlates inversely to RV/LV. D: blood flow (Q̇) of RCA correlates directly to RV/LV, which indicates that blood flow in RCA increases proportionally to RV growth during RVH. Values are means ± SD.
DISCUSSION
This is the first study to show a compromised endothelial function in RCA during RVH as verified in an ex vivo isovolumic myograph (18–20). Our findings suggest that the RCA endothelial dysfunction stems from eNOS uncoupling. The angiographic analysis of in vivo volume ratio suggests an increase in the basal tone of RCA during RVH. The increase in ROS production was also observed in the RCA of RVH. Although increased ROS production in hypertension and coronary artery disease has been well documented (4, 5, 14, 16, 25, 40), as has the predilection for vasospasm in hypertension and LV hypertrophy (6, 13, 26), this is the first study to suggest increased ROS production and compromised endothelial function in response to a gradual increase in blood flow under constant WSS conditions.
We observed chronic increase of blood flow in the RCA in PA banding-induced RVH. Despite the increase in RV pressure, systemic blood pressure in RCA was unchanged, indicating that coronary hypertension was not a factor in this study. The progression of RVH was monitored in the same pig longitudinally using angiography, which allowed each animal to serve as its own control. To our knowledge, this is the first porcine longitudinal model that reflects remodeling of coronary artery at different time points in the same animal. In this model, the increase in diameter cubed of RCA is proportional to the increase in blood flow, which is proportional to the increase in myocardial mass of RV, since RVH progresses slowly under the pressure overload. Therefore, WSS remained constant during increases in both diameter and blood flow in RCA during RVH. The constant WSS is also underscored by the unchanged expression of eNOS in contrast to increase of eNOS with elevated WSS (35).
In addition to WSS, other hemodynamic factors, especially the circumferential and axial distension on blood vessel, may be the stimuli for the biochemical, molecular, and functional responses of blood vessel (32). DSA measurement in this study clearly showed the progression of RCA expansion (Fig. 2), which is consistent with our previous observation; i.e., diameter increase and axial elongation simultaneously take place in the RCA during RVH (12). The axial elongation of RCA may result from dimensional remodeling (e.g., enlargement) of RV during RVH (12). It is well established that stretch-activated integrin, ion channels, and G protein-coupled receptors mediate cellular signaling and function in blood vessel. Endothelial dysfunction and increase in ROS production in this study are indeed similar to the pathological responses of blood vessels in hypertension that also entails stretch. An increase in ROS production is involved in changes of vasoreactivity (23), endothelial dysfunction (1, 24), and vascular remodeling (4, 36). ROS may reduce NO bioavailability by reaction with NO and, therefore, compromises endothelium-dependent vasorelaxation (4, 8).
The volume ratio has advantages over other clinical methods to quantify vascular geometric remodeling, including the following: 1) video-densitometric volume measurements are more accurate than angiographic edge detection of diameter and cross-sectional area measurements; 2) it is noninvasive compared with intravascular ultrasound, which is relatively expensive and requires the added procedure of an intravascular ultrasound catheter and potential injury to the endothelium. An increased volume ratio indicates significant geometric remodeling of RCA during RVH (Fig. 2C). With administration of vasodilators (nitroglycerin in the present study), the basal vascular tone can be estimated as the differences of volume ratio with and without the vasodilator. The analysis of in vivo volume ratio suggests a progression of elevated basal tone in the RCA during RVH (Fig. 2C), which may predispose the vessel to vasospasm. The data from ex vivo vasoreactivity (SNP-induced vasorelaxation) using isovolumic myograph suggest that vascular smooth muscle of RCA is the same in sham and RVH groups (Fig. 5). Therefore, we conclude that the increased basal tone is likely from endothelial dysfunction, i.e., eNOS uncoupling.
The upregulation of oxidative stress (Figs. 3 and 4) may also compromise endothelial function by oxidizing BH4 (an eNOS cofactor) to form uncoupled eNOS, which produces ROS instead of NO (38). As an important cofactor of eNOS, BH4 plays a critical role in endothelial function (38). In the present study, the result shows that BH4 can reverse the endothelial dysfunction of RCA by ex vivo administration of BH4 (Fig. 5A), which suggests eNOS uncoupling. But ex vivo administration of apocynin (an antioxidant and inhibitor of NOX) did not restore the endothelial function (Fig. 5A). This implies that the deficiency of BH4 in the RCA is not acute oxidization of ROS and needs further study.
In blood vessel, NOX, xathine oxidase, mitochondria, and eNOS uncoupling are recognized as the sources of ROS. Among them, NOX has been identified as a major source of superoxide in blood vessels in response to pathological stimulation (hypertension, hypercholesterolemia, and diabetes) and mechanical stimulation (5, 7, 8). In this study, we examined the expression of NOX in RCA using Western blot and chemiluminescence analysis. The upregulation of expression of NOX underscores its role in RCA remodeling during RVH. The result of BH4 in ex vivo studies implies that eNOS uncoupling is one of the sources of ROS in the RCA during RVH. The administration of inhibitors of various oxidases and in vivo manipulation of BH4 requires additional study.
The interaction of ROS between RCA and RV during RVH is likely to be small, since our measurement of ROS in RV tissue showed a relatively small increase (<8%) during RVH compared with the changes in the vessel tissue. Ethidium (the product of ROS reacting with DHE) lacks the specificity to detect ROS, since DHE may be reduced by light, oxidization, etc. Hence, we used three approaches to confirm elevation of ROS in RCA after 4-wk PA banding.
In this model, we established stretch rather than WSS as the major stimulus. Accordingly, we chose DSA rather than conventional implantable probe-based or Doppler wire methods based on several reasons. The video densitometry-based DSA is independent of geometry or velocity profile and is less invasive to the vessel. Ultrasonic flow wire provides flow velocity as opposed to volumetric flow rate. The flow rate can be calculated if the velocity is measured from the centerline, which is often not the case. The flow rate calculation also requires the assumption of laminar flow (a condition that is not necessarily true in RCA) and an accurate area measurement. DSA was selected over transonic flow probe in this study, since implantation of a flow probe around the RCA presented a technical challenge; i.e., access to the RCA is difficult from a left lateral thoracotomy. Even when surgical implantation of the flow probe was successful, as was the case in several test animals, the implant may induce an inflammatory response in the RCA, which contributes strong oxidative stress. Additionally, the flow probe itself may cause a focal stenosis when vessel growth is retarded by the flow probe. Microsphere techniques were not used to measure flow or profile, because they require withdrawal of blood per measurement. Furthermore, any degradation of microspheres lodged in the capillary bed within the 1-mo measurement period would underestimate flow. Finally, the change in heart weight makes the normalization of flow (perfusion) inaccurate. The DSA technique has been validated in vivo against an ultrasonic flow probe with a discrepancy of 4% (30).
Conclusion.
We confirmed that WSS remains constant in this model of RVH and implicated stretch as the major stimulus. ROS production and NOX content increased significantly in this RCA model of RVH. Endothelial function of RCA was compromised after 4 wk of RVH, and eNOS uncoupling was implicated in the endothelial dysfunction. In vivo analysis suggests an increased basal tone in the RCA during RVH and, therefore, increases the potential risk of vasospasm.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL055554-12.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Carlos Linares, Brian Dang, Jamie Berke, and Eric Budiman for excellent technical expertise.
REFERENCES
- 1. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bleske BE, Hwang HS, Zineh I, Ghannam MG, Boluyt MO. Evaluation of immunomodulatory biomarkers in a pressure overload model of heart failure. Pharmacotherapy 27: 504–509, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Botham MJ, Lemmer JH, Gerren RA, Long RW, Behrendt DM, Gallagher KP. Coronary vasodilator reserve in young dogs with moderate right ventricular hypertrophy. Ann Thorac Surg 38: 101–107, 1984 [DOI] [PubMed] [Google Scholar]
- 4. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res 97: 533–540, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels. J Clin Invest 110: 203–208, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Qt Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res 80: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 105: 1656–1662, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Hoshio A, Kotake H, Mashiba H. Significance of coronary artery tone in patients with vasospastic angina. J Am Coll Cardiol 14: 604–609; discussion 610–602, 1989 [DOI] [PubMed] [Google Scholar]
- 10. Huo Y, Linares CO, Kassab GS. Capillary perfusion and wall shear stress are restored in the coronary circulation of hypertrophic right ventricle. Circ Res 100: 273–283, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Kai H, Mori T, Tokuda K, Takayama N, Tahara N, Takemiya K, Kudo H, Sugi Y, Fukui D, Yasukawa H, Kuwahara F, Imaizumi T. Pressure overload-induced transient oxidative stress mediates perivascular inflammation and cardiac fibrosis through angiotensin II. Hypertens Res 29: 711–718, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Kassab GS, Imoto K, White FC, Rider CA, Fung YC, Bloor CM. Coronary arterial tree remodeling in right ventricular hypertrophy. Am J Physiol Heart Circ Physiol 265: H366–H375, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Koshiba K, Hoka S. Clinical characteristics of perioperative coronary spasm: reviews of 115 case reports in Japan. J Anesth 15: 93–99, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Landmesser U, Harrison DG, Drexler H. Oxidant stress–a major cause of reduced endothelial nitric oxide availability in cardiovascular disease. Eur J Clin Pharmacol 62, Suppl 13: 13–19, 2006 [Google Scholar]
- 15. Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation 106: 3073–3078, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Laurindo FR, Pedro Mde A, Barbeiro HV, Pileggi F, Carvalho MH, Augusto O, da Luz PL. Vascular free radical release. Ex vivo and in vivo evidence for a flow-dependent endothelial mechanism. Circ Res 74: 700–709, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Lu X, Kassab GS. Vasoactivity of blood vessels using a novel isovolumic myograph. Ann Biomed Eng 35: 356–366, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Lu X, Guo X, Karathanasis SK, Zimmerman KM, Onyia JE, Peterson RG, Kassab GS. Rosiglitazone reverses endothelial dysfunction but not remodeling of femoral artery in Zucker diabetic fatty rats. Cardiovasc Diabetol 9: 19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu X, Kassab GS. Assessment of endothelial function of large, medium, and small vessels: a unified myograph. Am J Physiol Heart Circ Physiol 300: H94–H100, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu X, Kassab GS. Nitric oxide is significantly reduced in ex vivo porcine arteries during reverse flow because of increased superoxide production. J Physiol 561: 575–582, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manohar M. Transmural coronary vasodilator reserve, and flow distribution during tachycardia in conscious young swine with right ventricular hypertrophy. Cardiovasc Res 19: 104–112, 1985 [DOI] [PubMed] [Google Scholar]
- 23. Matsubara T, Nakazawa M, Yoshida Y, Imai S, Suzuki K, Hori T, Konno T, Higuchi K, Tamura Y, Yamazoe M, Izumi T, Aizawa Y. Increasing vasoconstrictor response to ergonovine with oxidative injury in canine coronary artery. Coron Artery Dis 8: 1–7, 1997 [DOI] [PubMed] [Google Scholar]
- 24. McIntyre M, Bohr DF, Dominiczak AF. Endothelial function in hypertension: the role of superoxide anion. Hypertension 34: 539–545, 1999 [DOI] [PubMed] [Google Scholar]
- 25. McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285: H2290–H2297, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Mohri M, Takeshita A. Coronary microvascular disease in humans. Jpn Heart J 40: 97–108, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Molloi S, Zhou Y, Kassab GS. Regional volumetric coronary blood flow measurement by digital angiography: in vivo validation. Acad Radiol 11: 757–766, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Molloi S, Berenji GR, Dang TT, Kassab G. Assessment of vasoreactivity using videodensitometry coronary angiography. Int J Cardiovasc Imaging 19: 271–279, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Molloi S, Kassab GS, Zhou Y. Quantification of coronary artery lumen volume by digital angiography: in vivo validation. Circulation 104: 2351–2357, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Molloi S, Ersahin A, Tang J, Hicks J, Leung CY. Quantification of volumetric coronary blood flow with dual-energy digital subtraction angiography. Circulation 93: 1919–1927, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Murray PA, Vatner SF. Reduction of maximal coronary vasodilator capacity in conscious dogs with severe right ventricular hypertrophy. Circ Res 48: 25–33, 1981 [DOI] [PubMed] [Google Scholar]
- 32. Oeckler RA, Kaminski PM, Wolin MS. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res 92: 23–31, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Schachinger V, Zeiher AM. Quantitative assessment of coronary vasoreactivity in humans in vivo. Importance of baseline vasomotor tone in atherosclerosis. Circulation 92: 2087–2094, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Schwarzacher S, Weidinger F, Krejcy K, Raberger G. Assessment of changes in vasomotor tone in vivo using intravascular ultrasound. J Pharmacol Toxicol Methods 28: 143–147, 1992 [DOI] [PubMed] [Google Scholar]
- 35. Tuttle JL, Nachreiner RD, Bhuller AS, Condict KW, Connors BA, Herring BP, Dalsing MC, Unthank JL. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol 281: H1380–H1389, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84, 2007 [DOI] [PubMed] [Google Scholar]
- 37. van Wolferen SA, Marcus JT, Westerhof N, Spreeuwenberg MD, Marques KM, Bronzwaer JG, Henkens IR, Gan CT, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J 29: 120–127, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Vásquez-Vivar J, Kalyanaraman B, Martásek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic Res 37: 121–127, 2003 [DOI] [PubMed] [Google Scholar]
- 39. White FC, Nakatani Y, Nimmo L, Bloor CM. Compensatory angiogenesis during progressive right ventricular hypertrophy. Am J Cardiovasc Pathol 4: 51–68, 1992 [PubMed] [Google Scholar]
- 40. Zhu XY, Daghini E, Chade AR, Rodriguez-Porcel M, Napoli C, Lerman A, Lerman LO. Role of oxidative stress in remodeling of the myocardial microcirculation in hypertension. Arterioscler Thromb Vasc Biol 26: 1746–1752, 2006 [DOI] [PubMed] [Google Scholar]