Abstract
There is not a clinically available technique for measuring the physiological traits causing obstructive sleep apnea (OSA). Therefore, it is often difficult to determine why an individual has OSA or to what extent the various traits contribute to the development of OSA. In this study, we present a noninvasive method for measuring four important physiological traits causing OSA: 1) pharyngeal anatomy/collapsibility, 2) ventilatory control system gain (loop gain), 3) the ability of the upper airway to dilate/stiffen in response to an increase in ventilatory drive, and 4) arousal threshold. These variables are measured using a single maneuver in which continuous positive airway pressure (CPAP) is dropped from an optimum to various suboptimum pressures for 3- to 5-min intervals during sleep. Each individual's set of traits is entered into a physiological model of OSA that graphically illustrates the relative importance of each trait in that individual. Results from 14 subjects (10 with OSA) are described. Repeatability measurements from separate nights are also presented for four subjects. The measurements and model illustrate the multifactorial nature of OSA pathogenesis and how, in some individuals, small adjustments of one or another trait (which might be achievable with non-CPAP agents) could potentially treat OSA. This technique could conceivably be used clinically to define a patient's physiology and guide therapy based on the traits.
Keywords: pathophysiology of sleep apnea, loop gain, pharyngeal closing pressure, upper airway, arousal threshold
obstructive sleep apnea (OSA) occurs because ventilation during sleep is insufficient to prevent ventilatory drive from reaching the arousal threshold, thereby causing a respiratory effort-related arousal. An important variable in this process is the level of ventilation during sleep. Patients with OSA have reduced ventilation during sleep due to partial or complete collapse of the pharyngeal airway. This will occur if the anatomical structures within or surrounding the airway produce a collapsing force that cannot be adequately compensated for by pharyngeal dilator muscles. Under passive conditions, when the pharyngeal muscles are atonic or hypotonic, the upper airway in patients with OSA collapses at a more positive pharyngeal lumen pressure than non-OSA individuals (12, 25). This suggests that the anatomical structures in patients with OSA produce a greater collapsing force than they do in non-OSA individuals. However, there is significant overlap, indicating that 1) some normal individuals adequately compensate for deficient anatomy, and 2) some OSA patients cannot adequately compensate despite minimally abnormal anatomy (19).
What are the factors responsible for “adequate compensation”? As Younes points out (38), it requires that an individual achieve a sustainable level of ventilation during sleep that is sufficient to prevent arousal (ventilation need not return to the eupneic level). Pharyngeal dilator muscle activity is certainly an important factor (13, 14), as it can counteract collapsing forces and allow more airflow through the upper airway. Other factors include a high arousal threshold, a low ventilatory demand, and a small increase in ventilatory drive for a given reduction in ventilation. Since arousals occur at a threshold level of ventilatory drive (2, 5), a high arousal threshold means that a lower level of ventilation can be tolerated and thus the pharyngeal dilator muscles need to do less work to increase ventilation to a sustainable level. By the same token, a low ventilatory demand, indicated by low eupneic ventilation on optimum continuous positive airway pressure (CPAP), means that a lower level of ventilation can be tolerated (38). Lastly, a small increase in ventilatory drive for a given reduction in ventilation below eupnea will also lower the ventilation required to prevent arousal.
In a particular patient, it would be helpful to know what level of ventilation is necessary for achieving stable breathing during sleep and how effective the pharyngeal muscles must be to overcome anatomical deficits. This would allow one to know what factors are important in causing OSA and how capable an individual is of preventing OSA from occurring. In this study, a noninvasive method for determining such information is presented. Specifically, four physiological traits are measured: 1) pharyngeal anatomy/collapsibility, 2) the ventilatory response-to-disturbance ratio (loop gain), 3) the ability of the upper airway to stiffen/dilate in response to an increase in ventilatory drive, and 4) the arousal threshold. This information could provide clinicians and patients with a broader range of treatment options if, for instance, it is found that small adjustments in one or another of these physiological traits could increase the likelihood of adequate compensation. This is fundamentally important as current treatment approaches are often unacceptable or ineffective (4, 16).
METHODS
Theoretical Description of the Measurements and the Model
The traits are measured by placing subjects on enough CPAP during sleep to completely open the pharyngeal airway (termed the holding pressure) and then dropping the pressure intermittently throughout the night to subtherapeutic levels for 3- to 5-min intervals.
Ventilatory response to disturbance measurement.
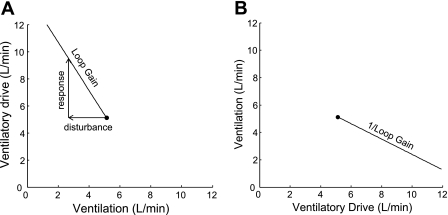
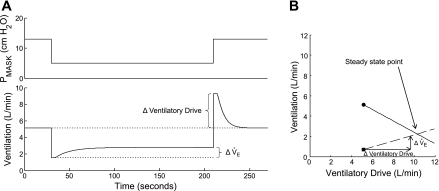
The ventilatory response-to-disturbance ratio is the loop gain of the respiratory control system. Figure 1 illustrates the concept of loop gain and how it can be measured from a CPAP drop. In this figure, a single 3-min CPAP drop is shown. During the first 30 s, CPAP is at the holding pressure and the airway is completely open. When CPAP is dropped to 5 cm H2O (in this example), ventilation decreases because the upper airway narrows. Thereafter, ventilation recovers partially due to airway stiffening and increased respiratory effort and achieves a new steady state that is below eupnea. The reduced ventilation on suboptimum CPAP [quantified as ΔVentilation (disturbance) in Fig. 1] leads to an accumulation of CO2 and ventilatory drive. The amount of ventilatory drive that has accumulated can be determined by returning CPAP to the holding pressure (thereby opening the airway) and measuring the ventilatory overshoot [ΔVentilation (response)]. Loop gain is the ventilatory response to disturbance ratio [Loop gain = ΔVentilation (response) ÷ ΔVentilation (disturbance)]. Figure 1 demonstrates that if ventilation is reduced by −2.3 l/min and is allowed to reach a steady state at this level, ventilatory drive will increase by 4.2 l/min, i.e., the steady-state loop gain is −1.8.1 Loop gain is a negative number because the response is in the opposite direction to the disturbance.
Fig. 1.
Measurement of loop gain from a continuous positive airway pressure (CPAP) drop. Top tracing is mask pressure (PMASK), and bottom tracing is breath-to-breath ventilation. See text for details.
To demonstrate how the different traits interact to produce OSA in a particular individual, they are plotted on a graph of ventilatory drive vs. ventilation. To put loop gain on this graph, the eupneic ventilation on CPAP is first plotted (see the dot in Fig. 2A). Then, vectors are drawn representing the ventilatory disturbance and the ventilatory response from Fig. 1. The line connecting these vectors is the loop gain, and thus loop gain is represented as a slope on this graph. To add the other traits to the model, the x-axis and y-axis in Fig. 2A are “flipped” to produce Fig. 2B. The reason for this will become clear in the next section.
Fig. 2.
Plot of loop gain. A: ventilation is on the x-axis and ventilatory drive is on the y-axis. Starting at the eupneic ventilation on optimum CPAP, the disturbance (a reduction in ventilation of −2.3 l/min, obtained from Fig. 1) is added, followed by the response (an increase in ventilation of 4.2 l/min). The slope of the line connecting the vectors is the loop gain. B: axes are flipped and the reciprocal of loop gain is plotted. Other traits will subsequently be added to B.
Pharyngeal anatomy/collapsibility measurement.
The upper airway “anatomy” is quantified as the ventilation on no CPAP (atmospheric pressure) at the eupneic level of ventilatory drive. This trait is symbolized as V̇0 (ventilation at zero nasal pressure). V̇0 is determined by recording the mask pressure and ventilation at the holding pressure and at the beginning of each pressure drop and then plotting them on a graph of ventilation vs. pressure. The data are fit with a straight line, and V̇0 is the y-intercept (Fig. 3A). This technique is similar to the pharyngeal critical pressure measurement (29) except that ventilation, rather than inspiratory flow, is plotted on the ordinate and the y-intercept, rather than the x-intercept, is used to quantify pharyngeal collapsibility. This approach allows the anatomy parameter to be incorporated into the model using units of liters per minute. Figure 3B shows the OSA model with the loop gain and anatomy traits plotted.
Fig. 3.
A: determination of V̇0. Ventilation at several levels of mask pressure is plotted (□) and fit with a straight line. The anatomy trait is the ventilation at zero mask pressure (■), which is 0.7 l/min in this example. B: model with the upper airway anatomy and loop gain plotted. To plot V̇0 in the model, the eupneic level of ventilatory drive is found on the x-axis (5.1 l/min) and a closed square is placed at a ventilation of 0.7 l/min.
Upper airway gain measurement.
During sleep, particularly in patients with OSA, the upper airway may partially or completely obstruct, thereby reducing ventilation. As a result, Pco2 and the ventilatory drive to breathe increase. The latter will stimulate the diaphragm and upper airway muscles, and ventilation may compensate to some extent. The amount of compensation depends mostly on the ability of the upper airway to stiffen/dilate in response to the increase in drive. This ability, which we term the “upper airway gain,” is quantified from a CPAP drop as shown in Fig. 4. In this figure, the drop in CPAP from 13 to 5 cm H2O leads to an abrupt reduction in ventilation from 5.1 to 1.5 l/min. As ventilatory drive increases, ventilation also increases but generally does not achieve eupneic levels because the airway is narrowed. The increase in compensatory ventilation (ΔV̇e in Fig. 4A) that occurs, divided by the increase in ventilatory drive (ΔVentilatory Drive), is the upper airway gain.2
Fig. 4.
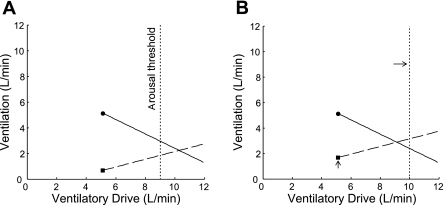
A: measurement of the upper airway gain from a CPAP drop. As in Fig. 1, a single 3-min drop is shown. The increase in ventilation across the drop (ΔV̇e) is divided by the increase in ventilatory drive (ΔVentilatory Drive) to get the upper airway gain. In this example, the upper airway gain is 1.3 l/min ÷ 4.2 l/min, which equals 0.31. The OSA model, with the addition of the upper airway gain, is shown in B. Starting at the anatomy point (■), the upper airway gain (dashed line) is plotted by drawing vectors for the increase in ventilatory drive and the resulting increase in ventilation (justification for plotting the upper airway gain starting at the anatomy point, V̇0, is given in the online Supplement Section III.A.). The intersection of the solid (1/Loop gain) and dashed (upper airway gain) lines is the predicted steady-state ventilation off CPAP (this intersection is termed the “steady-state point”). The model indicates that, in the absence of CPAP, ventilation will increase as a function of increasing ventilatory drive until it reaches a steady-state level at 2.2 l/min.
The upper airway gain can now be added to the model. Starting at the anatomy point (square in Fig. 4B), vectors representing the ΔV̇e and ΔVentilatory Drive from Fig. 4A are drawn. The dashed line connecting these vectors is the upper airway gain (see Section III.B. in the online supplement for justification for modeling the upper airway gain as a linear function of drive; Supplement Material for this article is available online at the J Appl Physiol website.). The intersection of the upper airway gain line and the 1/Loop gain line is the simultaneous solution to the equations for both lines, and it is the predicted steady-state ventilation that would occur during sleep in the absence of CPAP. Whether this level of ventilation is tolerated depends on the arousal threshold, which is described next.
Arousal threshold measurement.
The arousal threshold is defined as the ventilatory drive that produces arousal (2, 5). With this method, ventilatory drive during the CPAP drop is calculated using a dynamic model of the ventilatory control system (this dynamic model is different from the graphical OSA model depicted in Fig. 4B). From the same tracings used to determine loop gain, one can also estimate the circulation delay and time constant of the ventilatory control system (see Fig. 5A, the details of how the delay and time constant are estimated are described below and in the online Supplement Section I.B.). Knowing the gain, delay, and time constant allows the increase in ventilatory drive at any point in the CPAP drop to be calculated. Thus, when an arousal occurs, the ventilatory drive at the time of arousal can be determined (Fig. 5B).
Fig. 5.
Determination of the arousal threshold from a CPAP drop. A: same CPAP drop as that shown in Figs. 1 and 4A. As described in Fig. 1, the steady-state loop gain is calculated by dividing the response by the disturbance. The dynamics of the ventilatory control system (Delay and time constant) are estimated from the time course of the decline in ventilation after the overshoot. The dotted line is the calculated increase in ventilatory drive determined from a dynamic model of the ventilatory control system (described later in the text and in detail in the online Supplement Section I.B.). B: CPAP drop that produces an arousal. The solid line is the ventilation, and the dotted line is the calculated ventilatory drive. At 70 s, an arousal occurs. Ventilatory drive at arousal, which defines the arousal threshold, is 9 l/min.
The arousal threshold is added to the OSA model by placing a vertical line at the ventilatory drive that produces arousals (Fig. 6). The model is now complete and indicates that the subject will probably have OSA for the following reason: during sleep in the absence of CPAP, ventilation is initially 0.7 l/min. As ventilatory drive increases, ventilation increases (approximately) along the upper airway gain line. However, before it intersects the loop gain line, an arousal occurs. Therefore, according to the model, if the steady-state point (intersection of the dashed line and the solid line) is to the right of the arousal threshold line, then OSA will occur. If the steady-state point is to the left of the arousal threshold line, then OSA will not occur. Figure 6B shows how small manipulations in the traits might bring the steady-state point into the stable region (to the left of the arousal threshold line).
Fig. 6.
A: OSA model with all 4 traits plotted. Arousal threshold (dashed line) is added by moving along the x-axis and placing a vertical line at 9 l/min. The model indicates that, off CPAP, an arousal will occur before stable breathing can be achieved, i.e., the subject will have OSA. Diagram B illustrates the potential clinical usefulness of the model and suggests that slight adjustments in the anatomy point (moving the square vertically upwards from 0.7 to 1.7 l/min, as might occur with weight loss or pharyngeal surgery) and the arousal threshold (shifting the dotted line to the right by 1 l/min, as might be achievable with a sedative) may move the intersection of the solid and dashed lines from the right (unstable region) to the left (stable region) of the arousal threshold line and eliminate or substantially reduce apnea.
Of note, the OSA model in Fig. 6 is a steady-state model. There is no time axis, and hence the dynamics of the ventilatory control system (delay and time constant) cannot be easily depicted. Consequently, the frequency of cycling and other dynamic features cannot be construed. There are strengths and weaknesses to this approach. The primary strengths are: 1) the steady-state loop gain is important for determining whether stable (steady state) ventilation can occur during sleep without arousals, and 2) the traits can be plotted on a graph that allows their interrelationships to be appreciated. The major weakness is that, under certain conditions, the dynamics can affect the interpretation of the steady-state model. These conditions are described in the online Supplement Section I.C.
Subjects
The normal controls were recruited from the community and had an apnea-hypopnea index (AHI) <10/h during supine non-rapid eye movement (NREM) sleep. CPAP-adherent OSA patients with an AHI >10/h during supine NREM sleep were recruited from our clinical sleep center. Adherence was defined as a compliance factor >5 h/night for >2 mo. Subjects were on no medications that could affect respiration or muscle control. Individuals with concurrent sleep disorders such as periodic limb movements (periodic limb movement arousal index >10), narcolepsy, or parasomnia were excluded. Other exclusion criteria included the following: renal failure, neuromuscular disease or other major neurological disorders, uncontrolled diabetes, heart failure, central sleep apnea or Cheyne-Stokes respiration, uncontrolled hypertension, thyroid disease, or any other unstable medical condition. The age range was 21–65 yr.
This study is part of a large “apnea phenotyping” protocol in which we had planned to measure loop gain using proportional assist ventilation (PAV; Refs. 20, 21, 41) and pharyngeal critical pressure, pharyngeal muscle responsiveness, and arousal threshold using CPAP drops. Each of these measurements was to be made in an individual over 2 study nights, the first on PAV and the second on CPAP. However, obtaining precise loop gain measurements with PAV proved difficult. As a result, different methods for disturbing the ventilatory control system were explored, which led to the current methodology. In this study, 14 subjects (4 normal controls, 10 OSA patients) studied using this new technique are presented. Four of the subjects had repeat measurements on a second night. The study was approved by the Institutional Review Board at Brigham and Women's Hospital. All subjects gave informed, written consent before participating.
Equipment and Techniques
Standard polysomnography.
Standard polysomnography was performed with at least 4 h of supine sleep to confirm the presence or absence of OSA. EEG, chin electromyogram (EMG), and electrooculogram signals were monitored for sleep staging. Airflow was measured using thermistors and nasal pressure. Chest and abdominal motion (piezo-electric bands), EKG, anterior tibialis EMG, and arterial oxygen saturation were also measured. All signals were acquired on the Alice 5 data acquisition system (Philips Respironics, Murrysville, PA). Apneas and hypopneas were defined using American Academy of Sleep Medicine guidelines (1), and the AHI provided is the value determined during supine NREM sleep.
CPAP drops.
On a subsequent night, subjects presented to the research laboratory for measurements of the physiological traits. EEG, chin EMG, and electrooculogram were monitored for sleep staging. Airflow was measured with a pneumotachometer (Hans-Rudolph, Kansas City, MO) and pressure transducers (Validyne, Northridge, CA) attached to a sealed nasal mask. Mask pressure was monitored with a second Validyne pressure transducer referenced to atmospheric pressure. Arterial oxygen saturation was monitored at the finger (BCI, Waukesha, WI). Signals were sampled at 125 Hz and recorded with both a Nihon Kohden data acquisition system (Tokyo, Japan) and Spike 2 software (Cambridge, UK).
Subjects slept in the supine position and breathed through the nasal mask connected via a breathing circuit to a positive/negative pressure source (modified CPAP machine; Philips Respironics). The mouth was taped shut to reduce leaks. Once asleep, CPAP was set to the holding pressure, defined as the mask pressure required to eliminate hypopneas, snoring, and flow limitation (flattened inspiratory flow). After several minutes of stable breathing at the holding pressure, CPAP was abruptly decreased for 3–5 min and then returned to the holding pressure for another 3–5 min. These maneuvers were repeated multiple times throughout the night to varying levels of CPAP or continuous negative airway pressure in random order. If awakening occurred during a drop, the pressure was returned to the holding pressure until sleep resumed and subsequent drops could be performed. The physiological traits were estimated from data obtained during supine NREM sleep.
Data analysis
Loop gain.
The raw flow signals were integrated to get volume. Unintentional (e.g., mask) leaks were removed by fitting a regression line to the volume signal. The slope of this line is the leak, which was subtracted from the flow signal before reintegrating it to get the corrected tidal volume. This was done for each level of mask pressure.
The loop gain, time constant, and delay (as labeled in Fig. 5A) were estimated from the last 60 s of a drop (in which CPAP was at a subtherapeutic level) plus the subsequent 3 min immediately succeeding the drop (in which CPAP was at the holding pressure). In order for a drop to be considered for loop gain measurement, ventilation during the last 60 s of the drop had to be significantly less (P < 0.1) than eupneic ventilation on optimum CPAP. Also, no arousals could occur during this interval. Each signal meeting these criteria was scaled so that the disturbance magnitude [ΔVentilation (disturbance) in Fig. 1] equaled 1 l/min. This scaling was done so that the different drops (which had different disturbance amplitudes) could be ensemble averaged to reduce noise (see the online Supplement Section I.A. for more details). The ensemble averaged signal was fit to the model equation below, which is a transfer function model written as a function of complex frequency (the model is described in detail in the online Supplement Section I.B.).
GL(s) is the loop gain as a function of complex frequency. The s is the symbol used to denote complex frequency. ΔV̇drive(s) is the change in ventilatory drive, or the response, as a function of complex frequency. ΔV̇e(s) is the change in ventilation, or the disturbance, as a function of complex frequency. GL is the steady-state loop gain, which is the ventilatory response to a steady-state disturbance. This is the ratio defined in Fig. 1. D is the circulation delay between the pulmonary capillaries and the peripheral chemoreceptors. In the complex frequency domain, time delays are modeled as exponential functions, hence the term e−sD. τ is the time constant for the ventilatory control system. To estimate the steady-state loop gain (GL) and dynamics (D and τ), we need to know the input, ΔV̇e(s), and the output, ΔV̇drive(s), to the equation. ΔV̇e(s) is obtained from the measured flow signal. ΔV̇drive(s) is determined by extrapolating the ventilatory overshoot backwards, as depicted in Fig. 7 [more detail about how ΔV̇e(s) and ΔV̇drive(s) were obtained is given in the online Supplement Section I.A.]. The parameters (GL, D, and τ) were determined by inputting ΔV̇e(s) into the equation and then “tuning” GL, D, and τ using least squares regression to make the model output fit the ΔV̇drive(s) signal. This was accomplished using Matlab's System Identification Toolbox (Natick, MA).
Fig. 7.
Back extrapolation from the peak of the overshoot is used to construct the ventilatory drive signal (dotted line) during the last 60 s of the CPAP drop (from 150 to 210 s). After the CPAP drop, from 210 s onwards, the ventilation matches the ventilatory drive (because the airway is open and there is no longer any impediment to ventilation) and the dotted and solid lines equal one another. There are now 2 signals: the solid line, which is the pneumotach-measured ventilation signal that is the input to the transfer function model, ΔV̇e(s); and the dotted line which is the derived ventilatory drive signal that is the output of the model, ΔV̇drive(s). Online Supplement Section I.A. describes in detail the justification for this “back extrapolation” procedure.
Pharyngeal anatomy/collapsibility.
To estimate the ventilation at zero nasal pressure, V̇0, a graph was constructed like the one in Fig. 3A using the following: 1) the ventilation at the holding pressure (obtained from each 1-min segment of data before each CPAP drop); and 2) the mask pressure and ventilation of the second and third breath of each pressure drop. The first breath was not used as it often is not reduced as much as breaths 2 and 3, possibly due to lung volume effects (24). Breaths 4, 5, or 6 were not used because the ventilatory drive would likely have increased by this time, and we wanted V̇0 to reflect the ventilation at eupneic ventilatory drive. The data were then fit with a straight line, and the y-intercept was taken as V̇0.
Upper airway gain.
The upper airway gain was calculated from runs that did not end in arousal. The upper airway gain is a ratio (ΔVentilation ÷ ΔVentilatory Drive), as shown in Fig. 4A. The numerator (ΔVentilation) is the increase in ventilation across the drop, and it was calculated by subtracting the mean ventilation on the last three breaths of the drop by the mean ventilation of breaths 2 and 3 of the drop. The denominator (ΔVentilatory Drive) is the increase in ventilatory drive across the CPAP drop, and it was calculated by subtracting the mean ventilation of the first two breaths of the ventilatory overshoot (after CPAP was returned to the holding pressure) by the eupneic ventilation on optimum CPAP (see the online Supplement Section III for a detailed illustration of this calculation). The different responses were averaged to determine the mean upper airway gain in an individual.
Arousal threshold.
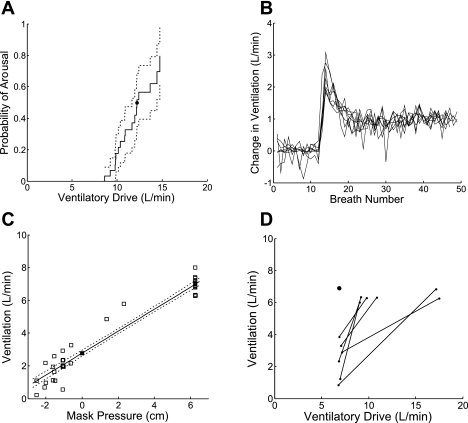
Arousals, defined as a high frequency shift in EEG for >3 s, were scored by an experienced technologist, with particular attention paid to the start and end of each arousal. To estimate the arousal threshold, the increase in ventilatory drive was calculated using the GL, D, and τ. Because many drops do not end in an arousal, and because the ventilatory drive achieved on these drops is sometimes higher than those that do end in arousal (arousal threshold is variable), the arousal threshold was estimated using survival analysis (40) (see the online Supplement Section II). From a survival analysis plot like the one shown in Fig. 8A, the arousal threshold was taken as the ventilatory drive at which there is a 50% probability of arousal.
Fig. 8.
A: example of a survival analysis plot used for determining the arousal threshold. Arousal threshold is defined as the ventilatory drive at which there is a 50% probability of arousal (marked by the dot). The 90% confidence intervals (calculated using Greenwood's formula; Ref. 8) are indicated by the dotted lines. In this patient, the estimated arousal threshold is 12.2 l/min. B: ventilation signals used for estimating loop gain in one subject. The last portion of each drop (breaths 1–12) and the subsequent 3 min (breaths 13–49) are plotted. Change in ventilation, rather than absolute ventilation, is plotted on the y-axis. Disturbance magnitudes have been normalized to 1 l/min. C: representative graph used for determining V̇0. The open squares at 6 cm H2O are the mean ventilations at the holding pressure (eupneic ventilation). The other open squares are the ventilations at various levels of mask pressure. Data are fit with a straight line and 90% confidence intervals. V̇0 is the ventilation at zero mask pressure (■). D: plot showing the change in ventilation from the beginning of the drop (leftmost dots) to the end of the drop (rightmost dots) as a function of increasing ventilatory drive for one subject. Each pair of dots is connected with a straight line. In this patient, ventilation increases as ventilatory drive increases, i.e., the slope of the upper airway gain is positive. Eupneic ventilation is marked by the single dot at 6.9 l/min.
RESULTS
The subjects' characteristics are shown in Table 1. Four individuals (subjects 1–4) did not have significant OSA in NREM sleep. Subject 3 used CPAP at home for REM-related OSA. The average CPAP used at home was 9 ± 3 cm H2O. Most subjects were middle aged (47 ± 12 yr old) and obese (body mass index = 34 ± 7 kg/m2). There was a large range of AHIs from 0 to 115 episodes/h.
Table 1.
Subject characteristics
| Subject | Age, yr | Sex | BMI kg/m2 | CPAP,* cmH2O | AHI,† episodes/h |
|---|---|---|---|---|---|
| 1 | 31 | F | 28 | NA | 0 |
| 2 | 50 | M | 24 | NA | 1 |
| 3 | 58 | F | 42 | 6 | 4 |
| 4 | 63 | F | 29 | NA | 8 |
| 5 | 50 | M | 33 | 8 | 15 |
| 6 | 20 | M | 31 | 7 | 17 |
| 7 | 34 | M | 35 | 9 | 54 |
| 8 | 60 | M | 31 | 12 | 80 |
| 9 | 39 | F | 46 | 17 | 112 |
| 10 | 56 | M | 41 | 7 | 115 |
| 11 | 47 | F | 38 | 9 | 12 |
| 12 | 57 | M | 26 | 9 | 48 |
| 13 | 52 | M | 32 | 4 | 52 |
| 14 | 43 | F | 45 | 8 | 95 |
| Means | 47 | 34 | 9 | 44 | |
| SD | 12 | 7 | 3 | 42 |
Values are means ± SD. BMI, body mass index; CPAP, continuous positive airway pressure; AHI, apnea-hypopnea index; NA, not applicable.
Home use.
Supine, non-rapid eye movement sleep.
Figure 8 shows representative examples of raw data used to determine the physiological traits in several subjects. Figure 8B displays the changes in ventilation when CPAP was returned to the holding pressure in one subject. These data were used for calculating loop gain. Multiple drops to different levels of nasal pressure were performed in this individual, but the signals have been scaled (such that the difference between eupneic ventilation and the reduced ventilation during the drop is 1 l/min) and shifted (so that the baseline is 0 l/min). The signals from each run overlay one another, indicating that loop gain is linear in the ranges tested. To reduce the noise before estimating the loop gain, delay, and time constant, these signals were ensemble averaged.
Figure 8C is the ventilation at each level of nasal pressure (including the holding pressure) for a single subject. These data were used for calculating V̇0. In this individual, 21 CPAP drops were performed. The holding pressure was 6 cm H2O, which produced 21 data points at this pressure level (this is the eupneic ventilation). The open squares at each pressure level are the average of the second and third breaths of each CPAP drop. The V̇0 in this patient is 2.8 l/min.
Figure 8D displays the changes in ventilation as a function of increasing ventilatory drive in one subject. These data were used for calculating the upper airway gain. The dots on the left correspond to the ventilation on breaths 2 and 3 of each drop, and the dots on the right correspond to the ventilation for the last three breaths of the drop. Thus there are two dots connected by a line for each drop. The average upper airway gain in this patient is 0.64.
Model Results for Each Subject
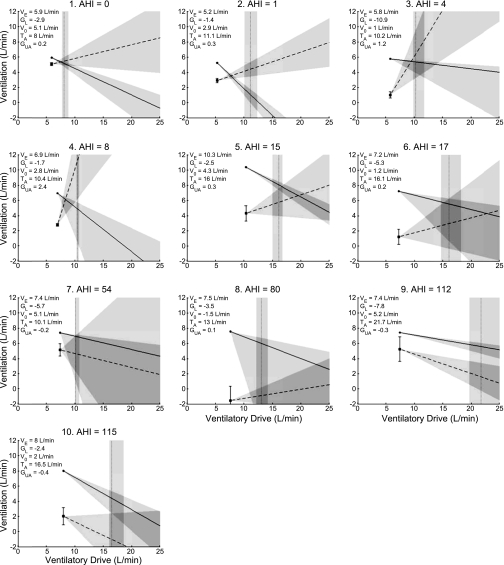
The model diagrams in Fig. 9 suggest that OSA can occur, or not occur, for different reasons in different individuals [subjects 1–10 are shown in Fig. 9 and subjects 11–14 (repeatability data) are shown in Fig. 10]. Subject 1 has a low/normal loop gain, a V̇0 that is only slightly below eupneic ventilation, and a positively sloped upper airway gain, all of which protect against OSA. Thus, while the arousal threshold is the lowest of the 10 individuals shown here (which would increase the probability of having respiratory effort-related arousals), subject 1 does not have OSA. In subject 2, the combination of a low loop gain and positive upper airway gain is probably the most important trait preventing OSA. Subject 3 has a very high loop gain, a low V̇0, and a low arousal threshold. If only these three traits were measured, one might predict that this patient has OSA. However, the upper airway gain is very positive and clearly protective in this individual. Subject 4 also has a very positive upper airway gain, which in combination with a low loop gain, is enough to overcome the poor anatomy and low arousal threshold.
Fig. 9.
Model results and AHI for subjects 1–10 (subjects 11–14 are shown in Fig. 10). As in the previous diagrams, eupneic ventilation is marked by the dot, V̇0 by the square, the reciprocal of loop gain by the solid line, the upper airway gain by the dashed line, and the arousal threshold by the vertical dotted line. Values for each trait are listed in the top left of each graph. VE, ventilation on optimum CPAP; GL, loop gain; V0, ventilation at zero nasal pressure and eupneic ventilatory drive; TA, arousal threshold in l/min; Gua, upper airway gain. The 90% confidence intervals are marked by shading.
Fig. 10.
Repeatability data in subjects 11–14 (labeled A–D). Night 1 is on the left and Night 2 is on the right. See text for details.
Subjects 5 and 6 have mild OSA. The possible cause of OSA in subject 5 is interesting. Note that the anatomy point (V̇0) in this subject is greater than in the preceding three individuals. Also, the loop gain is low, the arousal threshold is high, and the upper airway gain is positively sloped. It would be expected, therefore, that this patient would not have OSA. However, the eupneic ventilation is substantially elevated, thereby making it harder for the loop gain line to intersect the upper airway gain line in the stable region (to the left of the arousal threshold line). In this regard, the eupneic ventilation plays a similar role to the V̇0. In subject 6, the relatively high loop gain and low V̇0 are probably the important traits that contribute to mild OSA in this patient.
Subjects 7–10 have severe OSA. In subject 7, the loop gain and upper airway gain are variable and not well characterized by a mean slope. Nevertheless, it seems that the mostly negative upper airway gain and low arousal threshold are contributing to OSA in this individual. Note that the anatomy points are similar in subjects 7 and 1, yet the AHIs are substantially different. Subject 8 has a normal loop gain, a slightly positive upper airway gain, and a normal arousal threshold. However, the V̇0 is markedly reduced and has a negative value. While it is impossible to have negative ventilation, a negative V̇0 means that the airway will be closed off of CPAP and will remain closed until the ventilatory drive increases enough to reopen it (which is ∼20 l/min in this individual). In subjects 9 and 10, the major mechanism causing OSA is the negative upper airway gain. Such a response makes the possibility of achieving stable breathing during sleep virtually impossible, regardless of the other traits. Note that subject 9 has a normal V̇0 and a high arousal threshold, and subject 10 has a low loop gain and a high arousal threshold. Nevertheless, both clearly have OSA. These diagrams illustrate the importance of interpreting the physiological traits in relationship to one another.
Repeatability of the measurements.
Figure 10 shows night-to-night repeatability data in four subjects. In subject A, the traits are similar on both nights except for the arousal threshold, which is 3.2 l/min higher on night 2. In subject B, the parameters are virtually the same on both nights. Subject C has an ∼1 l/min higher ventilation on night 2, and all of the other traits are slightly higher as well, but not by much, and the physiology is generally the same: there is a negative upper airway gain without bad anatomy (V̇0 is 1.5–2 l/min less than eupneic ventilation), a moderately high loop gain, and a medium arousal threshold. In subject D, the traits mostly agree except for V̇0, but again the major physiology (mild anatomical abnormality with significantly negative upper airway gain) is captured. These data suggest that the measurements of the traits are generally repeatable from night to night.
DISCUSSION
In this study, a noninvasive technique for measuring several physiological traits that contribute to the pathogenesis of OSA is presented. The technique involves raising and lowering CPAP and measuring the resultant changes in ventilation. This is a relatively simple methodology that could be used in a clinical laboratory. A model of OSA pathogenesis that incorporates the measured traits is also presented. The model illustrates in a concise diagram how the traits interact to produce or prevent OSA.
The purpose for developing this technique is to provide clinicians and patients with additional potential treatment options. Currently, most OSA patients are prescribed CPAP, which is effective but has an acceptance rate of only ∼50% (4, 16). If, however, the contribution of each trait could be determined in a particular patient, then it might be possible to target the abnormal traits and treat OSA without CPAP in some individuals. For example, upper airway surgery and a sedative might be effective in a patient with a small/narrow upper airway and a low arousal threshold. If the ventilatory control system is also unstable, then adding oxygen or acetazolamide (which stabilize the ventilatory control system; Refs. 17, 22, 26, 32, 35) might be useful as well. Also, if drugs that stimulate the upper airway muscles are developed, they will likely need to be combined with other therapies to fully treat the OSA.
A number of investigators have already studied the effects of non-CPAP therapies [e.g., uvulopalatopharyngoplasty (30), oral appliances (10, 23), oxygen (6, 7, 18, 33), acetazolamide (11, 28, 31, 34, 37), and sedatives (3, 9, 27)] on apnea severity and, in a limited fashion, the physiological traits themselves. These studies were performed in largely unselected populations. In most cases, the agents, when tested, altered/improved the trait; however, the effect on AHI was generally modest, although some patients exhibited more complete responses. We believe that better matching of the agent (or combinations of agents) with the mechanisms causing OSA will lead to better results.
As an example of how the model might help in this process, consider the model diagrams in Fig. 9 again. In subject 5, the only abnormal trait is the high ventilatory demand. Thus it might be possible for this patient to achieve stable breathing during sleep by lowering the metabolic rate. In subject 6, lowering the loop gain and raising V̇0 using some of the interventions described above might eliminate OSA. Subject 7 has a low arousal threshold. This would seem a reasonable therapeutic target. However, unless the negative upper airway gain is also addressed (if future drugs make this possible), raising the arousal threshold is unlikely to be effective. Similarly, lowering the relatively high loop gain in subject 9 would probably also need to be combined with an improvement in the upper airway gain (the upper airway gain is undoubtedly an important trait that needs to be more completely understood). Lastly, subjects 8 and 10 probably require CPAP, as it is unlikely that small adjustments in the traits could allow the patients to achieve stable breathing. Clearly, knowledge regarding how much a particular trait could be adjusted would be helpful in making this decision.
Limitations
There are a number of limitations that must be kept in mind when considering this study. 1) In this study, the “validation” of the overall approach is whether or not the complete set of measured traits predicts OSA (or no OSA) when incorporated into the model. This was accomplished, as we correctly predicted the presence or absence of OSA in all 14 subjects. However, we recognize that each measurement should be validated independently, and this is planned in future experiments. 2) All of the determinations in this study were obtained from supine, NREM sleep, and thus they cannot be extrapolated to REM sleep. Measurement of traits during REM sleep is challenging due to REM-related breathing variability and insufficient time in REM sleep. However, such testing may be needed. 3) The measurements are noisy, which is likely due to intrinsic variability in the physiology as well as measurement noise. In most cases, multiple measurements were made to “average out” the noise. However, the requirement that ventilation reach a steady state during the CPAP drop (to measure loop gain, see Fig. 1) limited the number of measurements that could be made in a single night. The model would be improved if the traits could be estimated from shorter drops, which would allow for more measurements and greater confidence in the estimates. 4) The frequency of events as a measure of OSA severity might not be predicted well by a steady-state model such as the one described in this study. Thus, while it can be used to predict if someone will have OSA, it might not correlate well with the AHI in all individuals. Despite these limitations, this approach may have both diagnostic and potentially therapeutic value. Future studies of the effect of non-CPAP agents on the traits are needed, as is testing of combination therapy in which agents are matched with the abnormal traits.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 5R01-HL-048531-16, R01-HL-085188, R01-HL-090897, K24-HL-093218, and P01-HL-095491 and American Heart Association Grants 0840159N and 0575028N. D. Eckert is supported by a NHMRC of Australia Overseas Biomedical Fellowship (510392).
DISCLOSURES
Andrew Wellman is a consultant for Philips. Danny Eckert and Amy Jordan are consultants for Apnex Medical. Atul Malhotra receives research and/or consulting income from Philips, Apnex Medical, Cephalon, Sepracor, Pfizer, Merck, Ethicon, Medtronic, SGS, SHC, and Apnicure. David White is Chief Medical Officer for Philips.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Karen Stevenson, Lauren Hess, Scott Smith, and Louise Dover for technical assistance with the studies.
Footnotes
The steady-state loop gain is different from the loop gain “at the frequency associated with a phase angle of 180°,” which is what our laboratory and others have previously reported (15, 36, 41). The loop gain at the frequency associated with a 180° phase angle is a function of ventilatory control system dynamics (e.g., circulation delay, time course of the increase in CO2 after a change in ventilation). The steady-state loop gain, as the name implies, is not dependent on dynamics. Rationale for using the steady-state loop gain in the OSA model, and not the “dynamic” loop gain, is given in the online Supplement Section I.C. Note, however, that a model is presented later in the text that would allow one to calculate the loop gain at any frequency, i.e., the dynamic loop gain.
Ventilation during a CPAP drop (divided by the increase in ventilatory drive) could increase for reasons other than pharyngeal stiffening/dilation, namely from changes in breath timing or the inspiratory flow shape (39). However, it is referred to as the upper airway gain for simplicity.
REFERENCES
- 1. American Academy of Sleep Medicine International classification of sleep disorders: Diagnostic and coding manual (2nd ed.). Westchester, IL: American Academy of Sleep Medicine, 2005 [Google Scholar]
- 2. Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep 20: 654–675, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Berry RB, Kouchi K, Bower J, Prosise G, Light RW. Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med 151: 450–454, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Engelman H, Wild MR. Improving CPAP use by patients with the sleep apnea/hypopnea syndrome (SAHS). Sleep Med Rev 71: 81–99, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis 142: 295–300, 1990 [DOI] [PubMed] [Google Scholar]
- 6. Gold AR, Bleecker ER, Smith PL. A shift from central and mixed sleep apnea to obstructive sleep apnea resulting from low-flow oxygen. Am Rev Respir Dis 132: 220–223, 1985 [DOI] [PubMed] [Google Scholar]
- 7. Gold AR, Schwartz AR, Bleecker ER, Smith PL. The effect of chronic nocturnal oxygen administration upon sleep apnea. Am Rev Respir Dis 134: 925–929, 1986 [DOI] [PubMed] [Google Scholar]
- 8. Greenwood M. The natural duration of cancer. In: Reports on Public Health and Medical Subjects. London: Her Majesty's Stationery Office, 1926, p. 1–26 [Google Scholar]
- 9. Heinzer RC, White DP, Jordan AS, Lo YL, Dover L, Stevenson K, Malhotra A. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J 31: 1308–1312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inazawa T, Ayuse T, Kurata S, Okayasu I, Sakamoto E, Oi K, Schneider H, Schwartz AR. Effect of mandibular position on upper airway collapsibility and resistance. J Dent Res 84: 554–558, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Inoue Y, Takata K, Sakamoto I, Hazama H, Kawahara R. Clinical efficacy and indication of acetazolamide treatment on sleep apnea syndrome. Psychiatry Clin Neurosci 53: 321–322, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol 82: 1319–1326, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Jordan AS, Wellman A, Heinzer RC, Lo YL, Schory KE, Dover L, Gautam S, Malhotra A, White DP. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax 62: 861–867, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jordan AS, White DP, Lo YL, Wellman A, Eckert DJ, Yim-Yeh S, Eikermann M, Smith SA, Stevenson KE, Malhotra A. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep 32: 361–368, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol 53: 644–659, 1982 [DOI] [PubMed] [Google Scholar]
- 16. Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, Redline S, Henry JN, Getsy JE, Dinges DF. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea [see comments]. Am Rev Respir Dis 147: 887–895, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Lai J, Bruce EN. Ventilatory stability to transient CO2 disturbances in hyperoxia and normoxia in awake humans. J Appl Physiol 83: 466–476, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Martin RJ, Sanders MH, Gray BA, Pennock BE. Acute and long-term ventilatory effects of hyperoxia in the adult sleep apnea syndrome. Am Rev Respir Dis 125: 175–180, 1982 [DOI] [PubMed] [Google Scholar]
- 19. McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol 105: 197–205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meza S, Mendez M, Ostrowski M, Younes M. Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J Appl Physiol 85: 1929–1940, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Meza S, Younes M. Ventilatory stability during sleep studied with proportional assist ventilation (PAV). Sleep 19: S164–166, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. Am J Respir Crit Care Med 165: 1251–1260, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Oliven R, Tov N, Odeh M, Gaitini L, Steinfeld U, Schwartz AR, Oliven A. Interacting effects of genioglossus stimulation and mandibular advancement in sleep apnea. J Appl Physiol 106: 1668–1673, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Owens RL, Malhotra A, Eckert DJ, White DP, Jordan AS. The influence of end-expiratory lung volume on measurements of pharyngeal collapsibility. J Appl Physiol 108: 445–451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol 102: 547–556, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Reite M, Jackson D, Cahoon RL, Weil JV. Sleep physiology at high altitude. Electroencephalogr Clin Neurophysiol 38: 463–471, 1975 [DOI] [PubMed] [Google Scholar]
- 27. Rosenberg R, Roach JM, Scharf M, Amato DA. A pilot study evaluating acute use of eszopiclone in patients with mild to moderate obstructive sleep apnea syndrome. Sleep Med 8: 464–470, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Sakamoto T, Nakazawa Y, Hashizume Y, Tsutsumi Y, Mizuma H, Hirano T, Mukai M, Kotorii T. Effects of acetazolamide on the sleep apnea syndrome and its therapeutic mechanism. Psychiatry Clin Neurosci 49: 59–64, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Schwartz AR, O'Donnell CP, Baron J, Schubert N, Alam D, Samadi SD, Smith PL. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med 157: 1051–1057, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Schwartz AR, Schubert N, Rothman W, Godley F, Marsh B, Eisele D, Nadeau J, Permutt L, Gleadhill I, Smith PL. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis 145: 527–532, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Sharp JT, Druz WS, VDS, Diamond E. Effect of metabolic acidosis upon sleep apnea. Chest 87: 619–624, 1985 [DOI] [PubMed] [Google Scholar]
- 32. Simakajornboon N, Beckerman RC, Mack C, Sharon D, Gozal D. Effect of supplemental oxygen on sleep architecture and cardiorespiratory events in preterm infants. Pediatrics 110: 884–888, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Smith PL, Haponik EF, Bleecker ER. The effects of oxygen in patients with sleep apnea. Am Rev Respir Dis 130: 958–963, 1984 [DOI] [PubMed] [Google Scholar]
- 34. Tojima H, Kunitomo F, Kimura H, Tatsumi K, Kuriyama T, Honda Y. Effects of acetazolamide in patients with the sleep apnoea syndrome. Thorax 43: 113–119, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wagenaar M, Teppema L, Berkenbosch A, Olievier C, Folgering H. Effect of low-dose acetazolamide on the ventilatory CO2 response during hypoxia in the anesthetized cat. Eur Respir J 12: 1271–1277, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz E, Schory KE, Edwards JK, White DP. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med 170: 1225–1232, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Whyte KF, Gould GA, Airlie MA, Shapiro CM, Douglas NJ. Role of protriptyline and acetazolamide in the sleep apnea/hypopnea syndrome. Sleep 11: 463–472, 1988 [DOI] [PubMed] [Google Scholar]
- 38. Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med 148: 645–658, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol 105: 1389–1405, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol 103: 1929–1941, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med 163: 1181–1190, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.