Abstract
Peripheral arterial disease (PAD) results in a failure to adequately supply blood and oxygen (O2) to working tissues and presents as claudication pain during walking. Nitric oxide (NO) bioavailability is essential for vascular health and function. Plasma nitrite (NO2−) is a marker of vascular NO production but may also be a protected circulating “source” that can be converted to NO during hypoxic conditions, possibly aiding perfusion. We hypothesized that dietary supplementation of inorganic nitrate in the form of beetroot (BR) juice would increase plasma NO2− concentration, increase exercise tolerance, and decrease gastrocnemius fractional O2 extraction, compared with placebo (PL). This was a randomized, open-label, crossover study. At each visit, subjects (n = 8) underwent resting blood draws, followed by consumption of 500 ml BR or PL and subsequent blood draws prior to, during, and following a maximal cardiopulmonary exercise (CPX) test. Gastrocnemius oxygenation during the CPX was measured by near-infrared spectroscopy. There were no changes from rest for [NO2−] (152 ± 72 nM) following PL. BR increased plasma [NO2−] after 3 h (943 ± 826 nM; P ≤ 0.01). Subjects walked 18% longer before the onset of claudication pain (183 ± 84 s vs. 215 ± 99 s; P ≤ 0.01) and had a 17% longer peak walking time (467 ± 223 s vs. 533 ± 233 s; P ≤ 0.05) following BR vs. PL. Gastrocnemius tissue fractional O2 extraction was lower during exercise following BR (7.3 ± 6.2 vs. 10.4 ± 6.1 arbitrary units; P ≤ 0.01). Diastolic blood pressure was lower in the BR group at rest and during CPX testing (P ≤ 0.05). These findings support the hypothesis that NO2−-related NO signaling increases peripheral tissue oxygenation in areas of hypoxia and increases exercise tolerance in PAD.
Keywords: nitrite, nitric oxide, exercise, peripheral arterial disease
peripheral arterial disease (PAD) is a form of cardiovascular disease (CVD) caused by atherosclerotic occlusions that impair blood flow to the lower extremities. PAD affects ∼27 million people in Europe and North America (7). Approximately one-third of PAD patients suffers from intermittent claudication, which is defined as ischemic leg pain that occurs with walking and improves with rest (22). This inability to deliver oxygen (O2) at a rate to match demand in working tissues of the legs severely limits exercise tolerance and the ability to perform activities of daily living, reduces quality of life in PAD patients (44).
A diminished ability to produce nitric oxide (NO) is an early event in the process of atherosclerotic lesion formation (51) and is associated with risk factors for CVD, including PAD (9, 10, 47). During normal physiological conditions, NO bioavailability is a balance between production via endothelial NO synthase (eNOS) and consumption via various pathways—nitrosylation reactions, inactivation to nitrate (NO3−) or peroxynitrite, or oxidation to nitrite (NO2−). Of these species, plasma NO2− concentrations have been shown to best reflect acute changes in regional vascular NO bioavailability following both chemical [l-arginine and NG-monomethyl-l-arginine (36)] and physiological [hyperemia (2, 36)] stimuli. In fact, it is estimated that ∼70% of resting plasma NO2− is derived from eNOS activity in humans and other mammalian species (32).
Initially, it was believed these reserves of plasma NO2− were simply inert, but recent studies have now demonstrated an endocrine role for NO equivalents (8, 26), including NO2− (39, 42). These equivalents may be transported in the blood to areas of hypoxia, where they may then be converted back to NO and mediate vasodilation. Although the exact mechanism by which the reduction of NO2− to NO occurs is currently not clear, several alternate pathways have been described, including chemical acidification (55), xanthine oxidase (52), deoxyhemoglobin (HHb) (14), deoxymyoglobin (46), and mitochondrial enzymes (41).
A relatively easy method to increase plasma NO2− is through increasing dietary NO3− intake. Dietary NO3− is rapidly absorbed in the small intestine and has a plasma half-life of ∼5–6 h (39). Although much of it is eventually excreted in the urine, up to 25% of plasma NO3− is taken up by salivary glands, and concentrations of NO3− in saliva can be 20-fold higher than in plasma (37). Commensal bacteria in the mouth then reduces some of the NO3− to NO2− (38). The acidic environment of the stomach and small intestine causes some or all NO2− to be converted to other nitrogen oxides including NO. NO2− can be absorbed in the intestines and enter the blood directly or through the intermediacy of NO, which is then converted back (partially) to NO2−. Several recent studies in healthy, young subjects have shown increases in plasma NO2− above placebo (PL) of ≈100–300 nM (5, 6, 33, 34, 37, 50, 53) following various administrations of inorganic NO3−, including chronic (4–6 days) (5, 6, 33, 50) and acute (3 h) (50, 53) consumption of beetroot (BR) juice. Interestingly, these supplementations were accompanied by a reduction in systolic blood pressure (SBP) (5, 6, 28, 33, 35, 50) and diastolic BP (DBP) (5, 28, 35) and an increase in exercise tolerance (5, 6, 33, 50).
PAD is a condition of hypoxia in the peripheral tissues, and therefore, any intervention that improves blood flow and oxygenation to these areas could lessen or delay claudication pain and improve physical function and may be a significant treatment option. Additionally, the fact that NO2−-related NO signaling increases blood flow and targets areas of hypoxia (14, 19, 24, 25, 29, 49) supports the hypothesis that increasing plasma NO2− may increase peripheral blood flow where it is needed and possibly improve physical function through increased tissue oxygenation in PAD patients.
We have previously shown, in a cross-sectional approach, that changes in plasma NO2− following a maximal-graded cardiopulmonary exercise (CPX) test and endothelial function [brachial artery flow-mediated dilation (BAFMD)] were the most powerful predictors of exercise performance in subjects with risk factors for or with established PAD (1). We followed up that study by showing that after 3 mo of supervised exercise training, PAD subjects demonstrated statistically significant increases in exercise performance [claudication onset time (COT), peak walking time (PWT), and maximal O2 consumption (VO2peak)], along with significant changes in plasma NO2− following the CPX test (3). Most significantly, the degree of change in COT was significantly related to the change in plasma NO2− concentration. Exercise training, however, also increased BAFMD, which indicates that there was an up-regulation in vascular eNO production. This made it impossible to distinguish if changes in performance were related to 1) increased endothelial NO production and (subsequent oxidation to) NO2−, 2) greater reconversion of NO2− to NO in hypoxic tissues, or 3) both mechanisms' responsibilities.
Accordingly, to assess the effects of increased plasma NO2− in the absence of exercise training adaptations, the purpose of this study was to investigate the acute (up to 3.5 h) effects of BR juice vs. orange juice (PL) beverage ingestion on plasma NO3− and NO2− levels, exercise tolerance (COT, PWT) and tissue oxygenation [assessed with near-infrared spectroscopy (NIRS)] during a maximal-graded CPX, along with BAFMD and BP in subjects with diagnosed PAD. Specifically, the following hypotheses were tested: 1) BR would increase plasma NO2− concentration, 2) BR would delay COT and increase PWT during CPX testing, 3) BR would reduce gastrocnemius muscle tissue deoxygenation during CPX testing (as measured by NIRS), 4) BR would reduce resting BP, and 5) there would be no changes in ankle brachial index (ABI) or endothelial function (BAFMD) following BR.
MATERIALS AND METHODS
Subjects
Four male and four female subjects (three Caucasian, five African-American), age 67 ± 13 yr, height 172.2 ± 8.2 cm, mass 84.5 ± 16.5 kg, body mass index 28.6 ± 5.8, with an ABI in the incident leg of 0.64 ± 0.2, completed both visits of this study (ABI is the ratio of BP in the lower legs to the BP in the arms; a ratio of <0.9 is considered a diagnosis of PAD). All subjects had a history of stable intermittent claudication for 3 or more months and were recruited from Duke University Medical Center (Durham, NC) vascular clinics by physician referral. The procedures employed in this study were approved by the Duke University Medical Center Internal Review Board. Prior to participation, all subjects were informed of the experimental procedures, associated risks, and potential benefits of participation both in writing and verbally and gave their written informed consent to participate.
All subjects were receiving antiplatelet and lipid-lowering therapy unless medically contraindicated by their physician. There was no difference in subject medication regimens between visits. Exclusions were based on a past medical history of gangrene, impending limb loss, or osteomyelitis; lower extremity vascular surgery, angioplasty, lumbar sympathectomy within 3 mo of enrollment, severe peripheral neuropathy, any condition other than PAD that limits walking, unstable angina, history of significant left-main or three-vessel coronary artery disease (>70% stenosis, unprotected by grafts), or recent myocardial infarction (6 wk); or chest pain during treadmill exercise, which appears before the onset of claudication, or >3 mm ST depression during exercise. Due to the high concentrations of potassium and phosphorus (1,697 mg and 170 mg, respectively) in 500 ml of BR juice (Biotta, Carmel IN), we excluded all subjects with any history of renal insufficiency. Additionally, to avoid any possible interference with the processing of NO3− or NO2− in the saliva or stomach, we instructed subjects to refrain from using antibacterial mouthwash products and excluded any subjects currently taking proton pump inhibitors.
Study Design
All subjects were instructed to arrive at the Frederick R. Cobb Non-Invasive Vascular Research Laboratory (Duke University) at 8 AM on two separate occasions, 7–14 days apart, in a fasting condition and holding medications shown to influence BP and endothelial function testing (including angiotensin-converting enzyme inhibitors, β, α, and Ca2+ channel blockers, etc., unless contraindicated). Subjects had refrained from exercise for 12 h and alcohol for 48 h. The study was an open-label, crossover study. The subjects were not aware of the experimental hypotheses to be tested but were informed that the purpose of the study was to compare the physiological responses with exercise following the consumption of two commercially available beverages. The laboratory staff members leading the vascular and CPX treadmill testing were unaware of the beverage consumed prior to each test.
Upon arrival to the clinic, subjects underwent a 10-min period of supine rest, followed by a BP measurement (in duplicate, using a stethoscope and sphygmomanometer) and a venous blood draw (5 ml). They were then randomly assigned to consume 500 ml of either NO3−-rich BR juice or PL (orange juice). We selected orange juice as the PL, because it is matched with BR juice for calories (200), carbohydrates (52 g), fat (negligible), and protein (4 g) content and is also similar in texture and is high in antioxidants. Biotta BR juice has an average listed content of NO3− of 1,500 mg/l. We measured 18,181 umol/l NO3− and 77.3 nmol/l NO2− in the batch used for this study. These values are in the middle of the ranges for NO3− used in other BR studies (5.1–45 mmol) (5, 6, 28, 33, 50). The PL contained 840 umol/l and 13.3 nmol/l, respectively. Subjects were then left to rest in a sitting position for 120 min to digest and process the beverage (28, 50, 53). There were no tolerance issues or side-effects noted for the BR or PL supplementations. Red urine and red stools, consistent with previous studies (6, 53), were noted.
After ∼120 min, a repeat BP measurement was taken, and a 20-gauge iv catheter was placed in the cephalic vein and 5 ml of blood drawn. Subjects then underwent vascular testing, including measures of ABI and endothelial function (BAFMD). Following this, they were given a low NO3− snack and allowed to take prescribed medications and rest for an additional 30 min prior to CPX testing. Medications were taken to maximize patient safety prior to maximal exercise testing, and the approach was the same for both treatment conditions.
At ∼180 min postbeverage consumption, subjects prepared and began the treadmill maximal CPX with NIRS monitoring of the oxygenation status of the gastrocnemius muscle. BPs were taken at rest, during every-other treadmill exercise stage, and every 2 min during recovery. Blood draws (5 ml) were taken at rest (3 h/pre), immediately upon test termination (peak), and 10 min into recovery.
Blood Sampling/NO Metabolite Measurement
All blood samples (baseline, 2 h, 3 h/pre, peak, and recovery) were immediately separated into 1 ml Eppendorf tubes containing 5 uL heparin (1–1,000 U/ml) and centrifuged at 5,000 g for 1 min. Plasma samples were then removed into separate tubes, snap-frozen in liquid nitrogen, and stored at −70°C until analysis.
All NO metabolite concentrations were measured (within 30 min of thawing) by chemiluminescence using Ionics/Sievers NO analyzer (NOA 280i), as per the manufacturer's instructions (Sievers Instruments, Boulder, CO). The reductant used for NO2− analysis was potassium iodide in acetic acid, which has the reduction potential to convert NO2− to NO but is insufficient to reduce any higher oxides of nitrogen such as NO3− and thus is relatively specific for NO2−. To obtain concentrations of total plasma nitrogen oxides, we used the same apparatus with a stronger reductant vanadium chloride in hydrochloric acid at 95°C. This stronger reductant reduces the sum of all nitrogen oxides with an oxidation state of +2 or higher, which is predominantly NO3− (μM) but also includes both NO2− (nM) and nitrosothiols (nM).
Maximal CPX Testing
All subjects underwent a maximal CPX with a 12-lead ECG and expired gas analysis on a treadmill. Expired gases were analyzed continuously using a TrueMax 2400 metabolic measurement system (ParvoMedics, Sandy, UT) and averaged in 15-s intervals. The Gardner protocol, which maintains 2 mph with a 2%-grade increase every 2 min, was used. The Gardner protocol is specifically designed for a claudication-limited PAD population (18). In addition to VO2peak, COT and PWT were measured.
NIRS
The oxygenation status of the gastrocnemius muscle of the leg with the worst PAD symptoms and ABI measures was monitored using a commercially available (“PortaMon”) NIRS system (Artinis Medical Systems B.V., The Netherlands). The system consists of a small portable unit that contains a light source, which emits near-infrared wavelengths of 850 and 764 nm and a detection probe (30 mm away) to measure the returning signals. The intensity of incident and transmitted light is transmitted in real time via Bluetooth to a laptop computer and recorded continuously at 10 Hz and used to estimate concentration changes from the resting baseline for deoxygenated [HHb] and oxygenated [HbO2] hemoglobin. Therefore, the NIRS data represent a relative change based on the optical density from the initial measure. The [HHb] signal is essentially blood volume insensitive during exercise (15, 21) and was therefore assumed to provide an estimate of changes in the fractional O2 extraction (16, 17, 21). Currently, the contribution of deoxygenated myoglobin to the NIRS signal is unclear, and therefore, the terms [HHb] and [HbO2] and their derivative total hemoglobin [(Hbtot)] refer to the combined concentrations of hemoglobin and myoglobin.
The PortaMon data collection unit was placed on the gastrocnemius muscle at the location where each subject typically felt the most claudication pain, wrapped in a black, light-absorbing cloth (to minimize the possibility that extraneous light could influence the signal), and secured in place using an elastic wrap/stocking (which was tight enough to prevent probe movement but not to restrict leg blood flow or venous return). The probe gain was set with the subject at rest in a seated position. NIRS data were collected continuously throughout the exercise protocols. The resulting NIRS data were reduced to a 1-Hz Excel data file and stored for later analysis.
Vascular Testing
All vascular testing was performed following 10 min of supine rest at ∼150-min postbeverage consumption prior to CPX testing. The ABI is the ratio of BP in the feet compared with the arms. Measurements were obtained on both the left and right side of each subject. A 5- to 7-MHz hand-held Doppler probe (Summit Doppler Systems, Golden, CO), coupled with a BP cuff (positioned proximal to the probe), was used to detect blood flow through the arteries in a similar manner to a stethoscope assessing SBP. On each arm, the brachial artery was occluded at the biceps, using an appropriate size BP cuff, while the Doppler probe was placed over the radial artery to detect blood flow. In the feet, the cuff was placed ∼3 cm above the medial malleolus to occlude the anterior tibial artery. The anterior tibial artery bifurcates in the foot into the dorsalis pedis and the posterior tibialis arteries. Both the dorsalis pedis and the posterior tibialis were measured separately via Doppler after occlusion of the anterior tibial artery using the BP cuff. The average of at least two separate measurements was taken at each artery. The ABI value for each leg was calculated by dividing the higher average dorsalis pedis or posterior tibialis value from that leg by the highest average radial artery value obtained (from either arm).
Brachial artery assessments were obtained on the left arm with the forearm extended and slightly supinated using high-resolution ultrasound and a 7.5-MHz linear array transducer (Accuson S2000, Siemens Medical Solutions, Malvern, PA). Measures were taken at baseline, during 5 min of forearm occlusion, and continuously on r-wave trigger for 2 min following cuff release [hyperemia; for further details and reproducibility data, see refs. (13, 54)]. The percent change in brachial artery diameter was calculated by: (peak posthyperemia diastolic diameter − baseline diastolic diameter/baseline diastolic diameter) × 100.
Data Analysis Procedures and Statistics
NIRS curve fitting.
To characterize the rise in HHb during the CPX testing, the data were fitted to a single exponential two-parameter empirical model (Sigma plot, version 9.0) described by the equation: y = a × [1 − exp (−b × t)], where a represents the amplitude, b is a time constant, and t is time. The fitting window was started at the point where time = 0 (exercise start time) and stopped at the end of the CPX test stage one (× = 120 s). The r2 for the curve fit was 0.94 and 0.98 for BR and PL, respectively. Differences between curves were detected by calculating an F statistic. Significant effects were further explored using z-score calculations for a and b.
The [HbO2] and [Hbtot] responses did not approximate an exponential and were therefore not modeled. We assessed changes in these parameters (and in [HHb]) at baseline (just prior to exercise start), 100 s into stage one of the CPX, 100 s into stage two of the CPX, and at the time of COT and PWT for each subject.
Statistical Analysis
Statistical analyses were performed using SPSS for Windows (version 15.0). Differences in the BP, VO2peak, COT, PWT, and NIRS-derived variables between conditions were analyzed with two-tailed, paired-samples t-tests. Alterations in plasma [NO2−] and [NO3−] were determined via a two-tailed, two-way (supplement × time) repeated-measures ANOVA. Significant effects were further explored using simple contrasts, with the α-level adjusted via a Bonferroni correction. Correlations were assessed using Pearson's product moment correlation coefficient. Data are presented as means ± SD, unless otherwise stated. Statistical significance was accepted when P ≤ 0.05.
RESULTS
Venous Plasma NO3− and NO2− Concentrations
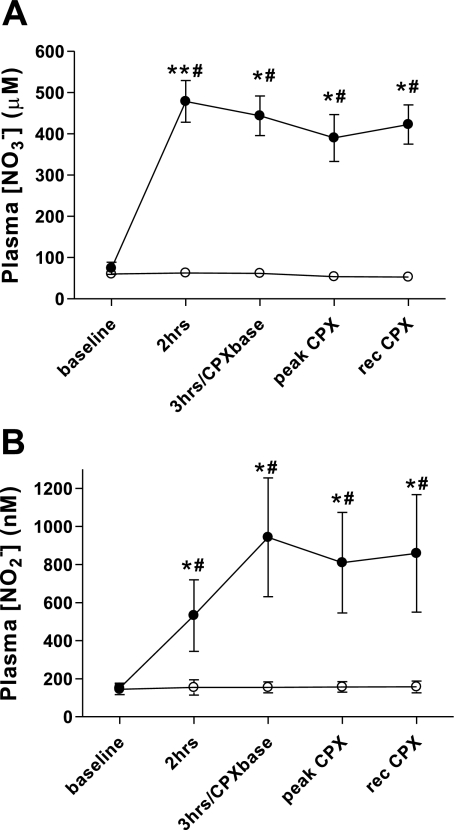
The venous plasma NO3− and NO2− concentrations at all sample points (baseline, 2 and 3 h postbeverage, at peak exercise, and following 10 min of recovery) are shown in Fig. 1, A and B. There were no changes in either plasma NO3− or NO2− levels following PL. Following BR, plasma NO3− reached a peak concentration at 2 h and remained elevated throughout the testing. Plasma NO2− was elevated above baseline at 2 h but reached a peak level at 3 h and remained elevated throughout the testing.
Fig. 1.
Plasma nitrate (NO3−; A) and nitrite (NO2−; B) concentrations prior to (baseline) and following consumption of a high NO3− beetroot (BR; ●) or placebo (PL; ○) beverage. Three hours/cardiopulmonary exercise (CPX) base indicates the time point 3 h following beverage consumption, which was also just prior to commencement of the CPX test. Peak, immediately at time to exhaustion; rec, 10 min after time to exhaustion. Values are group mean ± SE. *significantly different from PL group, P ≤ 0.05; **significantly different from PL group, P ≤ 0.01; #significantly different from baseline, P ≤ 0.05.
Exercise Tolerance
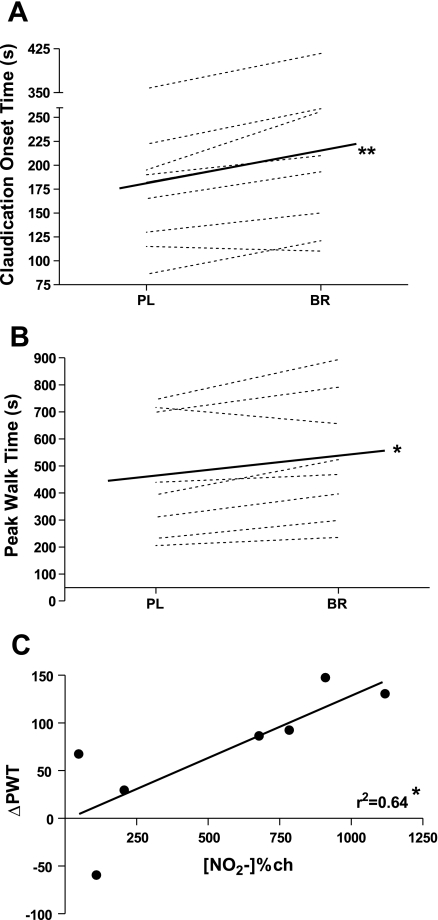
The mean and individual COT and PWT responses following PL and BR are shown in Fig. 2, A and B, respectively. Consumption of BR resulted in a 32-s (18%) increase in exercise time before the subject reported the onset of claudication pain (COT; Fig. 2A) and a 65-s (17%) increase in time to exhaustion (Fig. 2B) compared with PL. For each measure, only one of the eight subjects demonstrated a worse response following BR than PL. Interestingly, this was not the same person for both measures, and for COT, the value was 5 s, which can clinically be regarded as no change. Fig. 2C demonstrates a significant correlation between change in PWT (ΔPWT) and the percent change in plasma [NO2−] from pre-BR beverage to peak [NO2−] at 3 h (r2 = 0.64; P ≤ 0.05).
Fig. 2.
Absolute change in (A) claudication onset time and (B) peak walk time (PWT) during a maximal CPX following PL and BR beverage. (C) The relationship between change (ch) in plasma [NO2−] from baseline to peak (∼3 h) following BR beverage and change in PWT (ΔPWT). Values are group mean ± SE. *significantly different from PL group, P ≤ 0.05; **significantly different from PL group, P ≤ 0.01.
NIRS Measurements
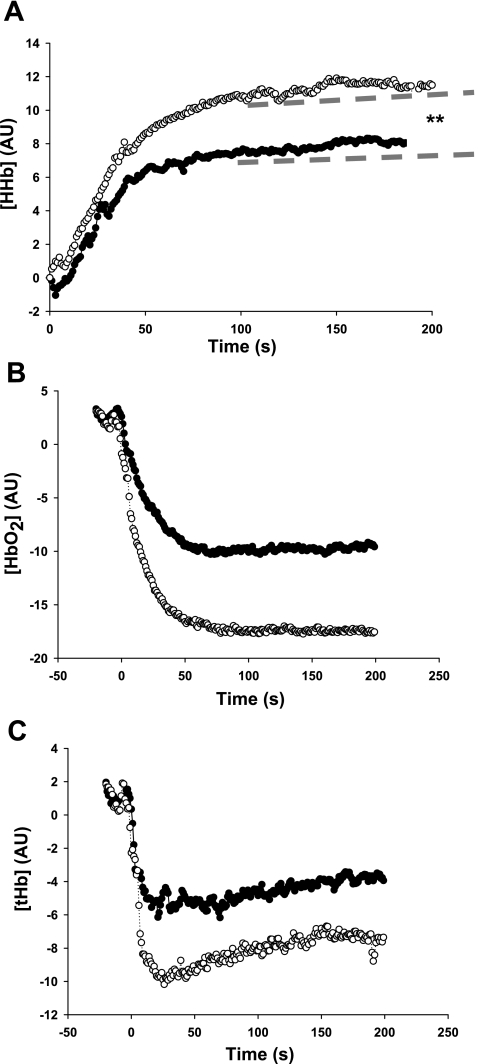
The [HHb,] [HbO2,] and [Hbtot] values measured during the exercise test at specific matched, absolute workloads—baseline, stage one (100 s), stage two (220 s), COT, and PWT—are shown in Table 1. The group mean responses are shown in Fig. 3, A–C. Comparison of the BR- vs. PL-fitted [HHb] curves generated an F statistic = 639 with a corresponding P value of ≤0.01, suggesting the two response curves were significantly different. Further analysis revealed a difference between curves for a (z-score = 20.68, P ≤ 0.01, indicated by the broken lines in Fig. 3A) but not for the b (z-score = 0.33, P = not significant). Overall, there was a 48% reduction in [HHb] peak-curve amplitude following BR, indicating that fractional O2 extraction was reduced (6, 40). This was also reflected in the [HHb] amplitude measures at 100 and 200 s into the exercise test of 44% and 53%, respectively (P ≤ 0.05). Comparisons of [HHb] at COT and PWT revealed significantly lower fractional O2 extraction in the BR group, despite the significantly increased performance (30% and 48%, respectively, P ≤ 0.01; see Table 1).
Table 1.
Near-infrared spectroscopy derived [HHb,] [HbO2,] and [Hbtot] dynamics during a maximal-graded CPX test following supplementation with BR or PL
| PL | BR | |
|---|---|---|
| [HHb] | ||
| Baseline (AU) | −1.38 ± 3.45 | −2.25 ± 3.64 |
| Stage One/100 s (AU) | 9.34 ± 5.41 | 5.25 ± 4.99* |
| Stage Two/220 s (AU) | 11.99 ± 6.27 | 5.53 ± 5.37* |
| Amplitudes at COT (AU) | 10.39 ± 6.08 | 7.29 ± 6.21† |
| Amplitude at PWT (AU) | 11.76 ± 9.42 | 6.04 ± 10.20† |
| [HbO2] | ||
| Baseline (AU) | −4.03 ± 7.42 | 2.60 ± 3.55 |
| Stage One/100 s (AU) | −17.48 ± 12.58 | −10.09 ± 10.00 |
| Stage Two/220 s (AU) | −17.98 ± 13.32 | −9.55 ± 12.52* |
| Amplitudes at COT (AU) | −17.55 ± 13.08 | −10.28 ± 11.57 |
| Amplitude at PWT (AU) | −17.41 ± 13.44 | −9.14 ± 13.01* |
| [Hbtot] | ||
| Baseline (AU) | −0.89 ± 9.35 | 2.60 ± 3.55 |
| Stage One/100 s (AU) | −20.59 ± 9.14 | −7.42 ± 11.76* |
| Stage Two/220 s (AU) | −20.24 ± 10.764 | −5.60 ± 14.02* |
| Amplitudes at COT (AU) | −7.15 ± 17.06 | −2.96 ± 14.31 |
| Amplitude at PWT (AU) | −5.65 ± 17.45 | −3.09 ± 12.28* |
Values are mean ± SD. HHb, deoxygenated hemoglobin concentration; HbO2, oxyhemoglobin concentration; Hbtot, total hemoglobin concentration; AU, arbitrary units; COT, claudication onset time; PWT, peak walk time; CPX, cardiopulmonary exercise; BR, beetroot; PL, placebo.
significantly different from PL group, P ≤ 0.05;
significantly different from PL group, P ≤ 0.01.
Fig. 3.
Group mean changes in the parameters of gastrocnemius muscle oxygenation measured by near-infrared spectroscopy during a maximal CPX following PL (○) and BR (●) beverage. The data represent 2 stages of the maximal CPX test (grade increased at 120 s). The average data are only shown up to 200 s, as this is the point at which some subjects reached PWT and had to stop. The dotted lines represent the difference in peak amplitude change for the fitted curve of (A) deoxyhemoglobin concentration [HHb] on data from stage 1 only, (B) oxyhemoglobin concentration [HbO2], and (C) total hemoglobin concentration [tHb]. Error bars are not shown for clarity (see Table 1 for further details). AU, arbitrary units. **significantly different from PL group, P ≤ 0.01.
The [HbO2] within the microvasculature was greater in the BR group matched for workload at stage two (220 s) and approached significance at PWT. The [Hbtot] data showed significantly higher values at stages one and two and PWT (see Table 1).
BP and Heart Rate Responses
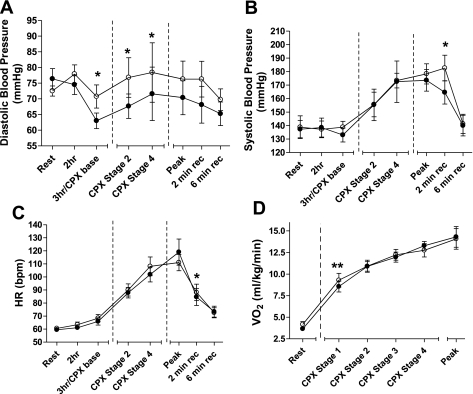
The group mean DBP and SBP, heart rate, and VO2peak responses for the BR and PL beverage treatments throughout the visits are shown in Fig. 4, A–D, respectively. Three hours postbeverage ingestion, BR had significantly decreased DBP compared with PL. This reduction was maintained during the CPX test at the submaximal workloads (these stages were matched for absolute work). At PWT and during recovery, there was no statistical difference in DBP between treatments, although it should be noted that these values were not matched for absolute work, as the BR group tended to exercise longer and reach a higher workload stage on the CPX test (see Fig. 2). Interestingly, despite no differences in SBP and heart rate at rest or during exercise testing, both of these measures were significantly lower in the BR group vs. PL, 2 min into exercise recovery (165 ± 24 vs. 183 ± 25s and 84 ± 18 vs. 88 ± 18s, respectively).
Fig. 4.
Group mean diastolic (A) and systolic (B) blood pressures, heart rate (HR; C), and oxygen consumption (VO2; D) prior to and following consumption of a BR (●) or PL (○) beverage. Three hours/CPX base indicates the time point 3 h following beverage consumption, which was also at rest just prior to commencement of the CPX. The dashed vertical lines represent the start and end of exercise. CPX stages 2 (2 mph/2%) and 4 (2 mph/6%) indicate stages of the CPX when measures were taken. Stages 2 and 4 are measures that are matched for work level. Peak indicates the measure taken immediately prior to test termination (which was at a different intensity for BR or PL). The measures taken at time points after time to exhaustion are 2- and 6-min recovery. Values are group mean ± SE. *significantly different from PL group, P ≤ 0.05; **significantly different from PL group, P ≤ 0.01.
The group mean values for relative VO2 at rest, the first four exercise test stages, and at peak exercise are shown in Fig. 4D. There was a significantly lower relative VO2 at the first stage of exercise for the BR group vs. PL (8.56 ± 1.79 vs. 9.29 ± 2.24 ml·kg−1·min−1; P ≤ 0.01). There was also a trend toward a lower resting VO2 in the BR group (3.65 ± 0.46 vs. 4.17 ± 0.92 ml·kg−1·min−1; P = 0.06).
Vascular Measures
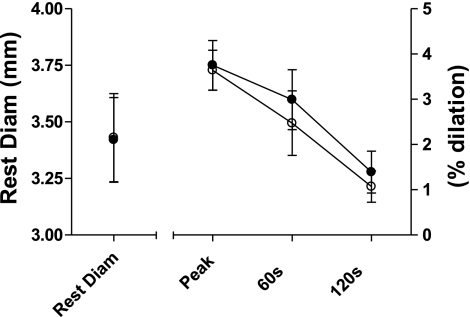
There were no changes in subjects' resting ABI measures between treatments, 0.63 ± 0.18 and 0.66 ± 0.15 for BR and PL beverages, respectively. Brachial artery resting diameters (Fig. 5) were not different between treatments at rest (BAFMD measures were taken at ∼150-min postbeverage consumption). Subjects also dilated similarly to the hyperemic stimulus, 3.75 ± 1.55% and 3.64 ± 1.25%, at peak dilation (at 42.6 ± 10.6 and 41.0 ± 10.39 s) for both BR and PL beverages, respectively, suggesting that vascular endothelial production of NO was not changed. This degree of dilation is consistent with previous studies in PAD populations (1, 3) and is considered a suboptimal vascular response (31).
Fig. 5.
Resting brachial artery diameters (Rest Diam; mm) and endothelial function (% dilation) to a hyperemic stimulus following consumption of a BR (●) or PL (○) beverage. Resting diameter is millimeters on the left y-axis; peak is maximal percent change in artery diameter from baseline regardless of time point; and 60 s and 120 s are the percent changes in artery diameter from baseline at specific time points on the right y-axis. Values are group mean ± SE.
DISCUSSION
The major findings of this study are that similarly to previous studies in healthy volunteers, consumption of a high NO3−-containing beverage (BR) raised circulating plasma NO2− concentrations in subjects with PAD. This rise was associated with an increase in both COT and PWT and a reduction in fractional O2 extraction at the working tissues (as measured by NIRS) during CPX testing compared with PL. These findings are consistent with our experimental hypothesis.
Additionally, we noted a decrease in DBP at rest and SBP and heart rate during recovery from maximal exercise. There were no treatment differences in ABI or endothelial function (BAFMD), suggesting that vascular stenosis and eNOS production were not changed.
Plasma NO3− and NO2− Concentrations
Acute supplementation with BR increased plasma [NO3−] approximately sixfold within 2 h of beverage consumption. These levels remained elevated for the remainder of the testing period, including during and following the CPX (Fig. 1A). Plasma [NO2−] also increased approximately sixfold, but although significantly greater than baseline/PL at 2 h (threefold increase), peak concentrations occurred at 3 h postbeverage consumption. These increases in [NO2−] are higher than those previously shown in healthy, young subjects (600 nM), although values of up to 1,500 nmol following 24 mmol KNO3 have also been observed (53). The [NO2−] were maintained throughout the duration of the testing (Fig. 1B). The timelines for plasma [NO3−] and [NO2−] are consistent with the literature in healthy, young subjects when administered BR (53), No NO3− (37), or KNO3 (28) and temporally support the idea of enterosalivary recirculation of NO3− and conversion to NO2− via oral commensal bacteria. Other studies have shown that this conversion process can be disrupted via a period of spitting out saliva (37, 53) or by using antibacterial mouthwash (20). It is possible that differences in saliva production, oral bacteria levels, and even digestion in an elderly (and/or PAD) population may influence conversion rates of NO3− in the beverage-to-plasma [NO2−].
Although BR juice contains several other components that may influence exercise performance, including K+, betaine, polyphenols, and antioxidants, two research groups recently used innovative study designs to specifically investigate if the inorganic NO3− was responsible for the observed exercise tolerance and BP effects. Kapil et al. (28) matched doses of KNO3 with potassium chloride (BR has a high K+ content), in addition to providing different doses of KNO3, whereas Lansley et al. (33) created a NO3−-depleted BR. They showed dose-dependent (28) decreases in BP and exercise tolerance (33) in the NO3−-consuming groups only.
Exercise Tolerance
Ingested BR increased COT and PWT by ∼18% and 17%, respectively (Fig. 2, A and B). To our knowledge, this is the first study to demonstrate a beneficial effect of increasing plasma [NO2−] on pain-free exercise time and peak exercise tolerance in a PAD population. Fig. 2C shows that there is a relationship between Δplasma [NO2−] from prebeverage to peak (3 h) at the BR visit and the ΔPWT between PL and BR visits. This suggests that plasma [NO2−] may directly influence exercise tolerance in subjects with PAD. We have previously reported a similar finding following 3 mo of supervised aerobic training, where those subjects that had the greatest improvements in plasma [NO2−] balance (production consumption) during the CPX test also had larger improvements in COT time (3).
To provide a clinical context to these improvements, we recently demonstrated improvements in both COT and PWT of 66% and 51%, respectively, following 3 mo of supervised exercise training (3). The current data show that approximately one-third of the functional benefits from chronic supervised exercise training was achieved acutely by high [NO3−] BR consumption.
Several previous studies in young, healthy subjects have shown increases in exercise time at set constant workloads of ∼15–25% (5, 6, 33) and a 2.4% increase in cycling peak-power output on a rapidly increasing (1 W/2 s) protocol (50). This increase was only observed following 15 days of NO3− supplementation (no changes seen after 2.5 h postacute ingestion or following 5 days of NO3− supplementation). It is difficult to compare improvements between constant exercise workloads and incremental test protocols, but data suggest that for a given intervention, constant work-rate tests produce a greater increase in exercise time. It has been shown in a chronic obstructive pulmonary disease population that an ∼20% increase in a maximal incremental work rate was the same as an ∼300% average increase in tolerance to a constant load test (43). Additionally, calculations in young, healthy subjects suggest that an 18% increase in our incremental PWT would be the equivalent to an ∼80% increase in time on a constant work-rate protocol (50). Regardless of the exact conversion rate, these findings suggest a greater functional benefit from NO3− supplementation in PAD populations compared with healthy, young subjects.
NIRS Measurements
A greater increase in exercise tolerance following increased plasma [NO2−] is logical in PAD subjects, given that intermittent claudication is caused by ischemia during exercise, and NO2− has been shown to facilitate hypoxic vasodilation. Accordingly, our data show that the consumption of BR changed indices of the gastrocnemius muscle oxygenation in the subjects' incident leg, as assessed by NIRS, compared with PL. The [HHb] response curve reflects changes in the balance between local O2 delivery and use and has been used previously as an index of muscle fractional O2 extraction (6, 16, 17, 21). Fig. 3A shows a significant difference between the two group mean-fitted [HHb] curves for BR and PL. Further analysis revealed a 44% lower peak amplitude (P ≤ 0.01) for the [HHb] curve but no difference in initial slope. Table 1 shows group mean differences for values matched at specific time points and demonstrates that [HHb] amplitude is lower across matched workloads (CPX stages) and at COT and PWT (which occurred at different points between visits). Studies in animal models suggest inhibition of eNOS increases muscle O2 extraction (30) but decreases mitochondrial O2 efficiency (45). Additionally, in humans, during heavy-intensity exercise, a greater VO2 “slow component” was shown following NOS blockade (27), which also suggests a greater O2 inefficiency. Taken together, these studies support the hypothesis of a regional NO effect from the BR supplementation.
Fig. 3, B and C, and Table 1 also show that the group mean values for [HbO2] and [Hbtot] were greater following BR at CPX stage one ([Hbtot] only), stage two, and PWT. The [HbO2] is an independent wavelength measure and complements the HHb data by suggesting that more of the hemoglobin in the region remained oxygenated during exercise stress. The [HHbtot] data are the sum of both [HHb] and [HbO2] data rather than an independent measure. The data tentatively indicate a relative increase in blood volume to the region and therefore, vasodilation (6) and perfusion following BR. Given that much of the blood volume is stored in the veins, the initial fall in [HHbtot] is most likely a reflection of venous emptying due to skeletal muscle contractions at the onset of exercise. NIRS [HHbtot] does not include any measures of actual limb blood flow and therefore, limits the interpretation of these data.
BP, Heart Rate, and VO2 Responses
A reduction in resting DBP (5, 28, 35, 53) and SBP (5, 6, 28, 33, 35, 50, 53) following consumption of a high NO3− beverage/diet in healthy subjects has been widely reported. To our knowledge, our data are the first to report a reduction in BPs in PAD subjects (Fig. 4, A and B).
Subjects held medications that have been shown to influence BP and vascular endothelial function for at least 12 h prior to resting measures (unless contraindicated). Subsequently, seven of the eight subjects were classified as prehypertensive or hypertensive at one or more of the baseline testing visits (11). BR consumption produced a significant reduction in resting DBP compared with PL at the time point when plasma NO2− reached a peak (3 h; Fig. 4A). This supports the idea that consumption of dietary NO3− may be an interesting avenue to investigate as effective treatment for hypertension in addition to current medication regimens. In fact, the Dietary Approaches to Stop Hypertension diet (4) contains ∼20 mmol NO3− daily (23) (approximately double the amount in our BR beverage) and reduced BP in both normal and hypertensive subjects.
To our knowledge, this is the first study to report a significant reduction in DBP following BR vs. PL during exercise stress measured at stage two (2 mph/2% grade) and stage four (2 mph/6% grade) of the CPX test. Beyond this point, over one-half of the PAD subjects had reached exhaustion. Note that all subjects are included in the analysis for peak and recovery data, although the workloads for BR were significantly higher than PL, as subjects lasted longer on the test. Despite this, the BR group had lower SBP and heart rate values at 2 min into recovery (Fig. 4, B and C). Taken together, these data suggest that the ability of BR to reduce BP in PAD is maintained even during exercise stress.
Several recent studies have demonstrated reductions in O2 cost to perform a submaximal workload following NO3− supplementation (5, 6, 33, 35, 50). For maximal efforts, the data are less clear. Two studies show a reduction in VO2peak in combined arm and leg exercise (34) and running (33), but two others show unaltered VO2peak during cycling (6) and knee-extension exercise (33). Our data show a trend toward a significant reduction in O2 cost at rest in PAD patients (P = 0.06) following BR vs. PL, which becomes significant at stage one of the CPX test (2 mph/0% grade; Fig. 4D). Several possible explanations for a reduced VO2 at matched workloads have been put forth, including 1) an improved regulation of mitochondrial O2 utilization, given that NO is known to be an inhibitor of cytochrome oxidase activity (12); 2) better O2 distribution in working tissues via partial inhibition of the mitochondria close to the feeding arteriole (48); and 3) reduction of ATP cost of force production (5). It is difficult to adequately detect if a reduction in O2 cost would persist at higher workloads in our study, as several patients reached maximal effort during the first two stages of the CPX test. Additionally, the impairment of blood flow to the lower extremities that characterizes PAD may also mean that whole-body VO2 consumption is not the best measure of exercise tolerance in this population.
Endothelial Function and ABI
There were no changes in ABI values, suggesting no structural changes in arterial stenosis between BR and PL. Additionally, there were no changes in endothelial function to a hyperemic stimulus (BAFMD; Fig. 5), which suggests no changes in eNOS production of NO or increased conduit vessel functionality. Taken together, these findings support the hypothesis that increased plasma NO2− is responsible for augmented oxygenation of the working tissues in this PAD study.
Summary and Conclusions
Acute supplementation of NO3− via a BR beverage increased plasma [NO2−] levels and increased exercise COT and PWT by almost one-fifth in subjects with PAD and intermittent claudication. This is a clinically significant increase in functionality for a disease state characterized by reduced physical function and quality of life and may present an easily administered and novel treatment option.
The mechanism by which this improvement occurred is not totally clear, but a reduction in gastrocnemius tissue deoxygenation (estimated by NIRS) and a reduction in BP suggest that increased tissue perfusion is the most likely explanation. Given that there were no changes in arterial endothelial function following BR, our findings support the hypothesis that NO2−-related NO signaling increases peripheral blood flow in areas of tissue hypoxia and increases exercise tolerance in subjects with PAD.
GRANTS
This work was supported by grants from Wake Forest University Translational Science Center and Duke University Claude D. Pepper Older American Independence Center (AG0287 from the National Institute on Aging) grants to J. D. Allen.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Drs. Gary Miller and Daniel Kim-Shapiro at Wake Forest University Translational Science Center for their support and advice in the initial study design. We thank Stephanie Decker for her phlebotomy skills and the clinical staff at the Duke Center for Living for their help when we needed it.
REFERENCES
- 1. Allen J, Miller E, Schwark E, Robbins J, Duscha B, Annex B. Plasma nitrite response and arterial reactivity differentiate cardiovascular health status and performance. Nitric Oxide 20: 231–237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen JD, Cobb FR, Gow AJ. Regional and whole-body markers of nitric oxide production following hyperemic stimuli. Free Radic Biol Med 38: 1164–1169, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Allen JD, Stabler T, Kenjale A, Ham KL, Robbins JL, Duscha BD, Dobrosielski DA, Annex BH. Plasma nitrite flux predicts exercise performance in peripheral arterial disease following 3 mo of exercise training. Free Radic Biol Med 49: 1138–1144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N, Simons-Morton D, McCullough M, Swain J, Steele P, Evans MA, Miller ER, Harsha DW. A Clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 336: 1117–1124, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol 109: 135–148, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, DiMenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol 107: 1144–1155, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Belch JJF, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, Creager MA, Easton JD, Gavin JR, III, Greenland P, Hankey G, Hanrath P, Hirsch AT, Meyer J, Smith SC, Sullivan F, Weber MA. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med 163: 884–892, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Cannon RO, III, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest 108: 279–287, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversable impairment of endothelium dependent dilation in healthy young adults. Circulation 88: 2149–2155, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgapoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National High Blood Pressure Education Program Coordinating Committee The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Cooper CE. Nitric oxide and cytochrome oxidase: substrate, inhibitor or effector? Trends Biochem Sci 27: 33–39, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery; a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003 [DOI] [PubMed] [Google Scholar]
- 15. De Blasi R, Cope M, Elwell C, Safoue F, Ferrari M. Noninvasive measurement of human forearm oxygen consumption by near infrared spectroscopy. Eur J Appl Physiol Occup Physiol 67: 20–25, 1993 [DOI] [PubMed] [Google Scholar]
- 16. DeLorey DS, Kowalchuk JM, Heenan AP, duManoir GR, Paterson DH. Prior exercise speeds pulmonary O2 uptake kinetics by increases in both local muscle O2 availability and O2 utilization. J Appl Physiol 103: 771–778, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Ferreira LF, Koga S, Barstow TJ. Dynamics of noninvasively estimated microvascular O2 extraction during ramp exercise. J Appl Physiol 103: 1999–2004, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc 23: 402–408, 1991 [PubMed] [Google Scholar]
- 19. Gladwin MT. Haldane, hot dogs, halitosis, and hypoxic vasodilation: the emerging biology of the nitrite anion. J Clin Invest 113: 19–21, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19: 333–337, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol 95: 149–158, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 113: e463–e654, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr 90: 1–10, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest 115: 2099–2107, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med 10: 1122–1127, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380: 221–226, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Jones AM, Wilkerson DP, Wilmshurst S, Campbell IT. Influence of l-NAME on pulmonary O2 uptake kinetics during heavy-intensity cycle exercise. J Appl Physiol 96: 1033–1038, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, MacAllister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension 56: 274–281, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol 26: 697–705, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Kindig CA, Gallatin LL, Erickson HH, Fedde MR, Poole DC. Cardiorespiratory impact of the nitric oxide synthase inhibitor l-NAME in the exercising horse. Respir Physiol 120: 151–166, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Kitta Y, Obata Je Nakamura T, Hirano M, Kodama Y, Fujioka D, Saito Y, Kawabata Ki Sano K, Kobayashi T, Yano T, Nakamura K, Kugiyama K. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol 53: 323–330, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med 35: 790–796, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol 110: 591–600, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic Biol Med 48: 342–347, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 191: 59–66, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA 98: 12814–12819, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med 37: 395–400, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol 2: 593–602, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008 [DOI] [PubMed] [Google Scholar]
- 40. McCully K, Halber C, Posner JD. Exercise induced changes in oxygen saturation in the calf muscles of elderly subjects with peripheral vascular disease. J Gerontol A Biol Sci Med Sci 49: B128–B134, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Nohl H, Staniek K, Sobhian B, Bahrami S, Redl H, Kozlov A. Mitochondria recycle nitrite back to the bioregulator nitric monoxide. Acta Biochim Pol 47: 913–921, 2000 [PubMed] [Google Scholar]
- 42. Pinder A, Pittaway E, Morris K, James P. Nitrite directly vasodilates hypoxic vasculature via nitric oxide dependent and independent pathways. Br J Pharmacol 158: 1523–1530, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Porszasz J, Emtner M, Goto S, Somfay A, Whipp B, Casaburi R. Exercise training decreases ventilatory requirements and exercise-induced hyperinflation at submaximal intensities in patients with COPD. Chest 128: 2025–2034, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Regensteiner JG, Hargarten ME, Rutherford RB, Hiatt WR. Functional benefits of peripheral vascular bypass surgery for patients with intermittent claudication. Angiology 44: 1–10, 1993 [DOI] [PubMed] [Google Scholar]
- 45. Shen W, Xu X, Ochoa M, Zhao G, Wolin M, Hintze T. Role of nitric oxide in the regulation of oxygen consumption in conscious dogs. Circ Res 75: 1086–1095, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res 100: 654–661, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995 [DOI] [PubMed] [Google Scholar]
- 48. Thomas DD, Liu X, Kantrow SP, Lancaster JR. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci USA 98: 355–360, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Faassen E, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro D, Kozlov A, Li H, Lundberg J, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin A, Weitzberg E, Zweier J, Gladwin M. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev 29: 683–741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol 299: R1121–R1131, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation 108: 2054–2059, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci USA 101: 13683–13688, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51: 784–790, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Welsch MA, Allen JD, Geaghan JP. Stability and reproducibility of brachial artery flow-mediated dilation. Med Sci Sports Exerc 34: 960–965, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Zweier J, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 8: 804–809, 1995 [DOI] [PubMed] [Google Scholar]