Abstract
Muscle rigidity and myotendinous junction (MTJ) deficiency contribute to immobilization in Duchenne muscular dystrophy (DMD), a lethal disease caused by the absence of dystrophin. However, little is known about the muscle passive properties and MTJ strength in a diseased muscle. Here, we hypothesize that dystrophin-deficient muscle pathology renders skeletal muscle stiffer and MTJ weaker. To test our hypothesis, we examined the passive properties of an intact noncontracting muscle-tendon unit in mdx mice, a mouse model for DMD. The extensor digitorum longus (EDL) muscle-tendon preparations of 2-, 6-, 14-, and 20-mo-old mdx and normal control mice were strained stepwisely from 110% to 160% of the muscle optimal length. The stress-strain response and failure position were analyzed. In support of our hypothesis, the mdx EDL preparation consistently developed higher stress before muscle failure. Postfailure stresses decreased dramatically in mdx but not normal preparations. Further, mdx showed a significantly faster stress relaxation rate. Consistent with stress-strain assay results, we observed significantly higher fibrosis in mdx muscle. In 2- and 6-mo-old mdx and 20-mo-old BL10 mice failure occurred within the muscle (2- to 14-mo-old BL10 preparations did not fail). Interestingly, in ≥14-mo-old mdx mice the failure site shifted toward the MTJ. Electron microscopy revealed substantial MTJ degeneration in aged but not young mdx mice. In summary, our results suggest that the passive properties of the EDL muscle and the strength of MTJ are compromised in mdx in an age-dependent manner. These findings offer new insights in studying DMD pathogenesis and developing novel therapies.
Keywords: passive properties, skeletal muscle, muscular dystrophy, dystrophin, myotendinous junction
dystrophin is a subsarcolemmal cytoskeletal protein (16). Absence of dystrophin leads to Duchenne muscular dystrophy (DMD), a severe muscle-wasting disease that affects 1 in 3,500 newborn boys. Dystrophin forms a complex with a series of transmembrane and cytosolic proteins. Throughout this molecular complex, dystrophin links the subsarcolemmal F-actin network to the extracellular matrix. It is generally agreed that the dystrophin complex stabilizes the sarcolemma during force transmission (reviewed in Ref. 10). In dystrophin-deficient muscle, the sarcolemma is damaged by muscle contraction (reviewed in Ref. 13). Eventually, muscle cells undergo necrosis and are replaced by fibrotic and/or adipose tissues.
The resistant force develops against the strain when a muscle is passively lengthened. This resistant force is referred to as the passive stress. Since the muscle is not actively contracting, the passive stress reflects the inherent (passive) properties of the muscle constituents such as the extracellular matrix, cytoskeletal proteins, and myofibrils. The passive properties of the muscle can be further defined by the elasticity (stiffness) and the viscosity (1, 20). Since fibrosis and inflammation are major pathological changes in dystrophin-deficient muscle, it is expected that the passive properties of the muscle would be altered in the absence of dystrophin. In support of this notion, it has been shown that muscle stiffness is markedly increased in DMD patients (8, 9). Here, we hypothesize that dystrophin-deficient muscle pathology profoundly influences the passive properties of muscle in mdx mice, a murine model for DMD.
The myotendinous junction (MTJ) is the link between the muscle and the tendon (27, 29). At the MTJ, fingerlike muscle protrusions invade into fibrous tendon tissue. The sarcolemma folds extensively at these fingerlike structures. This effectively increases the junctional area between muscle and tendon and provides a strong interface for force transmission. Interestingly, dystrophin is highly enriched at the MTJ (26). It has been reported that the ultrastructure of the MTJ is impaired in mdx mice and DMD patients (2, 15, 23). Here we hypothesize that the strength of the MTJ is weakened in mdx mice. As a consequence of this weakening, mdx muscle will fail at the MTJ when it is passively stretched.
In contrast to DMD patients, young mdx mice are mildly affected. Clinically obvious dystrophic symptoms are only seen in old mdx mice (4, 7). To test our hypotheses, we applied passive stretch to an intact extensor digitorum longus (EDL) muscle-tendon unit in 2-, 6-, 14-, and 20-mo-old male mdx as well as to age- and sex-matched normal control BL10 mice. We analyzed the stress-strain profile, hydroxyproline content, and muscle failure position. In addition, we examined MTJ structure by electron microscopy (EM).
MATERIALS AND METHODS
Evaluation of the passive properties of the EDL muscle.
All animal experiments were approved by the Animal Care and Use Committee of the University of Missouri and were in accordance with NIH guidelines. Dystrophin-deficient mdx mice and normal control BL10 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Only male mice were used in the study. Experimental mice were euthanized by cervical dislocation at the end of study. Mice were anesthetized via intraperitoneal injection of a cocktail containing 25 mg/ml ketamine, 2.5 mg/ml xylazine, and 0.5 mg/ml acepromazine at 2.5 μl/g body weight. The age and the body mass were recorded (Table 1). The animal was positioned on a custom-fabricated Plexiglas dissection board (22.9 cm × 17.8 cm × 1.3 cm) with a reservoir (12.7 cm × 7.6 cm × 0.6 cm) to collect excess buffer during muscle superfusion. The buffer was removed from the reservoir through a vacuum line. A Sylgard (World Precision Instruments, Sarasota, FL) ring (3.8 cm in diameter and 1.3 cm in height) was glued in the middle of the reservoir to secure the hindlimb. A radiant heat lamp was used to maintain the body temperature at 37°C. All exposed muscles were constantly superfused with Ringer buffer (pH 7.4; composition in mM: 1.2 NaH2PO4, 1 MgSO4, 4.83 KCl, 137 NaCl, 24 NaHCO3, 2 CaCl2, and 10 glucose).
Table 1.
Characterization of experimental mice
| Age, mo | n | Body Weight, g | TA Weight, mg | EDL Weight, mg | CSA, mm2 | Po, mN/mm2 |
|---|---|---|---|---|---|---|
| BL10 | ||||||
| 2 | 10 | 26.94 ± 0.34 | 47.07 ± 0.61 | 11.97 ± 0.35 | 1.89 ± 0.06 | 184.71 ± 5.32 |
| 6 | 10 | 32.03 ± 0.57 | 53.70 ± 1.41 | 13.90 ± 0.77 | 2.12 ± 0.12 | 185.38 ± 6.96 |
| 14 | 16 | 38.03 ± 0.43 | 55.70 ± 1.01 | 13.44 ± 0.20 | 2.06 ± 0.03 | 201.13 ± 7.43 |
| 20 | 10 | 37.44 ± 0.64 | 50.98 ± 1.51b | 13.00 ± 0.18 | 2.12 ± 0.03 | 182.03 ± 4.70 |
| mdx | ||||||
| 2 | 9 | 28.53 ± 0.79 | 66.06 ± 2.68a | 13.62 ± 0.49a | 2.32 ± 0.08 a | 147.53 ± 5.39a |
| 6 | 13 | 35.44 ± 0.42a | 77.40 ± 1.79a | 16.73 ± 0.42a | 2.57 ± 0.07a | 138.51 ± 5.67a |
| 14 | 22 | 34.25 ± 0.60 a | 64.36 ± 1.41a | 17.24 ± 0.48a | 2.61 ± 0.07a | 121.24 ± 7.29a |
| 20 | 19 | 31.12 ± 0.56 a,b | 56.61 ± 1.50a,b | 15.95 ± 0.33a,b | 2.42 ± 0.05a,b | 134.39 ± 5.90a |
Values are means ± SE; n = no. of mice. TA, tibialis anterior; EDL, extensor digitorum longus; CSA, cross-sectional area aSignificantly different from that of the age-matched BL10 controls. bSignificantly different from that of 14-m-old mice of the same strain. Po, normalized tetanic force.
While viewing through a stereomicroscope (Nikon, Melville, NY), the skin fascia and connective tissue were peeled off and the tibialis anterior (TA) muscle was gently removed and its mass was determined (Table 1). The distal and proximal EDL tendons were carefully exposed and tied with a 4-0 suture (SofSilk USSC Sutures, Norwalk, CT). The proximal tendon was secured to a 305B dual-mode servomotor transducer (Aurora Scientific, Aurora, ON, Canada), and the distal tendon was attached to a fixed post. The EDL muscle was submerged in a 30°C jacketed organ bath containing oxygenated (95% O2-5% CO2) Ringer buffer. After 10 min equilibration, the EDL muscle was stimulated at the optimal length (Lo). Active tetanic muscle force was recorded using the Lab View-based DMC program (version 3.12, Aurora Scientific) (Table 1). The entire muscle-tendon preparation was then subjected to a six-step passive stretch protocol. During these stretches, no electric stimulation was applied to the muscle. At each step, the EDL muscle was passively strained in the increment of 10% Lo at the rate of 2 cm/s (see Supplementary Fig. S1, available with the online version of this article) (17). In a subset group of 14-mo-old mice, the stretch was applied at a rate of 6 cm/s. The stress-strain response was recorded using the LabView-based DMC software (version 3.12) and analyzed by the DMA software (version 3.12), respectively (both from Aurora Scientific). After the final stretch (160% Lo), the EDL muscle was gently removed and pinned on a Sylgard plate and a whole mount muscle image was captured with a Nikon digital camera (Nikon, Melville, NY). At the end of each experiment, the distal and proximal tendons were removed and the EDL muscle mass was recorded (Table 1). The muscle cross-sectional area was calculated based on a muscle density of 1.06 g/cm3 and a fiber length-to-Lo ratio of 0.44 (6, 19).
The viscous property of the EDL muscle was determined by studying the stress-relaxation rate when the muscle was stretched and held at 110% Lo (12). The post-peak stresses were recorded at 0.1, 0.2, 0.5, 1 and 1.5 s. The relaxation rate at each time frame (from the peak to 0.1 s postpeak, from 0.1 to 0.2 s postpeak, from 0.2 to 0.5 s postpeak, from 0.5 to 1 s postpeak, and from 1 to 1.5 s postpeak) was calculated by dividing the difference in the stress with the time elapsed between two time points.
Electron microscopy (EM).
To preserve morphology, a separate set of muscles was used for the EM studies. The mice were anesthetized as described above. The EDL muscle and associated tendons were gently dissected out and pinned down on a Sylgard plate at the distal and proximal tendons. The EDL muscle was then covered with the primary fixative (2 % glutaraldehyde and 2% paraformaldehyde in 100 mM sodium cacodylate). Twenty minutes later, the EDL muscle was moved to a fresh primary fixative and incubated for at least 72 h at 4°C. After 3 × 15 min of wash with β-ME buffer (in mM: 100 sodium cacodylate, 130 sucrose and 10 β-mercaptoethanol), the EDL muscle was postfixed in a secondary fixative containing 1% OsO4 in β-ME buffer for 2 h. The muscle was washed 3 × 15 min in distilled water, dehydrated with acetone, and infiltrated in Epon/Spurr's resin for 4 days. Finally, the EDL muscle was cut in the middle and cured in Epon/Spurr's resin overnight at 60°C. Longitudinal thick sections (2 μm) were stained with toluidine blue. Longitudinal thin sections (85 nm) were stained with uranyl acetate and lead citrate. The thin sections were examined using a JEOL 1400 transmission electron microscope (Tokyo), and images were captured using a Gatan 895 digital camera (Gatan, Warrendale, PA).
Hydroxyproline quantification.
The hydroxyproline content was measured according to our previously published protocol with modification (5). Briefly, the EDL muscle was gently dissected from the hindlimb and secured on a Sylgard plate as described above. The proximal and distal tendons were trimmed away and the muscle was immediately frozen in liquid nitrogen. The muscle was lyophilized overnight and the dry mass was determined. The lyophilized muscle was hydrolyzed in 1 ml 6 N HCl for 3 h at 115°C and then neutralized with 10 N NaOH to the final pH of ∼7.5. The hydrolyzed muscle lysate was then oxidized with chloramine-T and reacted with p-dimethylamino-benzaldehyde and 60% perchloric acid. The absorbance was measured at 558 nm and the hydroxyproline concentration was determined using a standard curve.
Statistical analysis.
Data are presented as means ± standard error of the mean (SE). Statistical significance among multiple groups was determined by two-way ANOVA followed by Turkey-Kramer post hoc analysis using the SAS software (SAS Institute, Cary, NC). For two-group comparison, statistical significance was determined by Student's t-test. Difference was considered significant when P < 0.05.
RESULTS
Body mass, muscle mass, and active muscle force in experimental mice.
To compare the passive properties of the EDL muscle between BL10 and mdx mice, we studied young (2-mo-old), adult (6-mo-old), old (14-mo-old), and very old (20-mo-old) male mice. BL10 and mdx mice showed significant differences in the body mass at 6, 14, and 20 mo of age (Table 1). The muscle mass (TA and EDL) and the EDL muscle cross-sectional area were significantly higher in mdx mice at all ages. Very old mdx mice also showed significant body and muscle wasting compared with that of 14-mo-old mdx mice (Table 1). As expected, the specific active muscle forces were significantly reduced in mdx mice (Table 1).
The mdx EDL muscle developed significantly higher passive stresses at strains of 110–130% Lo.
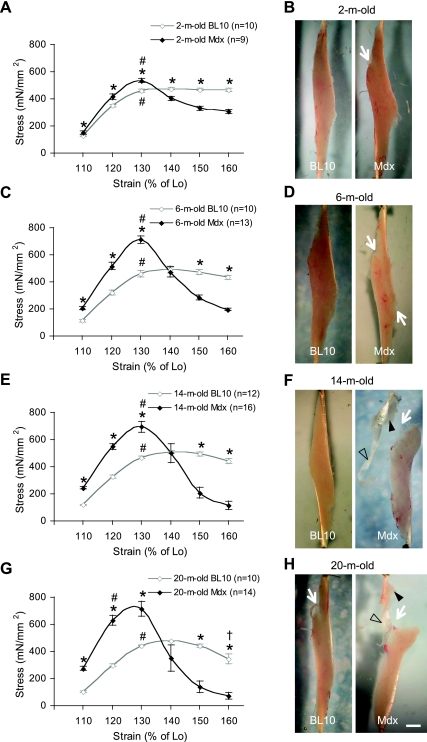
Compared with that of age-matched BL10, the mdx EDL muscle generated significantly higher stress when it was stretched to 110% of Lo (Fig. 1). There also appeared an aging effect in mdx mice. The differences between two strains were greater in older mice. Specifically, the stresses generated in 2-, 6-, 14-, and 20-mo-old mdx mice were 22, 84, 104, and 146% higher, respectively, than those of the same age BL10 mice at the strain of 110% Lo. While aging appeared to have nominal influence on the stress developed prior to the peak stress in BL10 mice, old mdx mice (14 mo old and 20 mo old) yielded significantly higher stresses than young mdx mice (2 mo old and 6 mo old) at the strains of 110 and 120% Lo (Fig. 1).
Fig. 1.
Age-matched comparison of the extensor digitorum longus (EDL) muscle stress-strain curves in BL10 and mdx mice. A, C, E, and G depict the stress responses to the strains of 110–160% of muscle optimal length (Lo) at the rate of 2 cm/s. *Stresses developed in mdx muscles significantly different from those of aged-matched BL10 at the same strain. #Strain at which the peak stress was generated. †Stress developed at 160% of Lo significantly different from that at 150% of Lo in 20-mo-old BL10 mice. At the end of the passive stretch protocol, macroscopic images of the muscle were obtained. B, D, F, and H are the representative muscle images at ages of 2, 6, 14, and 20 mo, respectively. In each panel, the BL10 muscle image is on the left side and the mdx muscle image is on the right side. The scale bar (1 mm) applies to all images. White arrows indicate the position of the muscle failure. Filled arrowheads indicate residual muscle attached to the tendon. Open arrowheads indicate the region of the tendon that is completely separated from the muscle.
At the strains of 120 and 130% Lo, mdx also generated significantly higher stresses (Fig. 1). The peak stress was achieved at the strain of 130% Lo in BL10 mice of all ages as well as in 2-, 6-, and 14-mo-old mdx mice. For very old (20 mo old) mdx mice, the numerically highest mean stress value was at the strain of 130% Lo. However, this was not significantly different from that at the strain of 120% Lo. Hence, the peak stress was shifted leftward in very old mdx mice. Interestingly, the overall peak stress value was not influenced by the age in either BL10 or mdx. Mdx showed an average peak stress of 644.3 ± 16.7 mN/mm2. This is significantly higher than that of BL10 (451.5 ± 8.3 mN/mm2, P < 0.0001) (Fig. 1).
The postpeak stresses dropped rapidly in the mdx but not BL10 EDL muscle.
In mdx mice, the stress declined significantly when the muscle was stretched beyond 130% of Lo. In 2-mo-old mdx mice, a 25% drop was seen from the strain of 130 to 140% of Lo. The magnitude of the drop became less afterward. In 6- to 20-mo-old mdx mice, a 30 to 60% drop was observed at each step of strain from 130 to 160% of Lo. In BL10 mice, a significant stress reduction was only found in the oldest age group (20 mo old) at the strain of 160% of Lo. For 2-, 6-, and 14-mo-old BL10 mice, the stress stabilized at the plateau after it attained the peak (Fig. 1).
Consistent with the stress-strain results, macroscopic examination also revealed different levels of muscle tearing in the stretched mdx EDL muscle. In 2-mo-old mdx mice, a partial tear was observed at the proximal end of the muscle (Fig. 1B, right panel). At 6 mo of age, the separation appeared to have crossed the entire muscle belly although there were still substantial attachments (Fig. 1D, right panel). In 14- and 20-mo-old mdx mice, the mdx EDL muscle was essentially torn apart except for a few residual connections (Fig. 1,F and H). In contrast, passive stretches resulted in minimal visible changes in the EDL muscles of 2-, 6-, and 14-mo-old BL10 mice. At these ages, the muscle remained intact (Fig. 1, B, D, and F). By 20 mo of age, partial tears similar to those seen in 2-mo-old mdx mice were observed at the proximal end of the muscle in BL10 mice (Fig. 1H, left panel).
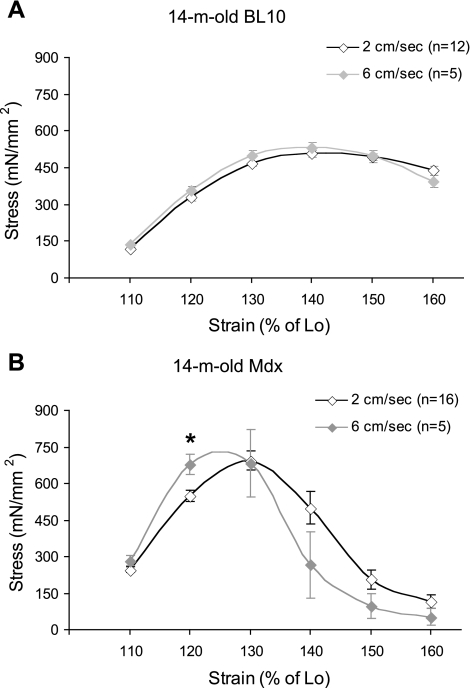
Increasing the stretch rate resulted in a leftward shift of the mdx stress-strain relation.
To further study the influence of dystrophin deficiency on the elastic property, we evaluated the stress-strain curve at a higher stretch rate in 14-mo-old mice. In 14-mo-old BL10 mice, the stress-strain curve was minimally altered when the rate was increased from 2 to 6 cm/s (Fig. 2A). However, we observed a dramatic leftward shift of the stress-strain curve when the increased stretch rate was applied to 14-mo-old mdx mice (Fig. 2B). At the higher rate, the peak stress was achieved at a lower strain. At the strain of 120% Lo, the stress developed at 6 cm/s was significantly higher than that at 2 cm/s (Fig. 2B).
Fig. 2.
Influence of the stretch rate on the stress-strain curve in 14-mo-old mice. A: change of the stretch rate from 2 to 6 cm/s did not significantly alter the stress response in BL10 mice. B: at the higher rate (6 cm/s), the stress-strain curve shifted leftward in mdx mice. *Stress is significantly different from that at the low rate (2 cm/s).
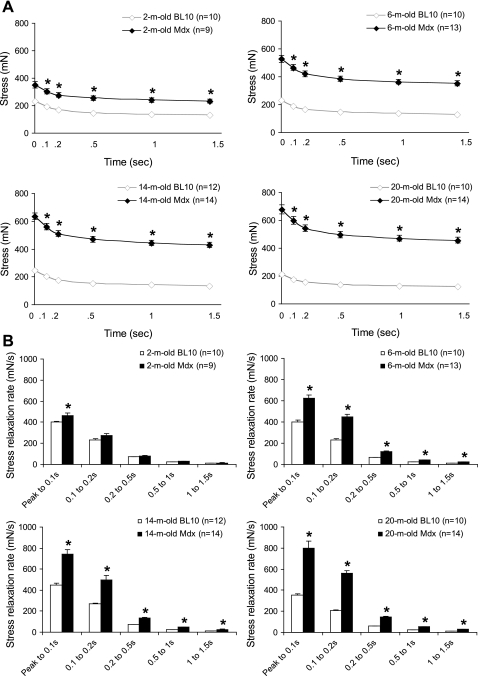
The viscous property of the mdx EDL muscle was altered.
The viscous property of the EDL muscle was determined by measuring the stress-relaxation rate while the EDL muscle was stretched to and held at 110% Lo (Fig. 3). We calculated the stress-relaxation rate from the peak stress (time 0) to 0.1 s, 0.1 to 0.2 s, 0.2 to 0.5 s, 0.5 to 1 s, and 1 to 1.5 s for all age groups. In 2-mo-old mice, the mdx EDL muscle showed slightly but significantly higher relaxation rate from the peak to 0.1 s. However, there were no significant difference between mdx and BL10 thereafter. In 6- to 20-mo-old mice, mdx consistently showed significantly higher stress-relaxation rate at all time frames measured. Interestingly, as mice got older, the difference between mdx and BL10 also became greater. For example, from the peak to 0.1 s, the relaxation rate of mdx mice were 14, 56, 65, and 125% higher than that of BL10 mice at 2, 6, 14, and 20 mo, respectively (Fig. 3B).
Fig. 3.
Age-matched comparison of the viscous property between BL10 and mdx at 110% of Lo. A: absolute stress decay at 0.1, 0.2, 0.5, 1, and 1.5 s after the peak stress (time 0). A rapid drop is observed within the first 0.2 s in both BL10 and mdx. B: stress relaxation rates at different time frames after the peak stress. *Significantly different from that of BL10.
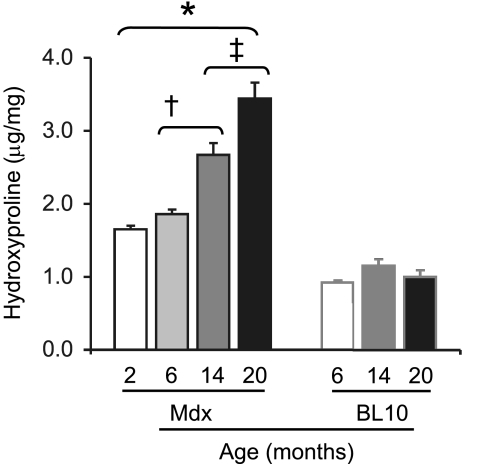
The mdx EDL muscle was significantly more fibrotic.
The amount of fibrotic tissue deposition in the EDL muscle was determined by hydroxyproline quantification (Fig. 4). Compared with mdx mice, the BL10 mice showed significantly lower hydroxyproline content. Interestingly, there was no significant difference in the hydroxyproline content among 2-, 14-, and 20-mo-old BL10 mice. The 6-mo-old mdx mice contained ∼12% more hydroxyproline than 2-mo-old mdx mice. However, the difference did not reach statistical significance. Compared with 6-mo-old mdx mice, the hydroxyproline content was increased by 42% in 14-mo-old mdx mice (P = 0.0003). The 20-mo-old mdx mice showed the statistically highest hydroxyproline level (P ≤ 0.006 compared with other groups). It was 84% higher than that of 6-mo-old mdx mice (Fig. 4).
Fig. 4.
Quantification of the hydroxyproline content. n = 7, 7, and 7 for 2-, 14-, and 20-mo-old BL10 mice; n = 9, 7, 7, and 7 for 2, 6, 14, and 20-mo-old mdx mice. *Significantly higher than that of BL10 mice. †Significantly higher than that of 2- and 6-mo-old mdx mice. ‡Significantly higher than that of 14-mo-old mdx mice.
Shifting of the muscle failure site correlated with structural changes at the MTJ in aged mdx mice.
In 2- and 6-mo-old mdx mice and 20-mo-old BL10 mice, tearing occurred within the proximal end of the muscle (Fig. 1). Interestingly, the failure site shifted toward the proximal MTJ in almost all 14- and 20-mo-old mdx mice. While there were still residual pieces of muscle attached to the proximal tendon, the far end of the proximal tendon appeared to have completely separated from the muscle (Fig. 1F and H, left panels).
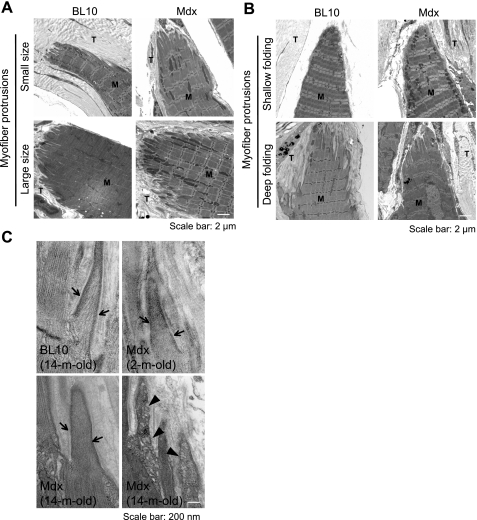
To determine whether MTJ insufficiency is responsible for the failure site shift in aged mdx mice, we examined the MTJ ultrastructure by EM. To avoid potential influence of the stretch protocol on the morphology, a separate set of muscles was used for the EM studies. We first examined the size and the shape of the digitlike muscle protrusions. We observed small and large bundles of protrusions in both BL10 and mdx mice in all age groups (Fig. 5A; Supplementary Fig. S3). Interestingly, at the tip of each protrusion some showed deep digitlike invasions while others were relatively flat. There was neither an age nor a disease-associated trend (Fig. 5B). Quantification of the invasion depth showed no significant difference between 14-mo-old BL10 and age-matched mdx mice (Supplementary Fig. S2).
Fig. 5.
Ultrastructure changes at the myotendinous junction (MTJ). A: muscle protrusions showed variable size. M, muscle; T, tendon. Scale bar, 2 μm. B: representative low-magnification EM photomicrographs showing variations in the depth of the digitlike folding at the muscle-tendon interface. M, muscle; T, tendon. Scale bar, 2 μm. C: representative high-magnification EM photomicrographs of the myofiber digitlike processings at the MTJ in 14-mo-old BL10 (top left panel), 2-mo-old mdx (top right panel), and 14-mo-old mdx (relatively normal, bottom left panel; degenerated, bottom right panel). Arrow, lateral condensation of the thin filament at the sarcolemma; arrowhead, vacuolar degeneration. M, muscle; T, tendon. Scale bar, 200 nm.
In 2- to 14-mo-old BL10 MTJ, thin filaments associate laterally with the sarcolemma. As a result, the membrane at the digitlike muscle protrusions appeared thick and dense (such as the one shown for 14-mo-old BL10 MTJ in Fig. 5C, top left panel). While sarcolemmal myofibril condensation was preserved at the MTJ in all young mdx mice (Fig. 5C, top right panel, an example of 2-mo-old mdx) and occasionally some old mdx mice (Fig. 5C, bottom left panel, an example of 14-mo-old mdx), this structural feature was lost in many MTJs in aged mdx mice (Fig. 5C, bottom right panel, an example of 14-mo-old mdx). In these cases, thin filaments were either partially or completely replaced by vacuole-like degenerative structures (Fig. 5C, bottom right panel).
DISCUSSION
Essentially all teenage DMD patients are wheelchair bound. Several factors may have contributed to immobilization. These may include muscle wasting, the loss of contractility, MTJ damage, and increased muscle stiffness. While pathogenic significance of muscle wasting and force loss have been well established, fewer studies have examined muscle passive properties and the MTJ strength. Herein, we hypothesize that dystrophin-deficient muscle pathology may alter muscle elasticity and viscosity and compromise the strength of the MTJ. To test this hypothesis, we examined the stretch-induced stress responses of noncontracting EDL muscle in 2-, 6-, 14-, and 20-mo-old male mdx and BL10 mice. We also quantified muscle fibrosis. We found that mdx muscle was stiffer and displayed an altered viscous property. Further, mdx muscle was significantly more fibrotic. We also observed structural alterations and weakened connection of the MTJ in 14- and 20-mo-old mdx mice.
Our results corroborated findings reported in DMD patients (8, 9). Cornu et al. (8, 9) compared muscle stiffness of the knee extensors and elbow flexors in healthy and DMD boys. They found that DMD muscles were significantly stiffer than normal muscles. As disease progresses, muscle stiffness was further increased (8, 9).
Mdx mice are the most widely used animal model in DMD studies. However, it has been debated whether the passive properties of mdx muscle are altered. Berquin et al. (2a) reported that 2-mo-old mdx EDL muscle generated higher stress than that of age-matched BL10 mice at a strain of 115% Lo. Unfortunately, only very few mice of unknown sex were studied (n = 5 for BL10 and n = 4 for mdx) and there was no statistical analysis on the data. Law et al. (17) compared EDL muscle passive stresses in 11-mo-old BL10 (n = 3) and mdx (n = 4) mice (sex not mentioned). The muscle was stretched to the point of complete tearing at the rate of 2 cm/s. Although the mdx muscle developed 22% higher stress than that of the BL10 EDL muscle at the failure point, the difference was not significant (17). Bobet et al. (3) examined passive stress of the EDL muscle in 20- to 21-mo-old female mdx and BL10 mice. The muscle was stretched in a 20°C organ bath at a rate of 10 cm/s for either 1 mm (∼8% of Lo) or 2.5 mm (∼20% of Lo). The mdx and BL10 muscles developed identical stresses when stretched for 1 mm (small stretch). For the 2.5 mm stretch, mdx muscles appeared to develop higher stresses but these were not statistically different (3). To study whether dystrophin deficiency affects the passive properties of the EDL muscles in very young mice, Wolff et al. (32) applied a single (or a series of single) very mild stretches at the strain of 105% Lo and the stretch rate of 1.5 Lo/s (∼0.15 cm/s) in 2-, 3-, 4-, and 5-wk-old mdx and BL6 mice. Comparison of the pre- and poststretch tetanic forces suggested that muscle was not damaged by this stretching protocol. Interestingly, mdx and BL6 mice showed similar passive properties under this experimental condition (32).
Our study confirmed and extended the preliminary findings by Berquin et al. (2a).Using a larger sample size, broader age range, and more comprehensive experimental approach, we demonstrated that 2- to 20-mo-old male mdx EDL muscles were significantly stiffer than age- and sex-matched BL10 EDL muscle. Law et al. (17) showed a similar trend in 11-mo-old mdx mice. We suspect that the small sample size used by Law et al. (17) may have limited their statistical power. Results from Bobet et al. (3) and Wolff et al. (32) are quite interesting. The differences in the sex (3), age (32), and assay temperature (3) are some obvious factors that may partially explain the discrepancy between our results and these reported by Bobet et al. (3) and Wolff et al. (32). Another important factor is the conditions used in passive stretch. In our study, the EDL muscle was stretched with an initial strain of 110% of Lo and the strain was then increased by an increment of 10% of Lo in subsequent stretches until it reached 160% of Lo. This allowed us to evaluate the full spectrum of stress development during mild, moderate, and extreme length changes. Our data suggest that the stress developed prior to the peak is directly proportional to the length stretched. Low strain resulted in low stress (Fig. 1). A similar trend was observed by Bobet et al. (3). The stresses developed at 2.5 mm stretch were 100% and 40% higher than those developed at 1 mm stretch in mdx and BL10, respectively (3). In the study of Wolff et al. (32), a very mild strain (105% of Lo) was applied at a very low stretch rate (∼0.15 cm/s). In our study, 2-mo-old mdx only yielded slightly higher stress than that of 2-mo-old BL10 at the strain of 110% of Lo although it is statistically significant (P = 0.037). Considering the age effect (the older mdx mice developed higher stress between the strain of 110 to 130% of Lo) and the stretch rate effect (the higher rate yielded a higher stress in mdx mice) (Fig. 1 and 2), the difference between our results and that of Wolff et al. (32) may likely be due to the difference in the mouse age and stretch rate used.
Skeletal muscle has both elastic and viscous properties in a passive state (11, 22). To study viscous properties, we quantified the stress-relaxation rate at a fixed strain length of 110% of Lo (Fig. 3) (21). Consistently, mdx muscle showed a significantly faster relaxation rate than that of age-matched BL10 muscle (Fig. 3). Further, older mdx muscles relaxed faster than that of the younger ones (Fig. 3). This result suggests that not only the elastic property of the mdx EDL muscle is altered; dystrophin-deficient muscular dystrophy also profoundly influences the viscous property of the muscle.
Massive muscle structural remodeling has been demonstrated in dystrophin-deficient mice. These include myofiber degeneration/regeneration, a switch of fast-twitch fibers to slow type, cytoskeleton alterations, inflammation, and fibrosis (14, 24, 30, 31). As an initial effort to investigate the mechanism(s) underlying the observed passive property changes in mdx muscle, we quantified the level of fibrosis (Fig. 4). Consistent with the increased mdx muscle stiffness, the hydroxyproline content was significantly increased in mdx muscle (Fig. 4). Further, aged mdx muscle was significantly more fibrotic than young adult mdx muscle (Fig. 4). These data suggest that muscle fibrosis may have at least partially contributed to the increased stiffness in mdx muscle.
It has been shown that dystrophin is highly enriched at the MTJ, the major force transmission structure between the muscle and the tendon (26). Previous EM studies in 1-wk-old to 6-mo-old mdx mice showed reduced junctional folding at the MTJ and the loss of the lateral association of thin filament to the sarcolemma (17, 18, 25, 28). Results from our passive stretch assay suggest that in young adult mdx mice, the MTJ was sufficiently strong to hold the tendon and muscle together under extreme strain (Fig. 1, B and D). However, as disease progressed in aged mice, we began to see muscle failure occurred at the proximal MTJ separating part of the tendon from the muscle. To understand the structural basis of this age-associated MTJ weakening, we performed a detailed EM study on the EDL muscle freshly isolated from a new set of muscles. These muscles were directly fixed and embedded for the EM study. Interestingly, we did not see a consistent pattern on either the size of muscle protrusion or the depth of MTJ folding. In both young and old, BL10 and mdx, we found interfaces that were small or large, shallowly or deeply invaginated (Fig. 5, A and B). Quantification of the folding depth in 14-mo-old mice failed to reveal a statistical difference between mdx and BL10 (see Supplementary Fig. S2, available with the online version of this article). In support of our observation, Miosge et al. (21a) have also shown that there is no difference at the MTJ folding in 3-mo-old BL10 and mdx mice.
We also examined lateral associations of the thin filament to the sarcolemma at the MTJ folding (Fig. 5C). Consistent with previous reports (17, 18, 25, 28), thin filaments condensed to the muscle cell membrane in BL10 mice irrespective of the age. Surprisingly, thin filament lateral associations were apparently intact in 2-mo-old mdx mice (Fig. 5C). Even in some regions in 14-mo-old mdx mice, we still detected thin filament lateral associations similar to those of 14-mo-old BL10 mice (Fig. 5C). Nevertheless, we observed substantial vacuolar degeneration in many muscle protrusions in 14-mo-old mdx mice (Fig. 5C). In these MTJs, thin filaments were either partially or completely replaced by vacuole-like structures and the sarcolemmal lining was disrupted. Essentially, the normal pattern of thin filament lateral association was lost in these degenerated MTJs. We speculate that these defective MTJ may have failed to hold muscle and tendon together and resulted in separation of the proximal tendon from the muscle (Fig. 1, E and F).
Conclusions.
Compared with that of the age-matched BL10 control, the mdx EDL muscle was significantly stiffer and the viscosity was also altered in mdx. Muscle failure was not observed in ≤14-mo-old BL10. In young (2 mo old and 6 mo old) mdx mice and very old (20 mo old) BL10 mice, muscle failure was found within the proximal end of the EDL muscle. However, the failure site shifted toward the proximal MTJ in old (14 mo old and 20 mo old) mdx mice. Electron microscopic examination revealed substantial MTJ degeneration in old but not young mdx mice. Taken together, we have demonstrated for the first time that the passive properties (viscosity and elasticity) of mdx muscle were significantly altered. Further, MTJ strength was weakened in aged mdx mice. These findings will not only help explain the severe dystrophic phenotype in aged mdx mice, but may also shed new light on our understanding of DMD clinical presentation. Future studies are needed to determine whether novel gene/cell/pharmacological therapies can halt the deterioration of the passive properties and improve function.
GRANTS
This work was supported by grants from the National Institutes of Health (NIH) (AR-49419, D. Duan), Muscular Dystrophy Association (D. Duan), and NIH Training Grant T90-DK-70105 (C. Hakim).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Isabella Zaniletti at the Biostatistics Group, Office of Research, University of Missouri for the help with two-way ANOVA analysis. We thank Drs. Kerry McDonald and Ron Terjung for helpful discussions and critical reading of the manuscript. We thank Marianne Abdo, Juveria Nayeem, and Alexandra Kellogg for technical assistance.
REFERENCES
- 1. Abbott BC, Lowy J. Stress relaxation in muscle. Proc R Soc Lond B Biol Sci 146: 281–288, 1956 [DOI] [PubMed] [Google Scholar]
- 2. Bell CD, Conen PE. Histopathological changes in Duchenne muscular dystrophy. J Neurol Sci 7: 529–544, 1968 [DOI] [PubMed] [Google Scholar]
- 2a. Berquin A, Schmit P, Moens P, Lebacq J. Compliance of normal, dystrophic and transplanted mouse muscles. J Biomech 27: 1331–1337, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Bobet J, Mooney RF, Gordon T. Force and stiffness of old dystrophic (mdx) mouse skeletal muscles. Muscle Nerve 21: 536–539, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Bostick B, Yue Y, Long C, Duan D. Prevention of dystrophin-deficient cardiomyopathy in twenty-one-month-old carrier mice by mosaic dystrophin expression or complementary dystrophin/utrophin expression. Circ Res 102: 121–130, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Bostick B, Yue Y, Long C, Marschalk N, Fine DM, Chen J, Duan D. Cardiac expression of a mini-dystrophin that normalizes skeletal muscle force only partially restores heart function in aged Mdx mice. Mol Ther 17: 253–261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71–82, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chamberlain JS, Metzger J, Reyes M, Townsend D, Faulkner JA. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB J 21: 2195–2204, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Cornu C, Goubel F, Fardeau M. Muscle and joint elastic properties during elbow flexion in Duchenne muscular dystrophy. J Physiol 533: 605–616, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cornu C, Goubel F, Fardeau M. Stiffness of knee extensors in Duchenne muscular dystrophy. Muscle Nerve 21: 1772–1774, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Ervasti JM. Dystrophin, its interactions with other proteins, and implications for muscular dystrophy. Biochim Biophys Acta 1772: 108–117, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Fenn WO, Garvey PH. The Measurement of the elasticity and viscosity of skeletal muscle in normal and pathological cases: a study of so-called “muscle tonus”. J Clin Invest 13: 383–397, 1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fung YC. Biomechanics: Mechanical Properties of Living Tissues. New York: Springer-Verlag, 1993, p. xviii, 568 p. [Google Scholar]
- 13. Goldstein JA, McNally EM. Mechanisms of muscle weakness in muscular dystrophy. J Gen Physiol 136: 29–34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanft LM, Rybakova IN, Patel JR, Rafael-Fortney JA, Ervasti JM. Cytoplasmic gamma-actin contributes to a compensatory remodeling response in dystrophin-deficient muscle. Proc Natl Acad Sci USA 103: 5385–5390, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hasegawa T, Matsumura K, Hashimoto T, Ikehira H, Fukuda H, Tateno Y. [Intramuscular degeneration process in Duchenne muscular dystrophy—investigation by longitudinal MR imaging of the skeletal muscles]. Rinsho Shinkeigaku 32: 333–335, 1992 [PubMed] [Google Scholar]
- 16. Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50: 509–517, 1987 [DOI] [PubMed] [Google Scholar]
- 17. Law DJ, Caputo A, Tidball JG. Site and mechanics of failure in normal and dystrophin-deficient skeletal muscle. Muscle Nerve 18: 216–223, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Law DJ, Tidball JG. Dystrophin deficiency is associated with myotendinous junction defects in prenecrotic and fully regenerated skeletal muscle. Am J Pathol 142: 1513–1523, 1993 [PMC free article] [PubMed] [Google Scholar]
- 19. Liu M, Yue Y, Harper SQ, Grange RW, Chamberlain JS, Duan D. Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol Ther 11: 245–256, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Magnusson SP. Passive properties of human skeletal muscle during stretch maneuvers. A review. Scand J Med Sci Sports 8: 65–77, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Minajeva A, Kulke M, Fernandez JM, Linke WA. Unfolding of titin domains explains the viscoelastic behavior of skeletal myofibrils. Biophys J 80: 1442–1451, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a. Miosge N, Klenczar C, Herken R, Willem M, Mayer U. Organization of the myotendinous junction is dependent on the presence of alpha7beta1 integrin. Lab Invest 79: 1591–1599, 1999 [PubMed] [Google Scholar]
- 22. Moss RL, Halpern W. Elastic and viscous properties of resting frog skeletal muscle. Biophys J 17: 213–228, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagao H, Morimoto T, Sano N, Takahashi M, Nagai H, Tawa R, Yoshimatsu M, Woo YJ, Matsuda H. [Magnetic resonance imaging of skeletal muscle in patients with Duchenne muscular dystrophy—serial axial and sagittal section studies]. No To Hattatsu 23: 39–43, 1991 [PubMed] [Google Scholar]
- 24. Prins KW, Humston JL, Mehta A, Tate V, Ralston E, Ervasti JM. Dystrophin is a microtubule-associated protein. J Cell Biol 186: 363–369, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ridge JC, Tidball JG, Ahl K, Law DJ, Rickoll WL. Modifications in myotendinous junction surface morphology in dystrophin-deficient mouse muscle. Exp Mol Pathol 61: 58–68, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Samitt CE, Bonilla E. Immunocytochemical study of dystrophin at the myotendinous junction. Muscle Nerve 13: 493–500, 1990 [DOI] [PubMed] [Google Scholar]
- 27. Tidball JG. Myotendinous junction: morphological changes and mechanical failure associated with muscle cell atrophy. Exp Mol Pathol 40: 1–12, 1984 [DOI] [PubMed] [Google Scholar]
- 28. Tidball JG, Law DJ. Dystrophin is required for normal thin filament-membrane associations at myotendinous junctions. Am J Pathol 138: 17–21, 1991 [PMC free article] [PubMed] [Google Scholar]
- 29. Trotter JA. Functional morphology of force transmission in skeletal muscle. A brief review. Acta Anat (Basel) 146: 205–222, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Webster C, Silberstein L, Hays AP, Blau HM. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell 52: 503–513, 1988 [DOI] [PubMed] [Google Scholar]
- 31. Williams MW, Bloch RJ. Extensive but coordinated reorganization of the membrane skeleton in myofibers of dystrophic (mdx) mice. J Cell Biol 144: 1259–1270, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wolff AV, Niday AK, Voelker KA, Call JA, Evans NP, Granata KP, Grange RW. Passive mechanical properties of maturing extensor digitorum longus are not affected by lack of dystrophin. Muscle Nerve 34: 304–312, 2006 [DOI] [PubMed] [Google Scholar]