Abstract
Increased flow in the distal nephron induces K secretion through the large-conductance, calcium-activated K channel (BK), which is primarily expressed in intercalated cells (IC). Since flow also increases ATP release from IC, we hypothesized that purinergic signaling has a role in shear stress (τ; 10 dynes/cm2) -induced, BK-dependent, K efflux. We found that 10 μM ATP led to increased IC Ca concentration, which was significantly reduced in the presence of the P2 receptor blocker suramin or calcium-free buffer. ATP also produced BK-dependent K efflux, and IC volume decrease. Suramin inhibited τ-induced K efflux, suggesting that K efflux is at least partially dependent on purinergic signaling. BK-β4 small interfering (si) RNA, but not nontarget siRNA, decreased ATP secretion and both ATP-dependent and τ-induced K efflux. Similarly, carbenoxolone (25 μM), which blocks connexins, putative ATP pathways, blocked τ-induced K efflux and ATP secretion. Compared with BK-β4−/− mice, wild-type mice with high distal flows exhibited significantly more urinary ATP excretion. These data demonstrate coupled electrochemical efflux between K and ATP as part of the mechanism for τ-induced ATP release in IC.
Keywords: MDCK-C11, collecting duct, shear stress, connexin, distal nephron
in the distal nephron of mammalian kidneys, high luminal flow stimulates secretion of ATP, which activates purinergic P2Y2 receptors (22, 30, 42). In principal cells (PC) of the distal nephron, extracellular ATP reduces Na reabsorption by inhibiting the epithelial Na channels (ENaC) (27, 34, 35, 52) and reduces K secretion by inhibiting the small-conductance K channel (SK) (29). Luminal ATP also inhibits AVP-stimulated osmotic water permeability in mouse collecting ducts (CD) (26, 41). These studies explain why mice with a deletion of the P2Y2 receptor (P2Y2-KO) have salt-resistant hypertension (40) and have led to the notion that ATP inhibition of Na and water reabsorption in the distal nephron (39) perpetuates the luminal flow that induces K secretion and extrusion of ATP.
Shear stress (τ) increases the release of ATP in a variety of epithelia, including renal (12, 33), bile duct (46), and Madin-Darby canine kidney (MDCK) (21) cells; however, it is unclear how renal epithelial cells “sense” τ. The primary cilium serves as a mechanosensor (32, 57) because it protrudes into the lumen 1–2 μm; however, τ also induces ATP secretion in nonciliated human bile duct epithelial cells (55). Moreover, MDCK cells exhibit pressure-dependent ATP secretion that is independent of cilia (36).
The intercalated cells (IC) of the distal nephron protrude ∼1–3 μm further into the lumen than PC, potentially enabling IC to serve as mechanosensors as well. In perfused rabbit cortical collecting ducts (CCDs), IC exhibit functional apical P2Y2 receptors and flow-induced increases in intracellular Ca2+ concentration ([Ca2+]i) (54). Furthermore, IC from isolated perfused murine CCDs generate threefold greater flow-induced ATP secretion, which is reduced in connexin 30-deficient mice (45) or inhibited by the P2 receptor blocker suramin.
We previously showed that high distal flow generated by a high-K diet causes a τ-dependent shrinking of IC in wild-type (WT) mice (16). However, the cell size reduction was attenuated in a mouse with a genetic deletion of the BK-β4 (BK-β4−/−), an ancillary subunit of the IC localized, large, Ca-activated K channel (BK-α/β4). These results were replicated in vitro in the MDCK-derived C11 cells (C11) (17), which predominantly express P2Y1 and P2Y2 receptors and are a model of acid/base-transporting IC (9). C11 undergo τ-induced volume decrease mediated by K efflux through BK-α/β4; however, anion efflux is required to maintain electrochemical balance.
The intracellular Cl− concentration ([Cl−]i) of IC-α is estimated at 35 mM, a concentration high enough for Cl to serve as the counteranion to balance the positive charge loss of K from the cell (5). However, the [Cl−]i in IC-β was measured at only 10 mM (5), a concentration that would be insufficient to balance the flow-induced loss of K. With up to four negative charges (38), ATP can serve as the negatively charged component to the K efflux.
The dependence of BK-α/β4-mediated K extrusion on the flow-stimulated release of ATP from IC can partially explain the enhanced Na reabsorption and fluid accumulation in mice with a genetic deletion (BK-β4−/−). We therefore hypothesize that high-flow, high-τ causes IC to extrude ATP via connexin channels and K via BK-α/β4, resulting in decreased cellular volume and increased tubular flow. Both in vitro and in vivo experiments using C11 and BK-β4−/−, respectively, suggest that BK-α/β4-mediated K extrusion from IC is a necessary component of ATP-mediated natriuresis in the distal nephron.
METHODS
Animal studies.
All animals were maintained in accordance with the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. At ∼12–20 wk of age, WT (C57BL/6, Charles River, Wilmington, MA) and BK-β4−/− mice (generously provided by R. Brenner) were given either normal mouse chow (0.6% K+, 0.32% Na+, control) or chow with a high K content (5.0% K+, 0.32% Na+, Harlan Teklad, Madison, WI) for 10 days. At all times, the animals had full access to water. Urine samples were collected several times a day using metabolic cages (Nalgene) as previously described (45). After treatment, fresh urine samples were collected at time of urination, immediately placed on ice, and centrifuged at 5,000 relative centrifugal force for 5 min at 4°C. An aliquot of 50 μl was taken and used for ATP measurements as described below. The kidneys were harvested and immediately fixed in Histochoice MB (Electron Microscopy Sciences, Hatfield, PA), and embedded in paraffin for sectioning as previously described (16).
Immunohistochemical staining.
Fluorescent immunohistochemical staining of kidney sections was performed as previously described (11). Primary antibodies, rabbit polyclonal anti-MnSOD (diluted 1:100, Millipore) and goat polyclonal anti-VATPase (diluted 1:100, Santa Cruz Biotechnology, Santa Cruz, CA), or species-specific IgG (negative control; diluted 1:100) was incubated overnight at 4°C. After washing, cells were incubated for 1 h at room temperature in the dark with the secondary antibody (donkey anti-rabbit IgG-conjugated Alexa Fluor 488 and donkey anti-goat IgG-conjugated Alexa Fluor 594, diluted 1:200) followed by nuclear staining with 0.25 μg/ml Hoechst 33258 for 10 min in the dark at 23°C. Coverslips were mounted onto microscope slides with Prolong Gold (Invitrogen, Carlsbad, CA) overnight, sealed with nail polish, and viewed on a Zeiss LSM 510 META Confocal microscope.
Cell culture.
C7-MDCK cells (passages 75–80, a generous gift of Dr. Bonnie L. Blazer-Yost of Indiana University-Purdue University, Indianapolis, IN) and C11-MDCK cells (passages 64–70, a generous gift of Dr. Hans Oberleithner, Münster University) were grown in high-glucose DMEM (Invitrogen) supplemented with 10% FBS, 1.0 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, and 25 μg/ml gentamicin under standard incubating conditions of 37°C, 95% humidity, 5% CO2.
Buffers, chemicals, drugs, and reagents.
Physiological saline solution (PSS) contained 135 mM NaCl, 5 mM KCl, 1 mM CaCl2, 2 mM MgCl2, and 10 mM HEPES, pH to 7.4 with NaOH. Distal tubule buffer, which is similar to mammalian distal tubule luminal fluid, contained 65 mM NaCl, 5 mM KCl, 5 mM urea, 1 mM CaCl2, 0.5 mM MgCl2, 0.4 mM MgSO4, supplemented with 3.3 mM NaHCO3, 5 mM HEPES, and 2 mM glucose, pH to 6.5, with HCl. Final osmolality was ∼150 mosmol/kgH2O, as measured by freezing-point depression (model 3250, Advanced Instruments, Norwood, MA). For calibration of the calcium imaging system, the high-calcium buffer contained 20 mM NaCl, 115 mM KCl, 3 mM CaCl2, 1 mM MgCl2, and 10 mM MOPS acid, pH to 7.05 with NaOH. The zero-calcium buffer contained 20 mM NaCl, 115 mM KCl, 3 mM EGTA, 1 mM MgCl2, and 10 mM MOPS acid, pH to 7.05 with NaOH. The calcium-free buffer contained 135 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1.08 mM EGTA, and 10 mM HEPES, pH to 7.4 with NaOH. PBS was obtained from Sigma (St. Louis, MO). Fura 2-AM, calcein-AM, and Pluronic F-127 were obtained from Invitrogen and resuspended in DMSO. Unless denoted, all other chemicals and solutions were obtained from Sigma.
Western blotting.
Standard Western blotting was performed as previously described (17) following the manufacturer's protocol (Bio-Rad Laboratories, Hercules, CA) except that RIPA buffer was replaced with PBS containing 0.5% SDS. Primary antibodies included anti-BK-β4 (rabbit polyclonal, diluted 1:500, Alomone Labs) and anti-β-actin (mouse monoclonal, diluted 1:5,000, Santa Cruz Biotechnology) with either goat (Santa Cruz Biotechnology) anti-rabbit IgG- or donkey anti-mouse (Santa Cruz Biotechnology) IgG-conjugated horseradish peroxidase (HRP) secondary antibody diluted 1:20,000–1:40,000. Expression of primary antibodies was quantified by densitometry.
siRNA.
Knockdown of the BK-β4 subunit in C11 was achieved as previously described (17).
Parallel plate flow system.
C7 or C11 cells were grown to confluency on glass coverslips and exposed to either static (control) or τ of 10 dynes/cm2 in a parallel plate flow chamber (PPFC) at 37°C for 30 min as previously described (17).
ATP measurement.
C11 cells were exposed to static or 10 dynes/cm2 in a PPFC at 37°C for 30 min. Afterward, both intracellular and secreted ATP were measured using an ENLITEN ATP Bioluminescence Detection Kit (Promega, Madison, WI). Briefly, after treatment, 2 ml of perfusate was collected into a tube containing 200 μl 20% TCA (2% final concentration) to prevent ATP degradation by ecto-nucleotidases, ecto-ATPase, and other enzymatic and hydrolytic activities. Cells were lysed with 2 ml ice-cold distilled H2O, scraped, vortexed, and centrifuged at 10,000 RCF for 5 min at 4°C. The supernatant was collected and used for both intracellular ATP and cation measurements as previously described (17). Both intracellular and extracellular ATP samples were then vortexed and neutralized with TAE buffer (Bio-Rad) to pH 7.7–7.8 following the manufacturer's protocol. ATP luminescence was measured by a Sirius Luminometer (Titertek Instruments, Huntsville, AL) at room temperature. ATP and cation measurements were normalized to DNA concentrations to adjust for cell loss during flow or variances in cell number between samples.
Calcium imaging.
C11 cells were grown on 22 × 22-mm glass coverslips (Fisher Scientific, Pittsburgh, PA) and incubated with fura 2-AM (7 μM) in PSS for 30–45 min at 37°C. After loading, coverslips were placed into a perfusion chamber (Warner RC-20H; Warner Instruments, Hamden, CT) and mounted on a Nikon Diaphot 300 inverted microscope. [Ca2+]i was measured as previously described (17).
Calcein imaging.
C11 cell volumes were measured with the volume-sensing dye calcein-AM as previously described (17). Fluorescence was measured in six cells at baseline and again 5 min after exposure to 10 μM ATP. Care was taken to ensure the optical section height was always thinner than the cell height (17).
Statistical methods.
Data shown in figures represent means ± SE. Unless otherwise denoted, data were analyzed by a t-test or Mann-Whitney rank sum test with the static controls, which were ran simultaneously using the same batch of cells, buffers, reagents, and incubation time. Calcium measurements were analyzed by two-way analysis of variance with Turkey's post hoc multiple comparisons test. P values <0.05 were considered significant. We performed statistical analyses using SigmaPlot 11.0 (Systat Software, Chicago, IL).
RESULTS
Purinergic receptors in C11.
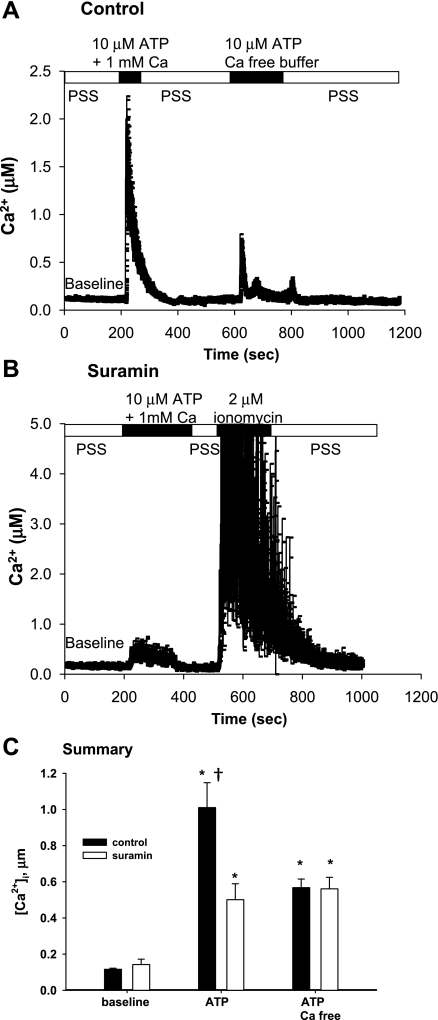
We examined the effects of purinergic signaling on [Ca2+]i in C11. As shown in Fig. 1, application of ATP significantly increased [Ca2+]i in the presence of external Ca2+. The removal of external calcium decreased the peak [Ca2+]i, elicited with 10 μM ATP, but was still well above baseline (Fig. 1A). The peak [Ca2+]i was substantially decreased by preincubation with 300 μM suramin (Fig. 1B). However, C11 still responded to ionomycin, indicating that a functional Ca2+ buffering system remained. A summary of the results is shown in Fig. 1C. At baseline, the [Ca2+]i, of C11 was 129 ± 37 nM (n = 12). In the presence of external Ca2+, ATP increased [Ca2+]i to a mean of 1,010 ± 64 nM (P < 0.002; n = 6) in the absence of suramin and to 501 ± 79 nM, (P < 0.002; n = 7) in C11 preincubated with suramin. In the absence of external Ca2+, ATP increased mean [Ca2+]i to 568 ± 71 nM (P < 0.005; n = 5), a value unaffected by suramin (561 ± 91 nM, P > 0.955; n = 3). These results suggested that ATP increases Ca2+ release from both internal stores and external entry pathways in C11, and activation of the purinergic pathway through suramin-sensitive P2 receptors is required for ATP-dependent extracellular calcium entry.
Fig. 1.
P2 signaling in C11. A: plot of intracellular Ca2+ concentration ([Ca2+]i) obtained from fura 2 imaging of C11 exposed to ATP in the presence or absence of 1 mM extracellular calcium. B: plot of [Ca2+]i of C11 preincubated with suramin and exposed to ATP in the presence of extracellular calcium and ionomycin. C: summary bar plot of average peak [Ca2+]i for each condition in A and B. *Significant (P < 0.005) difference from baseline. †Significant difference (P < 0.005) from suramin.
Co-dependence of ATP and BK-α/β4 for flow-induced K efflux.
The results in Fig. 2 show a comparison of ATP secreted from C11 and C7, a principal cell clone, under static and flow conditions using a PPFC. Under static conditions, both C7 and C11 secreted ATP and the quantities were not significantly different [0.53 ± 0.35 and 1.16 ± 0.11 ATP (pmol·μg DNA−1·min−1; n = 7), respectively]. Exposing cells to 10 dynes/cm2 flow τ led to a significant increase in secreted ATP for both C7 and C11 (2.92 ± 0.33 vs. 5.82 ± 0.54; P < 0.002; n = 7), with C11 secreting twice as much ATP as C7.
Fig. 2.
Flow-induced ATP efflux in C7 and C11. ATP secreted from C7 and C11 under static (control) and flow (10 dynes/cm2) conditions. Values are means ± SE. *Significant (P < 0.005) difference from static. †Significant (P < 0.005) difference between cell types.
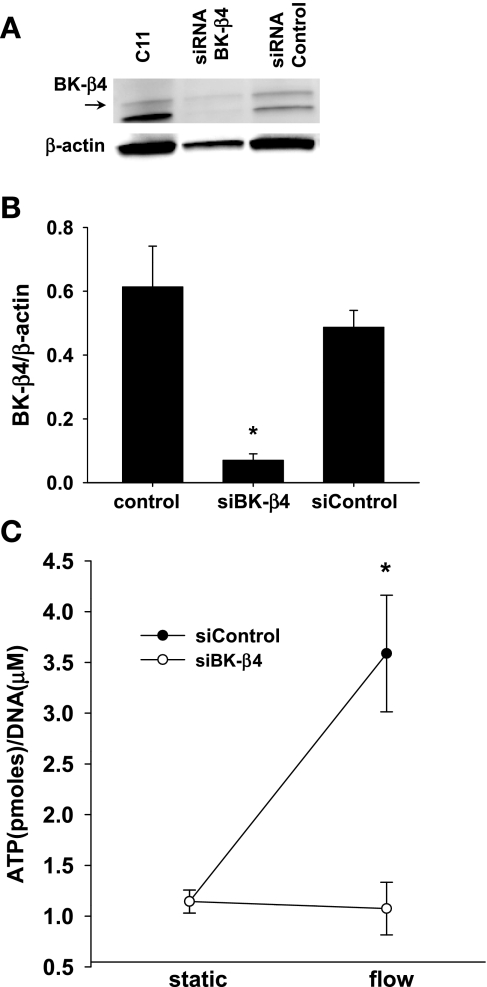
We examined the effect of silencing BK-β4 on ATP secretion from C11. As shown by Western blotting (Fig. 3A), BK-β4 siRNA successfully knocked down BK-β4 expression in C11 by 82% (Fig. 3B; n = 6). BK-β4 expression was unaffected by siRNA nontarget (Fig. 3, A and B; n = 6). Compared with static conditions, flow induced ATP secretion in C11 transfected with the nontarget siRNA (Fig. 3C, 1.14 ± 0.11 vs. 3.59 ± 0.58, P < 0.001; n = 7); however, flow did not induce ATP secretion when cells were transfected with BK-β4 siRNA, (Fig. 3C, 1.14 ± 0.11 vs. 1.07 ± 0.26, P > 0.72; n = 7).
Fig. 3.
Effects of large-conductance, calcium-activated K channel subunit BK-β4 small interference (si) RNA on ATP secretion in C11. A: Western blot analysis of protein from C11 that were untreated (C11), transfected with BK-β4 siRNA (siRNA BK-β4) or nontarget siRNA (siRNA control). B: BK-β4 siRNA silenced ∼82% of BK-β4 compared with normal conditions (P < 0.02; n = 6). C: flow-induced ATP release was blocked by BK-β4 siRNA (n = 7), but not by siRNA control (n = 7).
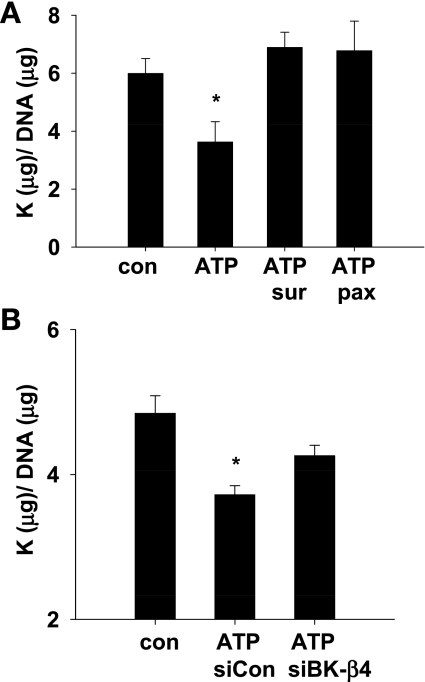
We previously showed with a PPFC that τ leads to K efflux and cell shrinkage in C11 (17). We examined whether K efflux, measured by K/DNA content, was dependent on purinergic signaling by exposing C11 to 10 dynes/cm2 in the presence and absence of suramin, a nonspecific blocker of P2 receptors. As shown in Fig. 4, flow induced K efflux from C11 (6.26 ± 0.32 vs. 4.89 ± 0.33, P < 0.02; n = 6), as we have shown previously (17); however, preincubating the cells with suramin for 30 min and then adding suramin to the perfusate inhibited the flow-induced K efflux (6.26 ± 0.32 vs. 5.65 ± 0.39, P > 0.265; n = 6).
Fig. 4.
Effects of suramin on flow-induced K efflux from C11 (n = 6). Symbols are the same as for Fig. 2.
Figure 5A illustrates with summary bar plots that purinergic signaling leads to BK-dependent K efflux. Under static conditions, the addition of 10 μM ATP decreased intracellular K/DNA content by 40% (5.99 ± 0.51 vs. 3.62 ± 0.71, P < 0.01; n = 8). This decrease was prevented by suramin (5.99 ± 0.51 vs. 6.88 ± 0.52, P > 0.2; n = 5) or 1 μM paxilline (5.99 ± 0.51 vs. 6.77 ± 1.02, P > 0.47; n = 3), a specific inhibitor of the BK channel (20).
Fig. 5.
ATP activation of BK-α/β4-mediated K efflux. A: under static conditions, the addition of ATP decreased C11 intracellular K/DNA content; this effect was blocked by suramin and paxilline (n = 5). B: ATP-induced K efflux was significantly decreased by siRNA BK-β4, but not by siRNA control (n = 4). *Significant (P < 0.02) difference from control.
The effect of silencing BK-β4 on ATP-mediated K efflux is shown in the summary bar plots in Fig. 5B. The addition of 10 μM ATP produced K efflux from C11 transfected with the nontarget siRNA compared with static (Fig. 5B, 4.84 ± 0.25 vs. 3.72 ± 0.13, P < 0.01; n = 4). However, ATP-dependent K efflux was attenuated in C11 transfected with BK-β4 siRNA (4.84 ± 0.25 vs. 4.26 ± 0.15, P > 0.15; n = 4). These data suggest that at least some of the ATP-dependent K efflux occurs through the BK channel and is dependent on the expression of BK-β4 subunit in C11.
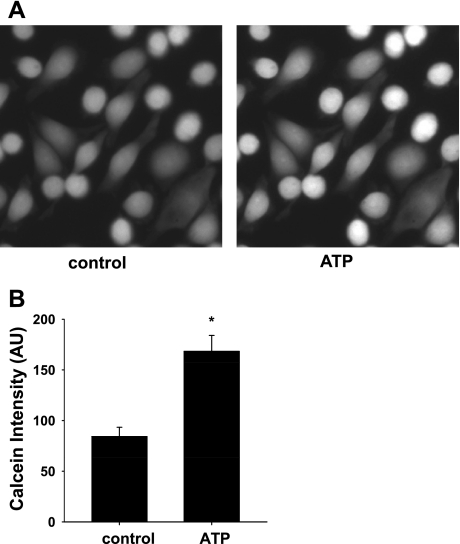
In a previous study, we showed that exposure of C11 to 10 dynes/cm2 led to K efflux and cell volume decrease (17). As shown in the typical experiment (Fig. 6A) and the summary bar plots (Fig. 6B), the addition of 10 μM ATP led to a significant increase in calcein intensity, demonstrating a decrease in cell volume (84.2 ± 8.2 vs. 168.3 ± 15.7, P < 0.001; n = 8).
Fig. 6.
Effects of ATP on BK-dependent cell volume decrease. A: representative images of calcein intensity of C11 before and after the addition of 10 μM ATP. The significant increase in calcein intensity indicated a decrease in cell volume compared with control (P < 0.01; n = 8). B: summary plot of calcein intensity before (control) and after ATP. *Significant difference from control.
ATP extrusion via connexin.
As shown in Fig. 7, addition of carbenoxolone, an inhibitor of connexins and pannexins (2, 8), blocked flow-induced ATP efflux. Carbenoxolone also blocked flow-induced ATP excretion (Fig. 7A, 1.49 ± 0.72 vs. 3.1 ± 0.76, P < 0.02, n = 10 for control and 1.49 ± 0.72 vs. 0.34 ± 0.76, P < 0.02, n = 7 for carbenoxolone). There was no significant flow-induced loss of intracellular ATP concentration for C11 incubated with carbenoxolone (Fig. 7B; 255.4 ± 18.5 vs. 260.4 ± 20.9, P > 0.871; n = 6); however, the ATP concentration significantly decreased in cells exposed to flow without carbenoxolone (Fig. 7B; 255.4 ± 18.5 vs. 182.2 ± 23.0, P < 0.05; n = 6). This decrease of ∼73 pmol·μg DNA−1·min−1 is close to the expected ATP secretion rate of 3.1 pmol·μg DNA−1·min−1 for 30 min (∼90 pmol·μg DNA−1·min−1). These data suggest coupled K and ATP efflux from C11 under τ = 10 dynes/cm2.
Fig. 7.
Effects of carbenoxolone on ATP and K secretion. Carbenoxolone blocks shear stress (τ)-induced ATP efflux (A) and intracellular ATP content (B). C: carbenoxolone block of τ-induced release of intracellular K (n = 6). Symbols are the same as for Fig. 2.
Under flow conditions, 25 μM carbenoxolone inhibited K efflux from C11 compared with the absence of carbenoxolone (Fig. 7C; 6.46 ± 0.34 vs. 5.39 ± 0.39, P < 0.05; n = 6).
BK-α/β4 dependency of flow-induced ATP efflux.
We examined the role of BK-β4 on ATP secretion in response to high luminal flow. WT and BK-β4−/− mice were placed on either a normal (0.6% K) or K-adapted (5% K; KA) diet for 10 days to induce high tubule flow, and urinary ATP excretion was measured. On a control diet, there was no significant difference between WT and BK-β4−/− mice (Fig. 8, 2.7 ± 0.7 vs. 5.7 ± 1.2 fmol/min, P > 0.82). However, ATP secretion was threefold greater in KA WT mice compared with BK-β4−/− mice (120.6 ± 13.3 vs. 41.6 ± 4.2 fmol/min, P < 0.001; n = 10).
Fig. 8.
Summary bar plots of urinary ATP secretion for wild-type (WT) and BK-β4−/− mice on control and high-K (HK) diets. *Significant (P < 0.005) difference from control. †Significant (P < 0.005) difference from WT.
DISCUSSION
This study examined the interaction between purinergic signaling and flow-induced K secretion via BK-α/β4 in C11. We showed that purinergic signaling leads to BK activation in C11, resulting in K and ATP efflux and cell volume decrease. Flow-induced τ release of K and ATP were interdependent; inhibiting ATP release prevented BK-α/β4-mediated K release, and blocking BK-α/β4 prevented the release of ATP. These results were replicated in vivo, with significantly greater rates of high flow-induced urinary ATP excretion in WT compared with β4-KO mice.
Purinergic signaling in C11.
Analysis of [Ca2+]i has been shown to be a reliable assay for active, purinergic signaling (4, 48). C11, which express abundant P2Y1 and P2Y2 receptors, with P2Y2 receptors on the apical membrane (1), exhibited increased [Ca2+]i, ATP release, K efflux, and cell volume decrease when exposed to 10 μM ATP. The dose of 10 μM ATP was chosen because it produces 80% of the maximal ATP-stimulated cell Ca response in MDCK cells (18), and 10 μM is within the range of ATP levels found in cystic fluid (43, 53).
ATP is found in the luminal fluid of the entire nephron (49) and binds with P2Y and P2X receptors to mediate flow-induced [Ca2+]i signaling in human distal nephron cells (58) and the perfused medullary thick ascending limb (TAL) (22). The magnitude of ATP-induced [Ca2+]i increase, ∼1 μM, with a maximum peak value of 2.2 μM, was unexpected. This magnitude is much greater than τ-mediated increases in [Ca2+]i previously reported for C11 (17). These results suggest either different mechanisms between τ-mediated and ATP-mediated [Ca2+]i increases or lower luminal ATP concentrations during previously reported τ-mediated events (49). Although the sharp increase in [Ca2+]i quickly dissipated, these [Ca2+]i values are high enough to activate BK-α/β4 (50).
We were also surprised by the level of ATP-evoked [Ca2+]i increase in the presence of suramin. Either 300 μM suramin does not completely inhibit P2 receptors as previously reported (36), or ATP was reduced to adenosine, leading to P1 receptor activation. The presence of other nucleotide receptors, such as cysteinyl leukotrienes (23), cannot be ruled out. Another possible explanation is that suramin interferes with fura 2 signaling (7, 22), which leads to the relatively high intracellular Ca levels measured in the presence of suramin and ATP.
Several previous studies have identified ATP as an indirect or direct regulator of K channels. MDCK cells release ATP in response to mild mechanical stimuli, leading to increased intracellular cAMP (33), which can activate BK channels. ATP increases K+ and HCO3− secretion in the gastrointestinal tract (25). In erythrocytes, P2 receptor activation of Ca-dependent K efflux results in regulatory volume decrease (28). ATP activates Ca-dependent K channels in African green monkey kidney cells (13, 14).
The Ca-sensitive intermediate potassium channel (IK), previously described in MDCK cells, activates after bending of the primary cilium (37). Because IK is activated by ATP (10), a role for IK in ATP-induced K efflux cannot be eliminated. However, in the present study, ATP-dependent K efflux was totally inhibited by paxilline, suggesting that the ATP-dependent K efflux is only through the BK channel.
τ-Induced ATP release and K efflux.
ATP release can be triggered by changes in either cell volume (3) or tubular flow (19, 22). In a normal human kidney epithelial cell line (HEK293 cells) (58), 10 dynes/cm2 produces significant ATP release as we observed for C11. In the distal nephron, high flow induces ATP excretion with the luminal microenvironment approaching 50 μM (45), which is five times the concentration used in our experiments.
We previously reported that exposure of C11 to 10 dynes/cm2 produced K efflux and cell volume decrease (17). However, we were surprised by the 50% reduction in cell volume caused by 10 μM ATP as measured by the 50% increase in calcein intensity (Fig. 6). Previously, we reported a 25% decrease in cell volume of C11 exposed to τ of 10 dynes/cm2 (16). Moreover, IC from mice on a high-K diet (high tubular flow rates) had a 20% decrease in IC volume compared with mice on a control diet (17). ATP is probably activating several pathways, many of which could be leading to transient changes in cell volume similar to the transient change in intracellular calcium (Fig. 1). Another possibility is that C11 are composed of both IC-α and IC-β cells, and maybe non-α/non β-IC cells. It is quite possible that one subset of C11 (IC-α, IC-β or IC-non-α/non-β) could be shrinking more in response to ATP than the other subsets.
Several pathways have been proposed for cellular ATP release (6, 38, 44, 59). As an organic anion, ATP can exit cells as a charge carrying current through large anion channels, such as connexins (24). Connexins are proteins that form gap junction channels called connexons (8) or hemichannels, which allow for molecular exchange between intra-intercellular environments. Connexins are expressed in IC (15) and can allow nonselective permeation of molecules up to 1 kDa (38). After a mechanical load, ATP is released via connexins in most cell types (17). Flow activation of BK results in cellular hyperpolarization, thereby increasing the electrochemical driving force for ATP exit via connexins.
Increased tubular flow triggers nucleotide release and both auto- and paracrine activation of tubular P2 receptors in both cultured bile ducts (56), and renal epithelia (16, 58). Fifty-six percent of the τ-induced K efflux in C11 can be blocked by 300 μM suramin, bringing into question whether the flow-induced K efflux is directly due to τ (i.e., mechanotransduction) or a secondary result of flow-induced ATP release and corresponding P2 receptor activation.
Co-dependence of K and ATP efflux.
In C11, 100% of the ATP-induced and 56% of the τ-induced K efflux were inhibited by 300 μM suramin, a nonspecific blocker of P2 receptors. Conversely, BK-β4 siRNA inhibited 51% of the ATP-induced and 87% of the τ-induced (22) K efflux. However, BK-β4 siRNA inhibited all of the τ-induced ATP release. Furthermore, ATP-induced, as shown in the present study, and τ-induced K efflux from C11, as shown in a previous study (55), were fully inhibited by 1 μM paxilline, a specific inhibitor of BK. These results suggest that τ-induced, BK-mediated K efflux has both P2-dependent and P2-independent components and τ-induced ATP release is dependent on K efflux. That K efflux is dependent on ATP efflux was revealed by application of carbenoxolone, an inhibitor of connexins and hemichannels (47). In C11, carbenoxolone inhibited 72% of the τ-induced K efflux and nearly all ATP release, suggesting dependence of K efflux on ATP extrusion via connexins.
Inhibition of ATP release by BK-β4 siRNA, suggests a regulatory role for the β4 subunit in C11. The BK-β4 subunit may enhance the sensitivity of the BK channel to either ATP or some downstream product of the purinergic signaling pathway.
ATP secretion enhances the K secretion/Na reabsorption ratio.
The physiological relevance of our findings could be related to the need to ensure high distal flow rates, with maximal K secretion and minimal Na reabsorption, when animals consume a high-K diet. High distal flow, required to maintain a low luminal K concentration for maximal K secretion, is initiated by K recycling in the medullary CCD with the high interstitial K concentration, causing inhibition of Na and Cl reabsorption in the TAL. However, the concentrating ability of the TAL is minimally affected. High flow could be augmented by releasing ATP, which inhibits Na and water reabsorption, at the same time K secretion is stimulated.
P2 receptor activation in principal cells results in inhibition of Na and water reabsorption via ENaC and aquaporin-2. Connexin 30, expressed on the apical membrane of IC in the connecting tubule and CCD, mediates ATP release into the lumen of CCDs from mice (45) and rabbits (31). Consistent with the notion that flow-induced ATP release inhibits ENaC-mediated Na reabsorption is the finding that mice with a deletion of the connexin 30 gene have a salt retention phenotype in response to acute increases in blood pressure (45).
The model of ATP as a local regulator of Na and K transport adequately describes experimental data for BK-β4−/− mice, which exhibit Na and water retention as well as decreased K secretion (16). The failure of BK-α/β4 in IC to activate in response to flow reduces the amount of ATP in the lumen, causing more Na and water retention. A reduction in BK-α/β4 activity results in less flow-induced reduction of IC volume with narrower tubule lumens, thereby reducing the volume for a K secretory gradient.
Summary and conclusion.
In IC, activation of P2 receptors leads to BK activation, resulting in decreased cell volume, and ultimately decreased tubule resistance. This dual-pathway model of P2-mediated ion transport provides the distal nephron a local mechanism for controlling fluid flow and ion transport.
GRANTS
This project was funding by National Institutes of Diabetes and Digestive and Kidney Diseases Grants RO1 DK49461 and RO1 DK73070 (to S. C. Sansom).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Akimova OA, Grygorczyk A, Bundey RA, Bourcier N, Gekle M, Insel PA, Orlov SN. Transient activation and delayed inhibition of Na+,K+,Cl- cotransport in ATP-treated C11-MDCK cells involve distinct P2Y receptor subtypes and signaling mechanisms. J Biol Chem 281: 31317–31325, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA 105: 18770–18775, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barrett JN, Magleby KL, Pallotta BS. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol 331: 211–230, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA 100: 4322–4327, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boettger T, Hubner CA, Maier H, Rust MB, Beck FX, Jentsch TJ. Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature 416: 874–878, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem 278: 1354–1362, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Conant AR, Fisher MJ, McLennan AG, Simpson AW. Characterization of the P2 receptors on the human umbilical vein endothelial cell line ECV304. Br J Pharmacol 125: 357–364, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Vuyst E, Decrock E, De BM, Yamasaki H, Naus CC, Evans WH, Leybaert L. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol Biol Cell 18: 34–46, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gekle M, Wunsch S, Oberleithner H, Silbernagl S. Characterization of two MDCK-cell subtypes as a model system to study principal cell and intercalated cell properties. Pflügers Arch 428: 157–162, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Gerlach AC, Syme CA, Giltinan L, Adelman JP, Devors DC. ATP-dependent activation of the intermediate conductance, Ca2+-activated K+ channel, hIK1, is conferred by a C-terminal domain. J Biol Chem 276: 10963–10970, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC. Hypertension of Kcnmb1-/- is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci USA 106: 11800–11805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol Cell Physiol 272: C1058–C1066, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Hafting T, Haug TM, Ellefsen S, Sand O. Hypotonic stress activates BK channels in clonal kidney cells via purinergic receptors, presumably of the P2Y subtype. Acta Physiol (Oxf) 188: 21–31, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Hafting T, Sand O. Purinergic activation of BK channels in clonal kidney cells (Vero cells). Acta Physiol Scand 170: 99–109, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol Regul Integr Comp Physiol 298: R1143–R1155, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holtzclaw JD, Grimm PR, Sansom SC. Intercalated cell BK-alpha/beta4 channels modulate sodium and potassium handling during potassium adaptation. J Am Soc Nephrol 21: 634–645, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holtzclaw JD, Liu L, Grimm PR, Sansom SC. Shear stress-induced volume decrease in C11-MDCK cells by BK-α/β4. Am J Physiol Renal Physiol 299: F507–F516, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hooper KM, Boletta A, Germino GG, Hu Q, Ziegelstein RC, Sutters M. Expression of polycystin-1 enhances endoplasmic reticulum calcium uptake and decreases capacitative calcium entry in ATP-stimulated MDCK cells. Am J Physiol Renal Physiol 289: F521–F530, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Hovater MB, Olteanu D, Welty EA, Schwiebert EM. Purinergic signaling in the lumen of a normal nephron and in remodeled PKD encapsulated cysts. Purinergic Signal 4: 109–124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, Storm JF. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci 21: 9585–9597, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Insel PA, Ostrom RS, Zambon AC, Hughes RJ, Balboa MA, Shehnaz D, Gregorian C, Torres B, Firestein BL, Xing M, Post SR. P2Y receptors of MDCK cells: epithelial cell regulation by extracellular nucleotides. Clin Exp Pharmacol Physiol 28: 351–354, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18: 2062–2070, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol 173: 1503–1510, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci 28: 4702–4711, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kerstan D, Gordjani N, Nitschke R, Greger R, Leipziger J. Luminal ATP induces K+ secretion via a P2Y2 receptor in rat distal colonic mucosa. Pflügers Arch 436: 712–716, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Kishore BK, Chou CL, Knepper MA. Extracellular nucleotide receptor inhibits AVP-stimulated water permeability in inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 269: F863–F869, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Lehrmann H, Thomas J, Kim SJ, Jacobi C, Leipziger J. Luminal P2Y2 receptor-mediated inhibition of Na+ absorption in isolated perfused mouse CCD. J Am Soc Nephrol 13: 10–18, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Light DB, Dahlstrom PK, Gronau RT, Baumann NL. Extracellular ATP activates a P2 receptor in necturus erythrocytes during hypotonic swelling. J Membr Biol 182: 193–202, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Lu M, MacGregor GG, Wang W, Giebisch G. Extracellular ATP inhibits the small-conductance K channel on the apical membrane of the cortical collecting duct from mouse kidney. J Gen Physiol 116: 299–310, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matos JE, Robaye B, Boeynaems JM, Beauwens R, Leipziger J. K+ secretion activated by luminal P2Y2 and P2Y4 receptors in mouse colon. J Physiol 564: 269–279, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol 289: F1304–F1312, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Ostrom RS, Gregorian C, Insel PA. Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. J Biol Chem 275: 11735–11739, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem 283: 36599–36607, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pochynyuk O, Bugaj V, Vandewalle A, Stockand JD. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am J Physiol Renal Physiol 294: F38–F46, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Praetorius HA, Frøkiær J, Leipziger J. Transepithelial pressure pulses induce nucleotide release in polarized MDCK cells. Am J Physiol Renal Physiol 288: F133–F141, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Praetorius HA, Frokiaer J, Nielsen S, Spring KR. Bending the primary cilium opens Ca2+-sensitive intermediate-conductance K+ channels in MDCK cells. J Membr Biol 191: 193–200, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal 5: 433–446, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Praetorius HA, Leipziger J. Fluid flow sensing and triggered nucleotide release in epithelia. J Physiol 586: 2669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Rouse D, Leite M, Suki WN. ATP inhibits the hydrosmotic effect of AVP in rabbit CCT: evidence for a nucleotide P2u receptor. Am J Physiol Renal Fluid Electrolyte Physiol 267: F289–F295, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Sausbier M, Matos JE, Sausbier U, Beranek G, Arntz C, Neuhuber W, Ruth P, Leipziger J. Distal colonic K+ secretion occurs via BK channels. J Am Soc Nephrol 17: 1275–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Schwiebert EM, Wallace DP, Braunstein GM, King SR, Peti-Peterdi J, Hanaoka K, Guggino WB, Guay-Woodford LM, Bell PD, Sullivan LP, Grantham JJ, Taylor AL. Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol Renal Physiol 282: F763–F775, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta 1615: 7–32, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol 20: 1724–1732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stiber JA, Zhang ZS, Burch J, Eu JP, Zhang S, Truskey GA, Seth M, Yamaguchi N, Meissner G, Shah R, Worley PF, Williams RS, Rosenberg PB. Mice lacking Homer 1 exhibit a skeletal myopathy characterized by abnormal transient receptor potential channel activity. Mol Cell Biol 28: 2637–2647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stoessel A, Himmerkus N, Bleich M, Bachmann S, Theilig F. Connexin 37 is localized in renal epithelia and responds to changes in dietary salt intake. Am J Physiol Renal Physiol 298: F216–F223, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Toma I, Bansal E, Meer EJ, Kang JJ, Vargas SL, Peti-Peterdi J. Connexin 40 and ATP-dependent intercellular calcium wave in renal glomerular endothelial cells. Am J Physiol Regul Integr Comp Physiol 294: R1769–R1776, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vekaria RM, Unwin RJ, Shirley DG. Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol 17: 1841–1847, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Wang B, Rothberg BS, Brenner R. Mechanism of beta4 subunit modulation of BK channels. J Gen Physiol 127: 449–465, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang X, Pluznick JL, Settles DC, Sansom SC. Association of VASP with TRPC4 in PKG-mediated inhibition of the store-operated calcium response in mesangial cells. Am J Physiol Renal Physiol 293: F1768–F1776, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Wildman SS, Marks J, Turner CM, Yew-Booth L, Peppiatt-Wildman CM, King BF, Shirley DG, Wang W, Unwin RJ. Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol 19: 731–742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilson PD, Hovater JS, Casey CC, Fortenberry JA, Schwiebert EM. ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys. J Am Soc Nephrol 10: 218–229, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Woda CB, Leite M, Jr, Rohatgi R, Satlin LM. Effects of luminal flow and nucleotides on [Ca2+]i in rabbit cortical collecting duct. Am J Physiol Renal Physiol 283: F437–F446, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Woo K, Dutta AK, Patel V, Kresge C, Feranchak AP. Fluid flow induces mechanosensitive ATP release, calcium signalling and Cl- transport in biliary epithelial cells through a PKCzeta-dependent pathway. J Physiol 586: 2779–2798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Woo K, Sathe M, Kresge C, Esser V, Ueno Y, Venter J, Glaser SS, Alpini G, Feranchak AP. Adenosine triphosphate release and purinergic (P2) receptor-mediated secretion in small and large mouse cholangiocytes. Hepatology 52: 1819–1828, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xu C, Rossetti S, Jiang L, Harris PC, Brown-Glaberman U, Wandinger-Ness A, Bacallao R, Alper SL. Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+ signaling. Am J Physiol Renal Physiol 292: F930–F945, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xu C, Shmukler BE, Nishimura K, Kaczmarek E, Rossetti S, Harris PC, Wandinger-Ness A, Bacallao RL, Alper SL. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol 296: F1464–F1476, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol 9: 945–953, 2007 [DOI] [PubMed] [Google Scholar]