Abstract
Past simulations of oxidative ATP metabolism in skeletal muscle have predicted that elimination of the creatine kinase (CK) reaction should result in dramatically faster oxygen consumption dynamics during transitions in ATP turnover rate. This hypothesis was investigated. Oxygen consumption of fast-twitch (FT) muscle isolated from wild-type (WT) and transgenic mice deficient in the myoplasmic (M) and mitochondrial (Mi) CK isoforms (MiM CK−/−) were measured at 20°C at rest and during electrical stimulation. MiM CK−/− muscle oxygen consumption activation kinetics during a step change in contraction rate were 30% faster than WT (time constant 53 ± 3 vs. 69 ± 4 s, respectively; mean ± SE, n = 8 and 6, respectively). MiM CK−/− muscle oxygen consumption deactivation kinetics were 380% faster than WT (time constant 74 ± 4 s vs. 264 ± 4 s, respectively). Next, the experiments were simulated using a computational model of the oxidative ATP metabolic network in FT muscle featuring ADP and Pi feedback control of mitochondrial respiration (J. A. L. Jeneson, J. P. Schmitz, N. A. van den Broek, N. A. van Riel, P. A. Hilbers, K. Nicolay, J. J. Prompers. Am J Physiol Endocrinol Metab 297: E774–E784, 2009) that was reparameterized for 20°C. Elimination of Pi control via clamping of the mitochondrial Pi concentration at 10 mM reproduced past simulation results of dramatically faster kinetics in CK−/− muscle, while inclusion of Pi control qualitatively explained the experimental observations. On this basis, it was concluded that previous studies of the CK-deficient FT muscle phenotype underestimated the contribution of Pi to mitochondrial respiratory control.
Keywords: skeletal muscle, mitochondria, metabolic control, computational modeling
mitochondria are the principal source of ATP synthetic flux supporting energy balance in eukaryotic cells (1). In skeletal muscle, metabolic control of this flux is generally thought to involve a feedback control design, whereby mitochondria sense changes in cellular ATP turnover rate via changes in the cytoplasmic concentrations of the ATP hydrolysis products ADP and Pi ([ADP] and [Pi], respectively) (5). Kushmerick concluded on the basis of in silico studies that dynamic and stationary states of energy balance in fast twitch (FT) muscle were best explained by a model of mitochondrial ATP synthesis dependent only on [ADP] when a second-order transduction function for this particular mitochondrial substrate was used (15). This specific magnitude of the macroscopic mitochondrial [ADP] sensitivity was first proposed on the basis of analyses of steady-state data obtained from isolated mitochondria and human muscle (14) and recently confirmed by analysis of dynamic data on in vivo oxidative ATP metabolism obtained in human muscle (12).
Knowledge of the precise respiratory control mechanism in various striated muscle phenotypes is vital to support ongoing efforts, such as those undertaken by International Union of Physiological Sciences Physiome Consortium, to integrate the wealth of information gathered from mammalian biological systems using computational modeling (10). Experimentally validated computational models of mitochondrial ATP synthetic function, including its metabolic regulation, constitute crucial building blocks in any such eukaryotic “silicon cell” or organ-modeling effort. Availability of these models will benefit understanding of the role and impact of mitochondrial dysfunction in human disease (e.g., 30, 31), as well as discovery of novel therapeutic regimens to rescue cell and thereby organ function (31).
The enzyme creatine kinase (CK) plays a pivotal role in the [ADP] respiratory control model. This enzyme catalyzes the reversible phosphotransfer reaction between creatine and ATP in excitable tissues, including muscle (20). As such, the CK reaction constitutes a metabolic capacitance buffering the ATP/ADP ratio during rapid transitions in ATP turnover rate, resulting in a strongly dampened rise of cytosolic [ADP], both in terms of kinetics, as well as amplitude, during dynamic changes in metabolic activity (20). Conversely, elimination of CK activity in muscle should result in dramatically faster oxygen consumption kinetics during work jumps (22). Specifically, computer simulations have shown that muscle oxygen consumption kinetics in CK-deficient muscle in that case should be at least one order of magnitude faster than in wild-type (WT) muscle (15, 22, 25, 28). Experimental validation of this quantitative prediction of the strict ADP respiratory control model has, however, been lacking. Moerland and Kushmerick found only twofold faster oxygen consumption deactivation kinetics following tetanic contractions in mouse muscles lacking CK-catalyzed phosphotransfer activity as a result of chronic feeding of β-guanidinopropionic acid (β-GPA), a kinetically inert creatine analog (21). No information was obtained on the magnitude of change in oxygen consumption activation kinetics. In this same experimental model of CK inactivation, progressive, but incomplete, depletion of the creatine pool size by β-GPA feeding in rats was found to result in a maximally four-fold faster mitochondrial response time measured as the time constant of PCr changes in response to muscle stimulation (19). As such, this raises the question of whether these results invalidate the strict ADP respiratory control model for FT muscle.
Here, this problem was further investigated. Specifically, the impact of CK inactivation on FT muscle oxygen consumption kinetics was studied using a combination of experimental studies and model simulations. First, we measured oxygen consumption dynamics during electrical stimulation in superfused FT hindlimb muscles isolated from WT and transgenic mice deficient in the myoplasmic (M) and mitochondrial (Mi) CK isoforms (MiM CK−/−), respectively. Computational modeling and simulation were next used to study the theoretical effect of CK knockout on oxygen consumption kinetics, if in addition to second-order mitochondrial [ADP] sensitivity, new information on multi-site Pi stimulation of oxidative ADP phosphorylation (3) is taken into account. Simulations ran under conditions of high, clamped Pi concentration were performed to investigate this hypothesis. On the basis of the results of the measurements and simulations, it is concluded that Pi plays a significant role in feedback respiratory control in CK-deficient FT muscle.
METHODS
In Vitro Studies
Animals and muscle preparation.
Adult wild-type C57BL/6 mice were used as controls. Double knockout mice, deficient in cytosolic muscle type and sarcomeric mitochondrial CK (MiM-CK−/−), were generated in the laboratory of Dr. B. Wieringa (Nijmegen University, The Netherlands) by gene targeting, as described previously (23). Offspring obtained in the breeding program was genotyped by PCR analysis on a regular basis. All experimental procedures were approved by the Committee on Animal Experiments of the University Medical Center Utrecht and complied with the principles of good laboratory animal care. Mice (age 21–30 days) were killed by cervical dislocation and the extensor digitorum longus (EDL), a muscle composed for 99% of fast-twitch fibers (11), was dissected from both hindlegs and ligated using 5.0 silk suture (Ethicon, Norderstedt, Germany). This particular age of the animals was chosen to obtain muscles with a diameter of 1 mm or less. Tendon-to-tendon and muscle lengths were measured in situ.
Simultaneous recording of muscle oxygen consumption and force development.
Measurements were conducted, as described elsewhere (26). Briefly, rates of oxygen consumption (nmol O2·g muscle weight−1·min−1) were determined at 20°C, using a high-resolution oxygraph (Oroboros, Innsbruck, Austria) with a stirring rate of 500 rpm. One end of the muscle was mounted in a custom-made oxygraph stopper, and the other end was connected to an adjustable Harvard Apparatus 60–2995 force transducer (Harvard Instruments Limited, Edenbridge, UK), which allowed the muscle to be stretched to yield its maximum twitch force. Muscles were stimulated via platinum-wire electrodes by supramaximal pulses (0.5 ms duration; 6–15 V) by a Grass S88 dual-channel stimulator (Astro-med, West Warwick, RI, USA), and force signals were digitized at 1,000 Hz sampling rate. The measurement protocol started with 10 min of rest to record basal respiratory flux followed by 5 min at 0.5, 1.0, or 2.0 Hz. Episodes of muscle stimulation were separated by 10-min intervals, during which nonstimulated basal respiration of the muscle was recorded. Respiratory fluxes were corrected for chamber oxygen leak by measuring the exponential decay of Po2 in the oxygraph chamber at 20°C containing Ringer solution in mM: 116 NaCl, 25.3 NaHCO3, 4.6 KCl, 2.5 CaCl2, 1.16 KH2PO4, 1.16 MgSO4, at pH 7.4. Oxygen solubility of 5% CO2-95% O2 equilibrated Ringer medium was calculated according to Haller et al. (8) and oxygen electrode response times were constant at ∼4 s (tested prior to each experiment).
All measurements of muscle respiration were performed as randomized paired experiments with simultaneous measurement of one WT and one MiM-CK−/− muscle in a dual-chamber setup. All experiments were started after 30 min of equilibration. To avoid oxygen limitation of contraction, all measurements were performed at 20°C above a Po2 of 450 Torr. Chamber volume (∼5.2 ml) and muscle weight (blotted and tendon free) were determined after each experiment. Muscle diameters were estimated from muscle weight and length assuming 1 g of muscle has a volume of 0.7 ml.
Data acquisition, analysis, and statistics.
Oxygraph and force transducer data acquisition was performed with LabView software (National Instruments, Woerden, The Netherlands). Absolute muscle respiratory rates and time constants were calculated using Origin 6.0 (Microcal Software, Northampton, MA). Reported data are presented as arithmetic means ± SE. Statistical analyses were performed using a Student's unpaired t-test. Differences between MiM-CK−/− and WT muscle were considered significant if P < 0.05.
In Silico Studies
Simulations of radial intramuscular oxygen diffusion.
Simulations of radial intramuscular oxygen diffusion at 20°C for cylinders of diameters between 0.5 and 1 mm were performed using a program written in Borland Delphi code. Partial differential equations were numerically solved using a finite difference algorithm. We used the Grote and Thews value for the oxygen diffusion constant in striated muscle at 20°C of 1×10−6 cm·s−1 (7) that was previously used in a similar analysis by Mast and Elzinga (18). Intramuscular oxygen diffusion delay time constants of the measured oxygen consumption rate in the chamber were estimated for muscle diameters between 0.5 and 1 mm by curve-fitting of mono- and double-exponential functions to computed time courses of the peripheral oxygen consumption rate following a step change in intramuscular oxygen consumption rate of 180 nmol O2·g muscle−1·min−1. The time course was best described (not shown) by a double-exponential function of the form in Eq. 1:
| (1) |
An apparent diffusion delay time constant, TCdiffusion delay, was then calculated according to Eq. 2:
| (2) |
Simulations of oxygen consumption kinetics in WT and CK−/− muscle.
An adapted version (12) of the computational model of skeletal muscle oxidative ATP metabolism by Wu et al. (30) was used as a platform for all simulations. The model features a detailed biophysical model of mitochondrial ATP synthesis incorporating metabolic feedback regulation by ADP and Pi and is parameterized for 37°C (12). For the simulations of the in vitro experiments at 20°C, the model was reparameterized using a temperature scaling factor (TSF) to adjust the activity of selected enzymes in the model (Table 1). These enzymes were selected on basis of their Kolmogorov-Smirnov score in a previous parameter sensitivity analysis of the model with respect to maximal mitochondrial ATP synthesis flux (12). The TSF was calculated by comparing the measured oxygen cost per twitch in the experiments (see results below) to the reported in vivo ATP cost per twitch for WT mouse hindlimb muscle (22) using a Po2 ratio of 6 (30), yielding a TSF of 10. This corresponds to a Q10 for muscle metabolic rate of roughly 3.9, which is some twofold higher than typically found experimentally (13). Basal proton leak was then adjusted to 40% of total proton flux in resting conditions (4). The modified model parameters and their adjusted value are listed in Table 1. In the CK−/− simulations, the CK activity was set at 0 and the mitochondrial volume was increased 1.4-fold, according to measurements by Steeghs et al. (24). The same oxygen consumption cost per twitch was maintained on the basis of previous findings in MiM CK−/− muscles (26). In the simulations of mitochondrial activation, the ATPase rate was varied between 0.005 and 0.05 mM/s (step size 0.001 mM/s). The activation kinetics for oxygen consumption were quantified by fitting of a biexponential function to the simulated data using Eq. 1. A time constant for oxygen consumption activation, TCactivation, was then calculated using Eq 2. The time at which oxygen consumption attained 95% of its steady-state value (95AT) was then computed as 3 × TCactivation. The recovery kinetics of oxygen consumption were quantified as the time at which oxygen consumption flux had recovered to within 5% of the resting rate (95% recovery time; 95RT). The model was implemented in MATLAB (ver. 7.3, Mathworks, Natick, MA).
Table 1.
Model reparameterization for 20°C
| Parameter | Value | Value at 37°Ca | Unit |
|---|---|---|---|
| X_Hle (proton leak activity) | 5 | 200 | moles·s−1·M−1·mV−1· |
| (l mito)−1 | |||
| Resting ATPase activity | 0.0015e-3 | 0.015e-3 | moles/s |
| X_DH(dehydrogenase activity) | 0.026 | 0.26 | mol·s−1·M−1·(l mito)−1 |
| X_C1 (complex 1 activity) | 441 | 4405 | mol·s−1·M−2·(l mito)−1 |
| X_C3 (complex 3 activity) | 0.49 | 4.89 | mol·s−1·M−3/2·(l mito)−1 |
| X_C4 (complex 4 activity) | 6.8e-6 | 6.8e-5 | mol·s−1·M−1·(l mito)−1 |
| X_F1 (F1F0ATPase activity) | 100 | 1000 | mol·s−1·M−1·(l mito)−1 |
| X_ANT (ANT actitivity) | 0.0041 | 0.041 | moles·s−1·(l mito)−1 |
Ref. 12.
RESULTS
In Vitro Studies
Anatomical and mechanical characteristics of WT vs. MiM-CK−/− EDL muscle.
EDL muscle weights were significantly lower in MiM-CK−/− than WT mice [6.1 ± 0.5 mg (n = 6) vs. 10.9 ± 0.4 mg (n = 7), respectively]. CK−/− EDL muscle diameters estimated from muscle weight and length were 25% smaller than WT (0.3 mm vs. 0.4 mm, respectively). Twitch force per milligram of muscle wet-weight was the same for MiM-CK−/− and WT EDL muscles [2.9 ± 0.3 N/g (n = 7) vs. 3.4 ± 0.2 N/g (n = 6), respectively]. During serial stimulation at 1.0, no difference in fatigue rate was found for MiM-CK−/− muscles compared with WT over the first 20 s of stimulation (data not shown). After 250 s into the serial stimulation protocol, MiM-CK−/− EDL muscles exhibited significantly less fatigue than WT [86.1 ± 3.2% (n = 5) vs. 76.9 ± 2.3% (n = 5) of initial peak force, respectively]. These results agreed well with previous observations on the impact of MiM-CK knockout on the mechanical performance of mouse hindlimb muscle (23, 26). The reader is referred to a previous study for a more detailed description of their mechanical behavior (26). However, the improved fatigue resistance of the CK−/− FT muscle phenotype is particularly notable in the context of the present focus on the phenotypic energetics with regard to the predicted twofold larger drop of the free energy of ATP hydrolysis during serial twitch contractions compared with WT muscle (see Fig. 3 below). Similar surprisingly preserved mechanical performance against a highly reduced capacity for ATP free energy homeostasis was previously reported by Hancock et al. (9) for adenylate kinase 1-deficient mouse FT muscle. The interested reader is referred to the latter paper for an in-depth discussion of the relation between ATP free energy changes, calcium homeostasis, and muscle contractile function.
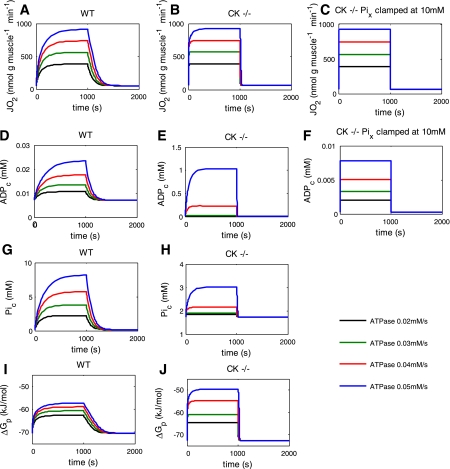
Fig. 3.
Computed timecourses of oxygen consumption (A–C), the cytoplasmic ADP (D–F), and Pi (G, H) concentrations, and the free energy of ATP hydrolysis (ΔGp; I, J) during simulated rest-stimulation-recovery experiments at four incremental ATP turnover rates in the network (0.02, 0.03, 0.04, and 0.05 mM/s, respectively) for three model configurations: 1) WT muscle (Fig. 3, A and D, G, and I); 2) CK−/− muscle (Fig. 3, B, E, H, and J); 3) CK−/− muscle with a constant intramitochondrial Pi concentration ([Pix]) of 10 mM (Fig. 3, C and F).
Oxygen consumption dynamics of WT and MiM-CK−/− muscle.
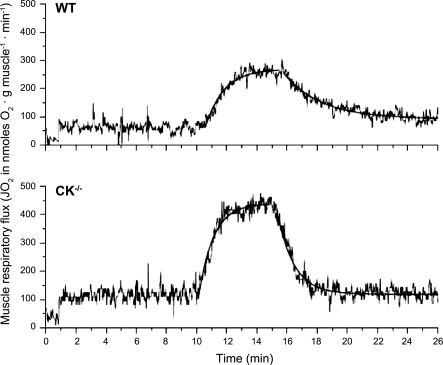
Figure 1 shows typical recordings of net changes in muscle oxygen consumption corrected for chamber leak rate from WT (top) and MiM-CK−/− (bottom) EDL muscle during a rest-stimulation-recovery experiment. At t = 10 min, oxygen consumption was activated by 5-min serial stimulation at 2 Hz. After cessation of electrical stimulation, the oxygen consumption rate returned to basal rate in both muscles. Inspection of the recordings from WT vs. MiM-CK−/− muscle identifies three main features: 1) a higher basal and stimulated steady-state oxygen consumption rate in MiM-CK−/− muscle compared with WT, 2) a similar rate of rise of oxygen consumption activation in both muscle phenotypes after the electrical stimulation was turned on, and 3) a considerably faster rate of return of oxygen consumption to basal rate in MiM-CK−/− muscle compared with WT after the electrical stimulation was turned off (Fig. 1). Also, it appeared that the recovery of oxygen consumption to baseline in WT more closely followed an exponential time course than in the MiM-CK−/− muscle phenotype (Fig. 1).
Fig. 1.
Typical results for the respiratory flux response during a rest-stimulation-recovery experiment for wild-type (WT) (top) and mitochondrial (Mi) creatine kinase isoforms (MiM-CK−/−) (bottom) extensor digitorum longus (EDL) muscle. The stimulation frequency was 2 Hz. Monoexponential best fits of the stimulation frequency transients from rest-to-active (0 to 2 Hz) and active-to-rest (2 to 0 Hz) are indicated as thickened line parts.
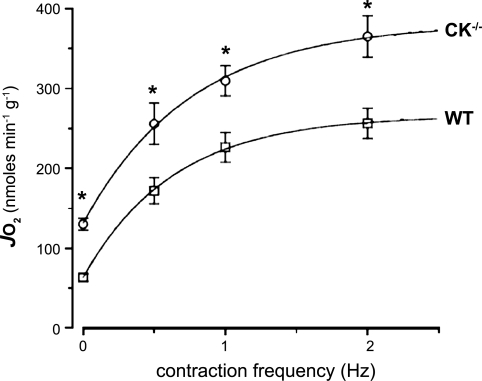
These qualitative trends in the dynamics of oxygen consumption activation and deactivation during step changes in ATP turnover rate for each muscle phenotype were objectified by quantitative analysis. Mono- and double exponential functions were fitted to the recorded net changes in muscle oxygen consumption (Fig. 1, solid lines). Statistically, no improvement of fit was obtained by using any higher order than one (not shown). Basal oxygen consumption in MiM-CK−/− muscle was found to be twofold higher than WT [134 ± 11 (n = 7) vs. 66 ± 9 (n = 6) nmol O2·min−1·g muscle−1, respectively; means ± SE]. The maximal steady-state oxygen consumption rate was estimated for each muscle phenotype by fitting a monoexponential function to the variation of steady-state oxygen consumption rate with contraction frequency, yielding estimates of 381 ± 12 and 266 ± 4 nmol O2·min−1·g muscle−1 for MiM-CK−/− and WT, respectively (means ± SE of regression; Fig. 2). These values were not significantly different from the highest measured steady-state oxygen consumption rates [365 ± 26 and 256 ± 19 nmol O2·min−1·g muscle−1 for MiM-CK−/− (n = 6) and WT (n = 7), respectively (2 Hz; means ± SE)] (Fig. 2). The oxygen cost per twitch at the experimental temperature of 20°C, as determined from the serial stimulation experiments at 1 Hz in WT muscle, was 4 nmol/g muscle. Assuming a Po2 ratio of 6, this value was 10-fold lower than the ATP cost per twitch for mouse hindlimb muscle measured in vivo (22).
Fig. 2.
Steady-state oxygen consumption (in nmol·min−1·g−1) of WT (n = 8) vs. MiM-CK−/− (n = 6) EDL muscle at rest and during serial stimulation at 0.5, 1, and 2 Hz, respectively. Values are expressed as means ± SE. *P < 0.05. The solid lines represent the fits of a monoexponential function to the data. Regression equations: WT: JO2 = 63.1 + 202.7 [1 − exp (−1.6/x)]; MiM-CK−/−: JO2 = 131.1 + 249.9 [1 − exp (−1.3/x)].
The mean values ± SE of the oxygen consumption activation and deactivation time constants (τon and τoff, respectively) for WT and MiM-CK−/− EDL muscles during stimulation at 1 and 2 Hz, respectively, are listed in Table 2. On average, the kinetics of activation of oxygen consumption in MiM-CK−/− muscle at these contraction frequencies were 30% faster than WT (Table 2). The kinetics of deactivation of oxygen consumption in WT were 380% slower than activation of oxygen consumption (Table 2). In MiM-CK−/− muscle, the oxygen consumption deactivation kinetics were 30% slower than the activation kinetics (Table 2).
Table 2.
Time constants of oxygen consumption activation and deactivation during work jumps and their ratio at two stimulation frequencies for WT and MiM-CK−/− EDL muscles
| Phenotype | Stimulation Frequency, Hz | τon, s | τoff, s | τon/τon |
|---|---|---|---|---|
| WT | 1 | 72.1 ± 5.1 | 255.5 ± 9.1 | 0.3 |
| (n = 8) | (n = 8) | |||
| 2 | 69.4 ± 3.6 | 264.4 ± 4.0 | 0.3 | |
| (n = 8) | (n = 8) | |||
| MiM-CK−/− | 1 | 53.1 ± 3.0* | 68.4 ± 4.6* | 0.8 |
| (n = 6) | (n = 6) | |||
| 2 | 54.0 ± 2.9* | 74.4 ± 4.0* | 0.7 | |
| (n = 5) | (n = 6) |
Listed values are expressed as means ± SE. τon, oxygen consumption activation; τoff, deactivation; MiM-CK, myoplasmic and mitochondrial creatine kinase isoform; EDL, extensor digitorum longus.
P < 0.05 compared with wild type (WT).
In Silico Studies
Simulations of radial intramuscular oxygen diffusion.
Analysis of the computed timecourse of peripherally measured oxygen consumption in response to a step change in intramuscular oxygen consumption revealed that radial intramuscular oxygen diffusion introduced a significant delay (i.e., on the order of tens of seconds) in the polarographic detection of changes in muscle oxygen consumption rate (data not shown). Furthermore, the analysis showed the expected steep dependence of the magnitude of this delay on muscle diameter (data not shown). Specifically, the intramuscular diffusion delay time constant for a cylindrical muscle of radius 0.5 mm was 35 s. For a 20% smaller muscle radius of 0.4 mm, the diffusion delay time constant dropped 40% to 21 s.
Simulations of oxygen consumption kinetics in WT and CK−/− muscle.
The computed resting values of selected metabolic state variables of the model at the imposed basal ATP turnover rate of 0.001 mM/s are given in Table 3 for the WT and CK knockout model configurations. Elimination of CK activity concomitant with a 40% increase in mitochondrial volume to simulate the CK−/− muscle phenotype (24) resulted in a higher resting [Pi] and basal oxygen consumption (Table 3).
Table 3.
Computed resting values of selected state variables for the WT and CK knockout model phenotypes and their ratios (T = 20°C)
| WT | CK−/− | CK−/−/WT | |
|---|---|---|---|
| JO2 (nmoles g muscle−1 min−1) | 50a | 62 | 1.2 |
| [PCr], mM | 37.4 | 35.4b | 0.9 |
| [Pi]i, mM | 0.2 | 1.7 | 8.5 |
| [ADP]i, μM | 7 | 0.4 | 0.1 |
The basal ATP turnover rate was set at 0.001 mM/s. The subscript i denotes the cytosolic compartment. PCr, phosphocreatine.
Initial condition, measured value.
Initial condition, value taken from Ref. 24.
Figure 3 shows the predicted time courses of oxygen consumption (Fig. 3, A–C), the cytoplasmic ADP concentration ([ADP]; Fig. 3, D–F), the cytoplasmic Pi concentration ([Pi]; Fig. 3, G and H), and the free energy of ATP hydrolysis (ΔGp; Fig. 3, I and J) during the simulated rest-contraction-recovery experiments for four incremental ATP turnover rates in three different model phenotypes: 1) WT muscle phenotype (Fig. 3, A, D, G, and I); 2) CK−/− muscle phenotype (Fig. 3, B, E, H, and J); 3) CK−/− muscle phenotype with a constant intramitochondrial Pi concentration (Pix) of 10 mM (Fig. 3, C and F). The oxygen consumption flux in the simulations attained values that were of the same order of magnitude as the measured oxygen consumption flux in contracting WT and CK−/− muscles (Fig. 3, A and B vs. Figs. 1 and 2, respectively). The free energy of ATP hydrolysis was predicted to drop maximally 13 kJ/mol during contraction for the WT phenotype over this range of ATP turnover rates (Fig. 3I), while for the CK−/− phenotype, the predicted maximal drop was nearly twofold bigger (23 kJ/mol; Fig. 3J). The latter was due to the large, unbuffered [ADP] accumulation in this phenotype (Fig. 3E), even causing cytoplasmic ATP to decrease by as much as 1 mM at the highest ATP turnover rate.
Over this dynamic range of metabolic activity, a dependence of the network response kinetics on the ATP turnover rate in the network was clearly discernable for both muscle phenotypes (Fig. 3). Notably, in the WT case, this rate dependence appeared to be continual, whereas in the CK−/− case, there appeared to be a threshold (Fig. 3, A, D, G and I vs. B, E, H, and J, respectively). In addition, in the WT case, the mitochondrial activation and deactivation kinetics both approximately followed exponential functions (e.g., Fig. 3A), whereas in the CK−/− case, the deactivation kinetics showed a more complex behavior (Fig. 3B). The CK−/− simulations performed under the condition of constant, high intramitochondrial Pi showed a near-instantaneous response of the network to changes in ATP turnover rate in the network (Fig. 3, C and F).
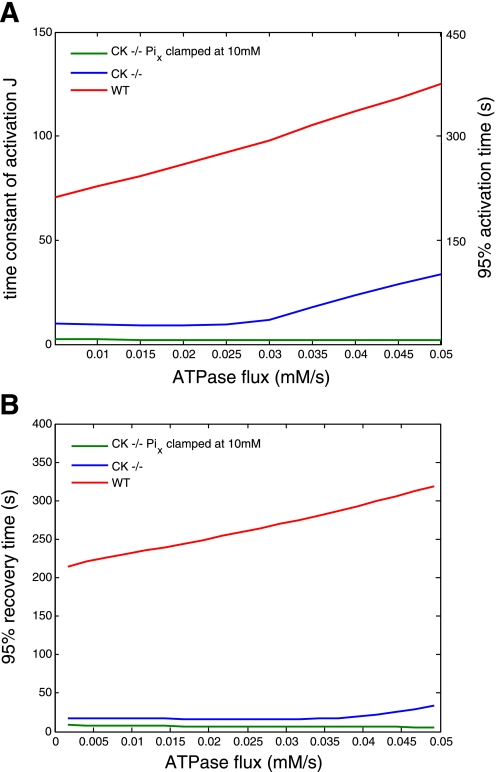
These qualitative trends in the simulation results were objectified by computation of the activation time constant (TCactivation) and 95% activation time (95AT), and 95% recovery time (95RT) of oxygen consumption (see methods) as a function of ATP turnover rate in the network for each model phenotype (Fig. 4, A and B, respectively). For the WT model phenotype, TCactivation (and thereby 95AT) (Fig. 4A) and 95RT (Fig. 4B) both increased quasi-linearly from 70 to 125 s, 210 to 375 s and 270 to 320 s, respectively, as the ATP turnover rate in the network was increased 10-fold from 0.005 to 0.05 mM/s. As such, the mitochondrial activation and deactivation kinetics for this particular model phenotype were nearly symmetrical over the entire studied range of ATP turnover rates. For the CK−/− model phenotype, this was only the case for ATP turnover rates below 0.03 mM/s. Specifically, in this range of metabolic rates, TCactivation, 95AT and 95RT in CK−/− were constant (9 s, 27 s, and 17 s, respectively) and 10- to 15-fold faster than WT (Fig. 4, A and B). At higher ATP turnover rates, TCactivation, 95AT and 95RT in CK−/− likewise increased quasi-linearly with ATP turnover rate to maximal values of 34 s, 102 s, and 34 s, respectively, at 0.05 mM/s (Fig. 4, A and B). In this particular velocity domain, the ratio of CK−/− TCactivation/WT TCactivation, therefore, decreased from 10 to only 4 (Fig. 4A). CK−/− 95RT remained up to 10-fold faster than WT 95RT (Fig. 4B). For the CK−/− simulations run under conditions of clamped, saturating mitochondrial Pi concentration, TCactivation, 95AT, and 95RT were constant and very fast (i.e., 2 s, 6 s, and 7 s, respectively) over the studied range of ATP turnover rates in the network (Fig. 4, A and B).
Fig. 4.
Computed oxygen consumption activation time constant and 95% activation time (A) and 95% recovery time (B) as a function of the ATP turnover rate in the network for the three model configurations: 1) WT, 2) CK−/−, and 3) CK−/− with [Pix] clamped at 10 mM. The kinetic parameters were derived from the computed time course of the oxygen consumption rate for ATP turnover rates between 0.005 and 0.05 mM for each model phenotype as described in methods.
A final set of simulations were run for the WT model configuration in which the creatine pool size, a thermodynamic model parameter, was altered. The aim of these simulations was to investigate previous findings of progressively faster PCr dynamics in rat FT muscle during cumulative β-GPA feeding (19). It was found that progressive, but incomplete, creatine pool size depletion in and by itself explained the experimental observations reported in that study (not shown) in agreement with Wu et al. (31).
DISCUSSION
Experimental Model
All experiments were conducted in isolated FT muscle as described in methods. It would have been of interest to compare the impact of CK knockout on mitochondrial kinetics in this skeletal muscle phenotype to the impact in the slow-twitch (ST) muscle phenotype. This was, however, not possible first and foremost because no skeletal muscle was available in the mouse that is both primarily composed of ST fibers, as well as suitable for ex vivo oxygen consumption measurements under superfused consitions. The soleus muscle that was studied in a previous investigation of the CK double-knockout phenotype (26), for example, is composed only for 50% of ST fibers (26) and, therefore, does not represent a suitable experimental model for ST muscle. A particular complication in the present study was the specific need to compare experimental results to quantitative model simulations. For the soleus muscle, this would have required parameterization and running of two separate mitochondrial models for the FT and ST fiber pools, respectively, that make up the soleus muscle. Moreover, there is evidence that respiratory control in ST muscle involves both feedback, as well as feedforward regulatory mechanisms (16). The latter control mechanism is not implemented in the model that was used as a platform for the simulations (12). On these grounds, it was selected to study only FT muscle.
The rate of mitochondrial oxygen consumption in the experiments was varied via the ATP turnover rate controlled by electrical stimulation of the muscle. Since the main focus of the investigation was the characterization of the mitochondrial response time in WT vs. CK-deficient FT muscles, it was important to minimize any anaerobic glycogenolytic ATP synthesis flux. A related concern was the fact that the latter may cause metabolic acidosis that, on the one hand, may negatively affect mitochondrial deactivation time (29) and, on the other hand, complicate any comparison of the experimental observations to model simulations, since the particular computational model of mitochondrial metabolism does not account for any significant extramitochondrial pH changes (12, 30). To limit any anaerobic glycogenolytic flux in the experiments, we first of all selected to apply serial twitch contractions rather than tetanic contractions. Second, the imposed frequency of twitch contraction was limited to 2 Hz. This upper limit was chosen on the basis of two prior observations in our previous studies in isolated superfused WT mouse EDL muscles at 20°C (J. A. L. Jeneson, R. W. Wiseman, and M. J. Kushmerick, unpublished observations; 11, 26) together with the reported observation that mechanical performance at this temperature is highly sensitive to metabolic acidosis (29). Firstly, maximal oxygen consumption flux was typically attained during serial stimulation at 2 Hz, with 1 Hz eliciting ∼80% of maximal flux. Secondly, the decline of mechanical performance measured by the drop in tension-time-integral was only 20% over 5 min of serial stimulation at 2 Hz (11, 26). In CK-deficient EDL muscle, mechanical performance at 2 Hz declined even less (26; this study). On this basis, we concluded that any anaerobic glycogenolytic flux and associated metabolic acidosis in the present experiments did not bias the main conclusion of the investigation. However, as discussed below, if at all, it may have contributed to the observed, ill-understood slow mitochondrial deactivation time in the WT phenotype compared with the CK−/− phenotype.
Muscle oxygen consumption was measured in vitro using an electrode that continuously monitored the oxygen content of the muscle bath. Therefore, a concern was that the finding of a much smaller kinetic effect of CK knockout than previously predicted was the trivial result of dynamic range limitations of this indirect assay of muscle respiration, due to the fact that the measured muscle oxygen consumption kinetics were dominated by intramuscular oxygen diffusion. Two lines of evidence indicate that this was not the case. First, we previously found that τon for mouse EDL muscles with a diameter of 0.9 mm was not significantly different from τon for muscles with a diameter of 0.6 mm (i.e., 86 vs. 82 s, respectively; WT Swiss-Webster mice; J. A. L. Jeneson, R. W. Wiseman, and M. J. Kushmerick, unpublished data). These time constants were measured using both the same setup, as well as experimental conditions, including temperature. For this very reason, mice aged between 21 and 30 days yielding WT muscle diameters of less than 1 mm rather than fully adult mice were used in the study. Second, the simulations of intramuscular oxygen diffusion that were performed in the present study indicated that for the typical WT EDL dimensions studied here (i.e., a diameter of 0.8 mm) intramuscular oxygen diffusion introduced a delay of 21 s in the time constant of changes in muscle bath oxygen content (see results). Together with a chamber mixing time constant of 4–6 s, this yielded a lower detection limit of 27 s for the activation time constant, τon, in WT muscle. Given the measured τon in WT muscle of 69–72 s (Table 2), the dynamical range of the assay to detect any acceleration of mitochondrial activation in the MiM-CK knockout phenotype was, therefore, on the order of 50 s (= τon_WT minus τdiffusion). In fact, the actual dynamic range of the assay was even larger since the diameter of the MiM-CK−/− muscles (and, therefore, also τdiffusion, albeit not proportionally) was 1.3-fold smaller than WT. The measured acceleration of oxygen consumption activation kinetics in MiM-CK−/− muscle was much less than 50 s (i.e., a mere 14 s; τon, Table 2). On the basis of both considerations, we concluded that the main experimental result of the investigation was not biased by intramuscular oxygen diffusion delay in the indirect assay of muscle respiration.
Computational Model
An adapted version (12) of the mathematical model of skeletal muscle oxidative ATP metabolism reported by Wu et al. (30) was used as the computational platform for the simulations. In this model, contemporary knowledge of mitochondrial sensing and transduction of ATP turnover changes in the cell via [ADP] and [Pi] feedback is implemented either mechanistically or phenomenologically (12). Any alternative respiratory control mechanisms, e.g., sensing and transduction to the mitochondrial oxidative phosphorylation pathway of cytosolic [Ca2+] changes (1), are, however, not implemented in this model. Second, the model was parameterized to test its performance at physiological temperature, i.e., 37°C (12). To simulate the experiments that were performed here at 20°C in isolated superfused mouse FT muscles, the model was reparameterized for this lower temperature as described in methods. The predicted basal concentrations of ADP and Pi for the reparameterized WT model configuration (Table 3) were in reasonable agreement with the measured values in WT mouse EDL muscle at 20°C (17). The model predictions of basal [Pi] and oxygen consumption for the CK−/− model phenotype were qualitatively likewise in agreement with the measured elevated values in hindlimb muscle of MiM-CK−/− mice (Ref. 23 and Fig. 2, respectively). The exact magnitude of elevation of resting oxygen consumption in this particular phenotype was, however, underestimated [125% (Table 3) vs. 200% (Fig. 2), respectively]. Therefore, the comparison of the experiment to model prediction in this study should be viewed within these limitations.
Experiment vs. Model
Agreement between experiment and model predictions with respect to differences in oxygen consumption dynamics between the CK−/− and WT FT muscle phenotypes was found on two points, including the central issue of the order of magnitude of the kinetic effect of CK knockout. Specifically, both in vitro as well as in silico, only a small effect was found over the tested range of respiration rates (Table 2 and Fig. 4, respectively). In addition, both experiment and simulation showed that the difference in oxygen consumption kinetics between the CK−/− and WT FT muscle phenotypes was larger with respect to mitochondrial deactivation than activation (Table 2 and Fig. 4, respectively). Less agreement between experiment and model was, however, found with respect to the relative speed of oxygen consumption activation vs. deactivation in individual FT muscle phenotypes. Specifically, τon in WT muscle was fourfold faster than τoff (Fig. 2 and Table 2), whereas the model predicted that these kinetic parameters should have the same value in this phenotype (Fig. 4). Likewise, Meyer (19) previously found faster τon than τoff for PCr dynamics in FT muscle of β-GPA-fed rats. It is unclear what caused this disagreement between experiment and simulation. A first explanation may be the different level of control of the ATP turnover rate between experiment and simulation. In the latter, this state variable immediately returned to basal rate at the onset of the recovery period, while in the former the exact ATP turnover rate during recovery was unknown. This was due to the fact that only the contractile ATP turnover rate was carefully controlled (i.e., by electrical stimulation) but not the ATP turnover rate associated with the subsequent relaxation process (i.e., cation pump activity). Any prolonged activity of the latter process could have contributed to the measured prolonged deactivation time compared with the simulations. However, no data were available to corroborate this explanation. An alternative explanation may be that significant metabolic acidosis did develop in WT muscles during the 300-s repetitive twitch contractions at 1 and 2 Hz. If so, it would then be likely that the process of mitochondrial deactivation rather than activation would be affected, in agreement with what was observed. Slowing down of oxidative ATP-driven energetic recovery of muscle following contractions, even in mild metabolic acidosis, has been commonly observed in vivo (27). Specifically, a phenomenological linear relation between recovery time and end-contraction intramuscular pH was found (27). Extrapolating this result to the lower temperature at which the present experiments were conducted, one would expect a longer τoff at 2 Hz than at 1 Hz. These values were, however, identical (Table 2), thus weakening the relevance of this metabolic acidosis explanation.
In CK−/− FT muscle τon was likewise faster than τoff, albeit less pronounced than in WT muscle (Table 2). For this phenotype, the model predicted that oxygen consumption activation should be significantly slower than deactivation (Fig. 4). The latter was a corollary of the model prediction that, in contrast to WT, oxygen consumption deactivation in this phenotype does not follow any exponential time course (Fig. 3B). Nonlinear curve-fitting analysis of the experimental recordings from MiM CK−/− FT muscle did not confirm this prediction (Fig. 1). However, this analysis was hampered by the high noise level of the chamber oxygen content recordings (Fig. 1). Finally, agreement between experiment and model with regard to any rate dependence of muscle oxygen consumption dynamics could not be verified (Fig. 4). This was due to the fact that the muscle oxygen consumption rates attained during serial contraction at 1 vs. 2 Hz were not significantly different in either phenotype (Fig. 2). Steady-state respiration rates at 0.5 Hz were significantly lower than at 1 or 2 Hz in both phenotypes (Fig. 2). However, any significant estimation of the mitochondrial response time constants at this low stimulation frequency was prevented by the high noise level of the chamber oxygen recordings referred to above.
Respiratory Control in CK-Deficient FT Muscle
Inherent limitations on both the accuracy of the experimental model and the chemical assay, as well as the computational model and its parameterization, prevented any firm conclusion to be drawn here on the complete set of respiratory control mechanisms that are active in CK-deficient FT muscle. For example, it was not possible to discriminate whether the lack of agreement between experiment and model regarding the relative speed of muscle oxygen consumption activation vs. deactivation in the CK−/− phenotype was due to either too much noise in the chamber oxygen recordings, preventing accurate characterization of the actual recovery kinetics, or an unrealistic computational model, giving rise to a faulty prediction. However, the investigation did yield enough evidence to conclude that inclusion of Pi stimulation of oxidative phosphorylation in a feedback control model of FT muscle respiration featuring macroscopic mitochondrial ADP ultrasensitivity was sufficient to account for the main differences between the measured oxygen consumption dynamics in MiM CK−/− vs. WT FT muscle. This can be explained as follows. In muscle, CK activity results in a relative amplification of any increase in [Pi] during contraction due to the fact that PCr is converted into Pi on a mol-to-mol basis, while any increase in [ADP] is dampened by two orders of magnitude (3). Elimination of CK activity reverses this modulation of respiratory control signal resulting in amplified [ADP] and dampened [Pi] changes during contraction. Dahlstedt et al. (6), for example, specifically exploited the latter metabolic effect of CK knockout to study the role of Pi accumulation in short-term muscle fatigue. Previous numerical studies of the impact of CK knockout on muscle respiration dynamics employing strict ADP feedback control formalisms either overlooked or neglected this effect of CK knockout on [Pi] control in muscle (15, 22, 25, 28). In the present study, this point was demonstrated by the CK−/− simulations run at a constant, saturating intramitochondrial Pi concentration. This particular condition transformed the combined ADP and Pi mode of the feedback respiratory control regime implemented in the model into the strict ADP control mode used in previous studies. Indeed, the model then reproduced the presently falsified past prediction of 1 to 2 orders of magnitude faster mitochondrial activation and deactivation kinetics compared with WT. The variation of steady-state oxygen consumption rate with twitch contraction frequency in the CK deficient vs. WT FT muscle phenotype (Fig. 2) may be viewed as a further illustration of the previous point. Underlying this relation is the increase in cytoplasmic [ADP] and [Pi] from increased ATP turnover during cyclic muscle contraction and relaxation. The magnitude of change in [ADP] and [Pi], respectively, at a particular stimulation frequency should differ tremendously between the WT and CK-deficient FT muscle phenotypes as shown by the simulations in Fig. 3. Yet, in both FT muscle phenotypes, the increase of oxygen consumption above basal rate with increasing contraction frequency more or less followed the same phenomenological monoexponential function (Fig. 2). This is irreconcilable with a strict ADP respiratory control model for both FT muscle phenotypes. Specifically, a previous analysis of metabolic control of mitochondrial ATP synthesis flux in muscle that did not consider any Pi control of respiration, concluded that flux control shifts from the contractile ATPase to mitochondria at high [ADP] (13). In view of the unchanged ATP cost per twitch in WT and CK−/− FT muscle (26) together with the predicted large differences in [ADP] and [Pi] dynamics during contraction between the phenotypes shown in Fig. 3, this would entail that increasing the contraction frequency between 0 and 2 Hz should result in a differential increase in oxygen consumption rate in CK−/− vs. WT FT muscle over this range of contraction frequencies. The fact that this flux instead varied in a similar manner with contraction frequency in both phenotypes (Fig. 2), therefore, lends further evidence that respiratory control in the CK−/− phenotype involves more than strict ADP control and includes at least Pi control.
Perspectives and Significance
The results and conclusions of the present investigation of the CK knockout FT muscle phenotype call for amendment of current understanding of respiratory control in rodent WT skeletal muscle. Specifically, it is hypothesized that Pi may contribute significantly to mitochondrial control and energy balance in this particular muscle phenotype at low respiration rates, including resting state. In view of the high ATP cost per twitch in this particular muscle phenotype (22), however, CK activity may well relieve any Pi limitation of respiration during active contraction resulting in an apparent dominant ADP control mode at moderate to high respiration rates. As for the ST muscle phenotype, it has been well documented that resting [Pi] in rodents is up to 10-fold higher than in FT muscle (16). As such, amendment of the feedback respiratory control hypothesis to include Pi regulation is likely specific only for the FT phenotype. Practical implementation of these conclusions in computational models of mitochondrial ATP synthetic function for FT vs. ST muscle phenotypes, however, do not require any use of alternative kinetic formulations of mitochondrial [ADP] and [Pi] sensing for each muscle phenotype. Proper muscle phenotype-specific model parameterization, including resting [ADP] and [Pi], as well as creatine and adenine nucleotide poolsizes (16), may be sufficient to capture muscle phenotypic mitochondrial sensitivities to [ADP] and [Pi] changes, underlying energy balance during contractile work. However, there is evidence that respiratory control in ST muscle fibers may also involve a feedforward regulatory mechanism (16).
ACKNOWLEDGMENTS
The authors acknowledge the generous support of Dr. B. Wieringa in supplying the transgenic mice and Dr. R. K. Porter in providing the Oroboros oxygraph apparatus. Also, the reviewers of the original manuscript are acknowledged and thanked for their valuable suggestions. This research was supported by research grants from the Council for Chemical Sciences of the Netherlands Organization for Scientific Research and the National Institutes of Health (Grant HL-072011). This work was carried out within the research program of the Netherlands Consortium for Systems Biology, which is part of the Netherlands Genomics Initiative/Netherlands Organization for Scientific Research.
Present address for J. A. L. Jeneson: Child Development & Exercise Center, Division of Pediatrics, Wilhelmina Kinderziekenhuis/University Medical Center Utrecht, Utrecht, The Netherlands.
REFERENCES
- 1. Balaban RS. Domestication of the cardiac mitochondrion for energy conversion. J Mol Cell Cardiol 46: 832–841, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blei ML, Conley KE, Kushmerick MJ. Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol 465: 203–222, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bose S, French S, Evans FJ, Joubert F, Balaban RS. Metabolic network control of oxidative phosphorylation: multiple roles of inorganic phosphate. J Biol Chem 278: 39155–39165, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Brand MD. The efficiency and plasticity of mitochondrial energy transduction. Biochem Soc Trans 33: 897–904, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Chance B, Im J, Nioka S, Kushmerick MJ. Skeletal muscle energetics with PNMR: personal views and historic perspectives. NMR Biomed 19: 904–926, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Dahlstedt AJ, Katz A, Westerblad H. Role of myoplasmic phosphate in contractile function of skeletal muscle: studies on creatine kinase-deficient mice. J Physiol 533: 379–388, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grote J, Thews G. [Requirements for the oxygen supply of heart muscle tissue.]. Pflügers Arch 276: 142–165, 1962 [PubMed] [Google Scholar]
- 8. Haller T, Ortner M, Gnaiger E. A respirometer for investigating oxidative cell metabolism: toward optimization of respiratory studies. Anal Biochem 218: 338–342, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Hancock CR, Brault JJ, Wiseman RW, Terjung RL, Meyer RA. 31P-NMR observations of free ADP during fatiguing, repetitive contractions of murine skeletal muscle lacking AK-1. Am J Physiol Cell Physiol 288: C1298–C1304, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Hunter P, Nielsen P. A strategy for integrative computational physiology. Physiology 20, 316–325, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Jeneson JA, de Snoo MW, Verlinden NA, Joosten BJ, Doornenbal A, Schot A, Everts ME. Treadmill but not wheel running improves fatigue resistance of isolated extensor digitorum longus muscle in mice. Acta Physiol (Oxf) 190: 151–161, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Jeneson JA, Schmitz JP, van den Broek NA, van Riel NA, Hilbers PA, Nicolay K, Prompers JJ. Magnitude and control of mitochondrial ADP sensitivity. Am J Physiol Endocrinol Metab 297: E774–E784, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jeneson JA, Westerhoff HV, Kushmerick MJ. A metabolic control analysis of kinetic controls in ATP free energy metabolism in contracting skeletal muscle. Am J Physiol Cell Physiol 279: C813–C832, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Jeneson JA, Wiseman RW, Westerhoff HV, Kushmerick MJ. The signal transduction function for oxidative phosphorylation is at least second order in ADP. J Biol Chem 271: 27995–27998, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Kushmerick MJ. Energy balance in muscle activity: simulations of ATPase coupled to oxidative phosphorylation and to creatine kinase. Comp Biochem Physiol B Biochem Mol Biol 120: 109–123, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Kushmerick MJ, Meyer RA, Brown TR. Regulation of oxygen consumption in fast- and slow-twitch muscle. Am J Physiol Cell Physiol 263: C598–C606, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Kushmerick MJ, Moerland TS, Wiseman RW. Mammalian skeletal muscle fibers distinguished by contents of phosphocreatine, ATP, and Pi. Proc Natl Acad Sci USA 89: 7521–7525, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mast F, Elzinga G. Time course of aerobic recovery after contraction of rabbit papillary muscle. Am J Physiol Heart Circ Physiol 253: H325–H332, 1987 [DOI] [PubMed] [Google Scholar]
- 19. Meyer RA. Linear dependence of muscle phosphocreatine kinetics on total creatine content. Am J Physiol Cell Physiol 257: C1149–C1157, 1989 [DOI] [PubMed] [Google Scholar]
- 20. Meyer RA, Sweeney HL, Kushmerick MJ. A simple analysis of the “phosphocreatine shuttle”. Am J Physiol Cell Physiol 246: C365–C377, 1984 [DOI] [PubMed] [Google Scholar]
- 21. Moerland TS, Kushmerick MJ. Contractile economy and aerobic recovery metabolism in skeletal muscle adapted to creatine depletion. Am J Physiol Cell Physiol 267: C127–C137, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Roman BB, Meyer RA, Wiseman RW. Phosphocreatine kinetics at the onset of contractions in skeletal muscle of MM creatine kinase knockout mice. Am J Physiol Cell Physiol 283: C1776–C1783, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Steeghs K, Benders A, Oerlemans FF, de Haan A, Heerschap A, Ruitenbeek W, Jost C, van Deursen J, Perryman B, Pette D, Bruckwilder M, Koudijs J, Jap P, Veerkamp J, Wieringa B. Altered Ca2+ responses in muscles with combined mitochondrial and cytosolic creatine kinase deficiencies. Cell 89: 93–103, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Steeghs K, Oerlemans FF, de Haan A, Heerschap A, Verdoodt L, de Bie M, Ruitenbeek W, Benders A, Jost C, van Deursen J, Tulsson P, Terjung R, Jap P, Jacob W, Pette D, Wieringa B. Cytoarchitectural and metabolic adaptations in muscles with mitochondrial and cytosolic creatine kinase deficiencies. Mol Cell Biochem 184: 183–194, 1998 [PubMed] [Google Scholar]
- 25. Sweeney HL. The importance of the creatine kinase reaction: the concept of metabolic capacitance. Med Sci Sports Exerc 26: 30–36, 1994 [PubMed] [Google Scholar]
- 26. Ter Veld F, Nicolay K, Jeneson JA. Increased resistance to fatigue in creatine kinase deficient muscle is not due to improved contractile economy. Pflügers Arch 452: 342–348, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Van den Broek NM, de Feyter HM, de Graaf L, Nicolay K, Prompers JJ. Intersubject differences in the effect of acidosis on phosphocreatine recovery kinetics in muscle after exercise are due to differences in proton efflux rate. Am J Physiol Cell Physiol 293: C228–C237, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Van Stiphout RG, van Riel NA, Verhoog PJ, Hilbers PA, Nicolay K, Jeneson JA. Computational model of excitable cell indicates ATP free energy dynamics in response to calcium oscillations are undampened by cytosolic ATP buffers. IEE Proc Syst Biol 153: 405–408, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Wiseman RW, Beck TW, Chase PB. Effect of intracellular pH on force development depends on temperature in intact skeletal muscle from mouse. Am J Physiol Cell Physiol 271: C878–C886, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Wu F, Jeneson JA, Beard DA. Oxidative ATP synthesis in skeletal muscle is controlled by substrate feedback. Am J Physiol Cell Physiol 292: C115–C124, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Wu F, Zhang J, Beard DA. Experimentally observed phenomena on cardiac energetics in heart failure emerge from simulations of cardiac metabolism. Proc Natl Acad Sci USA 106: 7143–7148, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]