Abstract
The processes that trigger severe muscle atrophy and loss of myosin in critical illness myopathy (CIM) are poorly understood. It has been reported that muscle disuse alters Ca2+ handling by the sarcoplasmic reticulum. Since inactivity is an important contributor to CIM, this finding raises the possibility that elevated levels of the proteins involved in Ca2+ handling might contribute to development of CIM. CIM was induced in 3- to 5-mo-old rats by sciatic nerve lesion and infusion of dexamethasone for 1 wk. Western blot analysis revealed increased levels of ryanodine receptor (RYR) isoforms-1 and -2 as well as the dihydropyridine receptor/voltage-gated calcium channel type 1.1 (DHPR/CaV 1.1). Immunostaining revealed a subset of fibers with elevation of RYR1 and CaV 1.1 that had severe atrophy and disorganization of sarcomeres. These findings suggest increased Ca2+ release from the sarcoplasmic reticulum may be an important contributor to development of CIM. To assess the endogenous functional effects of increased intracellular Ca2+ in CIM, proteolysis of α-fodrin, a well-known target substrate of Ca2+-activated proteases, was measured and found to be 50% greater in CIM. There was also selective degradation of myosin heavy chain relative to actin in CIM muscle. Taken together, our findings suggest that increased Ca2+ release from the sarcoplasmic reticulum may contribute to pathology in CIM.
Keywords: myosins, muscular diseases, ryanodine receptor calcium release channel, calpain
critical illness myopathy (CIM) is the most common cause of weakness in patients in the intensive care unit (25, 29). Patients often require prolonged ventilator support secondary to pulmonary muscle weakness and do not regain full strength for many months after their acute illness (11, 20). It is now appreciated that weakness following critical illness is the leading cause of disability in patients who survive acute respiratory distress syndrome (20, 22).
Most animal studies of CIM have been carried out in a rat model that involves pairing muscle inactivity, induced by denervation, with systemic administration of corticosteroids (50). The model was established to mimic the clinical situation in patients who develop CIM following infusion of neuromuscular blocking agents and corticosteroids (10, 36). A central issue is whether denervation of muscle mimics the effects of unloading muscle caused by neuromuscular blocking agents in patients. A recent study compared muscle treated with denervation alone to muscle treated with the combination of neuromuscular blockade and corticosteroids and found differences (42). We have previously found that the addition of corticosteroids to denervation has profound effects on muscle (47, 48), which suggests the differences were likely due to treatment with corticosteroids rather than difference between denervation and neuromuscular block. There is evidence suggesting that denervation has little effect other than loss of electrical activity on some muscle properties (34, 35, 62). Muscle mass is similarly reduced by denervation and disuse (67) (for a review see Ref. 14). Denervation and neuromuscular blockade have been directly compared and found to have nearly identical effects on skeletal muscle electrical properties (6).
There are a number of abnormalities that contribute to weakness in CIM. These include selective loss of myosin (10, 30, 31, 40, 43, 44, 53), disorganization of sarcomeres (10, 53), dramatic muscle atrophy (28), and electrical inexcitability of muscle (46, 49). All of these features occur in the steroid denervation rat model of the disease (38, 40, 48, 50). It thus appears that the steroid-denervation rat model of CIM recreates all of the contributors to weakness found in patients.
Despite a great deal of research, the inciting events that trigger the various contributors to weakness in CIM remain poorly understood. There are hints from different lines of work that suggest dysregulation of Ca2+ handling may be an early event in CIM. It has been found that muscle disuse (a contributor to development of CIM) alters Ca2+ handling by the sarcoplasmic reticulum (21). Ca2+ is elevated by dexamethasone treatment of skeletal muscle in vitro (23). Given these studies it appears highly likely that Ca2+ handling is abnormal in denervated muscle treated with corticosteroids.
There are multiple pathways that contribute to regulation of intracellular Ca2+ in skeletal muscle, including Ca2+ entry through voltage-gated L-type Ca2+ channels on the muscle surface (mediated by the CaV 1.2 isoform), Ca2+ release from the sarcoplasmic reticulum [mediated by the coordinated action of the voltage-gated calcium channel type 1.1 (CaV 1.1) and the RYR1], and Ca2+ entry via store-operated Ca2+ channels (mediated by ORAI 1) (12, 23, 24). We measured levels of Ca2+ handling proteins in these three pathways to determine whether increases in levels of proteins in any of these pathways might contribute to Ca2+ dysregulation in CIM. Our data suggest increased levels of proteins involved in Ca2+ release from the sarcoplasmic reticulum may contribute to development of severe atrophy in a subset of fibers in CIM.
MATERIALS AND METHODS
Animal protocols were approved by the Institutional Animal Care and Use Committee at Wright State University.
Induction of CIM.
Rat muscle was denervated by removing a 10-mm segment of the left sciatic nerve in isoflurane anesthetized adult female Wistar rats (250–350 g body wt). Buprenorphine was given subcutaneously for postoperative analgesia. Dexamethasone (4 mg·kg−1·day−1) was infused via an Alzet osmotic pump placed subcutaneously in the back (model 2 ml/1; Durect, Cupertino, CA) on the day of denervation. Control rats were treated with placement of osmotic pumps containing vehicle alone (50% DMSO, 15% ETOH, and 35% water). Steroid treatment induced severe weight loss that may be due, in part, to reduced food intake (51). Rats were given daily intraperitoneal injections of antibiotics (0.2 ml of 2.27% Baytril; Bayer, Shawnee Mission, KS). The injection of antibiotics substantially improved survival of steroid-treated rats. Seven days after surgery, rats were killed by carbon dioxide inhalation, and the tibialis anterior, soleus, and gastrocnemius muscles removed.
Preparation of muscle membranes.
Membranes were prepared from gastrocnemius muscles of control or CIM rats as reported previously (24). Muscles were harvested, weighed, frozen in liquid nitrogen, and stored at −70°C until use. Muscles were thawed on ice for ∼10 min and minced finely on a glass plate on ice prior to incubating in sucrose buffer with protease and phosphatase inhibitors for 15–30 min to allow the inhibitors to penetrate the muscle. Between 5 and 10 ml of buffer were used per gram of tissue. The sucrose buffer contained 0.3 M sucrose, 75 mM NaCl, 10 mM EGTA, 10 mM EDTA, 10 mM Tris, pH 7.4, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM iodoacetamide, 0.1 μg/ml pepstatin A, 10 μM leupeptin, 2.5 μM ALLN, and 1 mM sodium fluoride. Following the incubation, samples were homogenized in a PowerGen 700 tissue homogenizer at a setting of 6 for 30 s. Aliquots for whole muscle homogenate (0.5 ml) were removed, and the remaining sample centrifuged in a SM24 rotor at 4,500 g for 15 min. The supernatants were removed, centrifuged again, and the final supernatant centrifuged in a SW55 rotor at 138,000 g for 1 h. The pellets from this last step were resuspended in fresh sucrose buffer with inhibitors and used as the membrane fraction.
For purification of sodium channels, membranes were solubilized using NP-40 and the sodium channel fraction sequentially purified on DEAE-Sepharose and wheat germ agglutinin columns as described in (26). For all membrane preparation and channel purification procedures, all samples, equipment, and buffers were kept at 0–4°C.
Preparation of whole muscle homogenates.
Whole muscle homogenates were prepared from soleus, gastrocnemius, and tibialis anterior muscles. Aliquots taken for whole muscle homogenates were combined with 0.5 ml of 20% SDS, 100 mM Tris, pH 8.0, and incubated at 65°C for 20 min, vortexing vigorously at the beginning, halfway point, and end of the incubation. The homogenates were centrifuged at 13,700 g in a microfuge for 30 min, and the supernatants were taken as the whole muscle homogenate.
SDS-PAGE and Western blot analysis.
Membrane fractions and whole muscle homogenates were assayed for protein content using the Lowry protein assay, and equal amounts of protein were loaded for control and CIM samples for each protein assessed (40–100 μg per lane). SDS-PAGE was performed on Criterion 4–20% acrylamide gels (Bio-Rad), and Western blot transfers were carried out in a Criterion transfer apparatus using nitrocellulose as the blotting medium. Following transfer, the blots were washed with PBS/0.1% Tween (PBS/Tween) and the transfer efficiency assessed by staining with Ponceau S Red. After removing this stain by washing with PBS/Tween, the blots were blocked in 1% I-Block (Tropix) for 1 h to overnight, incubated with first antibody for 2 h, washed, incubated with mouse or rabbit secondary antibody conjugated to alkaline phosphatase (Tropix), and visualized using CDP-Star (Tropix) and a Fujifilm LAS-3000 closed-caption device camera.
For myosin/α-actin blots, the myosin signal in each lane was normalized to the α-actin in that lane since previous work has shown selective loss of myosin relative to α-actin in CIM (43). For all blots, the average of the signal in the control muscle was set at 100%, and individual control and CIM samples were calculated as a percentage of that. For membrane proteins, the signal for individual proteins was normalized to total membrane protein rather than a particular loading control. This approach was chosen, as it appeared that expression of many proteins are changed by the treatments used and total protein seemed a less biased normalization tool rather than relying on any one protein. Ponceau S Red staining indicated that the overall protein in the samples was similar (data not shown). Our approach to normalization appeared validated by the finding that some proteins went up (RYR1, RYR2, and CaV 1.1), while other proteins went down [CaV 1.2 and (ORAI type 1)], and levels of yet other proteins were not statistically changed (Nav 1.4 and β-tubulin).
The primary antibodies used in this study were obtained from the following sources: monoclonal to ryanodine receptor (RYR, Abcam), RYR1 and RYR2 (Alomone), CaV 1.1 (Abcam), CaV 1.2 (Alomone), ORAI Type I (ProSci), fast myosin II and α-actin (Abcam), slow myosin 1 (Sigma), β-tubulin (Abcam), α-fodrin (Abcam), calpain I and II (Calbiochem), and monoclonal LD3 to NaV 1.4 (Sigma).
Immunohistochemistry.
Tibialis anterior muscles were removed, fixed by placement in 4% paraformaldehyde for 1 h, cryoprotected in 15% sucrose solution overnight, and frozen in liquid nitrogen. Then 10-μm thick longitudinal sections were cut. Primary antibodies used against RYR1 (1:100 Alomone Labs), RYR2 (1:100 Alomone Labs), fast myosin II (Abcam), slow myosin 1 (Sigma), calpain II (Calbiochem), and Cav 1.1 (1:200 Abcam) were the same antibodies used for Western blot analysis. Labeling of primary monoclonal antibody against Cav 1.1, slow myosin I, fast myosin II, and calpain II was visualized using a Dylight 488-conjugated donkey anti-mouse secondary antibody (1:300; Jackson ImmunoResearch Laboratories). Primary antibody staining against RYR1 and RYR2 was visualized using a rhodamine-conjugated donkey anti-rabbit antibody (1:200, Jackson ImmunoResearch). The z-axis stacks of images at sequential focal planes (0.5 μm separation) of NMJs were obtained using a Fluoview FV 1000 confocal microscope and a ×60 oil objective (Olympus Optical). Illustrated images are flat-plane in-focus projections obtained from z-series images using Fluoview software.
Identification of fibers in which RYR1 levels were elevated was done qualitatively and then confirmed quantitatively. First, two independent reviewers examined muscle sections and were able to reproducibly identify the same fibers. To confirm qualitative impressions, fibers were imaged as described above and intensity was analyzed using Image Pro software. Image stacks were flattened using the flat-plane in-focus projections and the mean pixel intensity of each fiber was measured. For quantification of the number of fibers with elevated RYR1 all fibers in a single cross section of the entire tibialis anterior were counted.
Statistical analysis.
All Western blots and muscle weights were compared using the Student's t-test to determine statistical significance. Data are given as means ± SE. For analysis of rat-to-rat differences in the percent of fibers with elevated levels of RYR, Fisher's Exact test was used.
RESULTS
RYR protein is present at higher levels in CIM muscle.
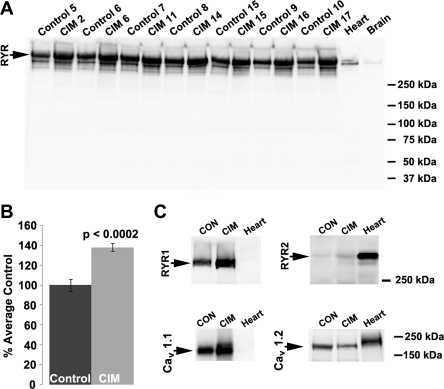
Membranes prepared from muscles of individual animals were screened in Western blot analysis with a monoclonal antibody that recognizes both RYR1 and RYR2 (Fig. 1A). There was an ∼40% increase in RYR expression in CIM (Fig. 1B). Using subtype-specific antibodies to further analyze which RYR isoform was contributing the most to this increase, indicated that the majority of the increase was due to increased expression of the skeletal muscle-specific RYR1 isoform, which was the most predominant RYR expressed in both control and CIM skeletal muscle, although there was increased expression of both RYR1 and RYR2 in CIM (Table 1). Given the close interaction between RYR and voltage-gated Ca2+ channels (12), the expression of Ca2+ channels was also determined in control and CIM muscle (Fig. 1C and Table 1). There was a concomitant increase of CaV 1.1 with RYR1, while expression of the CaV 1.2 decreased in CIM. Expression of calcium release-activated channels (ORAI, Type 1) also decreased in CIM (Table 1). Taken together, these data suggest that there is a parallel increase in CaV 1.1 and RYR1 that could lead to increased Ca2+ release from the sarcoplasmic reticulum.
Fig. 1.
Expression of the ryanodine receptor (RYR) increases in critical illness myopathy (CIM). A: skeletal muscle membranes prepared from individual control (Con) or CIM animals were analyzed in Western blot analysis using a monoclonal antibody to RYR. There was a consistent increase in RYR expression in CIM, as shown quantitatively in the graph in B. C: antibodies to individual subtypes of RYR indicate that most of the RYR was the type 1, normally expressed in skeletal muscle, although there was some expression of the type 2 receptor, normally found in cardiac tissue. The expression of the CaV 1.1 Ca2+ channel also increased in CIM, while expression of the CaV 1.2 Ca2+ channel, normally expressed in the surface membrane of type I and type II fibers (24), decreased. Quantification of the Western blots is shown in Table 1.
Table 1.
Altered expression of Ca2+ handling proteins in critical illness myopathy
| Protein Measured | Control | CIM |
|---|---|---|
| RYR1 | 100 ± 2.5 | 135 ± 6.5** |
| RYR2 | 100 ± 4.3 | 171 ± 13** |
| CaV 1.1 | 100 ± 5.1 | 133 ± 9.5** |
| CaV 1.2 | 100 ± 5.0 | 60 ± 7.3** |
| ORAI, type 1 | 100 ± 2.5 | 77 ± 4.9** |
| NaV 1.4 | 100 ± 7.6 | 97 ± 5.2 |
| β-Tubulin | 100 ± 5.8 | 117 ± 6.9 |
Data are means ± SE; control, n = 19; CIM, n = 16. Values are %average control ± SE. RYR1, ryanodine receptor type 1; RYR2, RYR type 2; CaV 1.1; voltage-gated Ca2+ channel type 1.1; CaV 1.2, CaV type 1.2; ORAI type 1, Ca2+ release-activated Ca2+ protein 1; NaV 1.4, voltage-gated sodium channel type 1.4.
P < 0.01 for CIM relative to control.
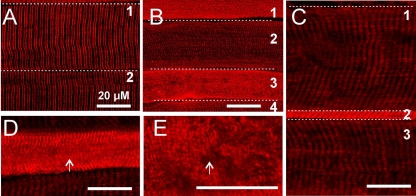
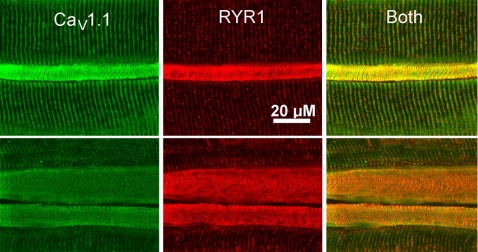
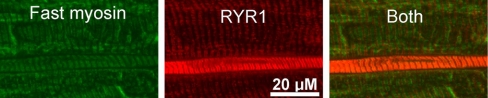
The elevation of RYR1 by Western blot analysis could be due to either uniform elevation of RYR in all muscle fibers or elevation in a subset of fibers. In control muscle, RYR1 staining was similar in intensity in all fibers and sarcoplasmic reticulum was organized into a series of parallel lines running perpendicular to the length of the fiber (Fig. 2A). In most CIM muscles (5/7) the increase in RYR1 staining was uniform such that no differences could be noted between fibers. However, in two of seven CIM muscles, there was a striking elevation of RYR1 in a subset (28/309) of fibers (Fig. 2, B–E, Table 2). On average, fibers with elevated intensity of RYR staining had an increase in pixel intensity of 228 ± 53% relative to other fibers in the same field. In the other five muscles only 4 of 777 fibers exhibited marked elevation in RYR1 staining. Fibers in which RYR1 was elevated were all abnormal in appearance. In some fibers with increased RYR1 there was severe disorganization of the sarcoplasmic reticulum such that no organization of RYR1 staining could be discerned (Fig. 2, B and E). In other fibers with elevated RYR1 there was severe atrophy (Fig. 2C). We found that the CaV 1.1 calcium channel was also increased in the subset of fibers with high levels of RYR1 (Fig. 3). Elevation of Ca2+ can trigger numerous pathologic processes, and it has been suggested that calpains and caspases, potent Ca2+-activated proteases, are activated in CIM (13, 52, 57, 58). We performed immunostaining and found that calpain II was elevated in muscle fibers with elevated RYR1 (Fig. 4).
Fig. 2.
Marked elevation of RYR1 in a subset of fibers. A: RYR1 staining in 2 fibers from the tibialis anterior muscle in a control rat. The staining is well organized into parallel stripes running perpendicular to the length of the fiber. B: RYR1 staining in 4 fibers from a tibialis anterior muscle with CIM. Fibers 2 and 4 have lower levels of RYR1 that are organized into stripes. Fibers 1 and 3 have very high levels of RYR 1 and have lost all organization. The RYR1 is present as spots rather than parallel lines. C: 3 fibers from a different tibialis anterior with CIM. Fibers 1 and 3 appear normal. Fiber 2 has high levels of RYR1 and is severely atrophied. Unlike fibers 1 and 3 in B with high levels of RYR1, the RYR1 in fiber 2 is still organized into stripes. D: a fiber from a CIM muscle in which RYR1 is organized into stripes at the edge of the fiber, but is disorganized in the center (arrows, D and E). E: high-power view of a region of disorganized RYR1 staining from the fiber in D.
Table 2.
Dramatic upregulation of RYR in a subset of fibers in a subset of rats
| CIM Rat No. | Number of Fibers with Dramatic Upregulation of RYR | No. of Normal Fibers |
|---|---|---|
| 1 | 13 | 130 |
| 2 | 0 | 157 |
| 3 | 1 | 181 |
| 4 | 0 | 162 |
| 5 | 15 | 151 |
| 6 | 1 | 124 |
| 7 | 2 | 149 |
Count of fibers with elevated levels of RYR on immunostained longitudinal sections of tibialis anterior muscle. Fisher's exact test revealed that there was a statistically significant difference in the %abnormal fibers between rats (P < 0.0001).
Fig. 3.
Voltage-gated calcium channel type 1.1 (CaV 1.1) and RYR1 are upregulated in the same fibers in CIM. Two fields from an individual tibialis anterior muscle double labeled for CaV 1.1 and RYR1 are shown. In the field at the top, a severely atrophied muscle fiber has markedly elevated levels of both CaV 1.1 and RYR1. Sarcoplasmic reticulum (SR) within the fiber is still organized into perpendicular stripes. In the lower field, 2 atrophied fibers have elevated levels of both CaV 1.1 and RYR1. The upper fiber with elevated CaV 1.1 and RYR1 has regions along the upper and lower edges in which SR organization is lost.
Fig. 4.
Calpain II is elevated in fibers with elevated RYR1. Shown is a field from a tibialis anterior muscle double labeled for calpain II and RYR1. In the field are two atrophied fibers that have elevated levels of both calpain II and RYR1. The normal fibers above and below the atrophied fibers have lower levels of calpain II.
Endogenous proteolysis is enhanced in CIM.
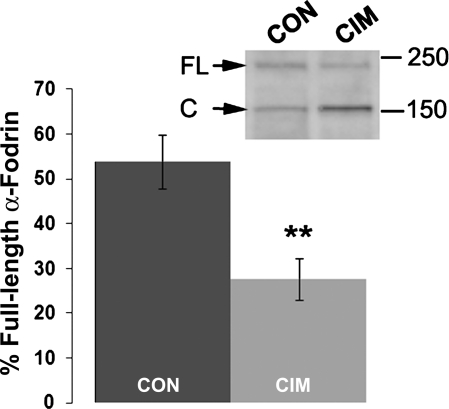
To determine whether endogenous calpain activity was elevated in CIM muscles in a functional test, proteolysis of the cytoskeletal protein α-fodrin, a well-known target of calpain (61), was analyzed by Western blot (Fig. 5). There was a 50% increase in the calpain-cleaved form of α-fodrin in CIM tissue, production of an ∼150-kDa fragment, consistent with increased calpain activity. Since cleavage of α-fodrin by caspases results in a cleavage product that runs at ∼120 kDa (61), these data implicate calpains, not caspases, in endogenous proteolysis of α-fodrin under these conditions.
Fig. 5.
Increased calpain-specific cleavage of α-fodrin in CIM muscle. Membranes from control or CIM muscles were resolved on 4–20% acrylamide gels and were analyzed by Western blot analysis. Consistent with published reports for calpain-mediated cleavage of α-fodrin (47), there was increased appearance of the 150-kDa cleaved band (C) relative to the full-length (FL) band (inset, Western blot analysis). For quantification, the amount of full-length product in each sample relative to the total of the full-length and cleaved for the sample was taken as the % of full-length α-fodrin. The average for 8 control samples and 8 CIM samples is shown in the graph (**P < 0.01).
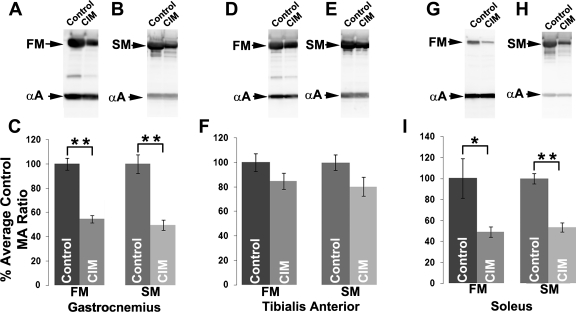
Calpains are also thought to initiate breakdown of myofibrillar proteins (9). One of the hallmarks of CIM is a selective degradation of myosin that results in a reduced myosin/actin ratio (10, 31, 53). We measured the myosin-to-actin ratio by Western blot analysis in control muscle and CIM muscles (Fig. 6). Consistent with reports from other laboratories, there was considerable reduction in the myosin-to-actin ratio for both fast myosin II and slow myosin I in both the gastrocnemius and soleus CIM muscles. However, in the tibialis anterior there was little reduction in the myosin-to-actin ratio for either myosin isoform (Fig. 6). Analysis of myosin expression in the gastrocnemius by immunohistochemistry indicated that the subset of extremely atrophied fibers with high levels of RYR1 expressed fast myosin II (Fig. 7). Staining with slow myosin labeled only a small percentage of fibers and did not label any of the fibers with high levels of RYR1 (data not shown).
Fig. 6.
Loss of myosin in the gastrocnemius and soleus, but not in the tibialis anterior in CIM. A: Western blot analysis of fast myosin (FM) and α-actin (αA) in control and CIM gastrocnemius muscles. B: Western blot analysis of slow myosin (SM) and α-actin (αA) in control and CIM gastrocnemius muscle. C: quantification of Western blot data from the gastrocnemius. All data is normalized to the myosin/actin (MA) ratio in control. D: Western blot analysis of fast myosin and α-actin in tibialis anterior. E: Western blot analysis of slow myosin and α-actin in tibialis anterior. F: quantification of Western blot data from the tibialis anterior. G: Western blot analysis of fast myosin and α-actin in soleus. H: Western blot analysis of slow myosin and α-actin in soleus. I: Quantification of Western blot data in the soleus. *P < 0.05, **P < 0.01. A total of n = 9 for the controls and CIM.
Fig. 7.
Fibers with elevated RYR1 contain fast myosin. Shown is a field from a tibialis anterior muscle double labeled for myosin II and RYR1. All 3 fibers in the field are positive for fast myosin. In the lower center of the field is a small fiber that stains brightly for RYR1 and is positive for fast myosin.
Consistent with the observed enhanced catabolic processes observed in CIM muscle, all muscles examined (gastrocnemius, soleus, and tibialias anterior) exhibited significant atrophy (50% by set weight, Table 3). Previous work indicated that fiber cross-sectional area decreased by a much greater degree in CIM muscle compared with control, denervation alone, or steroid treatment alone, indicating that the combination of corticosteroid treatment and denervation is necessary to induce this severe atrophy (48).
Table 3.
Atrophy of skeletal muscles in CIM
| Sample | % Body Weight Loss | Tibialis Anterior Muscle Weight, g | Soleus Muscle, Weight, g | Gastrocnemius Muscle Weight, g |
|---|---|---|---|---|
| Control | (0.6 ± 2.4) | 0.52 ± 0.009 | 0.15 ± 0.006 | 1.61 ± 0.030 |
| CIM | 23.9 ± 1.0** | 0.28 ± 0.012** | 0.08 ± 0.006** | 0.80 ± 0.021** |
Data are means ± SE, n = 9/group; numbers in parenthesis indicate weight gain.
P < 0.01.
RYR1 is modified in CIM.
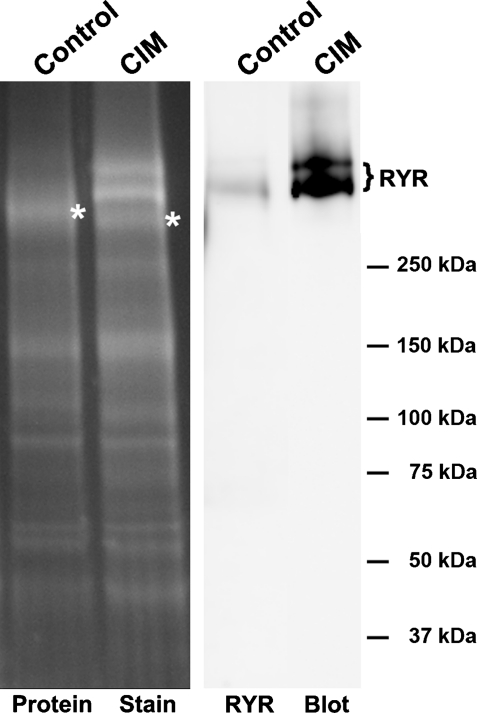
In addition to the changes in levels, we found evidence that RYR1 is sometimes modified in CIM. While purifying sodium channels from CIM muscle membranes, the RYR1 sometimes copurified in the sodium channel fraction (Fig. 8). That RYR1 was present in CIM but not control purifications suggests that some type of modification and/or protein association is different in CIM. We observed this striking copurification in two of five purifications, similar to the occurrence of a subset of fibers with very high RYR levels in two of seven muscles.
Fig. 8.
RYR behavior during purification is altered in a subset of CIM muscles. Left: the protein stain of the sodium channel fraction from control and CIM animals purified sequentially on DEAE-Sepharose and wheat germ agglutinin columns. *NaV 1.4 sodium channel bands in control and CIM samples. In the CIM sample, there is an additional prominent double band that is identified as the RYR in the Western blot analysis (right). All protein samples were resolved on 4–20% SDS-PAGE gels and analyzed by Western blot analysis and protein staining.
DISCUSSION
This study addresses both global and fiber-specific changes in patterns of expression of a subset of Ca2+ signaling proteins in CIM. In all muscles examined, there was an elevation in RYR1 channels in the muscle membrane fraction that was accompanied by a parallel increase in CaV 1.1. Additionally, there was a global increase in calpain-mediated cleavage of α-fodrin. On a more restricted level in terms of number of affected rats (2/7 CIM rats) and numbers of fibers (10% of fibers in affects rats), immunostaining demonstrated that fibers with the greatest elevation in CaV 1.1 and RYR1 had the greatest atrophy and disorganization of sarcomeres, raising the possibility that elevation of the levels of Ca handling proteins induced toxicity. Our findings suggest that increased Ca2+ release from the sarcoplasmic reticulum may play a role in triggering muscle pathology in CIM.
Our data demonstrate that proteins associated with Ca2+ release from the sarcoplasmic reticulum are elevated in CIM, while levels of Ca2+ handling proteins involved in entry of Ca2+ during muscle action potentials (Cav 1.2) and Ca2+ entry via store-operated channels (ORAI 1) were reduced. These data suggest that regulation of intracellular Ca2+ in CIM is highly likely to be altered. The upregulation of Cav 1.1 and RYR1 appeared to be due to coordinated regulation, since both proteins were coregulated at a global level, as demonstrated by increased expression in Western blot analysis, and on a fiber-by-fiber basis, as demonstrated by immunostaining. As it is well known that RYR1 and CaV 1.1 have a strong association in skeletal muscle (4, 12, 41, 54), it is not surprising that levels were coregulated; however, our study does not demonstrate a causal link.
There was also greater functional activity of calpain, as indicated by the increased appearance of a calpain-specific α-fodrin cleavage product in CIM muscle. The increased calpain activity likely initiates degradation of myosin heavy chain (17), although this degradation is likely completed by the ubiquitin-proteosome pathway, as it is the common pathway in all known triggers of muscle atrophy, including glucocorticoid treatment and denervation (7, 9, 17). The muscle atrophy in CIM, which is found uniformly in all muscles from all animals, is likely due in part to this increased muscle proteolysis. Taken together, our observations are consistent with a causal link between elevation of the Cav 1.1-RYR1 complex and myosin proteolysis and muscle atrophy.
In two out of seven of rats there was a subset of severely atrophied fibers that had very high levels of calpain, a finding that raises the possibility of future death of the fibers (55). In CIM there is a subset of patients who have modest elevations in serum levels of the muscle enzyme creatine kinase due to death of muscle fibers (8, 18, 46). The etiology of the death of muscle fibers in CIM has never been determined. Our data in rats suggest that a potential triggering mechanism is dramatic upregulation of Ca handling proteins in a subset of fibers. Future studies measuring Ca2+ handling proteins on a fiber-by-fiber basis in patient biopsies will determine whether this is the case.
In the rat model of CIM, muscle fibers are not electrically active, as they are denervated, and most of the fibers are electrically inexcitable so there is little of the spontaneous activity that normally occurs following denervation (48). Normally there is no Ca2+ release from the sarcoplasmic reticulum in the absence of electrical activity (12). This raises the question of why fibers with elevated levels of Cav 1.1 and RYR1 had the greatest atrophy and disruption of sarcomere organization. One possibility is that there is leak of Ca2+ from the sarcoplasmic reticulum in the absence of electrical activity in these fibers due to modification of the RYR1 protein or its protein association partners in CIM.
We found evidence that in addition to the increase in levels of RYR1, in a subset of muscles, there appeared to be modification of the channel that affected its binding to resins used for purification of sodium channels. The RYR is regulated at many levels such that a number of alterations might contribute to increased Ca2+ release from the sarcoplasmic reticulum. RYRs are associated with FKBP12 and FKBP12.6, also known as calstabin-1 and -2, which bind the RYR1 and RYR2, respectively, in a 1:1 stoichiometry (32, 37). The binding of the calstabins to the RYRs is regulated through a PKA-modified serine on the RyR receptor, Ser2844 for RYR1 and Ser2809 for RYR2, with hyperphosphorylation at these sites causing loss of calstabin binding and resulting in Ca2+ leak under resting conditions (32, 63). In addition to the PKA-modification of the RYRs, they are also covalently modified by S-nitrosylation, S-glutathionylation, and oxidation at several cysteines (1). Modification of the RYR1 at specific cysteines alters the probability of channel opening and association with other proteins, including calstabins and calmodulin (1, 5, 56). Collectively, these posttranslational modifications of RYRs could contribute to altered function of RYRs in CIM that results in Ca2+ leak in the absence of electrical activity.
As Ca2+ leak from the sarcoplasmic reticulum may contribute to development of CIM, we tested whether a drug that lessens sarcoplasmic reticulum Ca2+ release through RYRs might reduce CIM muscle pathology. We used azumolene, a more water-soluble analog of dantrolene, since it combined ease of use and efficacy (27, 68). Dantrolene has been shown to reduce muscle protein degradation early in the course of sepsis in rats in vivo (64, 66). Unfortunately at high doses, azumolene appears to increase mortality, and at lower doses, it did not significantly reduce either atrophy or loss of myosin (data not shown). Thus, preliminary efforts at treating CIM through reduction of Ca release from the sarcoplasmic reticulum were not encouraging.
Rat model of CIM.
Comparison of data from the tibialis anterior, gastrocnemius and soleus muscles addresses specific concerns about the rat steroid-denervation model of CIM employed in this study. Rats with lesions of the sciatic nerve still walk despite denervation of muscles below the knee. Lower leg muscles in rats with sciatic nerve lesions thus experience passive loading and unloading despite being denervated and thus do not mimic the situation in critically ill patients where muscles are continuously unloaded. However, this is not true for all muscles in the leg. While the gastrocnemius and soleus are weight-bearing muscles that experience load/stretch when the foot of a denervated rat leg is placed on the ground, the tibialis anterior is not a load-bearing muscle and thus never experiences loading/stretch after a sciatic nerve lesion (65). The only time the tibialis anterior experiences passive stretch is when the gastrocnemius and soleus contract, and this does not happen in a leg where the sciatic nerve has been lesioned. Thus the tibialis anterior in our rat model shares both loss of activity as well as unloading with critically ill patients. For all three muscles, there was a similar degree of atrophy, as indicated by the loss of wet weight (Table 3). Relative loss of myosin was least severe in the unloaded tibialis anterior muscle, while the gastrocnemius muscle and soleus muscle lost similar amounts of myosin. As the loaded gastrocnemius and soleus muscles were at least as severely affected as the unloaded tibialis anterior, our data are most consistent with the possibility that loss of muscle activity rather than unloading is the key risk factor for development of CIM in rat.
In the rat model we use, there are two contributing risk factors to CIM: steroid treatment and denervation. Previous work has shown that each of these treatments individually has profound effects on muscle. Steroid treatment induces severe muscle weight loss, reduces the cross-sectional area of type IIB and IIX myofibers, reduces expression of myosin IIx and IIB myosin, and reduces force produced by the myofibers (33, 39, 45, 59, 60). Denervation reduces expression of myosin IIx and IIB, increases net protein degradation via the ubiquitin-proteosome pathway, and increases and activates a subset of cell signaling factors involved in atrophy (2, 3, 15, 16). Previous work from our laboratory indicates that these two risk factors have a powerful synergistic effect (47, 48). One feature of the animal model we use that would be potentially more severe than in patients is the loss of trophic factors from nerve (19). It is our view that CIM is a common endpoint that can be induced by a number of triggers or risk factors in any given patient.
Perspectives and Significance
In this study, we demonstrate that levels of proteins involved in Ca2+ release from the sarcoplasmic reticulum are elevated in CIM. These findings suggest increased Ca2+ release from the sarcoplasmic reticulum may be an important contributor to development of CIM.
GRANTS
This work was supported by National Institutes of Health Grant NS-040826 (to M. M. Rich).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Experiments were carried out in the Wright State University Proteome Analysis Laboratory.
REFERENCES
- 1. Aracena-Parks P, Goonasekera SA, Gilman CP, Dirksen RT, Hidalgo C, Hamilton SL. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J Biol Chem 281: 40354–40368, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Argadine HM, Hellyer NJ, Mantilla CB, Zhan WZ, Sieck GC. The effect of denervation on protein synthesis and degradation in adult rat diaphragm muscle. J Appl Physiol 107: 438–444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Argadine HM, Mantilla CB, Zhan WZ, Sieck GC. Intracellular signaling pathways regulating net protein balance following diaphragm muscle denervation. Am J Physiol Cell Physiol 300: C318–C327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Avila G, Dirksen RT. Functional impact of the ryanodine receptor on the skeletal muscle L-type Ca2+ channel. J Gen Physiol 115: 467–480, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 15: 325–330, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berg DK, Hall ZW. Increased extrajunctional acetylcholine sensitivity produced by chronic post-synaptic neuromuscular blockade. J Physiol 244: 659–676, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Campellone JV, Lacomis D, Kramer DJ, Van Cott AC, Giuliani MJ. Acute myopathy after liver transplantation. Neurology 50: 46–53, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Costelli P, Reffo P, Penna F, Autelli R, Bonelli G, Baccino FM. Ca2+-dependent proteolysis in muscle wasting. Int J Biochem Cell Biol 37: 2134–2146, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Danon MJ, Carpenter S. Myopathy with thick filament (myosin) loss following prolonged paralysis with vecuronium during steroid treatment. Muscle Nerve 14: 1131–1139, 1991 [DOI] [PubMed] [Google Scholar]
- 11. De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, Raphael JC, Outin H, Bastuji-Garin S. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 288: 2859–2867, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Di Biase V, Franzini-Armstrong C. Evolution of skeletal type e-c coupling: a novel means of controlling calcium delivery. J Cell Biol 171: 695–704, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Giovanni S, Mirabella M, D'Amico A, Tonali P, Servidei S. Apoptotic features accompany acute quadriplegic myopathy. Neurology 55: 854–858, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Favier FB, Benoit H, Freyssenet D. Cellular and molecular events controlling skeletal muscle mass in response to altered use. Pflügers Arch 456: 587–600, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Geiger PC, Bailey JP, Zhan WZ, Mantilla CB, Sieck GC. Denervation-induced changes in myosin heavy chain expression in the rat diaphragm muscle. J Appl Physiol 95: 611–619, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Geiger PC, Cody MJ, Macken RL, Bayrd ME, Sieck GC. Effect of unilateral denervation on maximum specific force in rat diaphragm muscle fibers. J Appl Physiol 90: 1196–1204, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Gilson H, Schakman O, Combaret L, Lause P, Grobet L, Attaix D, Ketelslegers JM, Thissen JP. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 148: 452–460, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Helliwell TR, Coakley JH, Wagenmakers AJ, Griffiths RD, Campbell IT, Green CJ, McClelland P, Bone JM. Necrotizing myopathy in critically-ill patients. J Pathol 164: 307–314, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Hellyer NJ, Mantilla CB, Park EW, Zhan WZ, Sieck GC. Neuregulin-dependent protein synthesis in C2C12 myotubes and rat diaphragm muscle. Am J Physiol Cell Physiol 291: C1056–C1061, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348: 683–693, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Howell S, Zhan WZ, Sieck GC. Diaphragm disuse reduces Ca2+ uptake capacity of sarcoplasmic reticulum. J Appl Physiol 82: 164–171, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Hudson LD, Lee CM. Neuromuscular sequelae of critical illness. N Engl J Med 348: 745–747, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Itagaki K, Menconi M, Antoniu B, Zhang Q, Gonnella P, Soybel DI, Hauser CJ, Hasselgren PO. Dexamethasone stimulates store-operated calcium entry and protein degradation in cultured L6 myotubes through a phospholipase A2 -dependent mechanism. Am J Physiol Cell Physiol 298: C1127–C1139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeftinija DM, Wang QB, Hebert SL, Norris CM, Yan Z, Rich MM, Kraner SD. The Cav 1.2 Ca2+ channel is expressed in sarcolemma of type I and IIa myofibers of adult skeletal muscle. Muscle Nerve 36: 482–490, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khan J, Harrison TB, Rich MM, Moss M. Early development of critical illness myopathy and neuropathy in patients with severe sepsis. Neurology 67: 1421–1425, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Kraner S, Yang J, Barchi R. Structural inferences for the native skeletal muscle sodium channel as derived from patterns of endogenous proteolysis. J Biol Chem 264: 13273–13280, 1989 [PubMed] [Google Scholar]
- 27. Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F. Dantrolene–a review of its pharmacology, therapeutic use and new developments. Anaesthesia 59: 364–373, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Lacomis D, Giuliani MJ, Van Cott A, Kramer DJ. Acute myopathy of intensive care: clinical, electromyographic, and pathological aspects. Ann Neurol 40: 645–654, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Lacomis D, Zochodne DW, Bird SJ. Critical illness myopathy. Muscle Nerve 23: 1785–1788, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Larsson L. Acute quadriplegic myopathy: an acquired “myosinopathy”. Adv Exp Med Biol 642: 92–98, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Larsson L, Li X, Edstrom L, Eriksson LI, Zackrisson H, Argentini C, Schiaffino S. Acute quadriplegia and loss of muscle myosin in patients treated with nondepolarizing neuromuscular blocking agents and corticosteroids: mechanisms at the cellular and molecular levels. Crit Care Med 28: 34–45, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Lehnart SE, Wehrens XH, Marks AR. Calstabin deficiency, ryanodine receptors, and sudden cardiac death. Biochem Biophys Res Commun 322: 1267–1279, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Lewis MI, Monn SA, Sieck GC. Effect of corticosteroids on diaphragm fatigue, SDH activity, and muscle fiber size. J Appl Physiol 72: 293–301, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Lomo T, Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol 221: 493–513, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lomo T, Westgaard RH. Further studies on the control of ACh sensitivity by muscle activity in the rat. J Physiol 252: 603–626, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. MacFarlane IA, Rosenthal FD. Severe myopathy after status asthmaticus. Lancet 2: 615, 1977 [DOI] [PubMed] [Google Scholar]
- 37. Marks AR. Intracellular calcium-release channels: regulators of cell life and death. Am J Physiol Heart Circ Physiol 272: H597–H605, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Massa R, Carpenter S, Holland P, Karpati G. Loss and renewal of thick myofilaments in glucocorticoid-treated rat soleus after denervation and reinnervation. Muscle Nerve 15: 1290–1298, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Moore BJ, Miller MJ, Feldman HA, Reid MB. Diaphragm atrophy and weakness in cortisone-treated rats. J Appl Physiol 67: 2420–2426, 1989 [DOI] [PubMed] [Google Scholar]
- 40. Mozaffar T, Haddad F, Zeng M, Zhang LY, Adams GR, Baldwin KM. Molecular and cellular defects of skeletal muscle in an animal model of acute quadriplegic myopathy. Muscle Nerve 35: 55–65, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Nakai J, Dirksen RT, Nguyen HT, Pessah IN, Beam KG, Allen PD. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature 380: 72–75, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Norman H, Nordquist J, Andersson P, Ansved T, Tang X, Dworkin B, Larsson L. Impact of post-synaptic block of neuromuscular transmission, muscle unloading and mechanical ventilation on skeletal muscle protein and mRNA expression. Pflügers Arch 453: 53–66, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Norman H, Zackrisson H, Hedstrom Y, Andersson P, Nordquist J, Eriksson LI, Libelius R, Larsson L. Myofibrillar protein and gene expression in acute quadriplegic myopathy. J Neurol Sci 285: 28–38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ochala J, Larsson L. Effects of a preferential myosin loss on Ca2+ activation of force generation in single human skeletal muscle fibres. Exp Physiol 93: 486–495, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Polla B, Bottinelli R, Sandoli D, Sardi C, Reggiani C. Cortisone-induced changes in myosin heavy chain distribution in respiratory and hindlimb muscles. Acta Physiol Scand 151: 353–361, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Rich MM, Bird SJ, Raps EC, McCluskey LF, Teener JW. Direct muscle stimulation in acute quadriplegic myopathy. Muscle Nerve 20: 665–673, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Rich MM, Kraner SD, Barchi RL. Altered gene expression in steroid-treated denervated muscle. Neurobiol Dis 6: 515–522, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Rich MM, Pinter MJ, Kraner SD, Barchi RL. Loss of electrical excitability in an animal model of acute quadriplegic myopathy. Ann Neurol 43: 171–179, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Rich MM, Teener JW, Raps EC, Schotland DL, Bird SJ. Muscle is electrically inexcitable in acute quadriplegic myopathy. Neurology 46: 731–736, 1996 [DOI] [PubMed] [Google Scholar]
- 50. Rouleau G, Karpati G, Carpenter S, Soza M, Prescott S, Holland P. Glucocorticoid excess induces preferential depletion of myosin in denervated skeletal muscle fibers. Muscle Nerve 10: 428–438, 1987 [DOI] [PubMed] [Google Scholar]
- 51. Sakai Y, Kobayashi K, Iwata N. Effects of an anabolic steroid and vitamin B complex upon myopathy induced by corticosteroids. Eur J Pharmacol 52: 353–359, 1978 [DOI] [PubMed] [Google Scholar]
- 52. Showalter CJ, Engel AG. Acute quadriplegic myopathy: analysis of myosin isoforms and evidence for calpain-mediated proteolysis. Muscle Nerve 20: 316–322, 1997 [DOI] [PubMed] [Google Scholar]
- 53. Stibler H, Edstrom L, Ahlbeck K, Remahl S, Ansved T. Electrophoretic determination of the myosin/actin ratio in the diagnosis of critical illness myopathy. Intensive Care Med 29: 1515–1527, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Striessnig J, Murphy BJ, Catterall WA. Dihydropyridine receptor of L-type Ca2+ channels: identification of binding domains for [3H]+-PN200–110 and [3H]azidopine within the alpha 1 subunit. Proc Natl Acad Sci USA 88: 10769–10773, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sultan KR, Dittrich BT, Leisner E, Paul N, Pette D. Fiber type-specific expression of major proteolytic systems in fast- to slow-transforming rabbit muscle. Am J Physiol Cell Physiol 280: C239–C247, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Sun J, Xin C, Eu JP, Stamler JS, Meissner G. Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc Natl Acad Sci USA 98: 11158–11162, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Supinski GS, Callahan LA. Calpain activation contributes to endotoxin induced diaphragmatic dysfunction. Am J Respir Cell Mol Biol 42: 80–87, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Supinski GS, Wang W, Callahan LA. Caspase and calpain activation both contribute to sepsis-induced diaphragmatic weakness. J Appl Physiol 107: 1389–1396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Van Balkom RH, Zhan WZ, Prakash YS, Dekhuijzen PN, Sieck GC. Corticosteroid effects on isotonic contractile properties of rat diaphragm muscle. J Appl Physiol 83: 1062–1067, 1997 [DOI] [PubMed] [Google Scholar]
- 60. Verheul AJ, Mantilla CB, Zhan WZ, Bernal M, Dekhuijzen PN, Sieck GC. Influence of corticosteroids on myonuclear domain size in the rat diaphragm muscle. J Appl Physiol 97: 1715–1722, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Wang KK. Calpain and caspase: can you tell the difference?, by Kevin K. W. Wang: Vol. 23, p. 20–26. Trends Neurosci 23: 59, 2000 [DOI] [PubMed] [Google Scholar]
- 62. Wang X, Li Y, Engisch KL, Nakanishi ST, Dodson SE, Miller GW, Cope TC, Pinter MJ, Rich MM. Activity-dependent presynaptic regulation of quantal size at the mammalian neuromuscular junction in vivo. J Neurosci 25: 343–351, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wehrens XH, Lehnart SE, Reiken S, van der Nagel R, Morales R, Sun J, Cheng Z, Deng SX, de Windt LJ, Landry DW, Marks AR. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc Natl Acad Sci USA 102: 9607–9612, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Williams AB, Decourten-Myers GM, Fischer JE, Luo G, Sun X, Hasselgren PO. Sepsis stimulates release of myofilaments in skeletal muscle by a calcium-dependent mechanism. FASEB J 13: 1435–1443, 1999 [DOI] [PubMed] [Google Scholar]
- 65. Winiarski AM, Roy RR, Alford EK, Chiang PC, Edgerton VR. Mechanical properties of rat skeletal muscle after hind limb suspension. Exp Neurol 96: 650–660, 1987 [DOI] [PubMed] [Google Scholar]
- 66. Wray CJ, Sun X, Gang GI, Hasselgren PO. Dantrolene downregulates the gene expression and activity of the ubiquitin-proteasome proteolytic pathway in septic skeletal muscle. J Surg Res 104: 82–87, 2002 [DOI] [PubMed] [Google Scholar]
- 67. Zhan WZ, Sieck GC. Adaptations of diaphragm and medial gastrocnemius muscles to inactivity. J Appl Physiol 72: 1445–1453, 1992 [DOI] [PubMed] [Google Scholar]
- 68. Zhang Y, Rodney GG, Schneider MF. Effects of azumolene on Ca2+ sparks in skeletal muscle fibers. J Pharmacol Exp Ther 314: 94–102, 2005 [DOI] [PubMed] [Google Scholar]