Abstract
Blood pressure (BP) increases after menopause. However, the mechanisms responsible have not been elucidated. In this study we tested the hypothesis that 20-hydroxyeicosatetraenoic acids (20-HETE), produced by cytochrome P-450 (CYP450) ω-hydroxylase, contributes to the hypertension in a model of postmenopausal hypertension, aged female spontaneously hypertensive rats (PMR). 1-Aminobenzotriazole, a nonselective inhibitor of arachidonic acid metabolism, for 7 days, reduced BP in PMR but had no effect in young females. Acute intravenous infusion of HET-0016, a specific inhibitor of 20-HETE, over 3 h, also reduced BP in PMR. CYP4A isoform mRNA expression showed no difference in renal CYP4A1 or CYP4A3 but increases in CYP4A2 and decreases in CYP4A8. CYP4A protein expression was decreased in kidney of PMR compared with young females. Endogenous 20-HETE was significantly higher in cerebral vessels of PMR than young females (YF) but was significantly lower in renal vessels of PMR. Omega-hydroxylase activity in cerebral vessels was also higher in PMR but was similar in kidney vessels in both groups. In renal microsomal preparations, endogenous 20-HETE was not different in PMR and young females, but ω-hydroxylase activity was significantly lower in PMR than YF. The data with blockers suggest that 20-HETE contributes to postmenopausal hypertension in SHR. The data also suggest that cerebral production of 20-HETE may be increased and renal tubular production may be decreased in PMR, thus both contributing to their elevated BP.
Keywords: arachidonic acid metabolite, female, blood pressure
blood pressure (bp) increases in women following menopause. Hypertension is a major risk factor for cardiovascular disease, which is the leading cause of death in postmenopausal women. BP in aging women is also less well controlled than in men (15). Therefore, a better understanding of prohypertensive mechanisms in postmenopausal women may lead to better therapeutic options.
Arachidonic acid (AA) can be metabolized via cytochrome P-450 (CYP) enzymes to epoxyeicosatrienoic acids (EETs) and 20-hydroxyeicosatetraenoic acids (20-HETE) (13). 20-HETE is a potent vasoconstrictor, while EETs are vasodilators. Blockade of vascular 20-HETE has been reported to lower blood pressure in young male (SHR) (13). On the other hand, 20-HETE also inhibits Na+-K+-ATPase activity in the proximal tubule and thick ascending limb of Henle (TALH), and impaired renal production of 20-HETE is associated with Na retention and the development of hypertension in Dahl salt-sensitive rats (8, 13). Therefore, two mechanisms by which BP could increase after menopause is via a decrease in the intrarenal tubular production of 20-HETE or an increase in the vascular production of 20-HETE. The role that 20-HETE plays in mediating hypertension in females in general, and specifically in postmenopausal rats, has not been previously studied.
In the kidney, 20-HETE is produced in vascular smooth muscle cells by CYP ω-hydroxylase enzymes, mainly isoforms CYP4A1, CYP4A2, and CYP4A8, and to a lesser extent CYP4A3 (10, 13). All four isoforms have been found in male and female rat kidneys, but CYP4A2 is thought to be the major enzyme producing 20-HETE in the preglomerular vasculature in adult male rats (10). The isoform responsible for 20-HETE synthesis in the tubules is not clear since CYP4A2, CYP4A3, and CYP4A8 were all found in nephron segments of normotensive male and female rats by Ito et al. (10). Whether hypertensive females exhibit similar expression of the isoforms of CYP4A has not been determined.
We have previously characterized aged (18 mo) female spontaneously hypertensive rats (PMR) as a model of postmenopausal hypertension (5). In PMR, estrous cycling stops by 12 mo of age, and by 16 mo of age, BP is significantly higher than in young cycling female SHR (5). Recently, we showed that some, but not all, of the increases in BP in this model are mediated by activation of the endothelin and renin-angiotensin systems (RAS) (16, 17). However, blockade of each system individually does not normalize the BP in PMR, suggesting that other mechanisms may also contribute to postmenopausal hypertension in this model. 20-HETE has previously been shown to contribute to the vasoconstrictor actions of both endothelin and ANG II (2, 13).
Thus, the present study was performed to determine the role of 20-HETE in mediating the hypertension in a unique animal model of postmenopausal hypertension, the PMR. We hypothesized that 20-HETE would contribute to the elevated BP in PMR, either via an increase in vascular 20-HETE production or due to a reduction in renal tubular 20-HETE.
METHODS
Rats
Retired breeder PMRs, were received from the vendor (Taconic Farms; Germantown, NY) at 4–9 mo of age and were aged in the Laboratory Animal Facility of the University of Mississippi Medical Center. PMR were studied at 18 mo of age. Controls were young female SHR obtained at 2 mo of age and studied at 3 mo. All rats were maintained on standard rat chow (Teklad, Harlan Sprague Dawley, Indianapolis, IN) and tap water in an environment with 12:12-h light-dark cycles. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center, and studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals, National Institutes of Health.
Experimental Protocols
Effects of 1-aminobenzotriazole.
Radiotelemetry BP was monitored, as we have previously reported (18). Briefly, telemetry probes were implanted below the renal arteries using aseptic technique using inhaled anesthesia (isoflurane), and rats were allowed to recover for 2 wk. After that, mean arterial pressure (MAP) was recorded 24 h a day during a 3-day baseline period and then for 7 days while the rats received daily intraperitoneal injection of the nonspecific CYP 450 enzyme inhibitor, 1-aminobenzotriazole (1-ABT; Sigma, St. Louis, MO) in a dose of 50 mg·kg−1·day−1 or vehicle (0.9% NaCl). The effects of vehicle or ABT on MAP were compared in PMR (n = 6 or 7/group) and young female SHR (n = 5–6/group). This dose of 1-ABT has been previously shown to inhibit renal production of 20-HETE and EETs (3, 14).
Effects of HET-0016 on BP.
The effects of a more selective inhibitor of 20-HETE, N-hydroxy-N-(4-butyl-2 methylphenyl)formamidine (HET-0016; Cayman Chemical, Ann Arbor, MI) (12), on MAP was also determined in PMRs. A group of PMRs were anesthetized by isoflurane, and a catheter was placed in the femoral artery and vein. The catheters were exteriorized at the back of the neck, and after 4 days of recovery, MAP was recorded in conscious freely moving rats using Power LAB software, as we have previously reported (18). Following 30 min baseline MAP, PMR were given either HET-0016 (n = 10; 10 mg/kg bolus and then 1 mg·kg−1·h−1) or vehicle (n = 6; captisol 30% in saline) intravenously, and MAP was recorded over a 3-h period.
Preparation of renal microsomes.
Microsomal preparations were made from kidneys removed from young female rats and PMR (n = 6/group). Kidney tissue (0.5 g) was homogenized in 3 ml 100 mM potassium phosphate buffer (pH 7.4), with 250 mM sucrose and protease inhibitor cocktail (Sigma, St. Louis, MO). Following differential centrifugation, the supernatant fraction was discarded, and the pellet was resuspended in 500 μl 100 mM potassium phosphate buffer (pH 7.4), with 10 mM MgCl2, 33% glycerol, 500 mM EDTA, and 1 μM DTT. Protein concentration was determined by Bradford assay using a commercially available reagent (Bio-Rad, Richmond, CA) with BSA 2% as a standard.
Measurement of 20-HETE and ω-hydroxylase activity in cerebral and preglomerular blood vessels and renal microsomes of PMR and YF SHR.
Endogenous 20-HETE and ω-hydroxylase activity in cerebral and renal vessels and renal microsomes were assessed by HPLC-mass spectroscopy in tissues from young female SHR and PMR (n = 6/group). In our experience, the preglomerular microvasculature can be easily contaminated with tubular segments that have very high levels of 20-HETE synthesis. Thus, we also isolated cerebral microvessels to obtain an additional indication of microvascular 20-HETE production that was extrarenal. Both renal and cerebral vessels were isolated using 3% Evans blue and sieving methods, as described previously (4, 9). Endogenous 20-HETE levels were measured in vessels that were homogenized in 1 ml of ice-cold physiological salt solution. To determine ω-hydroxylase activity, isolated vessels were incubated for 30 min at 37°C in 1 ml of 100 mM potassium phosphate buffer (pH 7.4), containing 10 mM MgCl2 and 500 mM EDTA, 1 mM reduced NADPH, 40 μM cold arachidonic acid, and 2 μM indomethacin in a shaking bath, with 100% O2 superfusion. Reactions were terminated by acidification to pH 3.5 with formic acid, and the vessels were homogenized. Tissue samples were extracted twice with 3 ml of ethyl acetate, and organic layer dried under nitrogen. Samples were reconstituted in acetonitrile and analyzed using LC/MS/MS, as previously described (4), using an ABI Sciex 4000 Q TRAP system.
Measurement of mRNA expression of CYP4A isoforms with real-time RT-PCR in kidney of PMR and YF SHR.
Kidney cortical and medullary mRNA (n = 8 rats/group) was extracted with TRIzol Reagent (Molecular Research Center, Cincinnati, OH), resuspended in DEPC-H2O, DNase treated with DNA-free kit (Ambion, Austin, TX), and quantified by spectrophotometry, as we previously reported (17). Five micrograms of RNA were reverse transcribed with 0.5 μg T12VN primer and Superscript III (Invitrogen, Carlsbad, CA) in a final volume of 20 μl. The reaction was carried out for 60 min at 50°C and terminated by incubating at 75°C for 15 min. Primers for CYP4A1, CYP4A2, CYP4A3, and CYP4A8 have been previously described (11). Elongation factor I (EF-1) primers were used as controls. Real-time RT-PCR reactions contained 1 μl RT product, 0.1 μM each primer, 0.2 mM dNTPs, SYBR Green I (1:20,000 final concentration; Molecular Probes, Eugene, OR) and 1 μl Titanium Taq DNA polymerase (Clontech, Palo Alto, CA). Amplifications were performed in an iCycler real-time thermal cycler (Bio-Rad). Cycling conditions were 1 min at 95°C, followed by 50 cycles of 15 s at 95°C, 15 s at 60.0°C, and 60 s at 72°C. Fluorescence data were collected during the elongation step. After PCR amplification, the specificity of the PCR reaction was confirmed by melting temperature determination of the PCR products and electrophoretic analysis in 2% agarose gels. Standard curves were made with serial dilutions of pooled RT samples. Results are expressed as arbitrary units and standardized against EF-1 mRNA expression.
Western blot analysis of CYP4A in kidney microsomes.
Protein levels of CYP4A were determined in renal microsomal preparations from young female SHR and PMR (n = 6/group) by Western blot analysis, as we have previously described (17), using goat anti-CYP4A antibody (1:3,000; Daiichi, Tokyo, Japan) and donkey anti-goat secondary antibodies (1:15,000; Santa Cruz Biotechnology, Santa Cruz, CA). Mouse beta-actin antibody served as a loading control (1:10,000; Santa Cruz) with goat anti-mouse as secondary antibody (1:20,000; Santa Cruz).
Statistics.
Data are presented as means ± SE. Statistical analyses were performed with SigmaPlot v11 (Systat Software, San Jose, CA). MAP changes between young and old PMR with and without 1-ABT over 7 days were analyzed using a repeated-measures ANOVA followed by Student-Newman-Keuls post hoc comparisons. Differences in MAP with HET-0016 in PMR were analyzed by Student's t-test. Student's t-test was also used for comparison between young females and PMR in molecular studies and measurement of 20-HETE and omega-hydroxylase activities. A value of P < 0.05 was considered statistically significant.
RESULTS
Effects of 1-ABT on MAP in PMR and YF SHR.
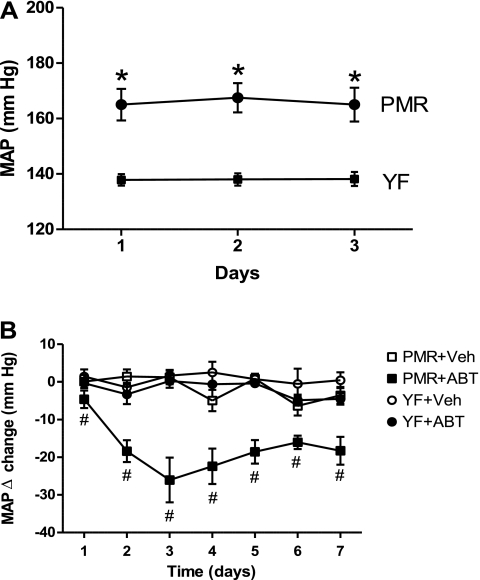
Telemetric MAP was significantly higher in PMR than young females during the 3-day baseline period (Fig. 1A) (166 ± 1 vs. 138 ± 1 mmHg, P < 0.05). Chronic treatment with 1-ABT reduced MAP significantly in PMR (average over 7 days =18 ± 3 mmHg), but had no effect on MAP in young female SHR (Fig. 1B).
Fig. 1.
A: Mean artierial pressure (MAP) during baseline period in female spontaneously hypertensive rats (PMR) and young female spontaneously hypertensive rats (YF SHR). B: effect of chronic 1- aminobenzotriazole (ABT) administration on MAP in PMR and young female SHR. Data are expressed as delta change ± SE. *P < 0.05 compared with young female SHR. #P < 0.05 compared with PMR controls.
Effect of HET-0016 infusion on MAP of PMR.
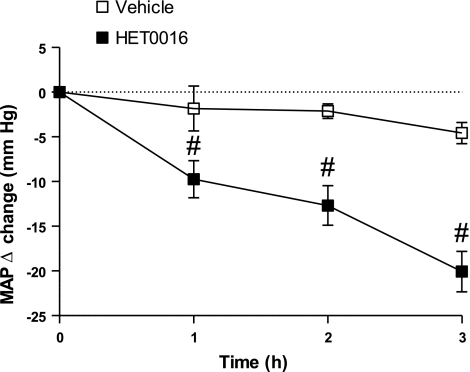
MAP in PMR controls and untreated PMR was 203 ± 3 mm and 200 ± 3 mmHg, respectively. Acute infusion of HET-0016, the specific inhibitor of 20-HETE synthesis, over 3 h, reduced MAP by 21 ± 3 mmHg in conscious, freely moving PMR (Fig. 2). Vehicle had no effect on MAP in PMR.
Fig. 2.
Effect of HET-0016 administration on MAP in PMR. Data are expressed as delta change in MAP compared with the baseline period. #P < 0.05 compared with untreated rats.
Expression (mRNA) of CYP 4A isoforms in kidney cortex and medulla in PMR and YF SHR.
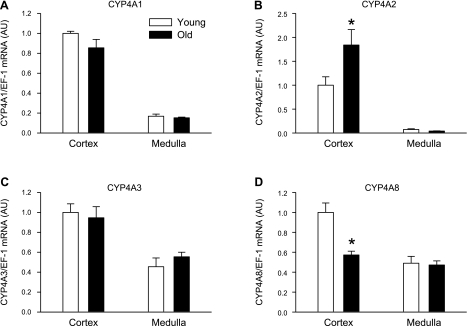
The results of these experiments are presented in Fig. 3. Expression of renal CYP4A1 and CYP4A3 mRNA was similar in cortex and medulla of PMR and young females (Figs. 3, A and C, respectively). Expression of CYP4A2 mRNA was significantly elevated in the renal cortex in PMR compared with young females and was in very low abundance and not different in medulla between the groups (Fig. 3B). In contrast, CYP4A8 mRNA expression was significantly decreased in the renal cortex of PMR compared with young female counterparts and was not different in the medulla between the groups (Fig. 3D).
Fig. 3.
mRNA levels of renal CYP 450 isoforms from PMR and young females by real-time RT-PCR. A and C: CYP4A1 and CYP4A3 mRNA expression were similar in PMR compared with YF SHR. B: CYP4A2 mRNA expression was significantly upregualted in PMR compared with YF SHR. D: CYP4A8 mRNA expression was significantly downregulated in PMR compared with YFSHR. Data are expressed as means ± SE. *P < 0.05 compared with young females.
Renal microsomal CYP4A protein expression.
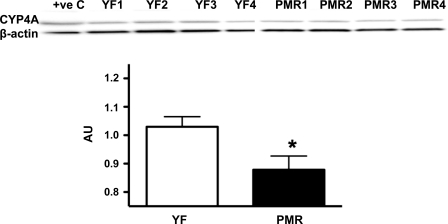
CYP4A protein expression in microsomal fractions was significantly lower in PMR than young females (Fig. 4).
Fig. 4.
CYP4A protein expression in young female SHR and PMR. Representative Western blot, and analyses of bands. Data are expressed at arbitrary units (AU) for each group and are presented as means ± SE. *P < 0.05 compared with young females.
Renal and cerebral vascular 20-HETE levels and ω-hydroxylase activity.
The results of these experiments are presented in Table 1. Endogenous 20-HETE levels in cerebral vessels were six-fold higher in PMR than young females. In contrast, endogenous 20-HETE in renal microvessels were 4-fold lower in PMR than young females. Omega-hydroxylase activity was also higher in cerebral vessels of PMR compared with young females but was not different in intrarenal microvessels between young and old females. Levels of endogenous 20-HETE in intrarenal microsomal fractions were not different between PMR and young females. However, ω-hydroxylase activity was significantly lower in microsomal fractions of PMR than young females.
Table 1.
20-HETE and ω-hydroxylase activities in cerebral and renal vessels and renal microsomes of YF and PMR
| 20-HETE Levels, pmol/mg |
ω-Hydroxylase Activity, pmol 20-HETE·min−1·mg−1 |
|||||
|---|---|---|---|---|---|---|
| Rats | Cerebral Vessels | Renal Vessels | Renal Microsomes | Cerebral Vessels | Renal Vessels | Renal Microsomes |
| YF (n = 6) | 0.138 ± 0.013 | 5.32 ± 1.20 | 6.40 ± 0.90 | 0.075 ± 0.007 | 0.905 ± 0.205 | 101.5 ± 5.2 |
| PMR (n = 6) | 0.821 ± 0.263 | 0.902 ± 0.064 | 6.20 ± 0.61 | 0.133 ± 0.020 | 1.28 ± 0.24 | 44.4 ± 2.8 |
| P value | <0.05 | <0.01 | NS | 0.05 | NS | <0.001 |
Values are expressed as means ± SE. YF, young females; PMR, female spontaneously hypertensive rats; NS, not significant.
DISCUSSION
BP increases after menopause in women, and hypertension is a major cardiovascular disease risk factor. The leading cause of death in postmenopausal women is cardiovascular disease (1, 7). The baseline study from the Women's Health Initiative showed that >60% of hypertensive postmenopausal women in the United States are currently treated with conventional pharmacological drugs, such as calcium channel blockers and diuretics, yet the hypertension was poorly controlled (15). These data suggest that more research is needed to elucidate the mechanisms that mediate postmenopausal hypertension to develop specific treatment protocols.
Previous studies have shown that the production of 20-HETE is altered in experimental and genetic models of hypertension, diabetes, pregnancy, and uremia (13). However, the role of 20-HETE in altering BP in an animal model of postmenopausal hypertension has not been studied previously. In the present study, we found that chronic infusion of 1-ABT, which inhibits the synthesis of both 20-HETE and EETs, lowered BP in PMR. It had no significant effect in young females. Similarly, acute administration of a specific inhibitor of 20-HETE, HET-0016, reduced MAP by about the same amount in PMR. These results are consistent with the view that an elevated vascular production of the vasoconstrictor 20-HETE contributes to the elevation in blood pressure in PMR.
To test this hypothesis, we measured endogenous 20-HETE and ω-hydroxylase activity in renal and cerebral arterioles, as well as in renal cortical microsomes. In addition, we measured the mRNA expression of CYP4A isoforms and the protein expression of CYP4A in kidney tissue. We found different levels of 20-HETE in cerebral and kidney vessels in PMR compared with young females. In cerebral microvessels, we found that endogenous 20-HETE levels were significantly higher in PMR than young females. But in kidney microvessels, 20-HETE levels were significantly lower in PMR than young females. One caveat to this finding is that renal microvessels can be contaminated with tubular segments that make much higher levels of 20-HETE, whereas 20-HETE is not thought to be made in other extra cerebral tissues than cerebral microvessels. On the basis of this, we measured cerebral 20-HETE and ω-hydroxylase activity levels to verify our findings of endogenous 20-HETE in kidney microvessels. It is possible that the renal microvessels of young females were contaminated with tubular segments. However, we doubt this is the case because the data were consistent between the groups, i.e., endogenous 20-HETE was much higher in all renal vascular samples from young females compared with PMR. Therefore, we believe that the renal microvascular 20-HETE levels are lower in PMR than young females. In support of higher 20-HETE in cerebral microvessels, ω-hydroxylase activity, the capacity to produce 20-HETE, was also higher in PMR than young females. In contrast, along with lower endogenous 20-HETE levels in renal microvessels of PMR, ω-hydroxylase activity was not different in renal microvessels of PMR and young females. These data suggest that the mechanism for the fall in BP with the 1-ABT and HET-0016 blockers, was due to a reduction in extrarenal 20-HETE vascular production.
In contrast to the renal vasculature, in renal cortical microsomes, mainly a tubular preparation, endogenous 20-HETE levels were similar, but the capacity to produce 20-HETE as measured by ω-hydroxylase activity was twofold lower in PMR than young females. These data support a role for reductions in 20-HETE synthesis in the renal tubules to contribute to the elevated BP in PMR compared with young females. The lower levels of CYP4A protein in the microsomes and the reduction in CYP4A8 in the cortex of PMR support these data. Since 20-HETE in renal tubules is antihypertensive due to its inhibitory effect on sodium reabsorption, a decrease in 20-HETE synthesis in renal tubules would be prohypertensive. In response to HET-0016 or 1-ABT, a further decrease in 20-HETE should cause an increase in BP. The reduction in BP with both blockers was ∼20 mmHg, leaving PMR with BPs still above normotensive levels. It is possible that with the blockers, the prohypertensive effect of reduced renal tubular 20-HETE was offset by the antihypertensive effect of increased extrarenal vascular 20-HETE.
In any case, the studies with the 20-HETE synthesis blockers do support a role for 20-HETE in contributing to the elevated BP in PMR. In previous studies, we have found that blockade of the RAS with losartan or the endothelin system with ETA receptor antagonists also does not normalize the BP in PMR (16, 17). Given the present data, we can hypothesize that multiple pathways contribute to the hypertension in PMR. Additional studies will be necessary to determine whether treatment with multiple blockers will reduce the BP in PMR to normotensive levels. Furthermore, the use of multiple blockers will also allow us to determine whether a component of the endothelin- or ANG II-mediated hypertension in PMR is mediated by 20-HETE, since both endothelin and ANG II can stimulate 20-HETE synthesis (13).
The expression of CYP4A isoforms has not been studied in female SHR previously. Studies by Ito and colleagues showed that there were sex differences in the expression of some of the CYP4A isoforms along the nephron (10). However, those studies were performed in Sprague-Dawley rats and the isoforms that were different between young and old female SHR, the 4A2 and 4A8, were found in similar areas of the nephron in both males and females.
Finally, because 1-ABT can also inhibit synthesis of EETs, it is possible that any effect to inhibit vasodilator EETs would offset the effect on BP. However, in preliminary studies, we have infused EET inhibitor, miconazole, in PMR and found no effect on BP (K. Yuan and J. F. Reckelhoff, unpublished observations). However, it is possible the EET levels could be already reduced in PMR. Further studies will be necessary to measure EET levels to determine whether they are, indeed, changed compared with young females.
Perspectives and Significance
The mechanisms responsible for postmenopausal increases in BP are not clear. However, women are at greatest risk for cardiovascular disease after menopause. An increase in BP is a significant risk factor for promoting cardiovascular disease, which is the leading cause of death and morbidity in aging women (1, 7). Our present data suggest that an increase in extrarenal vascular 20-HETE and a reduction in renal tubular 20-HETE, in part, contribute to the elevated BP in our model of postmenopausal hypertension. Identification of therapeutic targets specifically mediating hypertension in postmenopausal women may allow for specific treatment regimens that will improve their care and quality of life as they age.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
These studies were supported by National Institutes of Health Grants HL66072, HL51971, and HL69194 to J. F. Reckelhoff; HL085907, HL085907S1, and HL092284 to M. J. Ryan, and American Heart Association Scientist Development Grant 0830239N to LLY.
REFERENCES
- 1. Bentley-Lewis R, Koruda K, Seely EW. The metabolic syndrome in women. Nat Clin Pract Endocrinol Metab 3: 696–704, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Croft KD, McGiff JC, Sanchez-Mendoza A, Carroll MA. Angiotensin II releases 20-HETE from rat renal microvessels. Am J Physiol Renal Physiol 279: F544–F551, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Dos Santos EA, Dahly-Vernon AJ, Hoagland KM, Roman RJ. Inhibition of the formation of EETs and 20-HETE with 1-aminobenzotriazole attenuates pressure natriuresis. Am J Physiol Regul Integr Comp Physiol 287: R58–R68, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Dunn KM, Renic M, Flasch AK, Harder DR, Falck JR, Roman RJ. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 295: H2455–H2465, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fortepiani LA, Zhang H, Racusen L, Roberts LJ, 2nd, Reckelhoff JF. Characterization of an animal model of postmenopausal hypertension in spontaneously hypertensive rats. Hypertension 41: 640–645, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Fortepiani LA, Reckelhoff JF. Role of oxidative stress in the sex differences in blood pressure in SHR. J Hypertension 23: 801–805, 2005 [DOI] [PubMed] [Google Scholar]
- 7. He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP, Klag MJ, Whelton PK. Major causes of death among men and women in China. N Engl J Med 353: 1124–1134, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Hoagland KM, Maier KG, Roman RJ. Contributions of 20-HETE to the antihypertensive effects of Tempol in Dahl salt-sensitive rats. Hypertension 41: 697–702, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Imig JD, Zou AP, Stec DE, Harder DR, Falck JR, Roman RJ. Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am J Physiol 39: 217–227, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Ito O, Alonso-Galicia M, Hopp KA, Roman RJ. Localization of cytochrome P-450 4A isoforms along the rat nephron. Am J Physiol Renal Physiol 274: F395–F404, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Marji JS, Wang MH, Laniado-Schwartzman M. Cytochrome P-450 4A isoform expression and 20-HETE synthesis in renal preglomerular arteries. Am J Physiol Renal Physiol 283: F60–F67, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M, Kameo K. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol 133: 325–329, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Su P, Kaushal KM, Kroetz DL. Inhibition of renal arachidonic acid omega-hydroxylase activity with ABT reduces blood pressure in the SHR. Am J Physiol Regul Integr Comp Physiol 275: R426–R438, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Wassertheil-Smoller S, Anderson G, Psaty BM, Black HR, Manson J, Wong N, Francis J, Grimm R, Kotchen T, Langer R, Lasser N. Hypertension and its treatment in postmenopausal women: baseline data from the Women's Health Initiative. Hypertension 36: 780–789, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Yanes LL, Romero DG, Cucchiarelli VE, Fortepiani LA, Gomez-Sanchez CE, Santacruz F, Reckelhoff JF. Role of endothelin in mediating postmenopausal hypertension in a rat model. Am J Physiol Regul Integr Comp Physiol 288: R229–R233, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Yanes LL, Romero DG, Iles JW, Iliescu R, Gomez-Sanchez C, Reckelhoff JF. Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 291: R383–R390, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 296: F771–F779, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]