Abstract
The proper detection of environmental temperatures is essential for the optimal growth and survival of organisms of all shapes and phyla, yet only recently have the molecular mechanisms for temperature sensing been elucidated. The discovery of temperature-sensitive ion channels of the transient receptor potential (TRP) superfamily has been pivotal in explaining how temperatures are sensed in vivo, and here we will focus on the lone member of this cohort, TRPM8, which has been unequivocally shown to be cold sensitive. TRPM8 is expressed in somatosensory neurons that innervate peripheral tissues such as the skin and oral cavity, and recent genetic evidence has shown it to be the principal transducer of cool and cold stimuli. It is remarkable that this one channel, unlike other thermosensitive TRP channels, is associated with both innocuous and noxious temperature transduction, as well as cold hypersensitivity during injury and, paradoxically, cold-mediated analgesia. With ongoing research, the field is getting closer to answering a number of fundamental questions regarding this channel, including the cellular mechanisms of TRPM8 modulation, the molecular context of TRPM8 expression, as well as the full extent of the role of TRPM8 in cold signaling in vivo. These findings will further our understanding of basic thermotransduction and sensory coding, and may have important implications for treatments for acute and chronic pain.
Keywords: channel, pain, temperature
temperature discrimination is vital for the survival of most organisms, as failure to avoid nonoptimal temperatures can result in tissue damage or even death. While not as robust or precipitous as the sensation of heat, exposure to cold can produce strong and uncomfortable sensations and can be dangerous in terms of tissue damage (frostbite) and maintenance of core body temperature. Cold can also be therapeutic, as it is a common treatment strategy in response to injury. Thus, understanding of how cold, an external stimuli, is converted to neural activity, an internal signal, is an important question both biologically and clinically. Within the last decade and a half, a subset of genes of the transient receptor potential (TRP) family of ion channels has emerged as key mediators of temperature sensation with the preponderance of the thermal-sensitive channels involved in heat sensation (56). Cold, however, has been a relatively more controversial area with two distinct TRP ion channels, TRPM8 and TRPA1, implicated. Here we will discuss the former and our current understanding of its role in cold transduction, but consider how TRPA1 may also contribute to somatosensation. In consideration of each channel and its validity as a molecular cold transducer, we propose that three requirements need to be met: 1) the candidate gene must be expressed in cold-responsive sensory nerves that innervate organs such as the skin, 2) its activation by cold must lead to the generation of nerve impulses, and 3) it should confer cold sensation in vivo. We propose that only TRPM8 meets all three of these requirements.
The Search for the Cold Transducer
The study of cold transduction is by no means a new field. Although the identification of TRPM8 occurred within the last decade (57, 63), studies focusing on cold nerve fibers have been conducted for many years. For example, in the mid-20th century, Hensel and Zotterman published several classic papers demonstrating cold-evoked electrical impulses in the cat lingual nerve (33, 35–37). In addition, they elegantly linked the effect of menthol (the active ingredient in mint that evokes a psychophysical sensation of cold) to cold-responsive fibers, showing that menthol shifts the range at which these fibers fire action potentials toward warmer temperatures, rendering them more active at physiological temperatures (34). Furthermore, if the temperature of the tongue was maintained at 40°C, menthol-induced impulses were not observed, thereby suggesting that menthol stimulation is temperature dependent (34). These seminal experiments served as the first evidence of the existence of a cold receptor, specifically expressed within cold fibers, on which menthol was acting. In the next half century, several reports delved into the cellular mechanisms that account for menthol's actions on cold fibers, with several studies reporting that menthol inhibits voltage-dependent Ca2+ channels, which in turn inhibit Ca2+-dependent K+ conductances (76, 82). Nonetheless, a linkage to cold was not shown and the field searched for an explanation of how a nerve fiber could be electrically excited by lowering the temperature, a seemingly paradoxical situation in respect to the laws of thermodynamics.
With this last point in mind, one attractive and highly intuitive theory on the molecular basis of cold transduction was that cold inhibition of various molecular entities provoked nerve impulses (78). For example, it was shown that inhibition of the Na+/K+-ATPase increased the static discharge frequency of cold fibers at room temperature, thereby implicating it as a candidate molecular cold transducer (65). However, subsequent studies found that although inhibition of the Na+/K+-ATPase results in depolarization of cold-sensitive neurons, the magnitude of the depolarization was not sufficient to evoke nerve impulses (70). Similarly, inhibition of potassium conductances also plays a fundamental role in cold transduction. Initially, using patch-clamp recordings of cultured dorsal root ganglion (DRG) neurons, two groups reported that cooling inhibited potassium conductances that normally serve as excitability brakes to limit depolarization (70, 89). These intriguing observations generated the hypothesis that upon cooling, inhibition of these currents allows the cell to depolarize to a level sufficient to generate nerve impulses. Furthermore, cold-insensitive neurons could be made cold-responsive via inhibition of the slow transient potassium current (89). Thus, these results suggested that cold sensing was not the result of a specific cold transducer molecule but rather a balance of excitatory and inhibitory currents within a given cell that could render it cold sensitive (51, 89). Potassium channels clearly play modulatory roles in cold signaling; however, the identity of the specific channel(s) that mediate these excitatory current(s) had not been accounted for. Similarly, the two-pore K+ channels TREK-1 and TRAAK, which act as leak currents and are constitutively open and regulate membrane potential, have been proposed to regulate thermal sensitivity (53). In cold signaling, the loss of both channels in the TREK-1/TRAAK double knockout mice lowers the threshold for neural activation, thereby increasing the rate of firing (62). Therefore these channels likely play an important role in membrane excitability in sensory afferents, and their absence makes cold receptors more readily depolarized. Finally, members of the degenerin family of epithelial sodium channels (DEG/ENaC) have been shown to be potentiated by cold temperatures, yet thermosensory deficits in mice null for these channels have yet to be reported (4). Thus, cold clearly has profound effects on many cellular processes, such as protein and enzyme kinetics, as well as membrane fluidity, yet how cold uses these as a mechanism for neural activation remained obscure.
In later studies, stimulation of cultured DRG or trigeminal ganglion neurons by cold was shown to induce calcium influx that is facilitated by menthol (72, 81). Using an intracellular calcium indicator, Suto and Gotoh (81) reported that ∼13% of cultured DRG neurons respond to cold with increased intracellular calcium that was independent of extracellular sodium, strongly suggesting that this increase was due to Ca2+ influx via a cold-gated channel and not due to a depolarizing impulse leading to the activation of voltage-gated Ca2+ channels. This was confirmed electrophysiologically first in cultured DRG neurons (72), and then shortly thereafter in cells from the trigeminal ganglia (57). Reid and Flonta (72) using perforated-patch recordings of cold-sensitive DRG neurons, demonstrated that cold and menthol induce inward ionic currents and, similar to the observations of Hensel and Zotterman (34) 50 years earlier, that menthol potentiated these cold-evoked currents. Similarly, cold and menthol-sensitive trigeminal ganglion neurons were also shown to express a nonselective cation current that was activated by cold and menthol and had biophysical properties (outward rectification and ion selectivity) remarkably similar to that of TRPV1 and TRPV2, which at the time were the only known thermally sensitive ion channels (57). These studies were the first description of a cooling-activated ionic current and suggested that “inhibition of a background K+ current and of the electrogenic Na+/K+-ATPase” were secondary in the determination of cold-receptor activity (70). The next question was, exactly which molecule(s) mediated these cold- and menthol-gated currents?
TRPM8 Cloning, Modulation, and Mechanism In Vitro
An answer to this question immediately followed with the cloning of a cold and menthol receptor, TRPM8 (also called CMR1) (57, 63). TRPM8 (or Trp-p8) was originally discovered in a subtractive screen for molecules highly expressed in cancerous prostate cells, but neither its functional properties nor its expression in sensory ganglia were reported (87). In a remarkable convergence, two independent groups, using different experimental strategies, identified TRPM8 as a putative cold sensor in primary sensory afferents (57, 63). One approach was based on a genomic screen for TRP ion channels with robust expression in sensory ganglia (63). This intuitive strategy was based on the previous identification of the heat-sensitive channel TRPV1 in sensory neurons (18) and, in addition to TRPM8, was instrumental in the identification of other somatosensory-relevant TRP channels such as TRPA1 and TRPV3 (64, 77, 80, 94). The second strategy used an expression cloning paradigm similar to that used to identify TRPV1, in this case, a screen for trigeminal sensory neuron transcripts which could confer menthol- and cold-sensitivity to a heterologous cell-type (57). The hypothesis for this strategy stemmed from Hensel and Zotterman's original posit that menthol's action is “upon an enzyme, which is concerned in the thermally conditioned regulation of the discharge of the cold receptors” (34). Thus, if the molecular mediator of menthol's action were to be identified it, like that previously for capsaicin (18), would provide insights into cold transduction (57). Once cloned, the functional properties of recombinant TRPM8 channels were characterized and found to be remarkably similar to that observed for menthol- and cold-gated currents in cold-responsive afferents, including temperature sensitivity, menthol sensitivity, cation selectivity, channel rectification, and Ca2+-dependent adaptation (57, 63, 71). These results convincingly corroborated Hensel and Zotterman's hypothesis that menthol potentiated a cold receptor and directly showed an ionic mechanism that is stimulated by cold (57, 63).
Aside from menthol, a number of natural and synthetic chemicals have been shown to activate TRPM8 (29). Another plant-derived chemical eucalyptol was also found to activate the channel, albeit mildly, whereas the synthetic chemical icilin (AG-3–5) is a much more potent TRPM8 agonist, thus its designation as a “super-cooling” agent (57, 92). A number of menthol-derived WS compounds have been reported to gate the channel, with WS-12 being the most potent known agonist of TRPM8 to date (11). Although endogenous TRPM8 activators have been reported, such as lysophospholipids produced by the activity of calcium-independent phospholipases (iPLA2), the mechanism and purpose of nonthermal TRPM8 activation is unknown (3, 88). Overall, the effect of chemical activators of TRPM8 is to shift the temperature-gating curve of the channel so that it is more likely to open at warmer temperatures (72).
Another mode of TRPM8 modulation is the rapid calcium-dependent adaptation of the channel upon cold or agonist stimulation (57). Like many sensory systems, cold receptors adapt to prolonged cold stimuli, a process that is essential for signal discrimination in a changing thermal environment (16, 25). In vitro, recombinant TRPM8 channels also adapt or desensitize to prolonged cold stimulation in a manner that is Ca2+-dependent (57). The mechanism of adaptation was recently demonstrated to be a negative feedback loop due to cleavage of the membrane lipid phosphatidylinositol-(4,5)-bisphosphate (PIP2) via calcium-dependent activation of cytosolic PLC (24, 49, 73). Evidence suggests that influx of calcium ions through the channel activates Ca2+-sensitive PLCδ isozymes, likely PLCδ3 or -4 (24), which cleaves PIP2 in the inner leaflet of the plasma membrane into the second messengers diacylglycerol and inositol-(1,4,5)-trisphosphate (IP3). Since the direct association of PIP2 with TRPM8 is necessary for normal channel function, its enzymatic cleavage or removal by PIP2-scavenging molecules results in the reduction of TRPM8 currents (24, 49, 73). Subsequent stimulation of the channel is impaired until normal PIP2 levels are restored, which can be achieved by warming the cell to physiological temperatures and PIP2 resynthesis by PI-kinases (24, 49). As PLC is a common downstream effector protein of many cell surface signaling receptors (6), this form of regulation likely strongly influences cold signaling via TRPM8 (24). Specifically, if the rate of PIP2 breakdown is larger than synthesis, activation of TRPM8 channels would produce a less than optimal change in membrane potential, thereby necessitating a more robust stimulus for nerve activation. This may influence cold signaling in two ways. First, under pathological conditions that lead to increased PLC activity, cold receptors may require a more robust stimulus for activation. For example, cold- and menthol-evoked responses are diminished in DRG neurons treated with the inflammatory mediators such as bradykinin, prostaglandin E2, or histamine (48). Second, some cell types may have intrinsically lower PIP2 levels due to differential effects of breakdown/synthesis pathways. Thus, in this scenario, reduced TRPM8-mediated currents are present, thereby requiring a stronger stimulus for activation. Precedence for lower levels of functional TRPM8 expression has been reported in vitro for cold-sensitive cells with significantly colder thresholds for activation (51). Thus, one postulate for TRPM8 function in innocuous cool vs. noxious cold-signaling afferents could include differential levels of PIP2 and/or PIP2 synthesis and degradation pathways.
The physical mechanism of temperature sensing for TRPM8, as well as other thermosensitive TRP channels, is an area of intensive study. Both TRPV1 and TRPM8 do show some voltage-dependency in that each is characterized as having currents with strong outward rectification (18, 57, 85). These properties suggest that activation by temperature and voltage are intimately linked, as temperature has been shown to affect the maximum open probability of the channel in response to voltage changes, and a change in the channel's ability to sense voltage affects its thermal gating (12, 90, 91). For instance, neutralization of positively charged residues in the fourth transmembrane domain (S4) and the S4–S5 linker domain of the channel reduce the number of gating charges, suggesting these to be the site of a voltage sensor (91). However, there is evidence that temperature-, agonist-, and voltage-dependent gating are independent processes since distinct activation domains for each have been identified, suggesting that the effect of one gating mechanism acts on another in an allosteric fashion (12–14, 24, 55). For example, chimeric TRPM8 and TRPV1 channels suggest that the temperature sensor is a modular COOH-terminal domain and not associated with the S4–S5 domains previously linked to temperature and voltage sensing (14, 91). Similarly, PIP2- and PLC-mediated adaptation leads to a change in the voltage dependence of the channel but does not alter thermal sensitivity of TRPM8 channels (24). This, as well as recent evidence of the dissociation of thermal and voltage gating in TRPV1, suggests that the mechanisms of activation of TRPM8 by cold and by voltage, although related, are separate processes (24, 31, 90). It should be noted, however, that despite these distinct activation domains, certain TRPM8-specific antagonists have been shown to inhibit more than one process (46).
TRPM8 Confers Cold Sensation In Vivo
While the in vitro properties of TRPM8 were promising in its consideration as a cold sensor, it remained unknown whether TRPM8 was actually responsible for cold sensation in vivo (56, 68). In 2007, three independent groups created mouse lines in which the trpm8 genomic allele was disrupted, which, when bred to homozygosity, were null for functional TRPM8 channels (8, 21, 27). In a wide array of cellular and behavioral assays, these TRPM8 knockout mice were shown to have severe deficits in cold sensation and lacked cold allodynia and analgesia (8, 21, 27). Classical thermal behavioral assays include a heated or cooled plate from which the animal's latency to paw withdrawal is recorded as an indicator of thermal sensitivity. While this test is relatively robust for heat, rodent behavior on a cold plate is spurious at best. Indeed, compared with the hot plate test, the latencies to response in the cold plate test tend to be highly variable from group to group (23). Two groups reported no difference between TRPM8 knockout mice and their wild-type littermates when placed on cold plates held at 10, 0, −1, −5, or −10°C (8, 27), while a third group did find a significant difference on a 0°C cold plate test (21). Furthermore, the time to paw withdrawal at near freezing temperatures for wild-type mice ranged from 5 to 50 s (5, 20, and 50 s) between the three studies. These significant differences in animal behavior highlight the difficulty of these assays, and demonstrate the need for additional experimental paradigms.
With such variability in the cold plate assay, a variation on this approach using lightly restrained mice was reported recently (30). This method allows for easier measurements of both paws independently as only one is placed on a cold plate at a time. Additionally, this assay eliminates any confounds caused by whole body exposure to cold and subsequent reduction in mobility as seen in the cold plate assay. However, the acts of restraining and habituating the animals to being restrained can be problematic. Using this assay, Gentry et al. (30) found that TRPM8 knockout mice had significantly higher withdrawal latencies than wild type when their hindpaws were placed on a 10°C plate (wild type = ∼15 s, TRPM8 KO = ∼29 s), thus reaffirming that TRPM8 plays a role in cold sensation.
The use of the evaporative cooling assay, in which acetone is applied to the hindpaw, has further implicated TRPM8 in this process with two groups both showing reductions in acetone-evoked behaviors in TRPM8 knockout mice (8, 27). In addition, TRPM8 knockout mice appear to have altered thermal preference as seen by their spending the majority of their time in the 26–27°C range, differing significantly from the 30–31°C range seen in wild types, on a thermal gradient from 15 to 53.5°C (27).
The two-temperature choice assay has also proven to be a useful tool in characterizing the role of TRPM8 in cold sensation (Fig. 1). Mice are placed in a chamber and given a choice between two surfaces held at different temperatures. If both surfaces are maintained at 30°C, the optimal or thermoneutral surface temperature for normal mice (59), they will explore the entire chamber and spend an equal amount of time on each surface. If one surface is held at a cooler temperature, wild-type mice show a strong preference for 30°C by spending the majority of the time on that warmer surface. However, Bautista et al., (8) showed that TRPM8 knockout mice display no preference for the 30°C surface when the test plate is held at temperatures down to 15°C. Thus, mice lacking intact TRPM8 channels cannot discriminate between warm and putative innocuous cool temperatures. Once temperatures drop into the noxious range (10 and 5°C), TRPM8 knockouts display a preference for the warm side, albeit less than what is observed for wild-type mice (8). Data by Dhaka et al. (27) support these findings using a variety of temperature pairings. Since cool temperatures in the range of 15–30°C are generally considered innocuous, while lower temperatures are noxious, at first approximation these data suggest not only a TRPM8-dependent mechanism for innocuous cold transduction, but also the presence of a TRPM8-independent mechanism for noxious cold transduction. Yet, Colburn et al. (21) reported a clear effect of knocking out TRPM8 when the temperatures were set to room temperature (∼25°C) and 5°C with wild types showing a strong preference for the warmer temperature, whereas the knockouts showed no preference between the two.
Fig. 1.
Temperature preference and temperature avoidance in transient receptor potential (TRP)M8 knockout mice. In the 2-temperature discrimination assay, mice are placed in a chamber in which the temperature of the floor plates can be controlled. In the preference assay (A), the proportion of the testing period that the mice spend on each plate is measured with a 50% reading for any given plate indicating that the mouse does not discriminate between the 2 surfaces and spends equal amounts of time on each plate. Such is the case when both plates are set to 30°C for both wild-type (WT) and TRPM8 null (KO) mice. As the temperature of one of the plates is lowered, WT mice spend a greater proportion of the testing period on the 30°C plate, while KO mice display defects in thermal discrimination by showing no preference for the 30°C plate. KO mice do show some preference for the 30°C plate when the test plate is set to 5°C; however, their responses are still reduced compared with WT mice (8, 43). This can be explained by the presence of two partially overlapping drives: a cold discomfort avoidance drive, which is dependent on skin thermoceptors and increases as temperature decreases, and a second drive to maintain proper bodily temperatures by reducing the thermoregulatory burden, probably autonomic and perhaps dependent on the tonic activation of enteric warm receptors (thermoregulatory drive). At warm or mildly cool temperatures, this second drive does not significantly affect animal behavior, thus the behavior is driven solely by the discomfort drive. However, at noxious cold temperatures, this autonomic drive would engage and direct the behavior of both WT and KO mice to spend more time in the thermoregulation-favorable environment. Yet since the KO mice still show a deficit at noxious cold, this thermoregulatory drive would not be strong enough to totally compensate for the loss of the discomfort drive. If the same data are quantified for thermal avoidance (B), a clear picture of the role of TRPM8 in sensory discrimination emerges. Thermal avoidance is quantified as the number of times the mouse crosses the plate-plate boundary, with WT mice showing dramatically reduced numbers of crossings as the test plate temperature is lowered. The KO mice, on the other hand, continue crossing at a high rate regardless of the temperature of the test plate (43). Since the autonomic thermoregulatory drive in (A) would not be involved in crossing behavior, only the discomfort drive would be influencing this behavior. Since KO mice show no changes in crossings across the temperatures tested, this indicates that TRPM8 is responsible for the detection of both innocuous and noxious cold through the skin.
This incongruity suggests that interpretation of these results deserve a reexamination. Although two mouse lines lacking functional TRPM8 were shown to spend more time on the plate held at 30°C than the one held at 10°C or lower, it is not clear whether this preference for warmth is due to a drive to avoid a detected unpleasant stimulus, or perhaps, as we would suggest, results from a concurrent drive to seek/remain in a comfortably warm environment (Fig. 1A). Such a signal may be vital for proper maintenance of body temperature, and in the absence of this input (perhaps signaled through warm-tuned fibers) animals would actively seek a thermoneutral environment. TRPM8 knockout mice may not be able to discern the noxious cold signal, but are attracted to the thermoneutral 30°C surface, thus displaying a preference for the warmer side in the absence of any purely cold sensory behaviors. Indeed, in the above example from Colburn et al. (21), setting up the assay so the warmer side is set to a temperature below the thermoneutral point (25°C) abolishes the confound of this warmth-seeking drive.
Further support for our hypothesis comes from a subtly different interpretation of the two-temperature plate assay data that we recently reported (43). When the number of times an animal crosses from the 30°C surface to the test surface and back again is counted, wild-type mice show a precipitous drop in the number of crossing events as one plate is cooled (43). In this light, the two-temperature choice assay can be viewed as an operant conditioning assay, with the discomfort and/or pain from the cold surface serving as the punishment for sampling that part of the chamber, thus resulting in fewer excursions into that portion of the chamber for the remainder of the testing period regardless of a concurrent drive to seek a comfortably warm surface. Furthermore, in this paradigm, a single sampling of a stimulus within the noxious cold range could be sufficient to result in an immediate drop in the behavior, namely the exposure of the paws to the aversive cold surface. Indeed, when the test plate is held at 5°C, wild-type mice cross the threshold between the two surfaces an average of two times (from warm to cold and back again), indicating that one exposure to the aversive stimulus is sufficient to steeply reduce the sampling behavior (43). If TRPM8 was only responsible for signaling within the innocuous range of temperatures, it could be argued that as the temperature of the paw skin cools from warm to cool to noxiously cold, wild-type mice use the initial innocuous phase of skin cooling as a cue for the impending noxious stimulus, while TRPM8 mice would not have this information and thus not respond until skin temperatures reached the noxious range. If this were so, then we would expect the aversive stimulus to still be effective in this conditioning paradigm and the TRPM8 knockout mice should display reduced crossings with lower temperatures. However, TRPM8 knockout mice show no avoidance at even 5°C with a crossing rate equal to when both surfaces are held at 30°C, clearly indicating that the mice fail to sense the aversive stimulus (43). It is important to note two additional points: 1) this temperature (5°C) is indeed noxious, since experiments with wild-type mice have shown that, counterintuitively, the chill of 5°C is actually more aversive than the heat of 45°C; and 2) TRPM8 knockout mice are still able to sense and avoid noxious stimuli since they avoid a hot surface of 45°C (21). These data, along with the observation that even at 5°C TRPM8 knockouts show small but significant impairment in preference for 30°C than their wild-type counterparts, suggests that the preference quantification method of the two-temperature choice assay may be assessing multiple behavioral drives than simply cold sensory responses, and that TRPM8 does in fact mediate at least a significant component of noxious cold sensation.
Consistent with these results, chemical-evoked cooling and in vivo measures of neural activity also point to TRPM8 as a noxious cold transducer (27, 43). For example, intraperitoneal administration of the supercooling agent icilin produces a distinct “wet dog shake” behavior, characterized by prolonged grooming and shaking (92). Strikingly, this behavior is completely abolished in TRPM8 knockout mice (27). Similarly, hindpaw injections of icilin induce a robust nocifensive flinching and guarding response that is absent in TRPM8 nulls (43). Furthermore, neural activation of cold-sensing circuits, as measured by activation of the immediate early gene c-Fos in the spinal cord, was found to be TRPM8-dependent in response to menthol, icilin, and 0°C stimuli (43). Thus, a role for TRPM8 in noxious cold transduction appears to be likely, but why all aspects of noxious cold sensing, using current behavioral assays, cannot be accounted for by TRPM8 remains to be fully explained.
A related TRP channel, TRPA1, has been proposed as another noxious cold sensor, based on reports of its activation by icilin and noxious cold in vitro (42, 44, 80). However, this hypothesis has proven controversial (17, 39, 43, 56, 61, 99). Conflicting studies on the involvement of TRPA1 in the development of cold hypersensitivity have been reported by groups using the TRPA1 agonist cinnamaldehyde and mustard oil (28, 75). When cinnamaldehyde was applied topically to anesthetized rats no differences were seen in cold withdrawal threshold. Similarly, when mustard oil was topically applied, no increases in cold-evoked spikes were seen in spinal-wide dynamic range neurons. When cinnamaldehyde was injected into the rat paw, however, a significant drop in paw lick/jump latency was seen in response to both a 0°C and 5°C plate tests (86). Recent ex vivo skin nerve preparation studies in TRPA1-deficient mice showed no deficits in acute cold sensitivity, and cold-evoked behaviors were found to be either fully intact according to one group, or reduced in a female sex-specific manner by another (7, 44, 45). Furthermore, one group found that cultured TRPA1 knockout trigeminal neurons had ∼60% fewer cold-responding cells, while another found no difference compared with wild types (7, 42). An additional confound is that menthol has both agonistic and antagonistic actions on TRPA1 that are species specific (41, 93).
The discrepancies highlighted above may be due to the presence of functional TRPM8 channels in these animals and a partial overlap of TRPA1 and TRPM8 function. Moreover, if TRPA1 is indeed a noxious cold sensor, then the residual preference behaviors at noxious cold temperatures described above could be due to functional TRPA1 channels in the TRPM8 nulls. Thus an assessment of each channel's contribution to cold sensing was vital. Recently, mice lacking functional copies of both channels (double knockouts) were tested for behaviors relative to previous assays on the single-channel mutants (43). In the two-temperature choice assay, there were no differences between TRPM8 null and double knockout mice in either the preference or avoidance behaviors, demonstrating that the significant preference behaviors observed in TRPM8 nulls were not dependent on TRPA1 (8, 27). Furthermore, the nocifensive behaviors resulting from hindpaw injections of the cooling compound icilin were absent in TRPM8 nulls and double knockout animals, but retained in TRPA1-nulls. Therefore it is unlikely that TRPA1, although responsive to these agonists in vitro, serves as an acute cold sensor in vivo under normal conditions. Interestingly, however, cold-hypersensitivity behaviors resulting from hindpaw injections of menthol were shown to be lost in TRPA1-null mice, while retained in TRPM8 nulls (30, 43). As this is the first evidence showing TRPM8-independent menthol-mediated sensitization to cold, further work must be done to corroborate these findings. Nonetheless, a role for TRPA1 in cold hypersensitivity in chronic pain states is still possible and should be thoroughly explored (22).
Cold and Chronic Pain
Outside of acute thermosensation, TRPM8 also plays a role in cold hypersensitivity in conditions of chronic pain. After injury, painful stimuli become more intense (hyperalgesia) and normally innocuous stimuli become painful (allodynia). Both cold hyperalgesia and allodynia are well-documented phenomena in chronic pain patients, and behavioral assays testing induced models of chronic pain in animals have been developed (98). Rodents with induced chronic pain show both hyperalgesia and allodynia in a variety of thermal and mechanical behavioral assays, including tests of cold responses. Using the cold plate test, rats with neuropathic [using the chronic constriction injury model (CCI)] or inflammatory [using injection of complete Freund's adjuvant (CFA) into one hindpaw] pain show a marked increase in both the number and duration of paw withdraws from a noxious cold surface (2, 10, 38). In another model of neuropathic pain (spinal nerve ligation), rats exhibit a heightened response to normally innocuous evaporative cooling via the application of acetone to the affected hindpaw (19), as well as reduced latencies in paw withdrawals on a cold plate (2). Colburn et al. (21) directly investigated the role of TRPM8 in both the CCI and CFA chronic injury models using TRPM8 knockout mice. In both models, TRPM8 null mice exhibited significantly lower response intensities and durations in the evaporative cooling assay compared with injured wildtypes. Importantly, hypersensitivity to heat and mechanical stimuli remained intact in TRPM8 nulls, indicating that the deletion of TRPM8 specifically affected cold hypersensitivity and no other aspects of chronic pain hypersensitivity. Although differing results in the noxious cold plate test under normal conditions have been reported for TRPM8 null mice, the role of TRPM8 in cold hyperalgesia in this assay has not yet been reported (8, 21, 27).
Almost paradoxically, TRPM8 is also involved in cooling-mediated analgesia. Cold packs and cooling compounds, such as menthol, have long been used for their analgesic properties in treatment of both acute and chronic pain symptoms. In an elegant study, Proudfoot et al. (67) reported that activation of TRPM8 by moderate cooling or cooling chemicals results in analgesia in the CCI and CFA models. In mice treated with either topical or intrathecal menthol or icilin, thermal (heat) and mechanical hypersensitivities were nearly abolished ipsilateral to the injury. Intrathecal application of TRPM8-directed antisense oligonucleotides eliminated this analgesic effect, providing a direct link between TRPM8 and analgesia. These findings were further corroborated by Dhaka et al. (27) with studies in TRPM8 knockout mice using formalin, a compound which elicits acute followed by inflammatory pain. When wild-type mice were given a single hindpaw injection of formalin, they exhibited fewer nocifensive responses during both acute and inflammatory pain phases when placed on a cool 17°C surface. This effect was lost in injected TRPM8 knockout mice placed on a cool surface. Of note, Proudfoot et al. (67) observed that if analgesic cooling was more than moderate (i.e., < 15°C and therefore in the noxious cold range), then the stimulus was switched to a hyperalgesic effect. Again, it remains unclear whether the hyperalgesic effect of cold in chronic pain is due to TRPM8 or other receptors, but it will be intriguing to explore how this one channel is involved in such diverse actions as thermosensation, nociception, hypersensitivity, and analgesia.
How Can TRPM8 Mediate Both Innocuous Cool and Noxious Cold?
In humans, the sensation of cold has been shown to be mediated by both myelinated Aδ- and unmyelinated C-fibers (16). Psychophysical studies in humans have shown that the sensation of cool begins at temperatures below 30°C and becomes painful at temperatures below 15°C (60). Cold-sensitive Aδ fibers have been generally accepted to convey innocuous cool sensation, as selective block of these fibers significantly impairs cool temperature discrimination (50). Recent research, however, has shown a substantial amount of overlap between the cold responsive properties of what are considered to be nociceptive (C) and nonnociceptive (Aδ) fibers, and suggests a more complex view of cold sensation and the roles of specific fiber types (15). Furthermore, it has been suggested that the burning sensation associated with extreme cold is mediated by a class of polymodal cold-sensitive C-fibers which also respond to heat (15, 16). Other factors, including skin type and cooling rates have also been shown to further diversify cold sensation (32). How does TRPM8 fit into this seemingly complex picture of the different modalities of cold sensation? Although TRPM8 was originally cloned out of a sensory neuron cDNA library, its expression patterns in the body were unknown. Recently, the use of genetically encoded axonal tracers (such as GFP) has allowed the labeling of TRPM8-expressing afferents in vivo (26, 83). This technique has enabled the visualization of TRPM8-expressing neurons with robust GFP labeling in afferents projecting to the skin, where innervation can be sparse (83). Consistent with the broad range of cold-related behaviors reported, TRPM8+ neurons express both nociceptive and nonnociceptive markers as well as markers for both Aδ- and C-fibers. Furthermore, TRPM8 axon terminals can be observed in both the skin and the tooth, a highly cold-sensitive organ, in at least two distinct peripheral regions with unique reported pain-sensing characteristics, supporting the hypothesis that the overall population of TRPM8+ neurons is quite diverse in function, containing both nociceptors and nonnociceptors (40, 60, 83).
How is this diversity in expression phenotype correlated to function? As discussed above, sodium and potassium currents can affect a cell's ability to fire action potentials, and both the sodium channel Nav1.8 and Kv1 potassium channels have been implicated in cold responses (1, 51, 97). Nav1.8, a TTX-resistant sodium channel expressed in peripheral sensory neurons, is resistant to the cold-induced inhibition seen in all other sodium channels (97). Although cold does not cause this channel to fire action potentials directly, it reduces the voltage-activation threshold for the channel making it more sensitive to changes in membrane currents (such as those generated by TRPM8). Mice lacking the Nav1.8 gene, or whose Nav1.8-expressing neurons have been genetically ablated, show severe deficits in responses to cold stimuli as well as to mechanical stimuli and inflammatory hypersensitivity (1, 97). Menthol-evoked sensitization of cold fibers was retained, although significantly reduced, in the presence of TTX in wild-type mice, but completely abolished in Nav1.8-null mice (97). Indeed, we have found that greater than one-quarter of TRPM8-expressing neurons in the mouse are immunoreactive for Nav1.8 (R. Romanu, W. M. Knowlton, D. D. McKemy, unpublished observations). Thus these data suggest that TRPM8 and Nav1.8 function in noxious cold receptors, and that Nav1.8 likely acts as the second step in the cold transduction process, turning the initial currents generated by TRPM8 into action potentials.
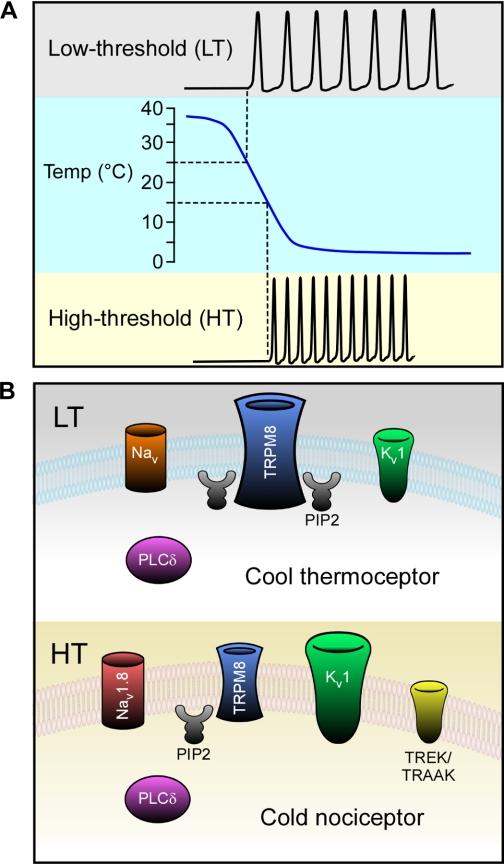
As for potassium channels, provocative data suggests that the relative expression of TRPM8 and hyperpolarizing potassium conductances are critical in determining a neuron's thermal threshold (51). Several groups have reported that cold-sensitive neurons in vitro fall into two loosely related categories, those with a low- (LT) or high-thermal (HT) activation threshold responding at either innocuous cool or noxious cold temperatures, respectively (Fig. 2A) (51, 69, 84). Madrid et al. (51) recently reported that menthol exerts a more prominent effect on LT cold-sensitive neurons compared with the HT population; a difference to which they postulated is due to decreased TRPM8 channel expression in the latter cell type. This same study also found a correlation between temperature threshold and the level of expression of a voltage-dependent slowly inactivating K+ current, termed IKD. Specifically, cells with a high temperature threshold expressed high levels of IKD currents, which were attributed to Kv1 channels. Thus, these results indicate that neurons which fall into the LT subtype and likely mediate responses to innocuously cool temperatures express high levels of TRPM8 but have low expression of a particular type(s) of Kv1 channel (51). Conversely, the expression ratios of TRPM8 and Kv1s are reversed in the HT subtype, thereby necessitating a more robust thermal stimulus to activate sufficient TRPM8 currents to overcome the excitability brake established by the K+ conductances (Fig. 2B). This attractive model provides a robust explanation for the range of cold responses that TRPM8 has been shown to mediate in vivo. However, the molecular identification of the critical components of the K+ conductances has yet to be elucidated, and the contribution of other ionic conductances (e.g., Nav1.8, TREK-1, TRAAK) that have been shown to be important for cold transduction have not been incorporated into this functionally distinct cellular model.
Fig. 2.
Model for innocuous cool vs. noxious cold TRPM8 afferent subtypes. Cold-sensitive neurons respond to different threshold stimuli and are characterized by differential molecular landscapes. A: low threshold (LT) cold-sensitive neurons respond to innocuous stimuli starting ∼25°C, while high threshold (HT) cold-sensitive neurons respond to noxious stimuli starting ∼15°C. B: LT neurons are predominantly controlled by heightened TRPM8-mediated currents due to high channel expression and/or activity regulated by high levels of phosphatidylinositol-(4,5)-bisphosphate (PIP2) (the substrate for PLCδ). Potassium brake currents associated with voltage-gated potassium channels belonging to the Kv1 family are reduced relative to HT neurons, resulting in more easily excitable cells. Unknown voltage-gated sodium channels may also be involved in LT cold transduction. HT neurons have reduced TRPM8-mediated currents due to low expression and/or activity facilitated by lower levels of PIP2. They are strongly influenced by heightened potassium brake currents coming from channels such as Kv1, TREK, and TRAAK. HT cold-sensing neurons also express the voltage-gated sodium channel Nav1.8 (a cold-insensitive channel), which directly facilitates action potential generation at lower temperatures where other sodium channels would be inhibited.
TRPM8 as a Therapeutic Target
TRPM8's involvement in the development of cold hypersensitivity has made it an ideal target for the treatment of various forms of chronic pathological cold pain. Diabetes is often accompanied by some forms of polyneuropathy as a result of the direct, toxic effects of glucose on nerve cells, and, in some cases, this can manifest itself as cold hyperalgesia (66). The chemotherapeutic drug oxaliplatin is also known to result in moderate to severe cold dysesthesia (5). Both of these conditions can be debilitating, and with no specific treatments available patients are often left with no options for directly alleviating their symptoms. Future development of TRPM8-specific antagonists could be the answer for these patients, but as it stands now, no published antagonist has been found without significant off-target effects (9, 46, 52, 54, 58).
Although outside the scope of this paper, it should be noted that TRPM8 has been shown to be expressed in a number of cell types in which there appear to be no thermosensory function: bladder, lungs, heart, and prostate, to name a few (74, 79, 96). The TRPM8 antagonist N-(3-aminopropyl)-2-{[(3-methylphenyl) methyl]oxy}-N-(2-thienylmethyl)benzamide hydrochloride salt has been shown to diminish the frequency of volume-induced bladder contractions in rat models of overactive and painful bladder syndrome, for instance (46). Additionally, TRPM8 expression has been shown to be upregulated in a number of cancers including prostate, breast, skin, colorectal, lung, and bladder, and could have additional therapeutic relevance in regard to their treatment (47, 87, 95). Although the main known function of TRPM8 lies in cold transduction, the expression of TRPM8 in tissues excluding sensory neurons leaves the door open for a multitude of other possible therapeutic applications. Before these avenues can be explored, however, research must first be done to determine its function in tissues of interest.
Conclusion
To date, TRPM8 is the only gene to meet all three requirements outlined above in the search for the molecular cold transducer. Not only is it expressed in a subset of sensory neurons sending afferents to the skin, but it responds to both cold and cold-mimetic chemicals such as menthol and icilin (26, 57, 63, 83). Unlike other molecules, cation influx through this channel in response to cold stimulation causes depolarization events large enough to generate nerve impulses (8, 72). TRPM8 is subject to calcium-based adaptation, as well as modulation by other ion channels expressed in the same neurons, allowing the tuning of neuronal responses to specific temperature ranges (1, 24, 49, 51, 97). Behavioral studies using TRPM8 knockout mice have identified TRPM8 as both an acute innocuous and noxious cold sensor and have shown its necessity for normal cold responses (8, 21, 27, 43). With the development of new cold behavioral assays, the extent to which TRPM8 accounts for many of the varied in vivo effects of cold is expanding (43). Fascinatingly, this single channel has been implicated in both injury-related cold hypersensitivity as well as the widely known analgesic effects of both mild cooling and menthol, a paradox that remains to be explained (21, 67). The development of more nuanced behavioral assays looking into the neural circuitry of cold signaling as well as specific TRPM8-antagonizing drugs will surely lead to critical insights into the workings of this enigmatic channel.
GRANTS
This work was supported by National Institutes of Health Grant NS-054069 to D. D. McKemy and W. M. Knowlton was supported by University of Southern California Cellular, Biochemical, and Molecular Sciences, National Institutes of Health Training Grant GM-067587-06.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 321: 702–705, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Allchorne AJ, Broom DC, Woolf CJ. Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol Pain 1: 36, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andersson DA, Nash M, Bevan S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci 27: 3347–3355, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Askwith CC, Benson CJ, Welsh MJ, Snyder PM. DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc Natl Acad Sci USA 98: 6459–6463, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Attal N, Bouhassira D, Gautron M, Vaillant JN, Mitry E, Lepere C, Rougier P, Guirimand F. Thermal hyperalgesia as a marker of oxaliplatin neurotoxicity: a prospective quantified sensory assessment study. Pain 144: 245–252, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 139: 267–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124: 1269–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol 141: 737–745, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33: 87–107, 1988 [DOI] [PubMed] [Google Scholar]
- 11. Bodding M. TRP proteins and cancer. Cell Signal 19: 617–624, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Brauchi S, Orio P, Latorre R. Clues to understanding cold sensation: thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc Natl Acad Sci USA 101: 15494–15499, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brauchi S, Orta G, Mascayano C, Salazar M, Raddatz N, Urbina H, Rosenmann E, Gonzalez-Nilo F, Latorre R. Dissection of the components for PIP2 activation and thermosensation in TRP channels. Proc Natl Acad Sci USA 104: 10246–10251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci 26: 4835–4840, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Campero M, Baumann TK, Bostock H, Ochoa JL. Human cutaneous C fibres activated by cooling, heating and menthol. J Physiol 587: 5633–5652, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Campero M, Serra J, Bostock H, Ochoa JL. Slowly conducting afferents activated by innocuous low temperature in human skin. J Physiol 535: 855–865, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caspani O, Heppenstall PA. TRPA1 and cold transduction: an unresolved issue? J Gen Physiol 133: 245–249, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 59: 369–376, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Chung MK, Caterina MJ. TRP channel knockout mice lose their cool. Neuron 54: 345–347, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 54: 379–386, 2007 [DOI] [PubMed] [Google Scholar]
- 22. da Costa DS, Meotti FC, Andrade EL, Leal PC, Motta EM, Calixto JB. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain 148: 431–437, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Daniels RL, McKemy DD. Mice left out in the cold: commentary on the phenotype of TRPM8-nulls. Mol Pain 3: 23, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem 284: 1570–1582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Darian-Smith I, Johnson KO, Dykes R. “Cold” fiber population innervating palmar and digital skin of the monkey: responses to cooling pulses. J Neurophysiol 36: 325–346, 1973 [DOI] [PubMed] [Google Scholar]
- 26. Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci 28: 566–575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron 54: 371–378, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Dunham JP, Leith JL, Lumb BM, Donaldson LF. Transient receptor potential channel A1 and noxious cold responses in rat cutaneous nociceptors. Neuroscience 165: 1412–1419, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eid SR, Cortright DN. Transient receptor potential channels on sensory nerves. Handb Exp Pharmacol 261–281, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Gentry C, Stoakley N, Andersson DA, Bevan S. The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity. Mol Pain 6: 4, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grandl J, Kim SE, Uzzell V, Bursulaya B, Petrus M, Bandell M, Patapoutian A. Temperature-induced opening of TRPV1 ion channel is stabilized by the pore domain. Nat Neurosci 13: 708–714, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harrison JL, Davis KD. Cold-evoked pain varies with skin type and cooling rate: a psychophysical study in humans. Pain 83: 123–135, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Hensel H, Zotterman Y. Action potentials of cold fibres and intracutaneous temperature gradient. J Neurophysiol 14: 377–385, 1951 [DOI] [PubMed] [Google Scholar]
- 34. Hensel H, Zotterman Y. The effect of menthol on the thermoreceptors. Acta Physiol Scand 24: 27–34, 1951 [DOI] [PubMed] [Google Scholar]
- 35. Hensel H, Zotterman Y. The persisting cold sensation. Acta Physiol Scand 22: 106–113, 1951 [DOI] [PubMed] [Google Scholar]
- 36. Hensel H, Zotterman Y. [Quantitative relations between the discharge of individual cold-receptors and the temperature]. Acta Physiol Scand 23: 291–319, 1951 [DOI] [PubMed] [Google Scholar]
- 37. Hensel H, Zotterman Y. The response of the cold receptors to constant cooling. Acta Physiol Scand 22: 96–105, 1951 [DOI] [PubMed] [Google Scholar]
- 38. Jasmin L, Kohan L, Franssen M, Janni G, Goff JR. The cold plate as a test of nociceptive behaviors: description and application to the study of chronic neuropathic and inflammatory pain models. Pain 75: 367–382, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Jyvasjarvi E, Kniffki KD. Cold stimulation of teeth: a comparison between the responses of cat intradental Aδ and C fibres and human sensation. J Physiol 391: 193–207, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci 27: 9874–9884, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA 106: 1273–1278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Knowlton WM, Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 150: 340–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50: 277–289, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci 29: 4808–4819, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lashinger ES, Steiginga MS, Hieble JP, Leon LA, Gardner SD, Nagilla R, Davenport EA, Hoffman BE, Laping NJ, Su X. AMTB, a TRPM8 channel blocker: evidence in rats for activity in overactive bladder and painful bladder syndrome. Am J Physiol Renal Physiol 295: F803–F810, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Li Q, Wang X, Yang Z, Wang B, Li S. Menthol induces cell death via the TRPM8 channel in the human bladder cancer cell line T24. Oncology 77: 335–341, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Linte RM, Ciobanu C, Reid G, Babes A. Desensitization of cold- and menthol-sensitive rat dorsal root ganglion neurones by inflammatory mediators. Exp Brain Res 178: 89–98, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci 25: 1674–1681, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mackenzie RA, Burke D, Skuse NF, Lethlean AK. Fibre function and perception during cutaneous nerve block. J Neurol Neurosurg Psychiatry 38: 865–873, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Madrid R, de la Pena E, Donovan-Rodriguez T, Belmonte C, Viana F. Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J Neurosci 29: 3120–3131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Madrid R, Donovan-Rodriguez T, Meseguer V, Acosta MC, Belmonte C, Viana F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J Neurosci 26: 12512–12525, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honore E. TREK-1 is a heat-activated background K+ channel. EMBO J 19: 2483–2491, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Malkia A, Madrid R, Meseguer V, de la Pena E, Valero M, Belmonte C, Viana F. Bidirectional shifts of TRPM8 channel gating by temperature and chemical agents modulate the cold sensitivity of mammalian thermoreceptors. J Physiol 581: 155–174, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Matta JA, Ahern GP. Voltage is a partial activator of rat thermosensitive TRP channels. J Physiol 585: 469–482, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain 1: 16, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Meseguer V, Karashima Y, Talavera K, D'Hoedt D, Donovan-Rodriguez T, Viana F, Nilius B, Voets T. Transient receptor potential channels in sensory neurons are targets of the antimycotic agent clotrimazole. J Neurosci 28: 576–586, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307: 1468–1472, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Morin C, Bushnell MC. Temporal and qualitative properties of cold pain and heat pain: a psychophysical study. Pain 74: 67–73, 1998 [DOI] [PubMed] [Google Scholar]
- 61. Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25: 4052–4061, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J 28: 1308–1318, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell 108: 705–715, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science 296: 2046–2049, 2002 [DOI] [PubMed] [Google Scholar]
- 65. Pierau FK, Torrey P, Carpenter DO. Mammalian cold receptor afferents: role of an electrogenic sodium pump in sensory transduction. Brain Res 73: 156–160, 1974 [DOI] [PubMed] [Google Scholar]
- 66. Pluijms W, Huygen F, Cheng J, Mekhail N, van Kleef M, Van Zundert J, van Dongen R. 18. Painful diabetic polyneuropathy. Pain Pract 11: 191–198, 2010 [DOI] [PubMed] [Google Scholar]
- 67. Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R. Analgesia mediated by the TRPM8 Cold receptor in chronic neuropathic pain. Curr Biol 16: 1591–1605, 2006 [DOI] [PubMed] [Google Scholar]
- 68. Reid G. ThermoTRP channels and cold sensing: what are they really up to? Pflügers Arch 451: 250–263, 2005 [DOI] [PubMed] [Google Scholar]
- 69. Reid G, Babes A, Pluteanu F. A cold- and menthol-activated current in rat dorsal root ganglion neurones: properties and role in cold transduction. J Physiol 545: 595–614, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Reid G, Flonta M. Cold transduction by inhibition of a background potassium conductance in rat primary sensory neurones. Neurosci Lett 297: 171–174, 2001 [DOI] [PubMed] [Google Scholar]
- 71. Reid G, Flonta ML. Ion channels activated by cold and menthol in cultured rat dorsal root ganglion neurones. Neurosci Lett 324: 164–168, 2002 [DOI] [PubMed] [Google Scholar]
- 72. Reid G, Flonta ML. Physiology. Cold current in thermoreceptive neurons. Nature 413: 480, 2001. [DOI] [PubMed] [Google Scholar]
- 73. Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci 8: 626–634, 2005 [DOI] [PubMed] [Google Scholar]
- 74. Sabnis AS, Shadid M, Yost GS, Reilly CA. Human lung epithelial cells express a functional cold-sensing TRPM8 variant. Am J Respir Cell Mol Biol 39: 466–474, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sawyer CM, Carstens MI, Carstens E. Mustard oil enhances spinal neuronal responses to noxious heat but not cooling. Neurosci Lett 461: 271–274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schafer K, Braun HA, Isenberg C. Effect of menthol on cold receptor activity. Analysis of receptor processes. J Gen Physiol 88: 757–776, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418: 186–190, 2002 [DOI] [PubMed] [Google Scholar]
- 78. Spray DC. Cutaneous temperature receptors. Annu Rev Physiol 48: 625–638, 1986 [DOI] [PubMed] [Google Scholar]
- 79. Stein RJ, Santos S, Nagatomi J, Hayashi Y, Minnery BS, Xavier M, Patel AS, Nelson JB, Futrell WJ, Yoshimura N, Chancellor MB, De Miguel F. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J Urol 172: 1175–1178, 2004 [DOI] [PubMed] [Google Scholar]
- 80. Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003 [DOI] [PubMed] [Google Scholar]
- 81. Suto K, Gotoh H. Calcium signaling in cold cells studied in cultured dorsal root ganglion neurons. Neuroscience 92: 1131–1135, 1999 [DOI] [PubMed] [Google Scholar]
- 82. Swandulla D, Carbone E, Schafer K, Lux HD. Effect of menthol on two types of Ca currents in cultured sensory neurons of vertebrates. Pflügers Arch 409: 52–59, 1987 [DOI] [PubMed] [Google Scholar]
- 83. Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci 27: 14147–14157, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thut PD, Wrigley D, Gold MS. Cold transduction in rat trigeminal ganglia neurons in vitro. Neuroscience 119: 1071–1083, 2003 [DOI] [PubMed] [Google Scholar]
- 85. Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543, 1998 [DOI] [PubMed] [Google Scholar]
- 86. Tsagareli MG, Tsiklauri N, Zanotto KL, Carstens MI, Klein AH, Sawyer CM, Gurtskaia G, Abzianidze E, Carstens E. Behavioral evidence of thermal hyperalgesia and mechanical allodynia induced by intradermal cinnamaldehyde in rats. Neurosci Lett 473: 233–236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res 61: 3760–3769, 2001 [PubMed] [Google Scholar]
- 88. Vanden Abeele F, Zholos A, Bidaux G, Shuba Y, Thebault S, Beck B, Flourakis M, Panchin Y, Skryma R, Prevarskaya N. Ca2+-independent phospholipase A2-dependent gating of TRPM8 by lysophospholipids. J Biol Chem 281: 40174–40182, 2006 [DOI] [PubMed] [Google Scholar]
- 89. Viana F, de la Pena E, Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci 5: 254–260, 2002 [DOI] [PubMed] [Google Scholar]
- 90. Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430: 748–754, 2004 [DOI] [PubMed] [Google Scholar]
- 91. Voets T, Owsianik G, Janssens A, Talavera K, Nilius B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol 3: 174–182, 2007 [DOI] [PubMed] [Google Scholar]
- 92. Wei ET, Seid DA. AG-3–5: a chemical producing sensations of cold. J Pharm Pharmacol 35: 110–112, 1983 [DOI] [PubMed] [Google Scholar]
- 93. Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J Neurosci 28: 9640–9651, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418: 181–186, 2002 [DOI] [PubMed] [Google Scholar]
- 95. Yamamura H, Ugawa S, Ueda T, Morita A, Shimada S. TRPM8 activation suppresses cellular viability in human melanoma. Am J Physiol Cell Physiol 295: C296–C301, 2008 [DOI] [PubMed] [Google Scholar]
- 96. Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin (TRPM)- and vanilloid-related (TRPV) channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290: L1267–L1276, 2006 [DOI] [PubMed] [Google Scholar]
- 97. Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 447: 855–858, 2007 [DOI] [PubMed] [Google Scholar]
- 98. Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol 429: 23–37, 2001 [DOI] [PubMed] [Google Scholar]
- 99. Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 10: 277–279, 2007 [DOI] [PubMed] [Google Scholar]