Abstract
Maintenance of reduced body weight in lean and obese human subjects results in the persistent decrease in energy expenditure below what can be accounted for by changes in body mass and composition. Genetic and developmental factors may determine a central nervous system (CNS)-mediated minimum threshold of somatic energy stores below which behavioral and metabolic compensations for weight loss are invoked. A critical question is whether this threshold can be altered by environmental influences and by what mechanisms such alterations might be achieved. We examined the bioenergetic, behavioral, and CNS structural responses to weight reduction of diet-induced obese (DIO) and never-obese (CON) C57BL/6J male mice. We found that weight-reduced (WR) DIO-WR and CON-WR animals showed reductions in energy expenditure, adjusted for body mass and composition, comparable (−10–15%) to those seen in human subjects. The proportion of excitatory synapses on arcuate nucleus proopiomelanocortin neurons was decreased by ∼50% in both DIO-WR and CON-WR mice. These data suggest that prolonged maintenance of an elevated body weight (fat) alters energy homeostatic systems to defend a higher level of body fat. The synaptic changes could provide a neural substrate for the disproportionate decline in energy expenditure in weight-reduced individuals. This response to chronic weight elevation may also occur in humans. The mouse model described here could help to identify the molecular/cellular mechanisms underlying both the defense mechanisms against sustained weight loss and the upward resetting of those mechanisms following sustained weight gain.
Keywords: obesity, set point, energy metabolism, POMC neurons
long-term maintenance of even modest reductions in body weight ameliorates or eliminates many of the comorbidities of obesity (10). The recidivism rate to obesity in formerly obese individuals is 75–85% (59), reflecting the potent metabolic and environmental pressures opposing long-term maintenance of a reduced body weight. We have previously shown that the maintenance of a 10% or greater reduction in body weight in both lean and obese humans is associated with a decrease in energy expenditure (EE) that is 15–20% below what can be accounted for by changes in body mass and body composition. This adaptive thermogenesis does not abate over time (46) and predominantly reflects increased mechanical work efficiency of skeletal muscle, decreased circulating concentrations of bioactive thyroid hormones, and reduced sympathetic autonomic nervous system tone (2, 32, 46, 54).
Leptin is an adipocyte-derived hormone whose circulating plasma concentrations are correlated with fat stores at usual (stable) body weight but which rapidly decline during food restriction and/or fasting (1, 52). We have proposed that central nervous system (CNS) energy homeostasis mechanisms respond asymmetrically (in a threshold-like mechanism) to changes in circulating plasma leptin concentrations (31). This asymmetry is evident in the demonstrations that reductions in circulating leptin concentrations in weight-reduced/food-restricted humans induces a strong leptin-reversible metabolic adaptation (EE reduced beyond expected per unit of metabolic mass) (45), while increases in plasma leptin concentrations as a result of weight gain do not provoke long-term changes in EE (52). Even 10-fold increases in circulating plasma leptin concentrations resulting from exogenous leptin administration do not invoke consistent increases in EE or decreases in energy intake (16). Regulatory pathways constituted by specific neurons and their connections in the hypothalamus (55) and brainstem (13, 21) provide the neural substrate for the proposed threshold mechanism that uses ambient leptin as a primary afferent signal (31). This threshold, set by genetic and developmental influences on its molecular and anatomic substrates determines a minimum circulating concentration of leptin (hence body fat) that is accepted by the CNS as sufficient to ensure reproductive capacity (1) and survival in circumstances of restricted access to food calories (31). The secular trend toward increasing prevalence of obesity and its continued resistance to long-term successful therapy (43, 59), suggest that increasing levels of body fatness are being defended and that structural/molecular changes in CNS regulatory regions for energy homeostasis may play a critical role in these changes. An important question in this context is whether the threshold for minimum adiposity can be reset upward by environmental factors, leading to physiological defense of an acquired increase in fat mass (FM).
In rodents, weight loss due to caloric restriction results in decreased EE per unit of metabolic mass (metabolic adaptation) consistent with the defense of body fat stores by CNS-mediated responses to circulating leptin and other signals (e.g., insulin), reflecting the status of somatic energy stores (5, 25, 29, 37–39, 42). Rats selected by breeding to be predisposed to diet-induced obesity defend higher body weights than diet-induced obese (DIO)-resistant rats (35) and readily regain lost weight following a switch back to ad libitum food access after a period of hypocaloric feeding (33) and have increased arcuate nucleus (ARC) expression of neuropeptide Y (NPY), a key anabolic neuropeptide released from leptin-sensitive neurons that increases food intake and decreases EE (34). Inbred mouse strains fed a high-fat diet gain different amounts of body fat that correlates with differential expression of genes in key regions of the ARC (20, 61).
The aim of the present study was to assess, in a mouse model, the physiological and molecular consequences of maintenance of increased body fat (by high-fat diet) and the subsequent adaptations following caloric restriction and maintenance of a reduced body weight. Our hypothesis was that such a chronic elevation in body fat would invoke changes in the structure of the hypothalamus resulting in an upward resetting of the threshold for minimum body fat. To assess the neural substrates for changes in EE and food intake in these circumstances, we analyzed excitatory and inhibitory synapses onto the cell bodies of proopiomelanocortin (POMC) neurons in the ARC, a cell population that is known to play a role in body weight regulation and whose synapses are leptin responsive. We hypothesized that prolonged maintenance of an elevated body weight by DIO followed by weight loss would result in mice that were hypometabolic compared with DIO and never-obese mice (CON), indicating that maintenance of an elevated body weight results in long-term upward resetting of a minimum threshold for body fat (31).We anticipated that the ratio of excitatory/total synapses onto the POMC neurons would be lower in the two weight-reduced groups, a phenotype similar to the decreases characterizing congenitally leptin-deficient mice (44).
MATERIALS AND METHODS
Animals and diets.
Eighteen-wk-old C57BL/6J male mice were obtained from Jackson Laboratory (Bar Harbor, ME). Sixteen DIO mice fed a high-fat diet starting at 6 wk of age (cat. no. D12492i, 60 kcal% fat; Research Diets) and 16 control diet-fed never-obese (CON) mice fed a low-fat diet also starting at 6 wk of age (cat. no. D12450Bi, 10 kcal% fat; Research Diets) were used for these studies (Fig. 1). Upon receipt, animals were kept in a pathogen-free barrier facility maintained at 22–24°C with a 12:12-h dark-light cycle (lights on at 0700 h). The mice were individually housed in plastic pens with corncob-based bedding, fed the same diet they had been provided at Jackson Laboratory and given ad libitum access to food (diet as specified) and water during a 30-day acclimatization period. The cages were equipped with feeding baskets especially designed to minimize food spillage. During this period, body weight and food intake were monitored every 2 to 3 days. The protocol was approved by the Columbia University Institutional Animal Care and Use Committee.
Fig. 1.
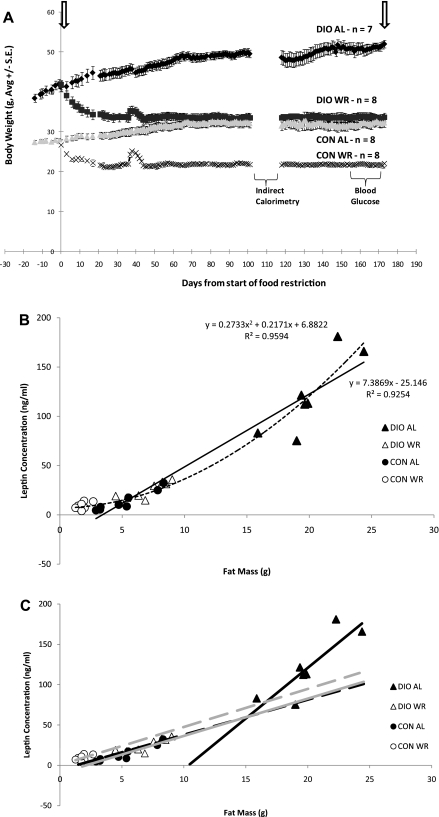
A: Means ± SE body weight (g) of 4 groups of 22-wk-old mice from start of food restriction protocol. Arrows represent 4-h fasted bleeding. Small upward inflections in body weights of diet-induced obese-weight-reduced (DIO-WR) and never-obese-WR control (CON-WR) mice (days 36–43) was due to a batch of corncob-based bedding containing corn kernels, a situation that was resolved by switching mice to wood-based bedding material on day 39. B: leptin (ng/ml) to fat mass (FM; g). Linear regression using DIO-ad libitum-fed (DIO-AL) and CON-AL mice; solid line. Nonlinear regression using all mice groups; dashed line. C: relationship between leptin and FM in DIO-AL (▴, black solid line), DIO-WR (▵, black dashed line), CON-AL (●, grey solid line), and CON-WR (○, grey dashed line) animals. DIO-AL: leptin = 12.6 (FM) to 130.3, r = 0.85, P = 0.014 ; DIO-WR: leptin = 4.3 (FM) to 5.4, r = 0.84, P = 0.009; CON-AL: leptin = 4.6 (FM) to 9.8, r = 0.95, P = 0.0003; CON-WR: leptin = 4.7 (FM) + 0.4, r = 0.53, P = 0.17. There was no statistically significant linear relationship of leptin to FM in CON-WR animals. There was no significant difference in the relationship of leptin to FM between DIO-WR and CON-AL groups. The slope of the regression was significantly greater and the intercept significantly lower in DIO-AL compared with DIO-WR and CON-AL.
Study design.
After the 30-day acclimatization period, mice in each group (DIO or CON) were paired by body weight (nearest body weight ± 0–1.7 g) and one member of each pair randomized to either an ad libitum fed group (DIO-AL or CON-AL) or a weight-reduced group (DIO-WR or CON-WR). There were eight mice in each of the four groups. Mice in the weight-reduced groups received 50% of their average ad libitum daily food intake until their body weight reached 80% of initial value (defined as day 0, Fig. 1A) at which time the mice were switched to 80% of their initial daily food intake. Subsequent adjustments in calories provided were made daily for the rest of the experimental period to maintain each mouse between 79 and 81% of initial (precaloric restriction) body weight (Fig. 1A). Weight-reduced mice (DIO-WR and CON-WR) had free access to water and were given one-third of their individual calculated food ration (±0.1 g) in the morning (07:45–08:15) and two-thirds of the food ration in the evening (18:30–19:00). All ad libitum-fed mice (DIO-AL and CON-AL) had free access to food and water throughout the day. In a subsequent study, seven DIO-AL mice and 12 DIO-WR mice were weight perturbed in the same manner as described above and the DIO-WR were subsequently switched to ad libitum access to the high-fat diet. Food intake (g) and metabolizable energy intake (kcal/24 h) were measured over the first 24 h (see Table 1).
Table 1.
Food intake (g/24 h) and metabolizable energy intake (kcal/24 h) in DIO-AL and DIO-WR during first 24 h after DIO-WR mice were given ad libitum access to high-fat diet (60% kcal from fat)
| Food Intake, g/24 h | Metabolizable Energy Intake, kcal/24 h | |
|---|---|---|
| DIO-AL, n = 7 | 3.5 ± 0.1* | 18.5 ± 0.6* |
| DIO-WR, n = 12 | 5.2 ± 0.2 | 27.0 ± 1.0 |
Data are means ± SE. Group means were compared by Student's t-test. DIO, diet-induced obese; AL, ad libitum fed; WR, weight-reduced.
P < 0.001.
Body weight, body composition, and food intake.
Body weight was measured (±0.1 g) daily before morning feeding using an Ohaus Scout Pro 200 g scale (Nänikon Switzerland, between 07:45–08:15). For ad libitum-fed mice (DIO-AL and CON-AL), body composition [FM, fat-free mass (FFM), and extracellular fluid] was measured by time domain NMR (Minispec Analyst AD; Bruker Optics, Silberstreifen, Germany) (15) before the morning feeding every 2–3 wk, before and after calorimetry measurements (see below), before start of the weight-reduction protocol, and on the day prior to death. Food intake was recorded daily for the WR mice and every 2 to 3 days for the AL mice (by weighing especially constructed feeding baskets designed to minimize spillage) during the entire weight perturbation experiment (Table 2).
Table 2.
Body weight, body composition, food intake, and energy expenditure
| Indirect Calorimetry Measurements (Days 132–144) |
||||
|---|---|---|---|---|
| DIO-AL, n = 7 | DIO-WR, n = 8 | CON-AL, n = 8 | CON-WR, n = 8 | |
| Body weight, g | 49.6 ± 1.2a | 33.3 ± 1.2b | 32.3 ± 1.4b | 21.7 ± 0.5c |
| Fat-free mass, g | 23.5 ± 0.6a | 20.0 ± 0.3b | 20.3 ± 0.3b | 15.0 ± 0.3c |
| Fat mass, g | 17.9 ± 0.8a | 6.7 ± 0.7b | 5.4 ± 0.9b | 1.7 ± 0.1c |
| Food intake, g/day | 3.1 ± 0.1b | 2.2 ± 0.0c | 3.4 ± 0.1a | 2.3 ± 0.1c |
| MEI, kcal/day | 16.1 ± 0.6a | 11.3 ± 0.2c | 13.1 ± 0.3b | 8.7 ± 0.2d |
| 24-Hour total energy expenditure, kcal/day | 14.2 ± 0.4a | 11.4 ± 0.2c | 12.3 ± 0.3b | 8.2 ± 0.3d |
| Resting energy expenditure, kcal/day | 11.4 ± 0.3a | 8.5 ± 0.3b | 9.0 ± 0.3b | 5.2 ± 0.4d |
| Nonresting energy expenditure, kcal/day | 2.9 ± 0.2b | 2.9 ± 0.1b | 3.4 ± 0.1a | 3.0 ± 0.2a,b |
| Respiratory quotient | 0.72 ± 0.01b | 0.71 ± 0.01b | 0.87 ± 0.02a | 0.85 ± 0.01a |
Data are means ± SE. Metabolizable energy intake (MEI) was calculated by multiplying food weight (g) by caloric density of respective diet (CON, 3.85 kcal/g: DIO, 5.24 kcal/g). CON, never-obese control. Data for any variable not marked by the same letter are significantly different by 2-way ANOVA with Tukey's post hoc analysis.
EE by indirect calorimetry.
EE was measured with a LabMaster-CaloSys-Calorimetry System (TSE Systems, Bad Homburg, Germany). Oxygen and CO2 measurements were taken every 14 min during a 72-h period while mice were maintained on their respective weight-maintenance feeding schedules. Because of possible initial stress related to transfer to the chambers, only the last 48 h of measurements were used to calculate total 24-h EE (TEE; expressed in kcal/day) and respiratory quotient (RQ = V̇co2/V̇o2). Resting EE (REE in kcal/day) was defined as the lowest 1-h period of EE, which coincided with the lowest 1 h of total ambulatory activity during the 48-h period, and this value was extrapolated to 24 h. Non-REE (NREE) was calculated as the difference between TEE and REE. Physical activity was measured by an infrared beam system integrated with the LabMaster System. Total activity (beam breaks) in x-, y-, and z-axis was stored every 14 min. The system is designed to differentiate between fine motor movement (defined as a single x- or y-axis beam break), ambulatory movement (defined as the simultaneous breaking of two adjacent x- or y-beams), and rearing, defined as the breaking of the z-axis infrared beam.
Calculations.
EE is proportional to body mass and composition (FFM and FM). We related TEE (kcal/day) of DIO-AL and CON-AL mice to both FFM and FM by multiple regression analysis. There was no significant effect of diet composition on TEE. We therefore pooled the data from ad libitum-fed mice to create a baseline regression equation relating TEE (kcal/24 h) to FFM and FM (g) (TEE = 0.34 * FFM + 0.06 * FM + 5.16, R2 = 0.66, P < 0.01). This equation was used to predict TEE for all mice following experimental weight perturbation as we have done in similar studies of human subjects (32, 52). The residuals (i.e., the difference between measured and predicted values) were calculated for each animal and were tested against the null hypothesis that they were equal to zero. Baseline regression equations relating REE (lowest 1-h period of EE extrapolated to 24 h) and NREE (NREE = TEE − REE) to FFM and FM, predicted REE and NREE values, and residuals were also calculated from data obtained by indirect calorimetry as described above (REE = 0.17 * FFM + 0.14 * FM + 4.96, R2 = 0.74; NREE = 0.18 * FFM - 0.08 * FM + 0.20, R2 = 0.53).
Serum hormone and metabolite profiles.
Before initiation of the weight reduction protocol and at time of death, blood glucose (by tail bleed) and circulating leptin, insulin, and bioactive thyroid hormone concentrations (by retroorbital bleed) were determined after a 4-h fast (see arrows on Fig. 1). Blood for hormone and metabolite assays was allowed to clot for 1 h at room temperature, spun at 4°C for 10 min at 1,000 g, and serum was collected and frozen at −80°C until time of assay. Leptin was assayed using Quantikine ELISA kit (R&D Systems, Minneapolis, MN); insulin using the Mercodia Ultrasensitive Mouse Insulin ELISA (Mercodia, Uppsala, Sweden); triiodothyronine (T3) and thyroxine (T4) using RIA at Hormone Assay and Analytical Services Core at Vanderbilt University (Nashville, TN); and thyroid-stimulating hormone by RIA at the National Hormone and Peptide Program (University of California at Los Angeles Medical Center, Torrance, CA). All assays were conducted according to manufacturers' protocols. HOMA2 (calculator developed by University of Oxford http://www.dtu.ox.ac.uk/index.php?maindoc=/HOMA/index.php) was used to estimate insulin resistance and insulin sensitivity (36).
Synaptic quantification on POMC neurons.
Animals were deeply anesthetized and then transcardially perfused with 50 ml of heparinized saline followed by 200 ml of fixation solution [4% paraformaldehyde 0.195% picric acid and 0.1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4)], and then brains were processed for immunolabeling for POMC for subsequent electron microscopic examination. Ultrathin sections were cut on a Leica ultra microtome, collected on Formvar-coated, single-slot grids and analyzed with a Tecnai 12 Biotwin electron microscope. The quantitative and qualitative analysis of synapse number was performed in an unbiased fashion as described earlier (11, 44). To obtain a complementary measure of axosomatic synaptic number, unbiased for possible changes in synaptic size, the dissector technique was used. On consecutive 90-nm-thick sections we determined the average projected height of the synapses and used ∼30% of this value as the distance between the dissectors. On the basis of this calculation, the number of axosomatic synapses was counted in two consecutive serial sections about 270 nm apart (reference and look-up sections) of seven perikarya profiles in each animal. Synapse characterization was performed at a magnification of 20,000. Symmetric and asymmetric synapses were counted on all selected neurons only if the pre- and/or postsynaptic membrane specializations were seen and synaptic vesicles were present in the presynaptic bouton. Synapses with neither clearly symmetric nor asymmetric membrane specializations were excluded from the assessment. The plasma membranes of selected cells were outlined on photomicrographs and their length was measured with the help of Scion image software (NIH). Plasma membrane length values measured in the individual animals were added, and the total length was corrected to the magnification applied. Synaptic densities were evaluated according to the formula NV = Q-/Vdis where Q- represented the number of synapses present in the reference section that disappeared in the look-up section. Vdis is the dissector volume (volume of reference), which is the area of the perikarya profile multiplied by the distance between the upper faces of the reference and look-up sections, i.e., the data are expressed as numbers of synaptic contacts per unit volume of perikaryon. The synaptic counts were expressed as numbers of synapses on a membrane length unit of 100 μm. We analyzed six POMC immunolabeled neurons per animal (DIO-AL, n = 6; DIO-WR, n = 8; CON-AL, n = 7; and CON-WR, n = 8).
Statistical analysis.
Data are expressed as means ± SE. Statistical analyses were performed using JMP (version 7; SAS, North Carolina). Where applicable, two-way ANOVAs were conducted using diet (DIO or CON) and treatment (WR or AL) as grouping variables. To determine whether the relationship between circulating leptin and FM differed among treatment groups, within-group regressions were performed relating leptin to FM (Fig. 1C) and then reanalyzed by ANCOVA by using group as a covariate for all groups wherein the relationship of leptin to FM was statistically significant (i.e., all groups except CON-WR). To ascertain that circulating leptin concentrations were reduced following weight loss, comparisons of absolute leptin concentrations were made between DIO-AL and DIO-WR and between CON-AL and CON-WR. To ascertain that any metabolic differences between DIO-WR and CON-AL groups were not due to lower circulating leptin concentrations in DIO-WR, a comparison of absolute circulating leptin concentrations was made between DIO-WR and CON-AL. Statistical significance was prospectively defined as Pα < 0.05.
RESULTS
Body weight and body composition.
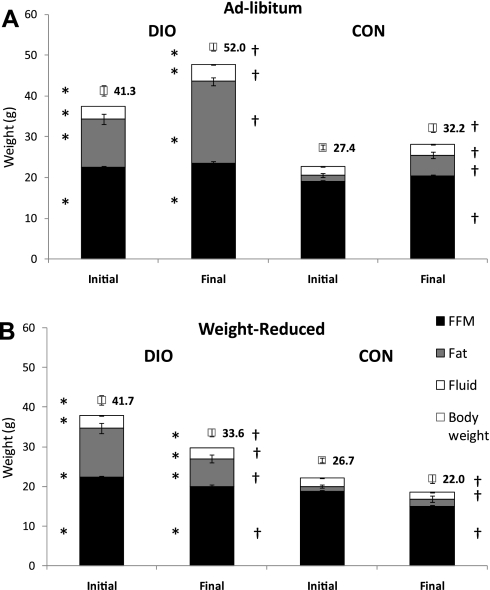
At the start of the weight-reduction phase of the study (day 0, mice aged 22 wk), DIO mice weighed 54 ± 3% more than CON-AL mice and had significantly higher fractional body fat (DIO, 29 ± 1%; CON, 5 ± 1% fat) (Figs. 1 and 2A). From day 0 to day 183 of the weight-reduction phase, both DIO-AL and CON-AL groups gained a significant amount of body mass (Figs. 1 and 2A). The increase in mass of both DIO-AL and CON-AL mice was primarily the result of increased FM (81 ± 4% of weight increment in DIO-AL and 79 ± 6% CON-AL). At time of death, DIO-AL body weight was 62 ± 3% higher than CON-AL body weight; 75 ± 3% of this excess weight was accounted for by increased FM. By design, caloric restriction (from day 0 to day 183) resulted in a 20% decrease in body weight in both DIO-WR and CON-WR groups. DIO-WR mice lost significant amounts of FM and FFM (FM accounted for 65 ± 4% of weight loss), whereas CON-WR mice showed a significant decrease only in FFM (FFM accounted for 87 ± 3% of lost weight). Weight and body composition of DIO-WR and CON-AL mice were not significantly different (Table 2 and Fig. 2, A and B).
Fig. 2.
Means ± SE body weight and body composition (g; fluid is defined as extracellular fluids) of DIO and CON mice either AL fed (A) or WR mice (B) at initial (day 0) vs. termination of experiment (n = 8 in all experimental groups except DIO-AL, n = 7). *P < 0.001, t-test vs.CON. at same time point, †P < 0.001, paired t-test vs. initial.
EE (TEE, REE, and NREE).
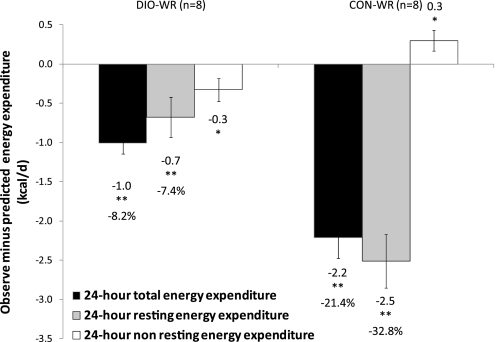
Absolute TEE and REE of DIO-AL mice were significantly higher than in CON-AL (Table 2). While DIO-AL mice were heavier and fatter than CON-AL mice, the relationships between TEE and REE and body composition (FM and FFM) were not significantly affected by diet composition. Residuals for 24-h TEE and of REE of WR mice were significantly below predicted (P < 0.001; Fig. 3), indicating that TEE and REE were reduced beyond what could be attributed to changes in body mass and composition. Residuals were calculated based on actual values minus those predicted based on FFM and FM in all AL mice. However, similar results were obtained regardless of whether residuals were calculated based on FFM alone or FFM and leptin (data not shown). In addition, the absolute values of TEE in DIO-WR mice were significantly lower than in CON-AL mice despite the near identity of body weight and body composition in these two groups (Table 2). REE of DIO-WR mice was 7.4 ± 2.7% lower than predicted, accounting for 67% of the reduction in total 24-h TEE (−0.7 kcal/day); in CON-WR mice, REE was 32.8 ± 4.5% lower than predicted. This decrease of REE (−2.5 kcal/day) in CON-WR exceeded the decrease in TEE (−2.2 kcal/day). The difference of 0.3 kcal/day is accounted for by an increase in NREE (0.3 kcal/day) due to increased locomotor activity probably related to food-seeking behavior as reflected in the measures of physical activity (see Physical activity and Fig. 4, A and C).
Fig. 3.
Means ± SE. Observed-minus-predicted 24-h total (black bars), 24-h resting (grey bars) and nonresting (white bars) energy expenditure (EE; kcal/24 h). Predicted values obtained by multivariate regressions relating 24-h total [total EE (TEE) = 5.16 + 0.34 * fat-free mass (FFM) + 0.06 * FM], 24-h resting EE (REE = 4.96 + 0.17 * FFM + 0.14 * FM) or non-REE (NREE = 0.20 + 0.18 * FFM − 0.08 * FM) EE with FFM and FM of AL-fed mice (DIO-AL + CON-AL). *P < 0.05, **P < 0.01, t-test of residuals vs. 0.
Fig. 4.
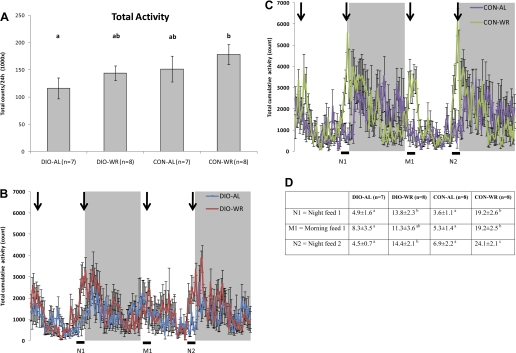
A: means ± SE 24-h ambulatory movement beam breaks (sequential breaking of two adjacent x- or y-beams). B and C: total cumulative ambulatory activity for each 14-min period. N, night; M, morning. Black bars at bottom are 1-h periods prior to feeding WR mice that are quantified in D. D: cumulative ambulatory activity for 1-h time period prior to feeding of WR mice (1,000 × beam breaks ± SE). Arrows represent the times at which the weight-reduced animals were fed. Data not marked by same letter are significantly different by two-way ANOVA with Tukey post hoc analysis.
NREE (NREE = TEE − REE) of CON-AL mice was significantly higher than the two DIO groups (Table 2). When adjusted for body mass and composition, residuals of NREE for DIO-WR mice were significantly decreased (−0.3 kcal below expected when adjusted for FFM and FM; P < 0.05), while NREE residuals were significantly increased in the CON-WR group (+0.3 kcal, P < 0.05).
Physical activity.
Total 24-h physical activity (ambulatory movement), measured by the TSE Systems infrared movement system, was highest in CON-WR and lowest in DIO-AL (Fig. 4A); these were the only groups that were significantly different from one another (P < 0.05) in this regard. DIO-WR mice and CON-AL mice had nearly identical 24-h total activity (Fig. 4A), yet the DIO-WR group had significantly lower NREE (2.9 ± 0.1 units vs. 3.4 ± 0.1, respectively; Table 2), indicating that the DIO-WR were expending ∼15% less energy per unit of movement than CON-AL although some of this decrease in NREE may be attributable to decreased thermic effect of feeding (TEF) (see discussion). Cumulative ambulatory activity rhythms (sum of every 14 min measuring period; Fig. 4B for DIO and Fig. 4C for CON) over 48 h show higher peaks of movement for WR mice, regardless of diet, in the 1-h period prior to AM and PM feeding times (see black bars on bottom of figures). Quantification of ambulatory activity in the 1-h periods prior to feeding of WR mice showed that WR mice have higher levels of ambulatory activity than AL mice, probably as a result of increased food-seeking behavior (see Fig. 4D) (40).
Leptin.
The key comparison is that of absolute circulating leptin concentrations in CON-AL and DIO-WR mice. If circulating leptin concentrations were significantly reduced in the DIO-WR mice compared with CON-AL mice, then the study would be biased toward our hypothesis: i.e., that DIO-WR mice will be hypometabolic and hypothyroid compared with CON-AL. In fact, the opposite was true, and circulating leptin concentrations were significantly higher in DIO-WR mice compared with CON-AL (by t-test comparison: Table 3). There was no effect of diet composition on circulating leptin concentrations since the regression equations relating leptin to FM are almost identical between the DIO-WR and CON-AL groups (Fig. 1C), and so the intergroup differences in circulating leptin concentrations are attributable to the higher FM of DIO-WR.
Table 3.
Serum hormone and metabolite data obtained during terminal bleed (days 173–179)
| DIO-AL, n = 7 | DIO-WR, n = 8 | CON-AL, n = 8 | CON-WR, n = 8 | |
|---|---|---|---|---|
| Leptin, ng/ml | 121.7 ± 14.9a | 25.1 ± 2.9b | 14.0 ± 3.6b* | 9.0 ± 1.3b* |
| Insulin, μg/l | 3.7 ± 0.7a | 1.6 ± 0.2b | 1.2 ± 0.2b | 0.6 ± 0.1b |
| Glucose, mg/dl | 125 ± 3a | 105 ± 3b | 110 ± 3b | 94 ± 2c |
| T3, ng/dl | 31.6 ± 5.3a | 16.8 ± 1.8b | 31.8 ± 8.1a | 15.7 ± 4.4b |
| T4, ng/ml | 45.8 ± 4 | 49.9 ± 2 | 46.1 ± 2.2 | 50.6 ± 2.6 |
| TSH, ng/ml | 283.1 ± 30.5 | 252.8 ± 35.5 | 221.7 ± 52.7 | 226.8 ± 42.6 |
| HOMA2 S | 10.1 ± 1.2a | 24.3 ± 4.3ab | 30.6 ± 4.5b | 60.2 ± 11.2c |
| HOMA2 IR | 11.1 ± 1.8a | 4.9 ± 0.7b | 3.8 ± 0.6bc | 2.0 ± 0.3c |
Data are means ± SE. T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone; HOMA S, HOMA insulin sensitivity; HOMA IR, HOMA insulin resistance. Data not marked by the same letter are significantly different by 2-way ANOVA with Tukey's post hoc analysis.
Student's t-test comparisons of CON-AL and CON-WR against DIO-WR.
Overall, leptin concentrations were highly correlated with total FM (by NMR) at the start and end of the experiment (r = 0.97 and 0.93, respectively, both P < 0.001; see Fig. 1B; data prior to weight stabilization of all four groups of animals are not shown). The best fit for the relationship of leptin to FM was nonlinear (r = 0.96, P < 0.0001), suggesting that the relationship between leptin and FM might differ at extremes of adiposity. To determine whether there were differences among groups in the relationship of leptin to FM, regressions relating leptin to FM for each of the four groups were made (see Fig. 1C). As reported by others (17, 34), DIO mice showed a disproportionately greater increase in circulating leptin concentrations relative to FM (Fig. 1C). There was no significant correlation of leptin and FM in the CON-WR animals, which is similar to what has been reported in studies of leptin in humans with extremely low FM (14, 18). The lack of significant difference in absolute circulating leptin concentrations between CON-AL and CON-WR probably reflects the nonlinearity of the relationship of leptin to FM in CON-WR animals.
Other hormones and metabolites.
Prior to the start of the weight loss protocols, circulating glucose and insulin concentrations were all significantly higher in DIO mice than CON mice (Table 4). Weight reduction resulted in significant decreases in circulating insulin, T3, and glucose concentrations in DIO-WR compared with DIO-AL mice. T3 concentrations significantly decreased in the CON-WR compared with CON-AL mice. Weight reduction, regardless of diet, significantly decreased circulating glucose concentrations and increased insulin sensitivity (HOMA2, Table 3).
Table 4.
Serum hormones and metabolites data obtained during initial bleed (day 0)
| DIO, n = 15 | CON, n = 16 | |
|---|---|---|
| Leptin, ng/ml | 74.4 ± 6.5a | 4.8 ± 1.6b |
| Insulin, μg/l | 3.7 ± 0.5a | 0.8 ± 0.1b |
| Glucose, mg/dl | 153.7 ± 3.8a | 121.7 ± 4.0b |
| T3, ng/dl | 60.8 ± 8.8a | 19.9 ± 2.8b |
| T4, ng/ml | 55.9 ± 2.0a | 48.2 ± 1.2b |
| TSH, ng/ml | 205.3 ± 32.5 | 170.2 ± 22.9 |
Values are means ± SE. Data not marked by the same letter are significantly different by 2-way ANOVA.
Synapses onto POMC neurons in the ARC.
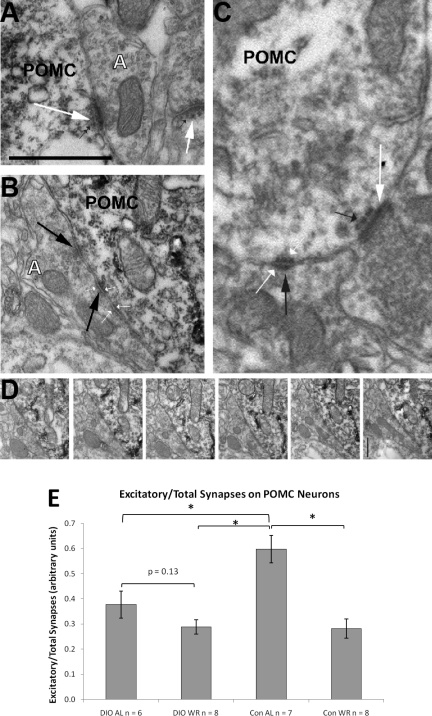
Fig. 5, A and B are examples of electron microscopy images of POMC cell bodies with either asymmetrical/excitatory synapses (Fig. 5A, large white arrows) or symmetrical/inhibitory synapses (Fig. 5B, large black arrows). Figure 5C is a magnified section showing both asymmetrical/excitatory (large white arrow) and symmetrical/inhibitory synapses (large black arrow). Small black arrow points to the specialization below the postsynaptic density of the asymmetrical contact (Fig. 5, A and C). Figure 5D represents consecutive serial sections of the symmetrical contact shown on in Fig. 5B. CON-AL mice had the highest excitatory/total synapse ratios of the four groups (Fig. 5E) indicating a predominance of excitatory synapses over inhibitory ones in these animals during a period (ad libitum feeding) of relative satiety. In the CON-WR and DIO-WR mice, there was a similar decrease in this ratio (−52% in DIO-WR and −53% in CON-WR compared with CON-AL mice; P < 0.01). In these groups of demonstrably more hungry animals, inhibitory synapses dominated over excitatory ones. DIO-AL mice also had decreased ratios of excitatory/total synapses (37% below CON-AL), revealing a predominance of inhibitory inputs on POMC perikarya at the time of relative satiety. DIO-WR mice had lower ratios than DIO-AL, although this difference did not quite reach statistical significance (P = 0.13).
Fig. 5.
A: sample of electron microscopy image showing asymmetrical/excitatory connections (large white arrows) onto proopiomelanocortin (POMC) cell body and unlabeled dendritic spine. Small black arrows point to an electron dense band below the postsynaptic membrane specialization, a characteristic sign of asymmetrical, excitatory synapses. B: sample of electron microscopy image showing a symmetrical/inhibitory connection onto POMC cell body (large black arrows). Small white arrows point to both pre- and postsynaptic thickening of the membranes typical of symmetrical synapses. C: an electron micrograph showing an asymmetrical (large white arrow) contact and a symmetrical contact (large black arrow) on a POMC-immunolabeled (light immunoperoxidase in cytosol) dendrite. Small black arrow points to the specialization below the postsynaptic density of the asymmetrical contact. Small white arrows point to both pre- and postsynaptic thickening of the membranes typical of symmetric synapses. D: consecutive serial sections of the symmetrical contact shown on B. Note that no electron dense band (a characteristic of asymmetrical, stimulatory synapses) ever appears below the postsynaptic density. Bar, xxx. E: mean ratio excitatory/total synapses on POMC cell bodies in the arcuate nucleus (arbitrary units ± SE): *P < 0.05. White A in panel A and B mark axon terminals. Bar scale on A represents 1 μm for A and B and 0.5 μm for C and D.
DISCUSSION
The major findings of this study are 1) as in humans, mice maintaining a reduced body weight (DIO-WR and CON-WR) show decreases in REE and TEE (adjusted for FM and FFM). Most notably, the DIO-WR animals defend a higher body weight following an extended period of DIO; 2) there are no significant differences in the relationships in TEE or REE and body weight/composition between CON-AL mice on a chow diet and DIO-AL mice maintaining an elevated body weight; 3) mice maintained at a reduced body weight, regardless of initial weight, have a significantly lower ratio of excitatory/total synapses onto POMC cell bodies than CON-AL fed animals, ratios that are similar to those observed in leptin deficient Lepob animals (44). These changes are accompanied by increased ad libitum food intake, i.e., increased hunger and food-seeking behavior (Table 1 and Fig. 4, B, C, and D), that has been documented in weight-reduced humans (48), mice (1), and rats (27, 37–38, 40). The relative hypometabolism and decreased excitatory input into hypothalamic POMC neurons in DIO-WR mice compared with CON-AL, despite higher leptin concentrations in the modified mice (Table 3), is consistent with the hypothesis that prolonged maintenance of an elevated body weight results in an upward resetting of the leptin threshold.
The magnitude of the decline in EE (both TEE and REE) following weight loss observed in the DIO-WR compared with the CON-AL group in this study is similar to those seen in humans (36). Interestingly, NREE was lower in DIO-WR compared with CON-AL (2.9 ± 0.1 SE vs. 3.4 ± 0.1 kcal/day, respectively) although they had similar body weights (Table 2; 33.3 ± 1.2 vs. 32.3 ± 1.4 g, respectively) and total activity counts (Fig. 4A), suggesting that the DIO-WR require less energy to accomplish similar amounts of activity (i.e., their skeletal muscles may be more efficient). Such an effect is, in fact, observed in weight-reduced human subjects (54).
In relating rodent data to human studies, it is important to consider the differences in the fractional contributions to EE, behavioral changes as a result of weight loss, and even definitions of the different components of TEE. TEE of weight-reduced obese humans (who have lost 10% or 20% of their initial body weight) and never-obese humans (who have lost 10% of their initial body weight), adjusted for body composition, is ∼15% below that predicted by the losses of FFM and FM (32). In humans, most of this relative decline in EE is attributable to an increase in skeletal muscle work efficiency (45, 54). In mice, our estimates of energetic cost of locomotion suggest that there may be an increase in activity-related efficiency following weight loss, but its contribution to the overall decline in TEE remains unclear. Unlike humans, the major component of decreased TEE in WR mice is decreased REE rather than NREE.
Comparisons of rodent and human TEF data are complicated by different definitions. In humans, TEF refers specifically to the energy expended during digestion in a sedentary subject (32), while in rodents this term includes postprandial changes in energy expended in physical activity (9), which may, in part, account for the lower NREE observed in DIO-WR vs. CON-AL mice. TEF in mice accounts for a significantly greater fraction of TEE (>15%) than in humans (<10%) (9, 32). It is possible that changes in TEF, either due to the decreased caloric intake of WR animals or to an actual decline in the fraction of caloric intake utilized in digestion, accounts for some of the observed declines in TEE and NREE. Given the significant decline in circulating concentrations of T3 in WR animals and the report that hypothyroidism are associated with a decrease in TEF in rats (22–23), it is possible that WR animals would expend a lower percentage of their ingested calories in TEF. However, studies of human subjects (32) and rats (9) who are being maintained at an ∼10–15% reduced weight have reported no changes in TEF, suggesting that maintenance of a reduced body weight is not associated with a significant decline in TEF expressed as a fraction of caloric intake.
Studies in humans suggest that there is no remission of the relative hypometabolism that accompanies the chronic maintenance of a weight-reduced state (46). Similarly, in rats, maintenance for 16 wk of a stable lower body weight was accompanied by a persistent hypometabolic phenotype and hyperphagia and weight regain once ad libitum feeding was resumed (38). This apparent irreversibility of the metabolic and behavioral consequences of sustained weight loss does not seem to occur following sustained weight gain; the data presented here suggest that prolonged elevation of body weight results in an upward resetting of defended levels of energy stores.
In the present study, long-term (16 wk) DIO-AL mice were not hypermetabolic (adjusted TEE) compared with CON-AL mice by ANCOVA or multivariate regression. In shorter-term overfeeding studies in rats (58) and humans (32), 10–15% increases in adjusted TEE are observed. Our data suggest that over longer periods of time, EE in weight-gained individuals returns to levels (adjusted for body mass and composition) that are comparable to those of individuals maintaining their usual (pregain) body weight. Such an inference is supported by the fact that weight-stable obese and nonobese humans have comparable adjusted EEs (32). Unlike mice that return to their usual body weight after short-term overfeeding and are then eumetabolic compared with their never-obese littermates, long-term DIO mice who were weight-reduced (DIO-WR) are hypometabolic compared with both DIO-AL and CON-AL mice, but are metabolically similar to CON-WR mice (Fig. 3). The reduction in EE in the weight-reduced DIO mice, to levels less than those of age, genotype, and body mass/composition-matched CON-AL mice, is consistent with our hypothesis that sustained maintenance of an increased body weight results in an upward resetting of the threshold for minimum body fat. It might be argued that the decline in EE of the DIO-WR is related to CNS effects that are specific to the high-fat diet. However, the same responses are seen in the CON-WR mice being fed a low-fat diet, and high-fat diets are certainly prevalent among human populations. Nevertheless, it would be interesting to examine the responses of DIO-WR to a lower-fat diet in terms of energy intake and expenditure.
Relevant to this issue, others have found that DIO mice settle at higher body weights (i.e., increased adiposity) than never-obese animals when switched from an ad libitum HFD to an ad libitum chow diet (56) (S. Corvera, personal communication). Chow-fed formerly DIO rats also resist weight reduction when fed a hypocaloric diet by becoming hypometabolic (like non-DIO weight-reduced rats) (56). Furthermore, mice that are switched from a high-fat diet to a low-fat diet and then back to the high-fat diet readily regain weight to levels similar before the diet switch (30).
Epidemiological observations of the increasing prevalence of obesity in humans (26, 43, 60) and long-term difficulties in sustaining even mild degrees of weight loss, suggest that the threshold for the minimal body weight that is metabolically defended may be elevated via maintenance of greater adiposity for prolonged periods of time and/or at specific time points during development. In both humans and rodents, weight reduction results in decreased concentrations of circulating leptin, T3, and insulin. In the present study, as expected, we detected significant effects of treatment (AL or WR) on insulin sensitivity as reflected by HOMA2 (36): CON-WR HOMA2 was 197% higher than CON-AL, and DIO-WR was 241% higher than DIO-AL, P < 0.05. Circulating concentrations of leptin are closely proportional to body FM in weight-stable mice and humans (4, 51). Leptin's capacity to reverse metabolic phenotypes seen in both rodents and humans, following weight loss and/or during caloric restriction and its effects on energy homeostatic processes in the brain, renders it a prime candidate as a mediator of metabolic adaptation under conditions of decreased somatic energy stores and/or negative energy balance (1, 45, 50). The higher circulating leptin concentrations relative to FM in DIO-AL mice and the loss of linearity in the relationship of leptin to FM in CON-WR mice are consistent with other studies of weight maintenance in rodents following overfeeding and underfeeding (12, 17). A nonlinear analysis improved the regression relating leptin to FM (r2 = 0.96, P > 0.001) when all groups were included, because it accounted for increased production of leptin per unit of FM in DIO-AL (see slope of linear regression in Fig. 1C). The differences in the relationship of leptin to FM during weight maintenance following extreme weight loss or gain are less pronounced than the striking decreases or increases in the ratio of leptin to FM observed in humans (52) and rodents (4) during dynamic weight loss or gain, respectively. The observation that the relationship between leptin and FM was not different between DIO-WR and CON-AL groups in this study (i.e., that they fell on similar regression lines: Fig. 1C) is an indication that these animals were, in fact, in similar states of energy balance. Since the regression of leptin on FM has a non-zero y-axis intercept, the ratios of leptin to FM, as used by some laboratories, are not appropriate for assessing leptin sufficiency/insufficiency as they reflect solely the slope but not the intercept of the relationship between the variables (see Fig. 1C for individual group regressions). Similar considerations dictate our use of multivariate regression to assess EE related to both FFM and FM as opposed to using ratios of TEE/FFM (24).
Thyroid hormone concentrations in blood correlate with EE by mechanisms that are not fully understood (6–7). T3 is increased during overfeeding (47, 57) and reduced during underfeeding and/or weight loss (47). Serum T3 in the CON-WR was decreased by 51% and by 47% in DIO-WR mice compared with their respective AL controls (Table 3). These changes in T3 concentrations following weight loss are similar to those noted in humans (47).
Chronic changes in leptin signaling have been associated with structural changes in the hypothalamus (44). These are plausible neural substrates for the consequent attenuations in energy intake and expenditure (28). Leptin deficient Lepob mice had decreased ratios of excitatory/total synapses onto POMC arcuate neurons compared with wild-type mice and exogenous leptin or estrogen normalized this phenotype (11, 44). In the DIO-WR and CON-WR mice we observed ratios of excitatory/total synapses onto POMC neurons that were 52% and 53% below those in ad libitum animals and comparable to those observed in the leptin-deficient Lepob mice (44). We have previously shown that the excitatory/total synaptic ratio positively correlates with POMC mRNA expression (19, 44). The comparability of these changes in DIO-WR and CON-WR animals supports our inference that the DIO-WR animals are now defending a higher level of body fat and that the reduced excitatory/total synapses onto POMC neurons constitute a signature of relative leptin deficiency. Consistent with the reduced excitatory tone in POMC neurons in the weight-reduced state, POMC mRNA in the arcuate is reduced in chronically food-restricted rats (27) and restored to fed levels by exogenous leptin. It is possible that opposite changes in orexigenic/anabolic NPY/AgRP neurons contribute to the phenotype (61). If these structural differences have functional consequences, a likely possibility given the physiology of leptin signaling in POMC neurons, the bioenergetics and endocrine profiles of the animals in this study could be accounted for (31). The bioenergetic/neuroendocrine, behavioral, and functional MRI responses of weight-reduced humans to low-dose exogenous leptin are consistent with this inference (49, 53). The DIO-AL animals had lower excitatory/total synapse ratios than CON-AL possibly reflecting effects of diet composition, the obese phenotype (i.e., increased leptin levels), or both. Feeding mice a high-fat diet reduces apparent arcuate leptin sensitivity as early as 6 days after switching to a high-fat diet (41). Enriori et al. (8) showed that decreases in leptin responsiveness in the ARC following diet-induced obesity could be reversed by decreasing the fat content of the diet. These effects may account for the smaller difference in excitatory/total synapse ratios observed between DIO-AL and DIO-WR ratios. There may be a floor to this ratio.
Perspectives and Significance
These data suggest that prolonged maintenance of an acquired elevation in body weight induces changes in energy homeostatic systems that lead to defense of a body weight higher than that dictated by genetic/developmental status of the animal. Structural changes in the ARC (and elsewhere) may play a role in upward resetting of defended body weight (3, 28, 44). Comparable processes, if present in humans, could account for some aspects of the secular trend to increasing obesity that clearly cannot be attributed to intercurrent genetic change. Understanding the neurobiological predicates of such an acquired upward resetting of the minimum defended level of adiposity threshold could provide novel approaches to the prevention and treatment of obesity. For instance, the observations presented here suggest that pharmacological elevation of melanocortinergic tone may be particularly effective in the prevention of weight regain in formerly obese subjects. These studies are also consonant with studies in humans indicating that the neurobiological responses to maintenance of a reduced body weight do not accommodate over time; i.e., that the physiology of the weight-reduced state persists.
GRANTS
This work was supported by National Institutes of Health Grants RO1-DK-066518, R01-DK- 080000, P30-DK-26687, P30-DK-63068, and ADA-1-08-RA-36, and a research grant from AstraZeneca. We gratefully acknowledge the financial and technical assistance of AstraZeneca.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We gratefully acknowledge the Vanderbilt University Hormone Assay and Analytical Services Core for performance of serum T3 and T4 assays.
M. Rosenbaum, T. L. Horvath, and R. L. Leibel contributed variously, and effectively equally, to the design of the studies and in the editing/writing of the manuscript.
REFERENCES
- 1. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Aronne LJ, Mackintosh R, Rosenbaum M, Leibel RL, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol Regul Integr Comp Physiol 269: R222–R225, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 7: 179–185, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Considine RV. Weight regulation, leptin and growth hormone. Horm Res 48, Suppl 5: 116–121, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Corbett SW, Stern JS, Keesey RE. Energy expenditure in rats with diet-induced obesity. Am J Clin Nutr 44: 173–180, 1986 [DOI] [PubMed] [Google Scholar]
- 6. Danforth E, Jr, Burger A. The role of thyroid hormones in the control of energy expenditure. Clin Endocrinol Metab 13: 581–595, 1984 [DOI] [PubMed] [Google Scholar]
- 7. Danforth E, Jr, Horton ES, O'Connell M, Sims EA, Burger AG, Ingbar SH, Braverman L, Vagenakis AG. Dietary-induced alterations in thyroid hormone metabolism during overnutrition. J Clin Invest 64: 1336–1347, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5: 181–194, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Even PC, Nicolaidis S. Adaptive changes in energy expenditure during mild and severe feed restriction in the rat. Br J Nutr 70: 421–431, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Franz MJ, Boucher J. Winning at weight loss. Small losses, big gains. Shedding even a little excess weight improves your health. Support is key to success. Diabetes Forecast 59: 41–42, 44, 2006 [PubMed] [Google Scholar]
- 11. Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 13: 89–94, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Ghanayem BI, Bai R, Kissling GE, Travlos G, Hoffler U. Diet-induced obesity in male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. Biol Reprod 82: 96–104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143: 239–246, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Grinspoon S, Gulick T, Askari H, Landt M, Lee K, Anderson E, Ma Z, Vignati L, Bowsher R, Herzog D, Klibanski A. Serum leptin levels in women with anorexia nervosa. J Clin Endocrinol Metab 81: 3861–3863, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Halldorsdottir S, Carmody J, Boozer C, LeDuc CA, Leibel RL. Reproducibility and accuracy of body composition assessments in mice by dual energy X-ray absorptiometry and time domain nuclear magnetic resonance. Intl J Body Compos Res 7: 147–154, 2009 [PMC free article] [PubMed] [Google Scholar]
- 16. Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282: 1568–1575, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Hoffler U, Hobbie K, Wilson R, Bai R, Rahman A, Malarkey D, Travlos G, Ghanayem BI. Diet-induced obesity is associated with hyperleptinemia, hyperinsulinemia, hepatic steatosis, and glomerulopathy in C57Bl/6J mice. Endocrine 36: 311–325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horlick MB, Rosenbaum M, Nicolson M, Levine LS, Fedun B, Wang J, Pierson RN, Jr, Leibel RL. Effect of puberty on the relationship between circulating leptin and body composition. J Clin Endocrinol Metab 85: 2509–2518, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J, Chowen JA, Perez-Tilve D, Pfluger PT, Bronneke HS, Levin BE, Diano S, Cowley MA, Tschop MH. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci USA 107: 14875–14880, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang XF, Han M, South T, Storlien L. Altered levels of POMC, AgRP and MC4-R mRNA expression in the hypothalamus and other parts of the limbic system of mice prone or resistant to chronic high-energy diet-induced obesity. Brain Res 992: 9–19, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Huo L, Maeng L, Bjorbaek C, Grill HJ. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology 148: 2189–2197, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Iossa S, Lionetti L, Mollica MP, Barletta A, Liverini G. Thermic effect of food in hypothyroid rats. J Endocrinol 148: 167–174, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Iossa S, Mollica MP, Lionetti L, Barletta A, Liverini G. Effect of a high-fat diet on energy balance and thermic effect of food in hypothyroid rats. Eur J Endocrinol 136: 309–315, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 59: 1657–1666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keesey RE, Corbett SW. Adjustments in daily energy expenditure to caloric restriction and weight loss by adult obese and lean Zucker rats. Int J Obes 14: 1079–1084, 1990 [PubMed] [Google Scholar]
- 26. Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 32: 1431–1437, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Kinzig KP, Hargrave SL, Tao EE. Central and peripheral effects of chronic food restriction and weight restoration in the rat. Am J Physiol Endocrinol Metab 296: E282–E290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 310: 679–683, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Korner J, Savontaus E, Chua SC, Jr, Leibel RL, Wardlaw SL. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol 13: 959–966, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Koza RA, Nikonova L, Hogan J, Rim JS, Mendoza T, Faulk C, Skaf J, Kozak LP. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet 2: e81, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leibel RL. The role of leptin in the control of body weight. Nutr Rev 60: S15–S19, S68–S87, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Levin BE, Dunn-Meynell AA. Defense of body weight against chronic caloric restriction in obesity-prone and -resistant rats. Am J Physiol Regul Integr Comp Physiol 278: R231–R237, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 283: R941–R948, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725–R730, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21: 2191–2192, 1998 [DOI] [PubMed] [Google Scholar]
- 37. MacLean PS, Higgins JA, Jackman MR, Johnson GC, Fleming-Elder BK, Wyatt HR, Melanson EL, Hill JO. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 290: R1577–R1588, 2006 [DOI] [PubMed] [Google Scholar]
- 38. MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Donahoo WT, Melanson EL, Hill JO. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 287: R1306–R1315, 2004 [DOI] [PubMed] [Google Scholar]
- 39. MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Peters JC, Hill JO. Metabolic adjustments with the development, treatment, and recurrence of obesity in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 287: R288–R297, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev 18: 171–195, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol 24: 1–10, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295: 1549–1555, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304: 110–115, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115: 3579–3586, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr 88: 906–912, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr 71: 1421–1432, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Rosenbaum M, Kissileff HR, Mayer LE, Hirsch J, Leibel RL. Energy intake in weight-reduced humans. Brain Res 1350: 95–102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rosenbaum M, Leibel RL. Leptin: a molecule integrating somatic energy stores, energy expenditure and fertility. Trends Endocrinol Metab 9: 117–124, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab 87: 2391–2394, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab 81: 3424–3427, 1996 [DOI] [PubMed] [Google Scholar]
- 52. Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab 82: 3647–3654, 1997 [DOI] [PubMed] [Google Scholar]
- 53. Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 118: 2583–2591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, Leibel RL. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol 285: R183–R192, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Shi H, Akunuru S, Bierman JC, Hodge KM, Mitchell MC, Foster MT, Seeley RJ, Reizes O. Diet-induced obese mice are leptin insufficient after weight reduction. Obesity (Silver Spring) 17: 1702–1709, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sims EA. Experimental obesity, dietary-induced thermogenesis, and their clinical implications. Clin Endocrinol Metab 5: 377–395, 1976 [DOI] [PubMed] [Google Scholar]
- 58. Tulp OL, Gregory MH, Danforth E., Jr Characteristics of diet-induced brown adipose tissue growth and thermogenesis in rats. Life Sci 30: 1525–1530, 1982 [DOI] [PubMed] [Google Scholar]
- 59. Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 21: 323–341, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci 331: 166–174, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Yu Y, Deng C, Huang XF. Obese reversal by a chronic energy restricted diet leaves an increased Arc NPY/AgRP, but no alteration in POMC/CART, mRNA expression in diet-induced obese mice. Behav Brain Res 205: 50–56, 2009 [DOI] [PubMed] [Google Scholar]