Abstract
It is well established that GABAergic inputs to the paraventricular nucleus of the hypothalamus (PVN) tonically suppress heart rate and the activity of several sympathetic nerves. However, whether GABA similarly inhibits PVN control of baroreflex function has not been previously investigated. To test this hypothesis, it was determined whether microinjection of the GABAA antagonist, bicuculline, into the PVN enhances the baroreflex in anesthetized female virgin rats. In addition, because GABAergic inhibition of PVN preautonomic neurons is decreased during pregnancy, it was also determined whether the effects of PVN bicuculline administration on baroreflex function were less in pregnant animals. In virgin rats, PVN microinjection of bicuculline increased (P < 0.05) baroreflex gain and maximum levels of heart rate (gain, from 1.6 ± 0.6 to 3.8 ± 1.3 bpm/mmHg; maximum, from 406 ± 18 to 475 ± 14 bpm) and of lumbar sympathetic nerve activity (gain from 2.6 ± 0.7 to 4.8 ± 1.6%/mmHg; maximum, 149 ± 32 to 273 ± 48%), indicating that PVN GABA normally suppresses baroreflex function. Pregnancy decreased heart rate baroreflex gain (pregnant, 0.9 ± 0.3 bpm/mmHg; virgin, 1.9 ± 0.2 bpm/mmHg; P < 0.05). Following PVN bicuculline administration in pregnant rats, smaller (P < 0.01) increments in baroreflex gain (pregnant, 0.6 ± 0.1 bpm/mmHg; virgin, 2.4 ± 0.9 bpm/mmHg) and maximum (pregnant, 33 ± 7 bpm; virgin, 75 ± 12 bpm; P < 0.05) were produced. Collectively, these data suggest that the PVN normally inhibits the baroreflex via tonic GABAergic inputs and that this inhibition is less during pregnancy.
Keywords: heart rate, baroreceptor reflex, lumbar sympathetic nervous system, γ-aminobutyric acid
the paraventricular nucleus (PVN) of the hypothalamus integrates multiple forebrain and brain stem inputs to regulate the autonomic nervous system via outputs that ultimately converge with focal brain stem nuclei, such as the rostral ventrolateral medulla, as well as preganglionic sympathetic neurons in the spinal cord (7, 27, 29, 32). While the contribution of the PVN and its connections to basal sympathetic tone at rest in normal animals is minimal, it appears to drive increased sympathetic activity in such pathophysiological states as hypertension, heart failure, and water deprivation (7, 27). Moreover, the PVN mediates, in part, autonomic responses to diverse homeostatic challenges, such as changes in food intake, blood volume, stress, and body temperature (29). However, whether and how the PVN can also influence the regulation of the baroreceptor reflex is currently unclear.
Of the PVN neurotransmitters and neuromodulators that effect these many functions, the tonically active inhibitory GABAergic inputs to preautonomic neurons are dominant (10, 28). Indeed, local pharmacological blockade of PVN GABAA receptors elicits profound increases in arterial pressure, heart rate (HR), and sympathetic activity and excites neurons that project to the brain stem or spinal cord (15, 18, 20, 22). Therefore, one aim of the present study was to test the hypothesis that this substantial GABAergic input also tonically inhibits baroreflex control of HR and sympathetic activity.
A secondary goal was to test whether the GABAergic inhibition can be altered, specifically during pregnancy. Pregnancy impairs baroreflex function by reducing baroreflex gain (BRG) and the maximum levels of sympathetic activity and HR achieved during acute severe hypotension (3). Yet, indirect evidence suggests that any PVN GABAergic suppressive influence on baroreflex function is lessened, thus counteracting the more primary inhibitory effect of pregnancy. First, pregnancy activates the renin-angiotensin system (3, 30), which has been shown to decrease PVN GABAergic inhibition of sympathetic activity and heart rate (17, 19, 20). Second, a recent study by Kvochina et al. (16) demonstrated that the increases in renal sympathetic nerve activity, HR, and arterial pressure following blockade of PVN GABAA receptors were less in anesthetized rats studied near term compared with nonpregnant animals. Therefore, the second aim of the present study was to test the hypothesis that GABAergic suppression of baroreflex function is reduced during pregnancy, by determining whether the increase in BRG following PVN bicuculline is blunted in pregnant rats.
METHODS
Animals.
Experiments were performed using female virgin (240–280 g) or pregnant (390–465 g) Sprague-Dawley rats (Charles River, Wilmington, MA). After arrival, all rats were housed in a room with a 12:12-h light-dark cycle and had free access to food (LabDiet 5001, Richmond, IN) and water; at least 5 days were allowed before any experimentation. Some rats were placed in a male rat's home cage, and vaginal cytology was examined daily. The presence of sperm was designated pregnancy day 0 (term is 21–22 days), and the female rat was returned to its home cage. All procedures were conducted in accordance with the National Institutes of Health's Guide for the Health and Use of Laboratory Animals and were approved by the Institutional (Oregon Health & Science University) Animal Care and Use Committee.
Surgery.
Throughout the surgery and experiment, body temperature was maintained at 37 ± 1°C using a rectal thermistor, heat lamp, and heating pad. Anesthesia was induced with 5% isoflurane in 100% oxygen. A tracheal tube was first inserted, so that the animals could be artificially ventilated, and a surgical plane of anesthesia was maintained with 2% isoflurane in 100% oxygen.
Femoral arterial and venous catheters were implanted for arterial pressure measurements and infusions, respectively. In some rats, after a midline abdominal incision, a bipolar stainless-steel electrode was placed around a lumbar nerve and secured using lightweight silicone material (Kwik-Sil, World Precision Instruments, Sarasota, FL). Rats were then positioned in the stereotaxic device (David Kopf, Tujunga, CA), with the skull flattened between bregma and lambda. A midline incision was made on the top of the skull, and an opening was burred through the bone on the midline caudal to bregma, to prepare for PVN microinjections or microinjections lateral or rostral to PVN. After completion of all surgical manipulations, an intravenous infusion of urethane (∼1.1 g/kg in 1 ml saline) was then administered over ∼30 min; beginning 10 min after initiating the urethane infusion, the gas anesthetic was slowly withdrawn, but artificial ventilation with 100% oxygen was maintained throughout the experiment. After completion of surgery and the urethane infusion, the rats were allowed to stabilize for ∼60 min before experimentation. The depth of anesthesia was periodically assessed by confirming the lack of response to tail or paw pinch. Additional urethane (0.2 g/kg) was occasionally administered intravenously when needed.
PVN microinjections.
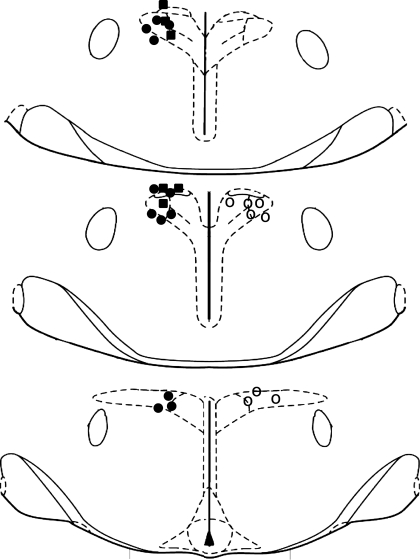
Single-barreled glass micropipettes (20- to 50-μm tip diameter) containing bicuculline were directed toward the PVN using the following coordinates (using bregma and the dorsal surface of the dura as zero): 1.8 mm caudal, 0.5 mm lateral, and 7.2–7.6 mm ventral. In addition, to document the anatomical specificity of the PVN injections, additional experiments were performed in which bicuculline was microinjected rostral or lateral to PVN. Coordinates lateral to the PVN were 1.8 mm caudal, 2.0 mm lateral, and 7.2 mm ventral, and coordinates rostral to the PVN were between 0.3 and 0.5 mm caudal, 0.5 mm lateral, and 7.2 mm ventral. Microinjections of bicuculline [60–70 nl of 1 mM/l in artificial cerebrospinal fluid (aCSF) containing (in mM): 128 NaCl, 2.6 KCl, 1.3 CaCl2, 0.9 MgCl2, 20 NaHCO3, and 1.3 Na2HPO4, pH 7.4] or the aCSF vehicle were made unilaterally over ∼3–7 s using a PicoPump (World Precision Instruments); the successful microinjection of drugs was verified by watching, through a microscope reticule, the movement of the fluid meniscus a distance calibrated to be ∼60 nl. At the conclusion of the experiment, ∼50 nl of 2.5% Alcian blue in 0.5 M sodium acetate was injected into the PVN using the same pipette and coordinates as for injections. The brain was removed and placed in 4% paraformaldehyde in PBS for at least 48 h. The hypothalamus was subsequently cut into 25-μm sections using a cryostat; sections were mounted on glass microscope slides and counter-stained with neutral red. Correct placement into PVN was indicated by dye centered just dorsally, ventrally, or within the nucleus, ∼1.8 to 2.2 mm caudal to bregma (Fig. 1).
Fig. 1.
Coronal sections of rat hypothalamus illustrating sites of bicuculline microinjection in virgin rats, in which baroreflex control of LSNA and HR were quantified (■), virgin rats in which baroreflex control of HR only was assessed (●), and pregnant rats (○). While injections were usually made on the left side, to enhance clarity, microinjection sites in pregnant rats are shown on the right side. Sections are (from top to bottom) −1.8, −1.88, and −2.12 from bregma.
Baroreflex function.
Baroreflex function curves were produced and recorded as previously described (26). Briefly, mean arterial pressure (MAP) was first rapidly decreased to ∼50 mmHg by intravenous infusion of nitroprusside (1 mg/ml; 20 μl/min). Then, MAP was slowly and smoothly raised to >175 mmHg over ∼3–5 min by both decreasing the rate of nitroprusside infusion and infusing phenylephrine at increasing rates (1 mg/ml; 1–35 μl/min). Curves were constructed from data obtained during the MAP upswing from 50 to 175 mmHg. MAP, HR, and lumbar sympathetic nerve activity (LSNA; band pass filtered between 100 and 3,000 Hz) were sampled at 2,000 Hz using a Biopac MP100 data acquisition and analysis system. Raw LSNA was rectified and integrated, and the LSNA, MAP, and HR data were grouped into 1-s bins, from which mean values were obtained. LSNA background noise was taken as the post mortem value and was subtracted from experimental LSNA values. LSNA was normalized to control LSNA before the experimental infusions were initiated (percentage of control). The sigmoidal baroreflex relationships between MAP and HR or LSNA generated in each experiment were fitted and compared using the Boltzman equation: HR or LSNA = (P1 − P2)/[1 + exp (MAP − P3)/P4] + P2. P1 is the maximum HR or LSNA, P2 is the minimum HR or LSNA, P3 is the MAP associated with the HR or LSNA value midway between the maximal and minimal HR or LSNA (BP50; denotes position of the curve on the x-axis), and P4 is the width (or mmHg over which the baroreflex operates), the coefficient used to calculate maximum gain, − (P1 − P2)/(P4 × 4), which is an index of the slope of the most linear part of the sigmoidal baroreflex curve.
Experimental protocols.
Baseline data were taken as the average of data collected 30 s prior to each baroreflex function assessment. Two control baroreflex curves were first determined, ∼30 min apart, to establish experimental stability. In most cases, results were within 10%, and the second curve was used for data analysis. In the first set of nonpregnant rats (n = 6) instrumented with recording electrodes for LSNA, bicuculline or aCSF was microinjected into PVN, and beginning ∼5 min after completing the injection, baroreflex function curves for control of LSNA and HR were regenerated. After at least 1 h, the other solution was microinjected, and a second curve was produced. In a second set of nonpregnant animals, following construction of control HR baroreflex curves, bicuculline was microinjected rostral (n = 5) or lateral (n = 5) to the PVN, and another curve was generated. Finally, in a third set of virgin (n = 12) and pregnant (n = 8; studied on gestational day 20) rats, HR baroreflex function curves were produced before and ∼5 min after completing a bicuculline microinjection. In four virgin and two pregnant rats in this set, aCSF was also microinjected into PVN (either before or after bicuculline administration), and baroreflex curves were produced as for the first set.
Data analysis.
The effects of PVN microinjection of bicuculline and aCSF in virgin rats on hemodynamics and HR/LSNA sigmoidal baroreflex parameters were determined using one-way ANOVA for repeated measures and the post hoc Newman-Keuls test. A paired t-test was used to assess differences between preinjection and postinjection responses in control virgin rats that received bicuculline lateral or rostral to PVN. Differences between and within the second set of virgin and pregnant rats in MAP, HR, and sigmoidal baroreflex curve parameters were determined using two-way ANOVA for repeated measures and the post hoc Newman-Keuls test; in the subset of virgin rats in which the aCSF vehicle was microinjected, differences were assessed using a paired t-test. P < 0.05 was considered statistically significant.
RESULTS
Effects of microinjection of bicuculline or aCSF into the PVN on baroreflex control of LSNA and HR in nonpregnant rats.
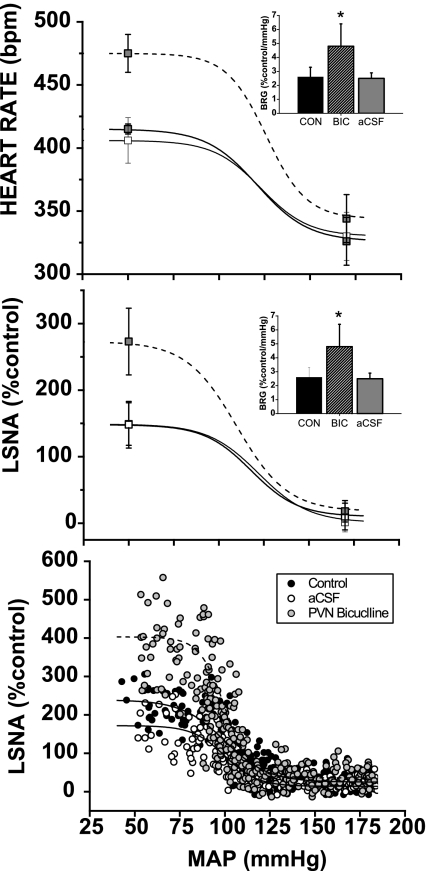
PVN bicuculline increased MAP, HR and LSNA (Table 1). In addition, gain of baroreflex control of HR and LSNA increased, as well as HR and LSNA baroreflex maximums, HR baroreflex minimum, and HR and LSNA baroreflex ranges (Fig. 2, Table 1). Neither HR and LSNA width nor BP50 were altered significantly (Table 1). Baroreflex control of HR and LSNA were also not changed following microinjection of the aCSF vehicle into the PVN (Fig. 2, Table 1).
Table 1.
Effect of PVN bicuculline and aCSF on basal values and baroreflex parameters
| Basal Values | MAP, mmHg | HR, bpm | LSNA, % |
|---|---|---|---|
| Control | 103 ± 5 | 367 ± 13 | 100 ± 0 |
| Bicuculline | 115 ± 5* | 430 ± 29* | 222 ± 16* |
| aCSF | 99 ± 4 | 367 ± 19 | 89 ± 12 |
| HR curves | HR Max, bpm | HR Min, bpm | HR Range, bpm | Width, mmHg | BP50, mmHg |
|---|---|---|---|---|---|
| Control | 406 ± 18 | 330 ± 19 | 76 ± 22 | 13.4 ± 2.2 | 123 ± 6 |
| Bicuculline | 475 ± 14* | 344 ± 19* | 132 ± 22* | 11.0 ± 1.9 | 126 ± 3 |
| aCSF | 415 ± 4 | 326 ± 19 | 89 ± 19 | 14.8 ± 1.8 | 121 ± 4 |
| LSNA curves | LSNA Max, % | LSNA Min, % | LSNA Range, % | Width, mmHg | BP50, mmHg |
|---|---|---|---|---|---|
| Control | 149 ± 32 | 1 ± 14 | 148 ± 38 | 12.6 ± 2.6 | 122 ± 7 |
| Bicuculline | 273 ± 48* | 18 ± 17 | 254 ± 44* | 12.7 ± 2.5 | 110 ± 7 |
| aCSF | 148 ± 35 | 10 ± 20 | 138 ± 38 | 12.4 ± 3.0 | 118 ± 6 |
MAP, mean arterial blood pressure; HR, heart rate; LSNA, lumbar sympathetic nerve activity; aCSF, artificial cerebrospinal fluid. Max, baroreflex maximum HR or LSNA; Min, baroreflex minimum HR or LSNA; BP50, see text.
P < 0.05, control vs. bicuculline; n = 6 virgin rats.
Fig. 2.
Microinjection of bicuculline (BIC) into paraventricular nucleus (PVN) increases gain of baroreflex control of heart rate (HR) (top panel and insert) and lumbar sympathetic nerve activity (LSNA; middle panel and insert and bottom representative experiment). CON, control; aCSF, artificial cerebrospinal fluid; BRG, baroreflex gain. *P < 0.05 compared with CON.
Effects of microinjection of bicuculline rostral or lateral to PVN.
In nonpregnant rats, microinjection of bicuculline in regions outside of PVN did not significantly alter HR baroreflex function (Table 2).
Table 2.
Effect of microinjections of bicuculline lateral or rostral to PVN on mean arterial pressure, heart rate and baroreflex parameters
| MAP, mmHg | HR, bpm | Gain, bpm/mmHg | Max, bpm | Min, bpm | Width, mmHg | BP50, mmHg | |
|---|---|---|---|---|---|---|---|
| Lateral Bicuculline (n = 5) | |||||||
| Control | 101 ± 3 | 364 ± 24 | 1.6 ± 0.2 | 366 ± 21 | 308 ± 31 | 8.2 ± 1.1 | 120 ± 3 |
| Bicuculline | 102 ± 4 | 371 ± 23 | 1.6 ± 0.3 | 389 ± 20 | 303 ± 33 | 13.3 ± 1.0 | 130 ± 3 |
| Rostral Bicuculline (n = 5) | |||||||
| Control | 107 ± 4 | 359 ± 8 | 1.8 ± 0.4 | 379 ± 19 | 309 ± 12 | 11.0 ± 1.3 | 123 ± 4 |
| Bicuculline | 106 ± 4 | 373 ± 12 | 1.7 ± 0.6 | 389 ± 24 | 323 ± 13 | 10.8 ± 1.8 | 122 ± 3 |
Max, Baroreflex maximum HR; Min, Baroreflex minimum HR.
Effects of pregnancy.
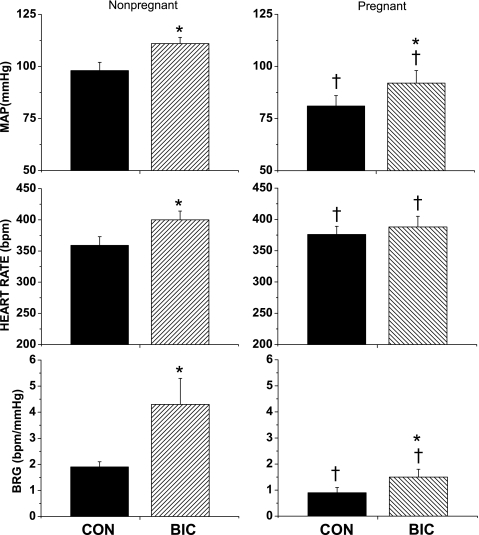
Pregnancy decreased MAP and increased HR (Fig. 3). In addition, baroreflex function was impaired as reflected by decreases in baroreflex gain (Figs. 3 and 4) and increases in width (Table 3); no other baroreflex parameters were significantly altered.
Fig. 3.
Effect of PVN BIC microinjection on mean arterial pressure (MAP), HR, and BRG. *P < 0.05, BIC compared with CON. †P < 0.05, pregnant compared with nonpregnant.
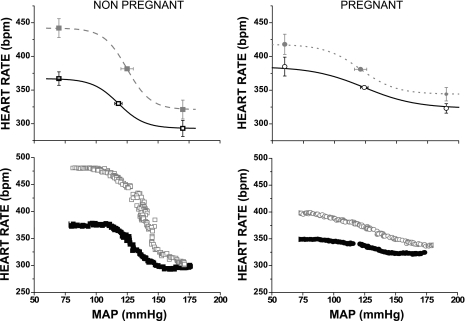
Fig. 4.
Effect of PVN microinjection of bicuculline (gray symbols and dashed lines) on baroreflex control of HR in pregnant and nonpregnant rats. Black lines and symbols are control curves.
Table 3.
Comparison of effects of PVN bicuculline on HR baroreflex parameters in pregnant and nonpregnant rats
| Max, bpm | Min, bpm | Range, bpm | Width, mmHg | BP50, mmHg | |
|---|---|---|---|---|---|
| Nonpregnant (n = 12) | |||||
| Control | 367 ± 10 | 293 ± 12 | 74 ± 8 | 10.5 ± 1.1 | 118 ± 3 |
| Bicuculline | 462 ± 14* | 321 ± 14* | 121 ± 13* | 9.5 ± 1.5 | 125 ± 5 |
| Pregnant (n = 8) | |||||
| Control | 385 ± 14 | 323 ± 7 | 62 ± 15 | 21.0 ± 3.6† | 124 ± 3 |
| Bicuculline | 418 ± 15*† | 344 ± 10 | 75 ± 8 | 14.3 ± 1.7†* | 121 ± 5 |
Max, baroreflex maximum HR; Min, baroreflex minimum HR.
P < 0.05, control vs. bicuculline;
P < 0.05, pregnant vs. nonpregnant.
Comparison of effects of microinjection of bicuculline into PVN between nonpregnant and pregnant rats.
As in the first experimental series, microinjection of bicuculline into the PVN in nonpregnant rats increased MAP, HR (Fig. 4) and baroreflex gain (Fig. 3). PVN bicuculline also increased maximum and minimum baroreflex HR and HR range, without affecting width or BP50 (Table 3).
In pregnant rats, microinjection of bicuculline into the PVN increased MAP and baroreflex gain, without significantly increasing HR (Figs. 3 and 4). Nevertheless, the increment in gain in pregnant rats (0.6 ± 0.1 bpm/mmHg) was less (P < 0.01) than in virgin rats (2.4 ± 0.9 bpm/mmHg). Bicuculline also increased maximum baroreflex HR and decreased width without significantly altering minimum baroreflex HR, HR range, or BP50 (Table 3). However, MAP, baroreflex gain, width, and maximum HR remained reduced compared with virgin rats (Figs. 3 and 4 and Table 3).
In the subset of pregnant and nonpregnant animals tested, as in the first experiment, microinjection of the aCSF vehicle into PVN did not appear to change basal values or baroreflex function (e.g., baroreflex gain in bpm/mmHg: virgin rats, 1.4 ± 0.2 to 1.3 ± 0.3, P > 0.50, n = 4; pregnant rats, 0.9 ± 0.4 to 1.1 ± 0.6, n = 2).
DISCUSSION
The purpose of the present study was to test the hypothesis that PVN GABAergic inputs tonically suppress baroreflex function and to determine whether this suppression is diminished during pregnancy. The major new findings are 1) blockade of PVN GABAA receptors via PVN microinjection of bicuculline enhances baroreflex control of HR and LSNA by increasing BRG and the reflex-induced maximal levels of HR and LSNA, and 2) the effects of bicuculline to improve baroreflex control of HR are smaller in late-pregnant rats. From these data, we conclude that the PVN normally inhibits the baroreflex via tonic GABAergic inputs and that this inhibition is less during pregnancy. Moreover, it would appear that the mechanisms by which pregnancy impairs baroreflex function do not involve greater GABAergic inhibition in the PVN.
While PVN GABA has been clearly established to tonically inhibit the basal activity of multiple sympathetic nerves (15, 27), a major goal of the present series of experiments was to test whether it also suppresses baroreflex function. Previous studies have suggested that the PVN may be involved in the regulation of the arterial baroreceptor reflex. Electrophysiological studies demonstrated that PVN neurons that project to the spinal cord are sensitive to changes in arterial pressure (23). Moreover, using c-Fos immunocytochemistry as an indirect index of neuronal activation, several previous reports suggest that PVN preautonomic neurons, albeit a small percentage, are stimulated by decreases in arterial pressure (2, 8). Patel and Schmid (25) further reported that PVN injection of lidocaine enhances lumbar sympathoinhibition in response to pressor stimuli (25). Conversely, in other studies, electrical stimulation of the PVN inhibited baroreflex responses (6, 11). However, the use of lidocaine or electrical stimulation may also have inhibited or stimulated fibers in passage, rather than influencing PVN neuronal activity directly. Whether the substantial GABAergic restraint of PVN preautonomic neurons also contributes to PVN regulation of the baroreflex has not been previously tested.
In the present study, we found that PVN microinjection of the GABAA antagonist, bicuculline, markedly increased gain of baroreflex control of HR and LSNA. In addition, the maximum levels of LSNA and HR achieved during severe hypotension were significantly increased by local blockade of PVN GABAergic influences. Importantly, neither PVN microinjection of the aCSF vehicle nor microinjection of bicuculline outside of the PVN significantly altered the baroreflex, indicating that the preparation was stable and that the effect of bicuculline was site specific. Therefore, we conclude that GABAergic inputs to the PVN tonically suppress baroreflex function and that release of this inhibition can enhance baroreflex gain and maximal levels of LSNA and HR.
Another major goal of this study was to determine whether PVN GABAergic inhibition of the baroreflex can be altered, specifically during pregnancy. We found that local blockade of PVN GABAA receptors only slightly enhanced baroreflex function in pregnant animals, and baroreflex function remained depressed in pregnant rats compared with virgin animals receiving PVN bicuculline. These results suggest that pregnancy reduces the inhibition of the baroreflex by endogenous GABA in PVN, which is consistent with a recent report that, during pregnancy in rats, PVN GABAergic inhibition of basal LSNA is also reduced (16). An alternative interpretation is that tonic excitatory drive of PVN preautonomic neurons, which would be revealed following blockade of PVN GABAA receptors (4, 5), is lessened. Arguing against this possibility, considerable previous work using multiple diverse approaches suggests that sympathetic tone is elevated during pregnancy (3). Moreover, in other pathophysiological states characterized by increased basal sympathetic tone, such as heart failure and hypertension, PVN GABA influences are also reduced (21, 22).
The mechanism by which pregnancy reduces the magnitude of PVN GABAergic inhibition was not investigated; however, previous work implicates ANG II. First, ANG II is increased during pregnancy (3, 30). Second, ANG II contributes to the increased basal levels of sympathetic activity in pregnant animals (24), indicating that angiotensin influences neural control of the circulation. Indeed, pregnancy appears to increase the relative contribution of PVN ANG II type 1 receptors to the support of arterial pressure and renal sympathetic nerve activity (16). Third, acute and chronic increases in ANG II decrease GABAergic function in PVN, possibly by presynaptic inhibition of GABA release (17, 19, 20).
In agreement with previous work in conscious and anesthetized animals (3), the current study demonstrated that pregnancy impairs gain of baroreflex control of HR. At least two mechanisms contribute to this. First, increased levels of the neurosteroid metabolite of progesterone, 3α-hydroxy-dihydroprogesterone (3α-OH-DHP), decrease the maximal level of sympathetic activity produced during hypotension by increasing GABAergic suppression of premotor neurons in the rostral ventrolateral medulla (3, 14). The present results demonstrating reduced GABA influences in PVN suggest that the effect of 3α-OH-DHP to enhance GABA actions in the PVN is minimal. Second, pregnancy decreases brain insulin, which contributes to the fall in BRG (1, 9). Insulin normally increases baroreflex gain via an action in the hypothalamus (26) and the PVN is involved (3). Insulin is an inhibitory neurotransmitter (31, 33); therefore, insulin would be expected to excite preautonomic neurons by disinhibition. The present results suggest that insulin does not enhance the baroreflex by inhibition of PVN GABAergic influences, since this mechanism would predict increased PVN GABAergic inhibition during pregnancy as brain insulin levels fall. Instead, the results suggest that the mechanisms by which pregnancy impairs the baroreflex do not involve greater PVN GABAergic inhibition.
Perspectives and Significance
The present results demonstrate that GABAergic PVN inputs tonically suppress the baroreflex and that this suppression is less during pregnancy, which may counteract the dominant effect of pregnancy to inhibit baroreflex control of HR and sympathetic activity. In addition to pregnancy, other conditions like heart failure and hypertension may be associated with impaired baroreflex function (12, 13). Interestingly, in these pathophysiological states, decreased PVN GABAergic influences on basal sympathetic tone have also been observed (21, 22). Therefore, we speculate that, in these conditions, like pregnancy, the suppression of baroreflex function does not involve enhanced GABAergic inhibition of PVN preautonomic neurons.
GRANTS
This work was supported in part by National Institutes of Health Grants HL70962 and HL088552 and a Grant-in-Aid from the American Heart Association, Pacific Mountain Affiliate.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Azar AS, Brooks VL. Impaired baroreflex gain during pregnancy in conscious rats: role of brain insulin. Hypertension 57: 283–288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Badoer E, Merolli J. Neurons in the hypothalamic paraventricular nucleus that project to the rostral ventrolateral medulla are activated by haemorrhage. Brain Res 791: 317–320, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Brooks VL, Dampney RA, Heesch CM. Pregnancy and the endocrine regulation of the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 299: R439–R451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen QH, Haywood JR, Toney GM. Sympathoexcitation by PVN-injected bicuculline requires activation of excitatory amino acid receptors. Hypertension 42: 725–731, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol 285: R1231–R1239, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Chen YL, Chan SH, Chan JY. Participation of galanin in baroreflex inhibition of heart rate by hypothalamic PVN in rat. Am J Physiol Heart Circ Physiol 271: H1823–H1828, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Dampney R, Horiuchi J, Killinger S, Sheriff M, Tan P, McDowall L. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol 32: 419–425, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Dampney RA, Polson JW, Potts PD, Hirooka Y, Horiuchi J. Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell Mol Neurobiol 23: 597–616, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Daubert DL, Chung MY, Brooks VL. Insulin resistance and impaired baroreflex gain during pregnancy. Am J Physiol Regul Integr Comp Physiol 292: R2188–R2195, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Decavel C, van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol 302: 1019–1037, 1990 [DOI] [PubMed] [Google Scholar]
- 11. Duan YF, Kopin IJ, Goldstein DS. Stimulation of the paraventricular nucleus modulates firing of neurons in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 277: R403–R411, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol 54: 375–385, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Grassi G. Sympathetic and baroreflex function in hypertension: implications for current and new drugs. Curr Pharm Des 10: 3579–3589, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Heesch CM, Foley CM. CNS effects of ovarian hormones and metabolites on neural control of circulation. Ann NY Acad Sci 940: 348–360, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Kenney MJ, Weiss ML, Haywood JR. The paraventricular nucleus: an important component of the central neurocircuitry regulating sympathetic nerve outflow. Acta Physiol Scand 177: 7–15, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Kvochina L, Hasser EM, Heesch CM. Pregnancy decreases GABAergic inhibition of the hypothalamic paraventricular nucleus. Physiol Behav 97: 171–179, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. LaGrange LP, Toney GM, Bishop VS. Effect of intravenous angiotensin II infusion on responses to hypothalamic PVN injection of bicuculline. Hypertension 42: 1124–1129, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li DP, Chen SR, Pan HL. Nitric oxide inhibits spinally projecting paraventricular neurons through potentiation of presynaptic GABA release. J Neurophysiol 88: 2664–2674, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci 23: 5041–5049, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li DP, Pan HL. Angiotensin II attenuates synaptic GABA release and excites paraventricular-rostral ventrolateral medulla output neurons. J Pharmacol Exp Ther 313: 1035–1045, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Li DP, Pan HL. Role of gamma-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther 320: 615–626, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand 177: 17–26, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Lovick TA, Coote JH. Electrophysiological properties of paraventriculo-spinal neurones in the rat. Brain Res 454: 123–130, 1988 [DOI] [PubMed] [Google Scholar]
- 24. O'Hagan KP, Skogg KA, Stevenson JB. AT1 receptor block does not affect arterial baroreflex during pregnancy in rabbits. Am J Physiol Heart Circ Physiol 280: H1996–H2005, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Patel KP, Schmid PG. Role of paraventricular nucleus (PVH) in baroreflex-mediated changes in lumbar sympathetic nerve activity and heart rate. J Auton Nerv Syst 22: 211–219, 1988 [DOI] [PubMed] [Google Scholar]
- 26. Pricher MP, Freeman KL, Brooks VL. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension 51: 514–520, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J Chem Neuroanat 38: 197–208, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol 332: 123–143, 1993 [DOI] [PubMed] [Google Scholar]
- 29. Schlenker EH. Integration in the PVN: another piece of the puzzle. Am J Physiol Regul Integr Comp Physiol 289: R653–R655, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Skinner SL, Lumbers ER, Symonds EM. Analysis of changes in the renin-angiotensin system during pregnancy. Clin Sci 42: 479–488, 1972 [DOI] [PubMed] [Google Scholar]
- 31. Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 3: 757–758, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Ann Rev Neurosci 6: 269–324, 1983 [DOI] [PubMed] [Google Scholar]
- 33. Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci 30: 2472–2479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]