Abstract
Mesenchymal stem cells (MSCs) may offer therapeutic benefit in the setting of sepsis and endotoxemia. Previous studies suggest that MSCs from female donors may possess better protective capabilities than their male counterparts. The present study examined whether female MSCs may offer a greater protective advantage in the setting of endotoxemic cardiac dysfunction compared with male MSCs. Adult male Sprague-Dawley rats were injected intraperitoneally with LPS and then treated with intraperitoneal injections of either saline, female MSCs, or male MSCs. Hearts and serum were then collected for analysis of myocardial function, myocardial protein, and myocardial and serum cytokines. Compared with male MSC or vehicle-treated animals, female MSC treatment resulted in greater preservation of myocardial function (P < 0.001). Serum and myocardial levels of all measured cytokines were comparable between rats given MSCs from male or female donors but substantially improved over rats given vehicle (P < 0.05). Reduced myocardial inflammation correlated with reduced levels of phosphorylated p38 MAPK expression in the myocardium of animals injected with MSCs of either sex (P < 0.05). The Bcl-xL/Bax ratio was increased to a greater extent following treatment with female MSCs vs. male MSCs (P < 0.05). Intraperitoneal administration of MSCs is effective in limiting myocardial inflammation and dysfunction in the rat endotoxemia model. Compared with treatment with their male counterparts, MSC treatment from female donors is associated with greater cardiac protection against acute endotoxemic injury.
Keywords: sex, sepsis, apoptosis, myocardial injury, mesenchymal stem cell
mesenchymal stem cells (mscs) represent a promising therapeutic modality for a variety of diseases. In sepsis, MSCs use paracrine signaling mechanisms to counteract the physiological derangements and multiorgan dysfunction created by an overwhelming systemic inflammatory response to invading pathogens (42, 43). They have been shown to prevent apoptosis, decrease systemic and local proinflammatory cytokines, augment anti-inflammatory mediators, preserve organ function, and upregulate bacterial killing in response to LPS and other inflammatory stimuli (14, 16, 24). They have been successfully employed in murine endotoxemia and cecal ligation and puncture models to decrease both the clinical symptoms and histopathologic severity of organ damage, increase survival times, and modulate the immune response (26, 43). Similar improvement can be noted in acute lung injury models using MSCs to combat the effects of overwhelming infection (16, 20).
Of interest, MSCs appear to exhibit sex-specific characteristics, which may impact their ability to protect injured tissue. Our group has noted sex differences in MSC function after exposure to LPS, hypoxia, and hydrogen peroxide, with increases in protective factors and decreases in proinflammatory factors noted in female-derived murine MSCs over that of male-derived MSCs (8). In addition, we have demonstrated that treatment with female MSCs improves myocardial functional recovery to a greater extent than male MSCs in a cardiac ischemia/reperfusion (I/R) injury model (6). This disparity in male and female MSC-protective capability may be due in part to differing levels of receptors or intracellular signaling enzymes that are activated in response to exogenous stressors, as well as the relative importance of these pathways in producing outcomes. In fact, studies involving tumor necrosis factor receptor 1 (TNFR1) ablation in MSCs exposed to LPS show an inherent resistance of female MSCs to the increased proinflammatory cytokine and decreased growth factor production of male MSCs (7).
The concept of a sex bias in disease has been previously recognized. Sex hormones have been shown to influence outcomes after trauma-hemorrhage, I/R injury, and sepsis (5, 19, 40). Increasing evidence suggests that sex steroids, specifically estrogen, may help normalize organ function and ameliorate the inflammatory response through paracrine actions on immune cells and host tissue (19, 37, 44). Indeed, studies involving males and ovariectomized females given exogenous estrogen support this hypothesis of a sex partiality in ischemia and sepsis (5, 40). Additional research has identified decreased activation of the proinflammatory p38 MAPK pathway in the female sex after both ischemic and endotoxemic insults and increased antiapoptotic mechanisms (15, 25, 38, 47).
However, it is unknown whether sex-specific MSCs can also ameliorate tissue injury in sepsis and provide cardioprotection. We hypothesized that treatment with female MSCs would improve myocardial functional recovery, decrease systemic and myocardial inflammation, and alter the myocardial profile to a more antiapoptotic state to a greater extent than treatment with male MSCs in a rat endotoxemia model. Furthermore, we hypothesized that this decrease in myocardial inflammation would be associated with MSC paracrine-mediated downregulation of the p38 MAPK pathway.
METHODS
Animals.
Adult male Sprague-Dawley rats weighing 250–300 g (Harlan, Indianapolis, IN) were fed a standard diet and placed in isolation for acclimation purposes for 1 wk prior to experimentation. The animal protocol was approved by the Institutional Animal Care and Use Committee of Indiana University, and humane care was provided according to the Guide for the Care and Use of Laboratory Animals (NIH publication 85Y23, revised 1996).
Bone marrow mesenchymal stem cell preparation.
Using previously described methods, we harvested MSCs from the femurs and tibias of male and female C57BL/6 mice (8–10-wk-old; Jackson Laboratory, Bar Harbor, ME) after euthanization and expanded for use between passages 3–6 (1). Briefly, epiphyses were removed and bony shafts were repeatedly flushed using complete media. Cells were strained through a 30-μm nylon mesh and centrifuged for 5 min at 300 rpm at 24°C; the supernatant was then discarded. The cell pellet was resuspended in 37°C complete media and cultured in a 25-cm2 flask under 5% CO2, 37°C, and 90% humidity conditions. MSCs preferentially adhered to the polystyrene surface; after 24 h, complete media were replaced, and changed every 3–4 days after. Cells were passaged at 90% confluence, replated in 75-cm2 culture flasks, and suspended in sterile saline at 2 × 106 cells/ml immediately prior to injection.
Cell surface marker and differentiation assessment.
Cell surface marker expression was analyzed via flow cytometry after passage 3. In concordance with known stem cell markers, greater than 90% of cells were found to be negative for hematopoietic markers CD45, CD11b, CD90, and CD117 and positive for stem cell markers Sca-1 and CD44 (data not shown) (1, 27, 29). Cells were also induced to differentiate into osteocytes, chrondrocytes, and adipocytes in culture (data not shown) (27).
Experimental groups.
Male rats were divided into four experimental groups and received two separate intraperitoneal injections at time 0 and time 1 h, respectively: 1) saline + saline (control) (n = 12), 2) LPS + saline (vehicle) (n = 12), 3) LPS + male MSCs (n = 12), and 4) LPS + female MSCs (n = 12). Doses were as follows: 1 ml normal saline, 20 mg/kg LPS (Salmonella typhimurium LPS; Sigma, St. Louis, MO) in 1 ml normal saline, 2 million male MSCs in 1 ml saline, 2 million female MSCs in 1 ml saline. LPS dose was determined on the basis of prior studies from this laboratory documenting its effectiveness in producing cardiac endotoxemia (22, 41). MSC dose was also determined to be the minimal effective dose based on previous laboratory data (41). Rats were killed at time 6 h. Serum from the right ventricle was collected for cytokine concentration determination prior to removing hearts for the Langendorff protocol.
Ex vivo isolated heart perfusion (Langendorff).
Hearts were subjected to the same protocol: 15 min equilibration and subsequent 5 min readings at end-diastolic pressure (EDP) pressures of 5, 10, 20, 30, and 40 mmHg. Rats were anesthetized (60 mg/kg ip pentobarbital sodium) and heparinized (500 U ip), and hearts were removed via median sternotomy and placed in a 4°C modified Krebs-Henseleit solution. The aorta was cannulated, pulmonary arteriotomy and left atriotomy were made, and a water-filled latex balloon was inserted through the left atrium to the left ventricle to measure EDP. The heart was perfused in the Langendorff mode and paced at 350 bpm. Pulmonary artery effluent was collected to measure coronary flow. Data were recorded using MacLab 8 preamplifier/digitizer software (AD Instruments, Milford, MA). Maximal positive and negative values of the first derivative of pressure (+dP/dt and −dP/dt, respectively) were calculated using PowerLab software (AD Instruments, Colorado Springs, CO). At end-perfusion, hearts were sectioned, snap-frozen in liquid nitrogen, and stored at −80°C for later analysis.
ELISA.
Homogenized heart tissue and serum samples that were taken from the right ventricle during rat death were centrifuged for 10 min at 12,000 rpm at 4°C, and supernatants were collected. Levels of IL-1β, IL-6, IL-10, and TNF-α production were measured by enzyme-linked immunosorbent assays (ELISA) using commercially available ELISA kits (R&D Systems, Minneapolis, MN). All tests on samples and standards were performed in duplicate per the manufacturer's instructions. Protein concentrations were measured using the Bradford protocol via biophotometer and used to standardize ELISA values. Results are reported as picograms per milligram protein.
Western blot analysis.
The heart was homogenized and lysed in a cold radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors (Sigma). The protein extracts (10 μg/lane) were then electrophoresed on a 4–12% Bis-Tris gel (Invitrogen, Carlsbad, CA), transferred to a nitrocellulose membrane, and blocked with 5% dry milk solution for 1 h. The membrane was then incubated with the primary antibodies p38 MAPK, phospho-p38 MAPK (Thr180/Tyr182), Bcl-xL, Bax (Cell Signaling Technology, Beverly, MA), or GAPDH (Biodesign International, Saco, ME) overnight at 4°C. Each incubation was followed by secondary horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG antibody incubation, and detection was determined by Supersignal West Pico stable peroxide solution (Pierce, Rockford, IL, USA). Films were scanned using an Epson Perfection 3200 Scanner (Epson America, Long Beach, CA).
Statistical analysis.
All reported values are means ± SE. Measures of myocardial function were compared using two-way ANOVA with post hoc Bonferroni test. Measures of myocardial protein and myocardial and systemic enzymes were compared using one-way ANOVA with post hoc Neuman-Keuls test. Results were considered statistically significant if P < 0.05.
RESULTS
Female MSCs offer greater preservation of myocardial function during endotoxemia.
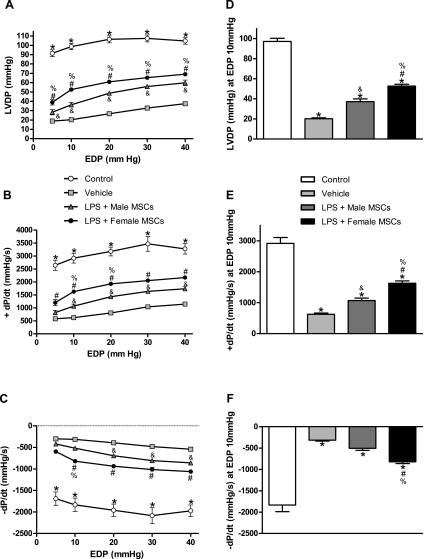
As expected, endotoxemic injury resulted in markedly depressed cardiac function in all groups (Fig. 1). Hearts treated with MSCs of either sex exhibited significant improvement in left ventricular developed pressure (LVDP), +dP/dt and −dP/dt compared with vehicle (Fig. 1, A–C). Control hearts demonstrated less depression of all myocardial functional parameters compared with treated groups. Furthermore, hearts treated with female MSCs exhibited greater preservation of myocardial function compared with rats treated with male MSCs or vehicle. LVDP, +dP/dt, and −dP/dt following treatment with female MSCs were 2.6 times greater than vehicle-treated animals (P < 0.001) and 1.4–1.6 times greater following treatment with male MSCs (P < 0.05) at a standard EDP of 10 mmHg (Fig. 1, D–F). In general, infusion of either male or female MSCs preserved cardiac function in endotoxemic hearts with female MSCs providing greater protection for all three parameters measured.
Fig. 1.
Changes in myocardial function over a range of end-diastolic pressures (EDP) following intraperitoneal injection of saline, male mesenchymal stem cells (MSCs), or female MSCs in rats pretreated with saline or LPS. LVDP (mmHg) over all EDPs measured (A) and at 10 mmHg (D); +dP/dt over all EDPs measured (B) and at 10 mmHg (E); -dP/dt over all EDPs measured (C) and at 10 mmHg (F). Endotoxemic rats given female MSCs had greater improvement in all functional parameters compared with endotoxemic rats given male MSCs or saline. Results are means ± SE. *P < 0.001 vs. all treated groups; #P < 0.01 vs. vehicle; &P < 0.05 vs. vehicle; and %P < 0.05 vs. LPS + male MSCs.
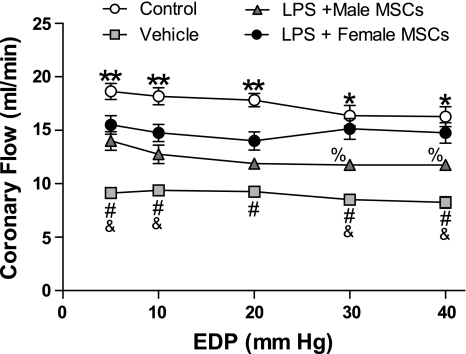
Coronary flow rates mimic myocardial function.
LPS injection depressed coronary flow rates in all treated groups with greater depression seen at higher EDPs of 30 and 40 mmHg (Fig. 2). The vehicle group experienced the greatest depression in flow rates over all EDPs and measured between 8.25 and 9.38 ± 0.5 ml/min vs. 11.75 and 14.0 ml/min ± 0.8 ml/min in the male MSC-treated group and between 14.0 and 15.5 ml/min ± 0.9 ml/min in the female MSC-treated group (P < 0.05). A significant difference was seen between male and female MSC-treated groups at EDPs of 30 and 40 mmHg only (P < 0.05). Coronary flow was preserved in the control group compared with the treated groups and ranged from 16.3 to 18.6 ml/min ± 0.9 ml/min over all EDPs measured. In general, as EDPs were sequentially increased over physiological levels of 10 mmHg, coronary flow became increasingly impaired within groups, with the exception of the female MSC-treated group.
Fig. 2.
Coronary flow rates over a range of EDPs following IP injection of saline, male MSCs, or female MSCs in rats pretreated with saline or LPS. **P < 0.05 vs. all treated groups; *P < 0.001 vs. vehicle or LPS + male MSCs; #P < 0.001 vs. LPS + female MSCs; &P < 0.05 vs. LPS + male MSCs; and %P < 0.05 vs. LPS + female MSCs.
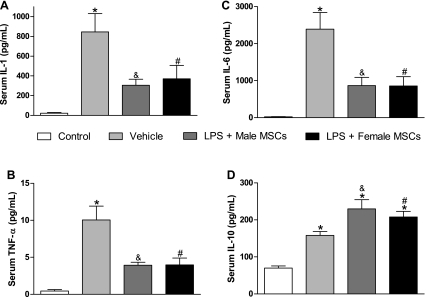
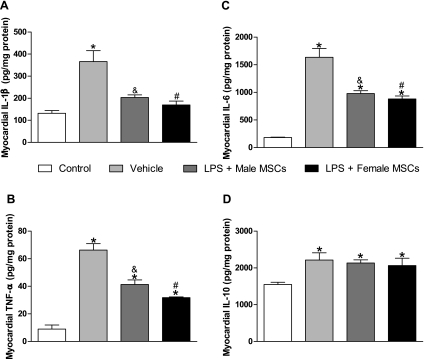
MSC injection of either sex is associated with decreased proinflammatory cytokine production and increased anti-inflammatory cytokine production.
Both systemic and myocardial production of proinflammatory IL-1β, IL-6, and TNF-α was reduced to an equal extent in endotoxemic rats treated with either male or female MSCs compared with vehicle (Fig. 3, A–C and Fig. 4, A–C). Control animals consistently expressed the lowest levels of all measured systemic and myocardial cytokines. Following female MSC injection, systemic and myocardial IL-1β levels were 370.9 ± 136 pg/ml and 169.8 ± 17 pg/mg protein, respectively, compared with 304.5 ± 61 pg/ml and 203.5 ± 12 pg/mg protein following male MSC injection and 844.9 ± 186 pg/ml and 366.5 ± 49 pg/mg protein in vehicles (P < 0.05). Similar results were observed for systemic and myocardial IL-6 and TNF-α. MSC treatment reduced proinflammatory serum cytokine levels to levels comparable with control, although a significant difference was observed between MSC treatment and control for myocardial IL-6 and TNF-α (P < 0.05). In addition, serum IL-10 levels were substantially elevated in MSC-treated rats compared with both control (P < 0.05) and vehicle (P < 0.05), although myocardial IL-10 levels were similar between MSC treatment and vehicle.
Fig. 3.
Serum levels of IL-1β, IL-6, TNF-α, and IL-10 following IP injection of saline, male MSCs, or female MSCs in rats pretreated with saline or LPS. Rats treated with MSCs of either sex had significantly decreased expression of IL-1β (A), TNF-α (B), and IL-6 (C) and significantly increased expression of IL-10 (D) compared with vehicle. *P < 0.05 vs. control; #P < 0.05 vs. vehicle; &P < 0.05 vs. vehicle.
Fig. 4.
Myocardial expression of IL-1β, IL-6, and TNF-α following intraperitoneal injection of saline, male MSCs, or female MSCs in rats pretreated with saline or LPS. Endotoxemic rats injected with male or female MSCs experienced significantly lower production of IL-1β (A), TNF-α (B), and IL-6 (C) compared with vehicle. MSC or vehicle treatment resulted in comparable significant increases in IL-10 production (D) compared with control. *P < 0.05 vs. control; #P < 0.05 vs. vehicle; &P < 0.05 vs. vehicle.
MSC injection of either sex is associated with comparable inhibition of myocardial p38 MAPK pathway.
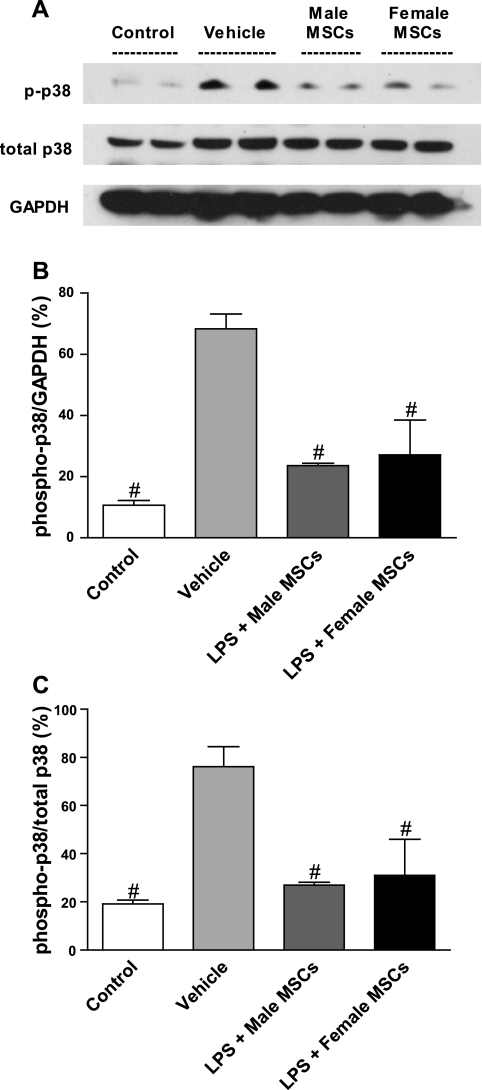
Myocardial p38 activation decreased in hearts treated with male or female MSCs compared with treated vehicles (Fig. 5, A–C). Phosphorylated p38 (p-p38) levels dropped by 60% in female MSC-treated animals (P < 0.05) and 68% in male MSC-treated animal (P < 0.05) vs. vehicle and corresponded to a 59% and 65% decrease in p-p38/total p38 levels, respectively (P < 0.05). There was no significant difference seen between MSC treatment or control. These findings suggest that sex-mediated MSC differences are not mediated by the p38 MAPK pathway in response to sepsis.
Fig. 5.
Activation of myocardial p38 following IP injection of saline, male MSCs, or female MSCs in rats pretreated with saline or LPS. Representative immunoblots of phosphorylated and total p38 and of GAPDH control (A). MSC-treated rats were found to have significantly lower myocardial levels of activated p38 enzyme as a whole (B) and as a fraction of total myocardial p38 (C) compared with vehicle-treated group. #P < 0.05 vs. vehicle.
Female MSC injection is associated with a greater myocardial antiapoptotic profile.
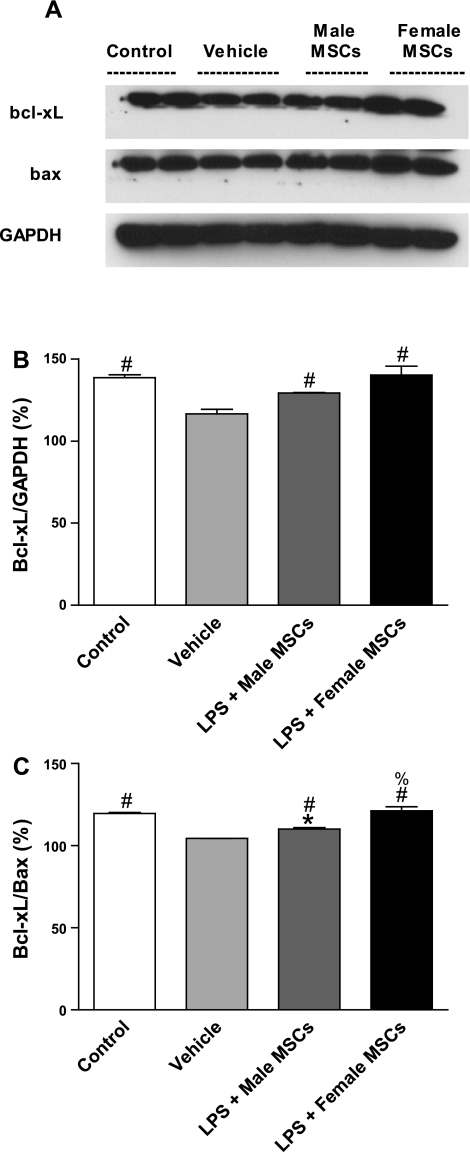
Myocardial Bcl-xL levels were significantly increased in rats treated with MSCs of either sex compared with vehicle and were comparable to control levels (Figs. 6, A and B). The Bcl-xL/Bax ratio was also increased in rats treated with MSCs of either sex compared with vehicle although female MSC-treated rats also exhibited a significantly higher ratio compared with male MSC-treated rats (Fig. 6C; P < 0.05). These findings suggest that sex-mediated MSC differences may, in part, be due to alteration of the myocardial apoptotic profile during sepsis.
Fig. 6.
Activation of myocardial Bcl-xL following intraperitoneal injection of saline, male MSCs, or female MSCs in rats pretreated with saline or LPS. A: representative immunoblots of Bcl-xL, Bax, and GAPDH control. Female MSC-treated rats were found to have significantly higher myocardial levels of activated Bcl-xL as a whole (B) compared with vehicle and as a fraction over Bax (C) compared with both male MSC and vehicle-treated groups. *P < 0.05 vs. control; #P < 0.05 vs. vehicle; %P < 0.05 vs. LPS + male MSCs.
DISCUSSION
The inherent advantage associated with the female sex regarding the cardiovascular response in sepsis is well documented and is recognized as a major factor in survival outcomes. Our results suggest that this same sex bias may increase the protective properties of MSCs against cardiac dysfunction in a rodent model of endotoxemia. In our study, while administration of female MSCs attenuated myocardial and systemic inflammation to a similar extent as administration of male MSCs, it improved myocardial functional parameters and beneficially altered the myocardial apoptotic profile beyond that seen with administration of male MSCs.
The myocardial depression observed in sepsis is thought to be due to the endotoxin-mediated release of inflammatory mediators that alters the intracellular milieu of cardiac myocytes with resultant cardiovascular compromise. Foremost among these inflammatory cytokines are TNF-α, IL-1β, and IL-6, which directly impact intracellular signaling cascades, mitochondrial function, and levels of oxidative stress to increase cardiac work, decrease contractility, and hasten myocyte injury and death (34, 45). Persistent myocardial dysfunction results in hemodynamic collapse and subsequent death.
The rodent endotoxemia model used in this study reliably mimics the cardiac depression and system-wide inflammatory response observed in human sepsis. While the cecal ligation and puncture model is more clinically relevant, the endotoxemia model allows for a more rapid rise in levels of inflammatory cytokines and in deterioration of cardiac function (33). As our aim was to determine whether the animals studied here responded favorably to either female or male MSC administration, this model provided a quicker, more efficient way to ascertain these changes. Indeed, all treatment groups experienced decreases in myocardial functional parameters across the board and elevated myocardial and serum levels of TNF-α, IL-1β, and IL-6. These changes were significantly blunted in both male and female MSC-treated rats compared with vehicle to a similar degree.
Prior studies have attempted to elucidate the properties by which MSCs appear to confer benefit in the septic host. Gene expression analysis in a rodent CLP model showed an association between MSC treatment, downregulation of systemic inflammatory genes and upregulation of genes involving phagocytosis, antigen presentation, and bacterial killing in innate immune cells (24). Other groups have used coculture experiments to provide evidence that MSCs mediate the majority of their effects through paracrine mechanisms (16, 43). Our laboratory has previously observed that MSC administration provides cardioprotection in an endotoxemia model and that this protection may, in part, be mediated by MSC-induced upregulation of IL-10 expression by peritoneal macrophages (41). The mechanism behind this hypothesis states that IL-10, an endogenous anti-inflammatory mediator, reduces the production of proinflammatory cytokines and limits the propagation of the inflammatory cascade by inhibiting the activation of peritoneal macrophages (14, 26). Less systemic inflammation results in less direct inflammation-induced insult to the endotoxemic heart. Indeed, our examination of serum IL-10 levels proved to be in concordance with this theory. In contrast, while levels of myocardial IL-10 were significantly elevated after both male and female MSC treatment compared with control, they did not differ from vehicle levels, which suggests that the myocardial injury sustained in our experiment was still in the earliest phases and that myocardial IL-10 levels were still in the process of rising. We anticipate that had the timing of the experiment been extended beyond 6 h, we would have seen an additional rise in myocardial IL-10 levels in rats treated with MSCs of either sex.
While MSC administration in general correlated with considerable improvements in endotoxemic myocardial function vs. vehicle, MSCs from female donors were associated with an additional 20.1% increase in LVDP, 25.4% increase in +dP/dt, and 25.5% decrease in −dP/dt on average over MSCs from male donors. In addition, the rates of coronary flow decreased in parallel to myocardial LVDP among all groups. Coronary flow rates were highest in the control group and lowest in the vehicle group, with evidence of equal preservation of flow among the MSC treatment groups, although they did not reach the level of controls. This greater preservation of myocardial function is likely not attributable to levels of myocardial or systemic proinflammatory cytokines, since MSC injection of either sex was associated with equivalent reductions in these parameters. Likewise, activation of the myocardial p38 MAPK pathway, which is required for proinflammatory cytokine production and contributes to the contractile dysfunction seen in the endotoxemia, was comparable among MSC treatment groups and substantially lower than vehicle levels (28, 39). Instead, this preferential improvement in myocardial function may be due to a more significant alteration in the myocardial apoptotic profile observed after treatment with female MSCs.
Prior studies have shown that females experience a distinct advantage in trauma hemorrhage, I/R injury, and sepsis (5, 19, 40). On the level of the stem cell, females have enhanced stem cell function and reparative capabilities. Female MSCs exposed to a variety of stressful conditions have greater protective factor and decreased inflammatory mediator production, as well as increased resistance to apoptosis compared with male MSCs (7, 8). In vivo animal models have pointed to improved organ function, as well as decreased tissue damage in the form of apoptosis, necrosis, and inflammation (6, 46).
Female MSCs appear to mediate some of these effects through pathways that differ from male MSCs. Wang and colleagues (7) demonstrated a reliance of male MSCs, but not female MSCs, on TNFR1 receptor expression for VEGF, TNF-α, and IL-6 production. Female MSCs also exhibit decreased apoptosis and increased levels of the protective factor VEGF in response to LPS and hypoxia compared with male MSCs (8). These findings suggest that female MSCs may inherently have a greater resistance to injury, which allows for their enhanced effects on host protection. Estrogen has been proposed as a possible mechanism to explain the protective advantage that females experience following several forms of acute injury and may also come into play at the stem cell level. Indeed, the addition of estradiol has been shown to upregulate both male and female MSC function, while testosterone may negatively affect both MSC and organ function (4, 9, 17, 31, 32). Furthermore, estrogen-preconditioned male MSCs have been shown to improve cardiac recovery following myocardial ischemia (12). However, the ability of female MSCs to provide enhanced protection in the absence of estrogen stimulation indicates that multiple factors are involved in activating the intracellular pathways involved in female stem cell activation.
One of these factors may ultimately involve alteration of the apoptotic profile in endotoxemic cardiomyocytes. Administration of LPS in our study significantly depressed the levels of antiapoptotic Bcl-xL in the myocardium, as well as decreasing the Bcl-xL/Bax ratio, thereby suggesting a shift to increased myocardial apoptosis through a combination of decreased expression of antiapoptotic proteins (such as Bcl-xL) and increased expression of proapoptotic proteins (such as Bax). This shift toward a proapoptotic state is a commonly observed pathophysiological mechanism leading to sepsis-induced myocardial dysfunction (10, 36). In contrast, administration of MSCs from male or female hosts preserved the level of Bcl-xL in the myocardium, while increasing the overall ratio of Bcl-xL to Bax. In addition, female MSC treatment was found to have a significantly increased Bcl-xL/Bax ratio over that of male MSC treatment and may be one reason why superior preservation of cardiac function was observed with female MSC treatment in our study. Indeed, the enhanced antiapoptotic properties associated with the female sex is but one reason why females have better outcomes following sepsis (30).
We would be remiss if we did not comment on the limitations of this study. Our study was designed to determine whether sex of mesenchymal stem cells had an effect on the heart in the setting of endotoxemia. However, we specifically chose to work solely with male rats to remove the influence of the female environment on final outcomes, so we do not know the effects of male MSCs on female rats. Our reasoning for this omission was to remove the influence of the female environment on cardiac function. Specifically, the so-called female advantage following sepsis has been partially attributed to the protective effects of estrogen. Removing this variable from the research environment allowed us to observe that female MSCs provided superior protection following endotoxemia irrespective of extraneous hormonal influences and suggests that there are inherent qualities in the female MSC that render it more effective in providing cardioprotection following endotoxemia over male MSCs.
While female MSCs have also been shown to have superior benefit over male MSCs in I/R injury, their potential in septic disease may arguably surpass their potential in I/R injury (6). Data examining several outcome parameters following coronary revascularization and coronary artery bypass grafting have actually shown females to have worse outcomes compared with males (18, 23). In contrast, males are more likely to experience detrimental outcomes following sepsis, including multiorgan dysfunction syndrome, more significant cytokine alterations, and higher mortality rates (13, 35). Estrogen plays an important role in maintaining the immune response following septic injury, but giving sick patients exogenous hormones entails its own risks. We've shown that female MSCs offer enhanced protection irrespective of estrogen stimulation, which suggests that the female advantage extends to the level of the stem cell. The potential of this treatment lies in the idea that MSCs can function dynamically at multiple levels and may be able to adapt to changes in the environment. Therefore, the potential of female MSCs to regulate inflammation across the breadth of sepsis may provide greater overall protection than in I/R injury, in which an ischemic hit at a specific timepoint is minimized to preserve myocardial function.
A second limitation involves our use of murine MSCs in a rat model, which has the potential for cross-reactivity. However, a number of studies has shown that the use of allogeneic and xenogenic MSCs does not appear to activate the immune system and can positively modulate local immune responses (2, 11, 21). In addition, both murine and human MSCs have been successfully transplanted long-term in rat hearts without evidence of immune rejection or immunosuppression (3, 21). Our laboratory has performed extensive research using murine MSCs in the rat model and has consistently observed the beneficial effects attributable to MSCs following both I/R and endotoxemic injuries (6, 12, 41). While rat MSCs could have been used to the same effect in this model, our main interest lies in the potential of targeted genetic manipulations of MSCs to increase their overall effectiveness in disease states. The main endpoint of this study was to conduct a baseline comparison of the inherent capabilities of male and female MSCs in an endotoxemic environment to determine whether a difference existed in their protective abilities, as our laboratory has previously shown for I/R injury. As a difference does indeed exist, we can now perform additional experiments incorporating genetically altered MSCs to good effect while comparing results with the known baseline function of female and male MSCs in endotoxemia.
Lastly, we paced the hearts as opposed to allowing them to beat spontaneously as would naturally occur. An inherent limitation of the ex vivo Langendorff protocol is its inability to mimic the physiological environment of an animal model. However, it does provide critical information on heart function through the control of specific independent variables. We chose to control EDP along a range of physiological and supraphysiological pressures to observe the compensatory abilities of the rat heart at pressures that could potentially be seen during sepsis. For similar reasons, we chose to control heart rate to focus on contractile function, since this is usually the deciding factor in maintaining systemic pressures. Had we not controlled for heart rate, we would not have been able to tease out whether a low LVDP was due to myocardial damage impeding contractile ability or to a too rapid heart rate that did not allow for sufficient filling times. Additionally, from a clinical perspective, septic patients are usually placed immediately on rate control medications to good effect, while their blood pressure tends to remain labile over a period of time. Therefore, controlling heart rate in our experiments allowed us to focus on the critical variables of heart function that determine whether heart failure will occur and which can affect survival rates in sepsis.
Taken together, these data suggest that mesenchymal stem cells are effective in limiting cardiac dysfunction in the setting of endotoxemia. Female MSCs provide greater protection over that seen with male MSC administration, in part, through increased normalization of the myocardial Bcl-xL/Bax ratio to facilitate greater preservation of cardiomyocyte function. Sex differences in the proinflammatory and anti-inflammatory response to septic injury do not appear to extend to the stem cell since myocardial and systemic cytokine production were equivalent following MSC treatment of either sex. However, the greater shift toward an antiapoptotic profile in female MSC-treated rats suggests that the benefits of female MSC treatment involve additional mechanisms beyond that of the well-subscribed sex hormone theory. Manipulating the beneficial properties of female MSCs may, therefore, provide another avenue through which to expand the potential role of stem cell use in sepsis.
GRANTS
This study was supported in part by the National Institutes of Health (Grants R01 GM070628, R01HL085595, F32HL092718, F32HL092719, and F32HL093987).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Abarbanell AM, Wang Y, Herrmann JL, Weil BR, Poynter JA, Manukyan MC, Meldrum DR. Toll-like receptor 2 mediates mesenchymal stem cell-associated myocardial recovery and VEGF production following acute ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 298: H1529–H1536, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105: 1815–1822, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Atoui R, Asenjo JF, Duong M, Chen G, Chiu RC, Shum-Tim D. Marrow stromal cells as universal donor cells for myocardial regenerative therapy: their unique immune tolerance. Ann Thorac Surg 85: 571–579, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Ba ZF, Wang P, Koo DJ, Zhou M, Cioffi WG, Bland KI, Chaudry IH. Testosterone receptor blockade after trauma and hemorrhage attenuates depressed adrenal function. Am J Physiol Regul Integr Comp Physiol 279: R1841–R1848, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, Bland KI, Chaudry IH. Gender differences in acute response to trauma-hemorrhage. Shock 24 Suppl 1: 101–106, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Crisostomo PR, Markel TA, Wang M, Lahm T, Lillemoe KD, Meldrum DR. In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power. Surgery 142: 215–221, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Crisostomo PR, Wang M, Herring CM, Markel TA, Meldrum KK, Lillemoe KD, Meldrum DR. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: role of the 55 kDa TNF receptor (TNFR1). J Mol Cell Cardiol 42: 142–149, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crisostomo PR, Wang M, Herring CM, Morrell ED, Seshadri P, Meldrum KK, Meldrum DR. Sex dimorphisms in activated mesenchymal stem cell function. Shock 26: 571–574, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Crisostomo PR, Wang M, Wairiuko GM, Morrell ED, Meldrum DR. Brief exposure to exogenous testosterone increases death signaling and adversely affects myocardial function after ischemia. Am J Physiol Regul Integr Comp Physiol 290: R1168–R1174, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Dhanantwari P, Nadaraj S, Kenessey A, Chowdhury D, Al-Abed Y, Miller EJ, Ojamaa K. Macrophage migration inhibitory factor induces cardiomyocyte apoptosis. Biochem Biophys Res Commun 371: 298–303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99: 3838–3843, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Erwin GS, Crisostomo PR, Wang Y, Wang M, Markel TA, Guzman M, Sando IC, Sharma R, Meldrum DR. Estradiol-treated mesenchymal stem cells improve myocardial recovery after ischemia. J Surg Res 152: 319–324, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frink M, Pape HC, van Griensven M, Krettek C, Chaudry IH, Hildebrand F. Influence of sex and age on MODS and cytokines after multiple injuries. Shock 27: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 58: 929–939, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Guerra S, Leri A, Wang X, Finato N, Di Loreto C, Beltrami CA, Kajstura J, Anversa P. Myocyte death in the failing human heart is gender dependent. Circ Res 85: 856–866, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179: 1855–1863, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Hong L, Sultana H, Paulius K, Zhang G. Steroid regulation of proliferation and osteogenic differentiation of bone marrow stromal cells: a gender difference. J Steroid Biochem Mol Biol 114: 180–185, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelsey SF, James M, Holubkov AL, Holubkov R, Cowley MJ, Detre KM. Results of percutaneous transluminal coronary angioplasty in women. 1985–1986 National Heart, Lung, and Blood Institute's Coronary Angioplasty Registry. Circulation 87: 720–727, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, Patel KM, Wairiuko GM, Markel TA, Meldrum DR. Sex differences in the myocardial inflammatory response to acute injury. Shock 23: 1–10, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 106: 16357–16362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacDonald DJ, Luo J, Saito T, Duong M, Bernier PL, Chiu RC, Shum-Tim D. Persistence of marrow stromal cells implanted into acutely infarcted myocardium: observations in a xenotransplant model. J Thorac Cardiovasc Surg 130: 1114–1121, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Markel TA, Crisostomo PR, Wang M, Herrmann JL, Abarbanell AM, Meldrum DR. Right ventricular TNF resistance during endotoxemia: the differential effects on ventricular function. Am J Physiol Regul Integr Comp Physiol 293: R1893–R1897, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Mehilli J, Ndrepepa G, Kastrati A, Nekolla SG, Markwardt C, Bollwein H, Pache J, Martinoff S, Dirschinger J, Schwaiger M, Schomig A. Gender and myocardial salvage after reperfusion treatment in acute myocardial infarction. J Am Coll Cardiol 45: 828–831, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 182: 1047–1057, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Meldrum DR, Wang M, Tsai BM, Kher A, Pitcher JM, Brown JW, Meldrum KK. Intracellular signaling mechanisms of sex hormones in acute myocardial inflammation and injury. Front Biosci 10: 1835–1867, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15: 42–49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 103: 1662–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Peng T, Zhang T, Lu X, Feng Q. JNK1/c-fos inhibits cardiomyocyte TNF-alpha expression via a negative crosstalk with ERK and p38 MAPK in endotoxaemia. Cardiovasc Res 81: 733–741, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 109: 1422–1432, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Raju R, Chaudry IH. Sex steroids/receptor antagonist: their use as adjuncts after trauma-hemorrhage for improving immune/cardiovascular responses and for decreasing mortality from subsequent sepsis. Anesth Analg 107: 159–166, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Ray R, Herring CM, Markel TA, Crisostomo PR, Wang M, Weil B, Lahm T, Meldrum DR. Deleterious effects of endogenous and exogenous testosterone on mesenchymal stem cell VEGF production. Am J Physiol Regul Integr Comp Physiol 294: R1498–R1503, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Ray R, Novotny NM, Crisostomo PR, Lahm T, Abarbanell A, Meldrum DR. Sex steroids and stem cell function. Mol Med 14: 493–501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock 13: 110–116, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 35: 1599–1608, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Schroder J, Kahlke V, Book M, Stuber F. Gender differences in sepsis: genetically determined? Shock 14: 307–310; discussion 310–303, 2000 [PubMed] [Google Scholar]
- 36. Sharma AC. Sepsis-induced myocardial dysfunction. Shock 28: 265–269, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Sperry JL, Minei JP. Gender dimorphism following injury: making the connection from bench to bedside. J Leukoc Biol 83: 499–506, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Wang M, Baker L, Tsai BM, Meldrum KK, Meldrum DR. Sex differences in the myocardial inflammatory response to ischemia-reperfusion injury. Am J Physiol Endocrinol Metab 288: E321–E326, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Wang M, Sankula R, Tsai BM, Meldrum KK, Turrentine M, March KL, Brown JW, Dinarello CA, Meldrum DR. P38 MAPK mediates myocardial proinflammatory cytokine production and endotoxin-induced contractile suppression. Shock 21: 170–174, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Wang M, Tsai BM, Reiger KM, Brown JW, Meldrum DR. 17-β-Estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J Mol Cell Cardiol 40: 205–212, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Weil BR, Manukyan MC, Herrmann JL, Wang Y, Abarbanell AM, Poynter JA, Meldrum DR. Mesenchymal stem cells attenuate myocardial functional depression and reduce systemic and myocardial inflammation during endotoxemia. Surgery 148: 444–452 [DOI] [PubMed] [Google Scholar]
- 42. Weil BR, Markel TA, Herrmann JL, Abarbanell AM, Kelly ML, Meldrum DR. Stem cells in sepsis. Ann Surg 250: 19–27, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol 293: L131–L141, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Yu HP, Chaudry IH. The role of estrogen and receptor agonists in maintaining organ function after trauma-hemorrhage. Shock 31: 227–237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care 15: 392–397, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Zeller CN, Wang Y, Markel TA, Weil B, Abarbanell A, Herrmann JL, Kelly ML, Coffey A, Meldrum DR. Role of tumor necrosis factor receptor 1 in sex differences of stem cell mediated cardioprotection. Ann Thorac Surg 87: 812–819, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Zhu H, Shan L, Peng T. Rac1 mediates sex difference in cardiac tumor necrosis factor-alpha expression via NADPH oxidase-ERK1/2/p38 MAPK pathway in endotoxemia. J Mol Cell Cardiol 47: 264–274, 2009 [DOI] [PubMed] [Google Scholar]