Abstract
Alterations in renal function contribute to Goldblatt two-kidney, one-clip (2K1C) hypertension. A previous study indicated that bioavailability of cytochrome P-450 metabolites epoxyeicosatrienoic acids (EETs) is decreased while that of 20-hydroxyeicosatetraenoic acids (20-HETE) is increased in this model. We utilized the inhibitor of soluble epoxide hydrolase cis-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (c-AUCB) and HET-0016, the inhibitor of 20-HETE production, to study the role of EETs and 20-HETE in the regulation of renal function. Chronic c-AUCB treatment significantly decreased systolic blood pressure (SBP) (133 ± 1 vs. 163 ± 3 mmHg) and increased sodium excretion (1.23 ± 0.10 vs. 0.59 ± 0.03 mmol/day) in 2K1C rats. HET-0016 did not affect SBP and sodium excretion. In acute experiments, renal blood flow (RBF) was decreased in 2K1C rats (5.0 ± 0.2 vs. 6.9 ± 0.2 ml·min−1·g−1). c-AUCB normalized RBF in 2K1C rats (6.5 ± 0.6 ml·min−1·g−1). HET-0016 also increased RBF in 2K1C rats (5.8 ± 0.2 ml·min−1·g−1). Although RBF and glomerular filtration rate (GFR) remained stable in normotensive rats during renal arterial pressure (RAP) reductions, both were significantly reduced at 100 mmHg RAP in 2K1C rats. c-AUCB did not improve autoregulation but increased RBF at all RAPs and shifted the pressure-natriuresis curve to the left. HET-0016-treated 2K1C rats exhibited impaired autoregulation of RBF and GFR. Our data indicate that c-AUCB displays antihypertensive properties in 2K1C hypertension that are mediated by an improvement of RBF and pressure natriuresis. While HET-0016 enhanced RBF, its anti-natriuretic effect likely prevented it from producing a blood pressure-lowering effect in the 2K1C model.
Keywords: renovascular hypertension, cytochrome P-450 metabolites, epoxyeicosatrienoic acids, hydroxyeicosatrienoic acids, renal functions, blood pressure, autoregulation, glomerular filtration rate, renal blood flow, cis-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid, HET-0016
the renin-angiotensin system (RAS) contributes to the pathophysiology of hypertension in the two-kidney, one-clip (2K1C) Goldblatt hypertensive rats (6, 32). While plasma angiotensin II (ANG II) is elevated during the developmental phase of hypertension, it becomes normalized in the later phase (5, 8, 11). These findings indicate that enhancement of systemic RAS cannot be the exclusive factor maintaining 2K1C hypertension (25, 32).
Previous studies demonstrated that renal blood flow (RBF) is attenuated in 2K1C rats, and, since RBF and glomerular filtration rate (GFR) are prerequisites for normal functioning of the pressure-natriuresis, this likely results in new adjustments of renal functional parameters. In fact, alterations in tubular ion reabsorption and reduced ability to autoregulate RBF and GFR were detected in this model (15, 33). It has been suggested that these alterations may contribute to the compromised ability of the nonclipped kidney to maintain normal sodium excretion at normotensive blood pressure (BP) and to the suppression of pressure-natriuresis (32, 34, 37). However, the mechanism(s) underlying this impairment are still poorly understood.
Cytochrome P-450 metabolites of arachidonic acid, including epoxyeicosatrienoic acids (EETs) and 20-hydroxyeicosatrienoic acid (20-HETE), play an important role in the regulation of renal tubular ion transport and renal and systemic vascular tone (1, 3, 10, 22, 36). EETs increase the open probability of large-conductance calcium-activated potassium channels (BKCa), leading to vasodilation (17, 42). Additionally, EETs inhibit epithelial sodium channel (ENaC), producing natriuresis (48). Therefore, decreased bioavailability of EETs as detected in the 2K1C animals (45) might be a contributing factor to the alteration of renal hemodynamics and sodium handling. Furthermore, production of 20-HETE is increased in this model (45). Because 20-HETE is a potent vasoconstrictor (31, 50, 56), it may contribute to increased vascular resistance and attenuated RBF.
Taken together, the available data indicate that cytochrome P-450 metabolites play an important role in the physiological regulation of renal tubular function and renal hemodynamics. Because 2K1C rats exhibit reduced bioavailability of EETs during the sustained phase of hypertension (45), we hypothesized that intrarenal deficiency of EETs contributes to the impaired renal hemodynamics and sodium excretory functions. Therefore, we employed the novel soluble epoxide hydrolase (sEH) inhibitor cis-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyl-oxy]-benzoic acid (c-AUCB) (16) to increase EETs bioavailability by blocking their degradation. We hypothesized that the antihypertensive effects are mediated by an improvement of renal hemodynamics and the pressure-natriuresis relationship. In view of the finding that 2K1C rats exhibit simultaneous elevation of intrarenal 20-HETE (45), we used HET-0016 to inhibit the production of 20-HETE and studied its effect on BP, renal hemodynamics, and sodium excretion.
METHODS
Our experimental protocol was submitted to and approved by the Committee for Animal Use of the Ministry of Health of Czech Republic. All Hannover Sprague-Dawley rats (HanSD) were bred at the Department of Experimental Medicine at IKEM from stock animals supplied from the Max Delbrück Center for Molecular Medicine of Berlin, Germany. All animals were fed standard rat chow (0.4% NaCl) and tap water ad libitum and were kept on a 12:12-h light-dark cycle.
2K1C goldblatt hypertensive rats.
Male HanSD rats (100–125 g) were anesthetized with a combination of tiletamine, zolazepam (Zoletil, 8 mg/kg; Virbac, Carros Cedex, France), and xylasine (4 mg/kg; Rometar Spofa, Czech Republic) administered intramuscularly. The right renal artery was isolated through a flank incision, and, as previously described (6, 11), a silver clip (0.25 mm in internal diameter) was placed on the renal artery. Sham-operated rats underwent the same surgical procedure except placing of the arterial clip.
Effect of c-AUCB and HET-0016 treatment on BP and sodium excretion in conscious rats.
On the 14th day postsurgery described above, sham-operated and 2K1C rats (total n = 24) were implanted with TA11PA-C40 radiotransmitters (Data Sciences, St. Paul, MN), as described previously (21, 43), to continuously monitor BP in conscious animals by a telemetric device. After 7 days of recovery, data acquisition was initiated to determine basal BP. Data were gathered as previously described (21, 43). In addition, rats were placed in metabolic cages for 24-h urine collections before and 48 h after initiating c-AUCB and HET-0016 treatment to assess daily sodium excretion in the following groups (n = 6–7 in each group): 1) untreated 2K1C rats, 2) c-AUCB-treated 2K1C rats, 3) HET-0016-treated 2K1C rats, 4) untreated sham-operated rats, 5) c-AUCB-treated sham-operated rats, and 6) HET-0016-treated sham rats. The sEH inhibitor c-AUCB was administered through drinking water. c-AUCB (26 mg) was dissolved in ethanol (5 ml) with cyclodextrin (150 mg), and, after 5 min of sonication, water was added to make up a final volume of 1 liter. Hydrogencarbonate (3 ml/l) was added to prevent the compound from precipitatation at low pH. On the 28th day after clip placement, c-AUCB treatment (26 mg/l) was initiated in 2K1C and sham-operated rats. It has been shown that these concentrations of c-AUCB inhibit sEH activity in in vitro as well as in vivo studies (16, 21). Plasma concentration of c-AUCB was determined by LC/MS analysis.
HET-0016 treatment was also initiated on the 28th day after clip placement. HET-0016 was dissolved in 10% lecithin solution on a heated stirring plate. HET-0016 was administered intraperitoneally for 2 days at a dose 10 mg·kg−1·day−1. This dose has been previously shown to block the production of 20-HETE (57).
Measurements of EETs and dihydroxyeicosatetraenoic acids.
EETs and dihydroxyeicosatetraenoic acids (DHETEs) were measured in the kidney samples employing a commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D, Detroit, MI), according to the manufacturer's suggestions (51, 53). Eicosanoids were extracted three times from kidney samples with ethyl acetate after acidification with acetic acid. After evaporation, saponification, and reextraction, the concentration of the stable EET metabolite 14,15-DHETEs was analyzed by a 14,15-DHETE ELISA kit (Spiecker; R&D).
Acute autoregulatory experiments.
To study renal autoregulation, a previously described protocol was employed (9, 46). Briefly, rats were anesthetized with thiopental (50 mg/kg ip) and placed on a thermoregulated surgical table to maintain body temperature at 370C. A tracheotomy was performed, and a PE-240 tube was inserted to maintain a patent airway. The tracheal cannula was placed inside an open plastic chamber; the animals breathed normal atmospheric air enriched by humidified gas containing a 95% oxygen-5% carbon dioxide mixture. This procedure improves stability of BP in anesthetized rats without indication of acidosis.
The right jugular vein was catheterized for fluid infusion and anesthetic administration. The left femoral artery was catheterized with a PE-50 catheter to allow continuous monitoring of arterial BP and blood sampling. Mean arterial pressure (MAP) was monitored with a pressure transducer (model MLT 1050) and recorded on the computer using a computerized data-acquisition system (PowerLab/4SP; AD Instruments). The left kidney was exposed via a flank incision, isolated from the surrounding tissue, and placed in a lucite cup, and the ureter was cannulated with a PE-10 catheter. The aortic adjustable clamp was placed on the aorta immediately above the junction of the left renal artery to regulate the level of renal arterial pressure (RAP). In addition, an ultrasonic transient-time flow probe (1RB; Transonic Systems, Altron Medical Electronic) connected to a Transonic flow meter was placed around the left renal artery, and RBF was recorded using a computerized acquisition system. During surgery, an isotonic saline solution containing bovine serum albumin (4%; Sigma Chemical, Prague, Czech Republic) was infused at a rate of 40 μl/min. After surgery, isotonic saline solution containing albumin (1% wt/vol) and polyfructosan (7.5%, Inutest; Laevosan, Linz, Austria) was infused at the same infusion rate.
After completion of the surgical procedures, an equilibration period of 1 h was allowed for the animals to establish steady state before initiating 30-min control urine collection at physiological RAP. Thereafter, 30-min urine collections were performed in 2K1C at reduced RAPs (125, 100, 90, and 80 mmHg). Similarly, in sham-operated rats, stepwise decreases of RAPs were performed. Ten minutes of equilibration time were allowed after each RAP reduction before urine collection. Blood samples were collected after the second and fourth urine collection to allow determination of GFR and renal sodium excretion. The same four experimental groups of rats were examined (n = 9–12).
Analysis of autoregulatory index.
For each RAP reduction, autoregulatory index (AI) was calculated based on the formula: AI = (RBF2-RBF1)/RBF1/(RAP2-RAP1)/RAP1. AI equal to zero suggest perfect autoregulation, and values greater than zero suggest less perfect autoregulation.
General analytical methods.
Urine volume was measured gravimetrically. Sodium and potassium concentrations were determined by flame photometry. Polyfructosan was measured colorimetrically. Polyfructosan was used as an index of the GFR. GFR and RBF per gram of kidney weight and fractional excretion of sodium and potassium were calculated using standard formulas. ANG II was measured in the decapitated rats (to avoid the effect of anesthesia) in the nonclipped kidney and plasma using previously published methods (43).
Statistical analysis.
All values are expressed as means ± SE. With Graph Pad Prism software (Graph Pad Software, San Diego, CA), one-way ANOVA and two-way repeated-measures ANOVA followed by the Student-Newman-Keuls test were employed when appropriate. Values exceeding the 95% probability limits (P < 0.05) were considered statistically significant.
RESULTS
Effect of c-AUCB treatment on systolic BP and sodium excretion in conscious rats.
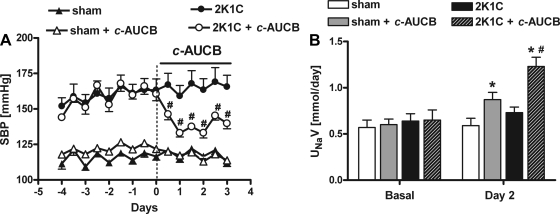
As previously established, 2K1C rats developed hypertension with systolic BP (SBP) averaging 161 ± 5 mmHg. Sham-operated rats remained normotensive (119 ± 3 mmHg). Although treatment with c-AUCB had no effect on SBP in sham-operated rats, it significantly decreased SBP in 2K1C rats; the maximal BP-lowering effect was attained 48 h after initiation of c-AUCB administration (−30 ± 2 mmHg, P < 0.05) (Fig. 1A).
Fig. 1.
Effect of cis-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (c-AUCB) on systolic blood pressure (SBP) and 24-h sodium excretion in conscious sham-operated and two-kidney, one-clip (2K1C) rats. A: SBP responses to c-AUCB (radiotelemetric recordings). B: 24-h sodium excretion measured on the 2nd day of c-AUCB administration in metabolic cages. UNaV, urinary sodium excretion. *P < 0.05 vs. basal values. #P < 0.05 vs. corresponding values from c-AUCB-treated rats.
The plasma concentration of c-AUCB reached 87 ± 10 ng/ml in the 2K1C rats and 69 ± 8 ng/ml in sham-operated rats.
The basal daily sodium excretion was not significantly different between groups and remained unaltered in untreated 2K1C rats as well as in sham-operated rats on tap water. However, c-AUCB administration elicited a marked increase in daily sodium excretion in 2K1C on the second day of treatment (Fig. 1B). c-AUCB also produced a slight natriuretic effect in sham-operated rats. While the regulation of sodium and fluid balance appears to be altered by c-AUCB, the treatment did not produce alterations of the hematocrit in these animals.
In addition, 14,15-EETs and 14,15-DHETEs were measured in the kidney samples by an ELISA kit; the EETs-to-DHETEs ratio was significantly smaller in 2K1C rats compared with sham rats (2.3 ± 0.2 vs. 6.9 ± 1). c-AUCB significantly enhanced the ratio in the 2K1C rats to 3 ± 0.1 but not in the sham rats (7.3 ± 1.3).
Finally, c-AUCB did not change plasma ANG II levels (32 ± 4 vs. 35 ± 6 and 38 ± 7 vs. 39 ± 6 fmol/ml) or ANG II levels in the nonclipped kidney (62 ± 6 vs. 59 ± 6 and 57 ± 4 vs. 63 ± 6 fmol/g) in sham and 2K1C rats, respectively.
Effect of HET-0016 treatment on SBP and sodium excretion in conscious rats.
As measured by radiotelemetry, administration of HET-0016 did not produce any effect on SBP in the 2K1C rats (171 ± 5 vs. 169 ± 10) nor in the sham rats (124 ± 7 vs. 122 ± 7). We also studied the effect of HET-0016 on basal daily sodium excretion using metabolic cages. Although HET-0016 treatment resulted in a reduction of sodium excretion in both 2K1C and sham rats, this reduction did not reach statistical significance (0.99 ± 0.1 vs. 1.32 ± 0.1 for 2K1C and 0.70 ± 0.2 vs. 0.93 ± 0.1 mmol/day for sham rats).
Acute autoregulatory experiments with sequential reductions of RAP.
Basal values of MAP, renal hemodynamics, and electrolyte excretion are summarized in Table 1. Although administration of c-AUCB did not alter MAP, RBF, and GFR in sham-operated rats, it significantly decreased MAP and normalized basal RBF without major effects on GFR in 2K1C rats. HET-0016 treatment did not affect MAP in 2K1C rats. However, it significantly enhanced both basal RBF and GFR in these rats. HET-0016 had no effect on the basal functional parameters in sham rats.
Table 1.
Basal values of mean arterial pressure, renal hemodynamics, and excretory function in experimental animals
| Group of rats | n | MAP, mmHg | RBF, ml · min−1 · g−1 | GFR, ml · min−1 · g−1 | UF, μl · min−1 · g−1 | UNaV, μmol · min−1 · g−1 | FENa, % |
|---|---|---|---|---|---|---|---|
| Sham | 11 | 113 ± 2 | 6.9 ± 0.3 | 1.5 ± 0.1 | 11.2 ± 1 | 1.2 ± 0.1 | 0.6 ± 0.1 |
| Sham + c-AUCB | 10 | 118 ± 3 | 7.4 ± 0.5 | 1.5 ± 0.1 | 13.1 ± 1.3 | 1.0 ± 0.2 | 0.5 ± 0.1 |
| Sham + HET-0016 | 6 | 111 ± 4 | 6.6 ± 0.7 | 1.5 ± 0.1 | 8.5 ± 1.7 | 0.9 ± 0.3 | 0.3 ± 0.1 |
| 2K1C | 12 | 163 ± 3# | 5.0 ± 0.2# | 1.4 ± 0.1 | 22.0 ± 3.6# | 3.7 ± 0.9# | 1.7 ± 0.3# |
| 2K1C + c-AUCB | 9 | 133 ± 1* | 6.5 ± 0.6* | 1.4 ± 0.1 | 24.7 ± 2.8# | 3.0 ± 0.7# | 1.5 ± 0.3# |
| 2K1C + HET-0016 | 9 | 161 ± 5 | 5.8 ± 0.2* | 1.7 ± 0.1* | 12.9 ± 3.0 | 1.3 ± 0.4* | 0.5 ± 0.2* |
Values are means ± SE. MAP, mean arterial pressure; RBF, renal blood flow; GFR, glomerular filtration rate; UF, urine flow; UNaV, absolute sodium excretion; FENa, fractional sodium excretion; c-AUCB, cis-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid; 2K1C, 2 kidney, 1 clip. There were significant differences in basal parameters between experimental groups, P < 0.05.
Significant difference between treated and nontreated group.
Significant difference between sham and 2K1C group.
Effect of c-AUCB on renal autoregulation.
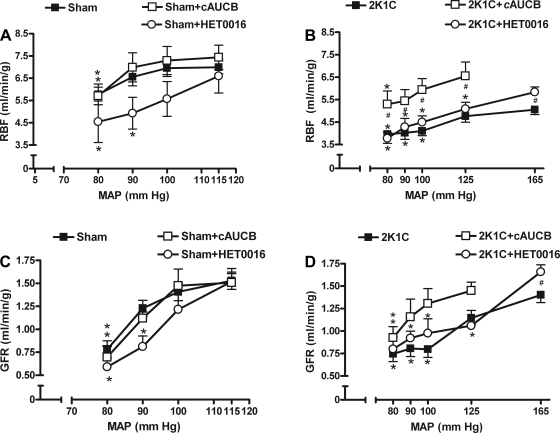
As shown in Fig. 2, A and C, sham-operated rats exhibited efficient autoregulation of both RBF and GFR up to the reduction of RAP to 90 mmHg, and c-AUCB had no effect on these parameters in these animals.
Fig. 2.
Effect of c-AUCB and HET-0016 on renal hemodynamic responses to stepwise renal arterial pressure (RAP) reductions. A and B: relationship between RAP and renal blood flow (RBF) in sham-operated (A) and 2K1C rats (B). C and D: the RAP and glomerular filtration rate (GFR) relationship in sham (C) and 2K1C (D) rats. MAP, mean arterial pressure. *P < 0.05 vs. basal values. #P < 0.05 vs. corresponding values from c-AUCB-treated rats.
However, reduction of RAP to 100 mmHg in 2K1C rats resulted in a significant decrease of RBF (Fig. 2B). In contrast, treatment with c-AUCB improved RBF at reduced RAP in 2K1C rats; RBF was significantly decreased only at 90 mmHg and below in the treated 2K1C rats. Moreover, RBF was significantly higher at all reduced RAPs in treated 2K1C rats compared with untreated 2K1C rats. A similar pattern has been detected with autoregulation of GFR; GFR was significantly attenuated in untreated 2K1C rats when RAP was reduced to 100 and below (Fig. 2D). With c-AUCB treatment, GFR was maintained stable up to a reduction of RAP to 90 mmHg. For each RAP reduction, we also calculated the AI to compare relative efficiency of autoregulation between treatment groups. c-AUCB significantly decreased AI of GFR in the 2K1C group (Table 2), indicating an improvement of autoregulation, but did not affect it in the sham group. It also did not significantly affect AIs of RBF in sham or in 2K1C group (Table 2).
Table 2.
AI was calculated to compare autoregulatory efficiency of RBF and GFR between treated and untreated animals when RAP was reduced by aortic clamp
| Sham | Sham + c-AUCB | Sham + HET-0016 | 2K1C | 2K1C + c-AUCB | 2K1C + HET-0016 | |
|---|---|---|---|---|---|---|
| RBF | ||||||
| AI 1 | 0.05 ± 0.2 | 0.19 ± 0.3 | 1.2 ± 0.5* | 0.51 ± 0.1 | 0.24 ± 0.2 | 0.67 ± 0.1 |
| AI 2 | 0.5 ± 0.3 | 0.46 ± 0.2 | 1.17 ± 0.2 | 0.29 ± 0.5 | 0.88 ± 0.2 | 0.83 ± 0.3 |
| AI 3 | 1.1 ± 0.3 | 1.5 ± 0.3 | 1.36 ± 0.5 | 0.43 ± 0.2 | 0.90 ± 0.3 | 0.76 ± 0.2 |
| GFR | ||||||
| AI 1 | 0.62 ± 0.2 | 0.80 ± 0.7 | 1.66 ± 0.7 | 1.05 ± 0.2 | 0.17 ± 0.4* | 1.10 ± 0.2 |
| AI 2 | 0.98 ± 0.6 | 1.12 ± 0.5 | 2.57 ± 1.5* | 0.3 ± 0.6 | 1.5 ± 0.5 | 1.7 ± 0.6 |
| AI 3 | 3.39 ± 0.4 | 3.57 ± 0.7 | 3.38 ± 1.6 | 0.6 ± 0.5 | 1.1 ± 0.6 | 0.8 ± 0.7 |
Values are means ± SE. AI, autoregulatory index; RAP, renal arterial pressure. AI was calculated for each RAP reduction, AI 1, AI 2, AI 3, and AI 4 represent the 1st, 2nd, 3rd, and 4th reduction, respectively. AIs were calculated from reductions in baseline to 100, 100 to 90, and 90 to 80 mmHg.
Significant difference between treated and nontreated group.
Effect of HET-0016 on renal autoregulation.
While sham rats exhibited an efficient autoregulation of RBF, HET-0016 treatment impaired this efficiency; when RAP was reduced to 90 mmHg, sham rats were no longer able to maintain RBF, as observed in the untreated group (Fig. 2A). AI of RBF in the sham group was significantly higher in the HET-0016-treated group compared with controls (Table 2), indicating an impairment of autoregulation of RBF by HET-0016. Similarly, in the 2K1C rats, the RBF was significantly reduced already at the first reduction to 125 mmHg in HET-0016-treated rats (Fig. 2B). However, although this was reflected in higher AI in this group, it was not statistically different from AI in the untreated group (Table 2).
The same pattern was observed with autoregulation of GFR; HET-0016-treated sham rats were not able to maintain GFR with a reduction of RAP to 90 mmHg (Fig. 2C). Also, GFR was significantly reduced at 125 mmHg in 2K1C rats in the HET-0016-treated group (Fig. 2D). Although the impaired autoregulation of GFR in both sham and 2K1C rats was reflected in higher AIs, the difference between AIs of treated and nontreated animals did not reach statistical significance except at the second reduction (AI 2) in the sham group (Table 2).
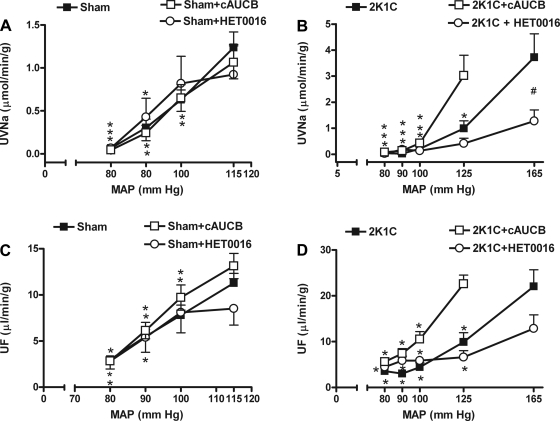
Effect of c-AUCB on sodium excretion and urine flow responses to reduced RAP.
Absolute sodium excretion and urine flow responses to reductions of RAP were not different between untreated and c-AUCB-treated sham rats (Fig. 3, A and C). Absolute sodium excretion and urine flow were also not significantly different between untreated and c-AUCB-treated 2K1C rats at basal RAP (Table 1). However, at reduced RAP levels, sodium excretion and urine flow in treated 2K1C rats were significantly greater compared with the nontreated 2K1C group (Fig. 3, B and D). c-AUCB shifted the pressure-natriuresis and pressure-diuresis curve to the left in 2K1C rats.
Fig. 3.
Effect of c-AUCB and HET-0016 on renal excretory responses to stepwise RAP reductions. A and B: relationship between RAP and absolute sodium excretion (UNaV) in sham-operated (A) and 2K1C (B) rats. C and D: the pressure-diuresis curves in sham (C) and 2K1C (D) rats. For clarity, the scale is different in sham than in 2K1C rats. *P < 0.05 vs. basal values. #P < 0.05 vs. corresponding values from c-AUCB-treated rats.
The fractional sodium excretion curve exhibited the same pattern as total sodium excretion in all four groups (data not shown).
Effect of HET-0016 on sodium excretion and urine flow responses to reduced RAP.
Although HET-0016 did not affect basal absolute sodium excretion and urine flow in sham rats, it significantly attenuated sodium excretion in 2K1C rats and shifted the pressure-natriuresis curve to the right (Fig. 3B). This was not accompanied by significant changes in urine flow (Fig. 3D and Table 1).
DISCUSSION
Our present study indicates that inhibition of sEH enzyme by c-AUCB produces natriuresis, normalizes RBF, and improves the slope of the pressure-natriuresis relationship in the 2K1C rats via increased endogenous EET levels. These improvements may greatly contribute to the antihypertensive effect of c-AUCB.
Our previous study emphasized the crucial role of EETs in the regulation of renal functions in the 2K1C rats; inhibition of EETs production significantly attenuated renal hemodynamic responses and sodium excretion (45). Recent findings indicate that deficiency in EETs contributes to the development and/or maintenance of ANG II-dependent forms of hypertension (4, 20, 59). Here we found that the EETs-to-DHETEs ratio indicative of EETs bioavailability was significantly lower in 2K1C hypertensive compared with sham rats. These data suggest increased conversion of EETs to DHETEs that have smaller biological potency than EETs (54, 55). A similar finding has been reported for human renovascular hypertension (30). Thus the present data and those of previous reports indicate that a reduction of EETs could contribute to the vascular and tubular abnormalities of the renovascular disease.
The current study determined that chronic c-AUCB treatment increased the EETs-to-DHETEs ratio, indicating increased EETs bioavailability. Indeed, c-AUCB and similar compounds have been previously shown to inhibit sEH in vivo and increase EETs in the kidney (14, 16, 21). In fact, plasma concentration of c-AUCB in our study was about 30 times higher than the IC50 of c-AUCB for the rat sEH enzyme (27). Furthermore, ANG II levels were not altered by c-AUCB, and thus the observed effect on BP and renal function might be attributable to increased endogenous EETs levels rather than alterations of RAS activity. In our study, we employed a commercially available ELISA kit to quantify 14,15-EETs. Although other regioisomers, including 5,6-EETs, 8,9-EETs, and 11,12-EETs, were likely increased by c-AUCB, we were not able to measure those simultaneously. Nevertheless, it has been reported that sEH inhibition results in comparable increases of all EET regioisomers; therefore, it is likely that c-AUCB elicited proportional increases in corresponding EET fractions. We chose to measure 14,15-EET because this regiosomer is the preferred substrate for the sEH enzyme and is known to be involved in the regulation of renal hemodynamics (22).
There are several mechanisms how increased EETs bioavailability could improve kidney function and lower BP. EETs directly stimulate BKCa and are potent vasodilators (1, 10, 17, 22, 36). They have been implicated as the endothelium-derived hyperpolarizing factor mediating nitric oxide and prostaglandin-independent vasodilatation (1). In the glomerular vasculature, EETs participate in the bradykinin-induced, endothelium-dependent vasodilatation and oppose ANG II-mediated constriction (17, 18, 20, 26). Current and previous studies indicated that RBF is reduced in the 2K1C rats (45). This could be because of EETs deficiency, resulting in increased renal vascular resistance. The increased EETs following c-AUCB treatment likely produced a vasodilatory effect and normalized RBF in our hypertension model.
Furthermore, c-AUCB also produced significant natriuretic effects. Thus additional antihypertensive mechanisms could be associated with effects on sodium reabsorption (1, 3, 22, 36, 48). It has been shown that EETs inhibit proximal tubular sodium reabsorption by blocking the sodium/hydrogen exchanger and ENaC in the cortical collecting duct (28, 48). Although we were not able to determine the specific transporter involved in c-AUCB-induced natriuresis, an improvement in tubular function was likely responsible for the leftward shift of the pressure-natriuresis in the 2K1C animals. Additionally, enhanced RBF could also contribute to the increase in sodium excretion and decrease in BP.
As opposed to sEH inhibition, 20-HETE inhibition by HET-0016 did not affect BP. However, our data agree with previous studies that, depending on the site of action, 20-HETE exhibits either prohypertensive vasoconstriction or antihypertensive natriuretic properties (23, 40, 50, 52, 56). Therefore, in vivo blockade of 20-HETE could produce counteracting effects on BP. RBF was increased by HET-0016 in 2K1C rats, suggesting decreased vascular resistance. Indeed, 20-HETE is a potent vasoconstrictor, and its overproduction in hypertension could contribute to increased vascular tone in the 2K1C rats. Inhibition of 20-HETE may therefore reduce the vascular resistance, resulting in increased GFR as observed in this study and previously (45). Additionally, basal sodium excretion was decreased, and the pressure-natriuresis curve was shifted to the right by HET-0016 in 2K1C rats. This agrees with the inhibitory effect of 20-HETE on sodium reabsorption (35, 39, 50, 52, 60).
In addition to basal functional parameters, we studied the effect of c-AUCB and HET-0016 on autoregulation of RBF and GFR to determine the role of EETs and 20-HETE in renal autoregulatory function in 2K1C hypertension. While c-AUCB appeared to improve the autoregulation of GFR and RBF in 2K1C rats (treated 2K1C rats were able to maintain stable RBF and GFR at RAP reduction 100 mmHg as opposed to nontreated), the calculated AI was only significantly improved for GFR (AI 1). AI for RBF did not differ between the treated and nontreated group, indicating that autoregulation per se was not radically improved by the treatment. These data suggest that, rather than improving autoregulation of renal hemodynamics, c-AUCB decreases BP through its effects on sodium transport and RBF, thus improving pressure-natriuresis. In contrast, autoregulation of RBF and GFR was impaired in HET-0016-treated 2K1C rats at RAP reduction to 125 (nontreated 2K1C maintained stable autoregulation at 125), and this was reflected in a higher AI value. Importantly, HET-0016 also impaired autoregulation of RBF in the sham group. These data are supported by previous findings that established the role of 20-HETE in RBF autoregulation (18, 61). Additionally, 20-HETE inhibition reduced basal sodium excretion in 2K1C rats. This observation indicates the 20-HETE operates to increase sodium excretion in hypertensive animals as previously suggested (49, 50, 52, 61).
Because of the antihypertensive properties of EETs and increased bioavailability of prohypertensive 20-HETE in 2K1C hypertension, it seems logical to investigate the combined effect of sEH and 20-HETE inhibition. However, combined EETs induction and 20-HETE inhibition on renal functions would involve antagonistic interactions (effect on natriuresis), complicating the data interpretation substantially. In addition, 20-HETE plays a critical role in the regulation of renal hemodynamics and sodium excretion (18, 49, 61), and its blockade in the kidney may counteract the antihypertensive effect of blockade in the vasculature. In the future, combined treatment of systemic inhibition of sEH and selective inhibition of 20-HETE in the vasculature could provide a great means to select the beneficial actions of 20-HETE that would synergistically interact with EETs, producing an improvement of renal functional parameters and the desired reduction of BP.
In summary, our data indicate that c-AUCB displays significant antihypertensive properties in 2K1C hypertension that are mediated by an improvement of RBF and sodium excretion. While HET-0016 also enhanced RBF, its antinatriuretic effect likely prevented it from producing a BP-lowering effect in the 2K1C model.
Perspectives and Significance
2K1C renovascular hypertension is an experimental paradigm for human renovascular hypertension. Impaired autoregulatory behavior and altered renal function, including decreased RBF and right-shifted pressure-natriuresis, could contribute to hypertension by compromising the kidney to achieve adequate excretory functions at normal pressures. Therefore, pharmacological interventions that increase RBF and decrease sodium reabsorption are promising treatment targets in hypertension. Our study indicates that inhibition of sEH has a great potential to become a new pharmacological treatment approach because of its beneficial effects on renal hemodynamics, sodium excretory responses, and BP-lowering effects.
GRANTS
L. Červenka was supported by EU (the Operational Program Prague) Competitiveness; project “CEVKOON” (#CZ.2.16/3.1.00/22126). This study was supported by a Marie Curie Fellowship from the European Commission Program PEOPLE (IRG 247847) and a postdoctoral fellowship from the Czech Science Foundation (GAČR; 303/10/P170) received by A. Sporková. This study was also supported by Grant No. 305/08/J006 awarded to L. Červenka by GAČR and by grant NS/10499-3 awarded to L. Červenka by the Internal Grant Agency of the Ministry of Health of the Czech and by the institutional financial support of the Institute for Clinical and Experimental Medicine (MZO 00023001). L. Kopkan is supported by Grant No. NS/9699-4 awarded by the Internal Grant Agency of the Ministry of Health of the Czech Republic and partially supported by a grant from the Grant Agency of the Academy of Science of Czech Republic (KJB 502030801). J. D. Imig is supported by National Institutes of Health Grants HL-59699 and DK-38226 and Advancing a Healthier Wisconsin. H. J. Kramer is supported by grants from the German Research Foundation (DFG), Bonn (Kra 433/14-2 and 436 TSE 113/50/0-1). S. H. Hwang was supported, in part, by the Howard Hughes Foundation and a fellowship from the National Institute of Environmental Health Sciences (NIEHS) Supported Basic Research Program. Partial support was provided by NIEHS Grant R37 ES-02710 and NIEHS Superfund Basic Research Program Grant P42 ES-004699 awarded to B. D. Hammock.
DISCLOSURES
B. D. Hammock and J. D. Imig are members of the Advisory Board of Arete Therapeutics.
REFERENCES
- 1. Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49: 590–596, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Capdevila JH, Falck JR, Imig JD. Role of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int 72: 683–689, 2007 [DOI] [PubMed] [Google Scholar]
- 4. CertíkováChábová V, Kramer HJ, Vaněčková I, Thumová M, Škaroupková P, Tesař V. The roles of intrarenal 20-hydroxyeicosatetraenoic and epoxyeicosatrienoic acids in the regulation of renal function in hypertensive Ren-2 transgenic rats. Kidney Blood Press Res 30: 335–346, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Cervenka L, Vaněčková I, Husková Z, Vaǒurková Z, Erbanová M, Thumová M. Pivotal role of AT1A receptors in the development of two-kidney, one-clip hypertension: study in AT1A receptor knockout mice. J Hypertens 26: 1379–1389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cervenka L, Wang CT, Mitchell KD, Navar LG. Proximal tubular angiotensin II levels and renal functional responses to AT1 receptor blockade in nonclipped kidneys of Goldblatt hypertensive rats. Hypertension 33: 102–107, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Cervenka L, Wang CT, Navar LG. Effects of acute AT1 receptor blockade by candesartan on arterial pressure and renal function in rats. Am J Physiol Renal Physiol 274: F940–F945, 1998 [DOI] [PubMed] [Google Scholar]
- 8. El-Dahr SS, Dipp S, Guan S, Navar LG. Renin, angiotensinogen, and kallikrein gene expression in two-kidney Goldblatt hypertensive rats. Am J Hypertens 6: 914–919, 1993 [DOI] [PubMed] [Google Scholar]
- 9. Erbanová M, Thumová M, Husková Z, Vaněčková I, Vaǒurková Z, Mullins JJ, Kramer HJ, Bürgelová M, Rakušan D, Červenka L. Impairment of the autoregulation of renal hemodynamics and of the pressure-natriuresis relationship precedes the development of hypertension in Cyp1a1-Ren-2 transgenic rats. J Hypertens 27: 575–586, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Fleming I. Vascular cytochrome P450 enzymes: physiology and pathophysiology. Trends Cardiovascular Med 18: 20–25, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Guan S, Fox K, Mitchell Navar LG. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one-clip hypertensive rats. Hypertension 20: 763–767, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, da Costa Goncalves Ach Huang, Huang Y, Luft FC, Gollasch M. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol 29: 54–60, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Hercule HC, Wang MH, Oyekan AO. Contribution of cytochrome P450 4A isoforms to renal functional response to inhibition of nitric oxide production in the rat. J Physiol 551: 971–979, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang H, Morisseau C, Wang J, Yang T, Falck JR, Hammock BD, Wang MH. Increasing or stabilizing renal epoxyeicosatrienoic acid production attenuates abnormal renal function and hypertension in obese rats. Am J Physiol Renal Physiol 293: F342–F349, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Huang WC, Ploth DW, Navar LG. Angiotensin-mediated alterations in nephron function in Goldblatt hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 243: F553–F560, 1982 [DOI] [PubMed] [Google Scholar]
- 16. Hwang SH, Tsai HJ, Liu Morisseau C JY, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem 50: 3825–3840, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Imig JD, Dimitropoulou C, Reddy DS, White RE, Falck JR. Afferent arteriolar dilation to 11,12-EET analogs involves PP2A activity and Ca2+-activated K+channels. Microcirculation 15: 137–150, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Imig JD, Falck JR, Inscho EW. Contribution of cytochrome P450 epoxygenase and hydroxylase pathways to afferent arteriolar autoregulatory responsiveness. Br J Pharmacol 127: 1399–1405, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imig JD, Falck JR, Wei S, Capdevila JH. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilatation in response to bradykinin. J Vasc Res 38: 247–255, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Imig JD, Zhao X, Falck JR, Wei S, Capdevila JH. Enhanced renal mircovascular reactivity to angiotensin II in hypertension is ameliorated by the sulfonimide analog of 11,12-epoxyeicosatrienoic acid. J Hypertens 19: 983–992, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim HI, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension 46: 975–981, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imig JD. Targeting epoxides for organ damage in hypertension. J Cardiovasc Pharmacol 56: 326–335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ito O, Roman RJ. Role of 20-HETE in elevating chloride transport in the thick ascending limb of Dahl SS/Jr rats. Hypertension 33: 419–423, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Jung O, Brandes RP, Kim IH, Schweda F, Schmidt R, Hammock BD, Busse R, Fleming I. Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension 45: 759–765, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Kohagure K, Endo Y, Ito O, Arima S, Omata K, Ito S. Endogenous nitric oxide and epoxyeicosatrienoic acids modulate angiotensin II-induced constriction in the rabbit afferent arteriole. Acta Physiol Scand 168: 107–112, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Liu J-Y, Tsai H-J, Hwang SH, Jones PD, Morisseau C, Hammock BD. Pharmacokinetic optimization of four soluble epoxide hydrolase inhibitors for use in a murine model of inflammation. Br J Pharmacol 156: 284–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Madhun ZT, Goldthwait DA, McKay D, Hopfer U, Douglas JG. An epoxygenase metabolite of arachidonic acid mediates angiotensin II-induced rises in cytosolic calcium in rabbit proximal tubule epithelial cells. J Clin Invest 88: 456–461, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Majid DSW, Navar LG. Blockade of distal nephron sodium transport attenuates pressure natriuresis in dongs. Hypertension 23: 1040–1045, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Minuz P, Jiang H, Fava C, Turolo L, Tacconelli S, Ricci M, Patrignani P, Morganti A, Lechi A, McGiff JC. Altered release of cytochrome P450 metabolites of arachidonic acid in renovascular disease. Hypertension 51: 1379–1385, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res 41: 175–193, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Navar LG, Zou L, Von Thun A, Wang CT, Imig JD, Mitchell KD. Unraveling the mystery of Goldblatt hypertension. News Physiol Sci 13: 170–176, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Ploth DW, Roy RN, Huang WC, Navar LG. Impaired renal blood flow and cortical pressure autoregulation in contralateral kidneys of Goldblatt hypertensive rats. Hypertension 3: 67–74, 1981 [DOI] [PubMed] [Google Scholar]
- 34. Ploth DW. Angiotensin-dependent renal mechanism in two-kidney, one-clip renal vascular hypertension. Am J Physiol Renal Fluid Electrolyte Physiol 245: F131–F141, 1983 [DOI] [PubMed] [Google Scholar]
- 35. Quigley R, Baum M, Reddy KM, Griener JC, Falck JR. Effects of 20-HETE and 19(S)-HETE on rabbit proximal straight tubule volume transport. Am J Physiol Renal Physiol 278: F949–F953, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Rostand SG, Lewis D, Watkins JB, Huang WC, Navar LG. Attenuated pressure natriuresis in hypertensive rats. Kidney Int 21: 331–338, 1982 [DOI] [PubMed] [Google Scholar]
- 38. Sakairi Y, Jacobson HR, Noland DT, Capdevila JH, Falck JR, Breyer MD. 5,6-EET inhibits ion transport in collecting duct by stimulating endogenous prostaglandin synthesis. Am J Physiol Renal Fluid Electrolyte Physiol 268: F931–F939, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Schwartzman M, Ferreri NR, Carroll MA, Songu-Mize E, McGiff JC. Renal cytochrome P450-related arachidonate metabolite inhibits (Na+ + K+)ATPase. Nature 314: 620–622, 1985 [DOI] [PubMed] [Google Scholar]
- 40. Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension 50: 123–129, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Spiecker M, Darius H, Hankeln T, Soufi M, Huesing A, Maisch B, Darryl C, Zeldin J, Liao JK. Risk of coronary artery disease associated with polymorphism of the cytochrome P450 epoxygenase CYP2J2. Circulation 110: 2132–2136, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sudhahar V, Shaw S, Imig JD. Epoxyeicosatrienoic acid analogs and vascular function. Curr Med Chem 17: 1181–1190, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vanourková Z, Kramer HJ, Husková Z, Vanecková I, Opocenský M, Chábová VC, Tesar V, Skaroupková P, Thumová M, Dohnalová M, Mullins JJ, Cervenka L. AT1 receptor blockade is superior to conventional triple therapy in protecting against end-organ damage in Cyp1a1-Ren-2 transgenic rats with inducible hypertension. J Hypertens 24: 2465–2472, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Von Thun AM, Vari RC, El-Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol Renal Fluid Electrolyte Physiol 266: F120–F128, 1994 [DOI] [PubMed] [Google Scholar]
- 45. Walkowska A, Škaroupková P, Husková Z, Vaǒurková Z, Čertíková Chábová V, Tesař V, Kramer HJ, Falck JR, Imig JD, Kompanowska-Jezierska E, Sadowski J, Červenka L. Intrarenal cytochrome P-450 metabolites of arachidonic acid in the regulation of the nonclipped kidney function in two-kidney, one-clip Goldblatt hypertensive rats. J Hypertens 28: 582–593, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol 279: F319–F325, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Wang D, Borrego-Conde LJ, Falck JR, Sharma KK, Wilcox CS, Umans JG. Contribution of nitric oxide, EDHF, and EETs to endothelium-dependent relaxation in renal afferent arterioles. Kidney Int 63: 2187–2193, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Wei Y, Lin DH, Kemp R, Yaddanapudi GS, Nasjletti A, Falck JR, Wang WH. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J Gen Physiol 124: 719–727, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Williams JM, Sarkis A, Lopez B, Ryan RP, Flasch AK, Roman RJ. Elevations in renal interstitial hydrostatic pressure and 20-hydroxyeicosatetraenoic acid contribute to pressure natriuresis. Hypertension 49: 687–694, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Williams JM, Murphy S, Burke M, Roman RJ. 20-Hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol 56: 336–344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang S, Lin L, Chen JX, Lee CR, Seubert JM, Wang Y, Wang H, Chao ZR, Tao DD, Gong JP, Lu ZY, Wang DW, Zeldin DC. Cytochrome P-450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor alpha via MAPK and PI3K/Akt signaling pathways. Am J Physiol Heart Circ Physiol 293: H142–H151, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yu M, Lopez B, Dos Santos EA, Falck JR, Roman RJ. Effects of 20-HETE on Na+ transport and Na+-K+-ATPase activity in the thick ascending loop of Henle. Am J Physiol Regul Integr Comp Physiol 292: R2400–R2405, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res 87: 992–998, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res 87: 992–998, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, Snapper JR, Capdevila JH. Regio- and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. J Biol Chem 268: 6402–6407, 1993 [PubMed] [Google Scholar]
- 56. Zhang F, Wang MH, Krishna UM, Falck JR, Laniado-Schwartzman M, Nasjletti A. Modulation by 20-HETE of phenylephrine-induced mesenteric artery contraction in spontaneously hypertensive and Wistar-Kyoto rats. Hypertension 38: 1311–1315, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Zhang Y, Wu JH, Vickers JJ, Ong SL, Temple SE, Mori TA, Croft KD, Whitworth JA. The role of 20-hydroxyeicosatetraenoic acid in adrenocorticotrophic hormone and dexamethasone-induced hypertension. J Hypertens 27: 1609–1616, 2009 [DOI] [PubMed] [Google Scholar]
- 58. Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol 15: 1244–1253, 2004 [PubMed] [Google Scholar]
- 59. Zhao X, Pollock DM, Zeldin DC, Imig JD. Salt-sensitive hypertension after exposure to angiotensin is associated with inability to upregulate renal epoxygenases. Hypertension 42: 775–780, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Zou AP, Drummond HA, Roman RJHypertension. Role of 20-HETE in elevating loop chloride reabsorption in Dahl SS/Jr rats. Hypertension 27: 631–635, 1996 [DOI] [PubMed] [Google Scholar]
- 61. Zou AP, Imig JD, Kaldunski M, de Mondellano PR, Sui Z, Roman RJ. Inhibition of renal vascular 20-HETE production impairs autoregulation of renal blood flow. Am J Physiol Renal Fluid Electrolyte Physiol 266: F275–F282, 1994 [DOI] [PubMed] [Google Scholar]