Abstract
Sutherlandia frutescens (sutherlandia), an African herbal supplement is currently recommended by the South African Ministry of Health for the treatment of AIDS patients. However, no reports yet exist delineating the effect of sutherlandia on pharmacokinetics of antiretroviral agents. Therefore, this investigation aimed at screening the effects of short term and chronic exposure of sutherlandia on oral bioavailability and pharmacokinetics of nevirapine (NVP), a non nucleoside reverse transcriptase inhibitor, in Sprague Dawley rats. NVP (6 mg/kg) was administered orally alone (control) and with co-administration of sutherlandia; short term (12 mg/kg single dose) and long term (12mg/kg, once a day for 5 days). No significant difference in the pharmacokinetic parameters of NVP was found upon short term co-administration of Sutherlandia. However, there was a 50% decrease (p < 0.05) in the AUC and Cmax values of NVP after 5 days of chronic exposure with Sutherlandia. In addition, quantitative RT-PCR studies demonstrated a 2–3 fold increase in the hepatic and intestinal mRNA expression of CYP3A2, relative to vehicle control. To further confirm, if this could translate into a clinically relevant pharmacokinetic interaction in patients, we tested this hypothesis employing LS-180 cells as an in vitro induction model for human CYP3A4. Ninety six hours post treatment, similar to positive control rifampicin (25µM), sutherlandia extract (300µg/mL) resulted in elevated m-RNA expression levels and functional activity of CYP3A4 (human homologue of rodent CYP3A2) in LS-180 cells. Taken together, these results suggest that a potential drug-herb interaction is possible when NVP is co-administered with sutherlandia frutescens, although this hypothesis still remains to be investigated in a clinical setting.
INTRODUCTION
Complementary and alternative medicines are widely co-administered with therapeutic agents in patients suffering from HIV, depression and other chronic disorders (Ernst et al., 1998; Lavretsky, 2009; Liu et al., 2009; Merenstein et al., 2008). Herbal supplements are known to possess various active constituents which can interact with efflux transporters such as P-glycoprotein (P-gp), multiple drug resistance associated proteins (MRP) and phase I drug metabolizing enzymes such as Cytochrome P4503A (CYP3A4), resulting in altered clearance and hence the bioavailability of concomitantly administered prescription drugs (Pal and Mitra, 2006). Oral absorption is primarily limited by a combined effect of efflux transporters and drug metabolizing enzymes (Benet et al., 2003). CYP3A4 is a major congener of the CYP 450 superfamily and is responsible for the metabolism of more than 60% of agents currently indicated in clinical practice. It is highly expressed in the liver and enterocytes. Inhibition or induction of intestinal and hepatic CYP3A4 activity can respectively increase or decrease the oral bioavailability of co-administered substrates and may alter the therapeutic outcome.
Sutherlandia frutescens (sutherlandia) is currently recommended by the South African Ministry of Health for the treatment of AIDS patients (Mills et al., 2005a). It is also prescribed for treatment of cancer, tuberculosis, diabetes, anxiety and clinical depression (Fernandes et al., 2004; Harnett et al., 2005; Ojewole, 2004; Sia, 2004). L-canavanine, GABA and D-pinitol are the primary known active constituents of sutherlandia but recently Fu et al. have isolated flavanol and cycloartanol glycosides form its aerial parts (Fu et al., 2008; 2010). The herbal product is manufactured in South Africa and is available in the form of 350 mg capsules and tablets. Though officially not approved by regulatory agencies in Europe and U.S., sutherlandia is distributed worldwide by a plethora of websites claiming it to be an immunity booster. Currently there are no reports of clinical trials available which suggest the efficacy of this product in AIDS or cancer patients. However, there have been several reports indicating reduction in viral load, induction of apoptosis and anti-proliferative effects in some in vitro cell culture based assays (Chinkwo, 2005; Tai et al., 2004). One study reported analgesic, anti-inflammatory and anti-diabetic effect of sutherlandia aqueous extract in various animal models (Ojewole, 2004). Hence, all these evidences indicate widespread use of sutherlandia across the globe and more than a probability of its co-administration with anti-retro viral agents, the first line treatment for AIDS patients.
The rationale for screening sutherlandia for its potential to cause clinically relevant drug-herb interactions was triggered by a recent publication by Mills et al. (Mills et al., 2005b). The authors concluded a two fold increase in the activity of Pregnane Xenobiotic Receptor (PXR) with an alcoholic extract of sutherlandia in a gene reporter based assay (Mills et al., 2005b). PXR is an orphan nuclear receptor that regulates the expression of CYP3A4 (Synold et al., 2001). It is highly expressed in liver and intestine, two key organs responsible for xenobiotic metabolism (Kliewer et al., 2002; Kliewer et al., 1998). This report prompted us to screen sutherlandia for its potential to interact with CYP3A2 expression following extended exposure in rats. NVP, a non-nucleoside reverse transcriptase inhibitor is commonly prescribed in South Africa for the treatment of HIV infection and has a similar metabolic profile in both rodent and humans (Riska et al., 1999) (Hoffmann et al., 2009; Janneh et al., 2009; Wen et al., 2009). Moreover, modulation of CYP3A activity can be time dependent, therefore short-term (for inhibition) and chronic exposure (for induction) of sutherlandia was tested in pharmacokinetic studies of NVP.
Given that sutherlandia has many pharmacological actions and is recommended for the treatment of various chronic disorders, the question still remains to be answered weather there is any potential for a pharmacokinetic drug-herb interaction. In this investigation, we have tried to answer this question by evaluating the effect of sutherlandia on modulation of CYP3A2 enzyme activity using rat model. In addition, to extrapolate the chronic effects of sutherlandia on metabolic activity of CYP3A in clinic, human adenocarcinoma cell line (LS-180) was employed as an in vitro induction model for human CYP3A4.
Materials & Methods
Chemicals
NVP oral suspension (Viramune®) was obtained from a local pharmacy store and sutherlandia capsules were a generous gift from Dr. William Folk (University of Missouri-Columbia). Dibenzepine and rifampicin were procured from Sigma Aldrich Ltd. (St. Louie, MO). All HPLC grade solvents were obtained from Fisher Scientific Co. (Pittsburgh, PA) and were of highest purity grade available.
Preparation of sutherlandia extract
1g of sutherlandia powder from capsules was extracted with 20mL of ethanol in a round bottom flask (RBF) with constant stirring for 2 hrs. After extraction, the contents were decanted in a 50mL centrifuge tube and centrifuged at 20,000rpm for 5 minutes. The supernatant was transferred into a tare weight RBF and the contents were evaporated using a rota-evaporator (Buchi, Switzerland). The residue was weighed and dissolved in distilled de-ionized water to make a stock of 5mg/mL and stored in −80°C until further use.
Cell culture and molecular biology reagents
Human colon adenocarcinoma cell line, LS-180 was obtained from American Type Culture Collection (ATCC) (Manassas, VA). Cell culture supplies: Dulbecco’s Modified Eagle’s Essential Medium (DMEM), Trypsin-EDTA solution, non-essential amino acids and Fetal Bovine Serum (FBS), were procured from Invitrogen (Carlsbad, CA). Penicillin, streptomycin, sodium bicarbonate and HEPES, were obtained from Sigma-Aldrich. Culture flasks (75 cm2 growth area) were procured from MidSci (St. Louis, MO). Ninety-six well culture plates (3.8 cm2 growth area per well) were obtained from Corning Costar Corp. (Cambridge, MA). Molecular biology reagents such as Trizol-LS® reagent was purchased from Invitrogen (Carlsbad, CA), oligo dT15 primer, Reverse transcription reagents and enzymes were procured from Promega (Madison, WI), OligoPerfect™ Designer (Invitrogen Corp. Carlsbad, CA) software was used to select the sequence of primers, SYBR® Green Quantitative RT-PCR Kit was purchased from Promega.
Animals
Male Sprague Dawley rats with jugular vein cannulated catheters, weighing 200−250 grams were procured from Charles River Laboratories International, Inc. (Wilmington, MA). The animals were used in accordance with the protocols approved by the University of Missouri-Kansas City (UMKC) Institutional Animal Care and Use Committee and housed in Laboratory Animal Care accredited facilities at UMKC. All animals were allowed to acclimatize for a minimum of 2 to 3 days before initiating the studies. Food and water were provided ad libitum. Rats were fasted overnight (10−12 hrs), with free access to water prior to the day of an experiment.
Cell line and growth conditions
LS-180 cells were selected as an in vitro model for induction studies. Cells were nourished with culture medium comprising DMEM supplemented with 10% FBS, HEPES, sodium bicarbonate, penicillin (100µg/mL), streptomycin sulphate (100µg/mL) and 1% (v/v) non-essential amino acids, adjusted to pH 7.4. Incubation took place in 75 cm2 culture flasks maintained at 37°C, in a humidified atmosphere of 5% CO2 and 95% relative humidity. Culture medium was replaced every alternate day. Cells were subcultured after 5−6 days (subculture ratio, 1:5) with triple express enzyme (Invitrogen) and plated at an appropriate density (250,000 cells/well for 12 well plates; 10,000 cells/well for 96 well plates) for each experiment. Passages 52−59 were employed for further studies.
Oral absorption studies
Dosing solution of NVP (Viramune® suspension, 6mg/kg) and sterile filtered aqueous extract (stock solution) of sutherlandia was diluted to desired concentration (12mg/kg) with distilled de-ionized water before dosing. Rats were divided into 3 groups (n=5−6/group). Three treatment regimens were selected in the oral absorption studies, i.e., (i) NVP alone (vehicle control), and (ii) NVP co-administered with sutherlandia (short term), (iii) NVP co-administered after 5 days of consecutive feeding of sutherlandia (long term) through oral gavage. The dosing volume was set at 0.8mL in all experiments. Blood samples (0.2 mL) were withdrawn from the cannulated jugular vein at pre-determined time intervals over the period of 8 hrs post dosing, and replaced with same volumes of 50U heparin (in saline) so as to prevent clotting of the catheter and to compensate for blood loss. Samples were collected in heparin coated micro-centrifuge tubes and centrifuged at 5000g for 7 min to collect the plasma which was stored at −80°C until further analysis. After the last time point, animals from each group were euthanized by injecting an excess dose of sodium pentobarbital (100mg/kg). Small intestine and liver lobes were harvested and stored at −80°C for further quantitative gene expression analysis. The dose selected for NVP and sutherlandia were similar to the recommended human doses.
Plasma sample analysis
Concentrations of NVP in rat plasma were analyzed by LC/MS-MS. Sample preparation was carried out by liquid–liquid extraction method with tert-butyl methyl ether (TBME) as the extracting solvent. Dibenzepine (150ng/mL) served as internal standard (IS). Quantitative analysis was carried out using previously published method (Laurito et al., 2002) with slight modifications. Briefly, 100µL of the plasma sample was mixed with 25 µL of internal standards (IS) solutions. Five hundred microliters of TBME was added and vortexed for 2 mins. For efficient separation of the aqueous and organic layers, samples were centrifuged at 10,000 g for 5 min. Subsequently, the organic layer was collected and dried in speed vacuum (Gene-vac DD-4X). The residue was reconstituted in 100µL acetonitrile/ammonium formate/formic acid (1:1:0.1, v/v) reconstitution mixture and 25µL was injected onto the LC/MS-MS for analysis. Extraction efficiencies for NVP and IS from plasma samples were approximately 90%.
LC/MS-MS QTrap® API-2000 mass spectrometer, (Applied Biosystems, Foster City, CA, USA) equipped with Agilent 1100 Series quaternary pump (Agilent G1311A), vacuum degasser (Agilent G1379A) and autosampler (Agilent G1367A, Agilent Technology Inc., Palo Alto, CA, USA) was employed to analyze plasma samples. HPLC separation was performed on an XTerra® MS C18 column 50 × 2.1mm, 5.0 µm (Waters, Milford, MA). The mobile phase consisted of 50% acetonitrile and 50% water with 0.1% formic acid, pumped at a flow rate of 0.3 mL/min. Analysis time was 5 min per run and NVP and dibenzepine eluted within 2–3 minutes. Multiple reaction monitoring (MRM) mode was utilized to detect the compounds of interest. The mass spectrometer was operated in the positive ion mode for detection of NVP. The precursor to product ions for all the analytes are as follows, NVP (m/z 267→226), dibenzepine (m/z 296→251). The operational parameters for the tandem mass spectrum for each analyte were obtained after running them in quantitative optimization mode. Turbo ion spray setting and collision gas pressure were optimized (IS voltage: ± 5500 V, temperature: 350°C, nebulizer gas: 40 psi, curtain gas: 30 psi). Limits of quantification were found to be 10–15 ng/mL for all the analytes. The method generated rapid and reproducible results.
Data treatment
Pharmacokinetic parameters for oral absorption studies, such as maximum plasma concentration (Cmax), time needed to reach maximum concentration (tmax) and area under the plasma concentration curve (AUC) were analyzed with noncompartmental analysis method using WinNonlin Professional v5.0 (Pharsight corporation, Mountain View, CA).
VIVID assay for determining CYP3A4 activity (Induction and inhibition)
After observing a significant pharmacokinetic drug-herb interaction from the oral absorption studies, it was important to elucidate if sutherlandia could also modulate the activity of CYP3A4 enzyme in a clinical setting, hence, Vivid CYP3A4 assay was employed. Following treatment of the LS-180 cells with sutherlandia (300µg/mL) or rifampicin (25µM in DMSO), as a positive control and 0.05% DMSO vehicle in the medium for 96h, medium was aspirated and cells were washed twice with phosphate buffer saline (PBS) at 37°C. The experiment was performed as published previously (Trubetskoy et al. 2005). Briefly, to initiate the vivid fluorescent assay, 50 µL of 100-µM vivid CYP3A4 red substrate diluted in warm PBS was added to each well resulting in a final concentration of 50 µM substrate and 0.5% acetonitrile. Fluorescent readings were monitored at 37°C after 30 minutes using a 96 well plate reader (Beckman Coulter® DTX 880) at an excitation wavelength of 535 nm, an emission wavelength of 595 nm, and CYP activity was measured as the rate of fluorescent metabolite production over the course of the reaction. Fold-induction was calculated as the activity observed after treatment with inducer compared with treatment with 0.1% DMSO (control).
A similar study was performed for CYP3A4 inhibition, concentrations ranging from 25ug/mL to 1500ug/mL of sutherlandia extract were selected for dose dependent inhibition. Ketoconazole (10µM, as suggested by the manufacturer) served as a positive control for inhibition of CYP3A4.
RTPCR and Quantification of m-RNA induction
Total RNA was isolated from cell lysate and rat tissue using Trizol-LS® reagent (Invitrogen, Carlsbad, CA) according to manufacturer’s protocol. Briefly, 1 µg of total RNA was reverse transcribed using standard protocol with MMLV Reverse Transcriptase (Promega, Madison, WI). After the first strand cDNA synthesis, 2 µL was used for further PCR analysis as per standard protocol using Taq polymerase (Promega,Madison, WI). Human primers used for the amplification CYP3A4 (Forward 5’-AAGACCCCTTTGTGGAAAAC-3’; Reverse 5’-AGAGAACACTGCTCGTGGTT-3’) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Forward 5’-GGGTGTGAACCATGAGAAGT-3’ Reverse 5’-TAGAGGCAGGGATGATGTTC-3’) All primers were designed using OligoPerfect™ Designer (Invitrogen Corp. Carlsbad, CA). For rat tissues the primers used were CYP3A2 (Forward 5’-TGCCGAGTAAGGCACCTCCT-3’; Reverse 5’-GACTGCATCCCGTGGCAGAA-3’) and GAPDH (Forward 5’-CATCATCTCCGCCCCTTCCG-3’ Reverse 5’-GCTTTCCAGAGGGGCCATCC-3’). Real-time PCR (qRTPCR) was conducted using an ABI 5700 GeneAmp Sequence Detection System (Perkin–Elmer, Applied Biosystems: Foster City, CA). Primers used for qRTPCR were CYP3A2 (Forward 5’-ACCACCAGCAGCACACTCTC-3’; Reverse 5’-GAGGTGCCTTACTCGGCAGG-3’) and GAPDH (Forward 5’-GGATGGCCCCTCTGGAAAGC-3’ Reverse 5’-GCTCTGGGATGACCTTGCCC-3’). The qPCR was carried out using SYBR® Green Quantitative RT-PCR Kit (Promega) as per manufacturer’s protocol. The specificity of the target amplification was tested by melting curve analysis (Wittwer et al., 2001; Zhang et al., 2002). Quantitation was carried out using comparative Ct method. Samples for qRTPCR were prepared at least in triplicate. Quantitative values were obtained above the threshold PCR cycle number (Ct) at which the increase in signal associated with an exponential growth for PCR products was detected. The relative mRNA levels in each sample were normalized according to the expression levels of GAPDH. An induction ratio (treated/untreated) was determined from the relative expression levels of the target gene using the equation: 2−ΔCt (ΔCt = Ct target gene – Ct GAPDH).
Cytotoxicity studies
To delineate whether the results obtained in the in vitro experiments were not due to the cytotoxic effects of sutherlandia, MTT assay (Promega) was performed according to manufacturer’s protocol (Paturi et al., 2010).
Statistical analysis
In vivo oral absorption studies and cellular accumulation studies were conducted at least in triplicate. Results from in vivo experiments are expressed as mean ± standard error (S.E). All other results are expressed as mean ± standard deviation (S.D). Student t test was applied to determine statistical significance between two groups, with p<0.05 being considered as statistically significant.
Results
Effects of sutherlandia on plasma pharmacokinetics of NVP
To determine if sutherlandia co-administration could alter pharmacokinetics of NVP, oral absorption studies were conducted in Sprague dawley rats. In case of short term co-administration of sutherlandia, the noncompartmental analysis of plasma-concentration time profiles (Fig.1, A) gave an AUC0-inf and Cmax values (see Table.1) of (60544.50±6579.32 min*ng/mL and 575.64±51.60 ng/mL for vehicle control whereas 62556.58±7996.58.55 min*ng/mL and 534.68±72.93 ng/mL respectively for NVP, showing no statistically significant difference between the two groups (p>0.05). However, five days pre-treatment with sutherlandia (Fig.1, B) significantly lowered the AUC0-inf of NVP from 60544.60 (±6579.32) min.ng/mL to 33000.60 (±7168.55) min.ng/mL (45% decrease) at an oral dose of 6mg/kg. Cmax also markedly got reduced from 575.64 (±51.60) ng/mL to 281.1(±62.80) ng/mL, indicating a 51% decrement (Fig.2). The time to reach maximum plasma concentration (tmax) changed from 60 min to 45 min. Chronic administration of sutherlandia, however, did not significantly affect the mean residence time (MRT) and the half life (t½) of NVP.
Fig. 1.
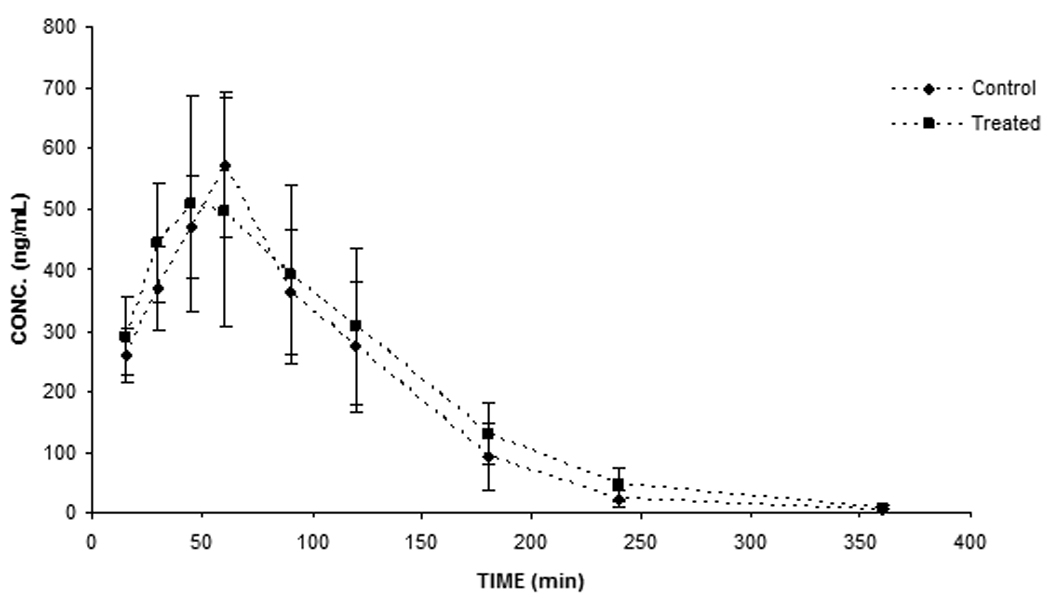
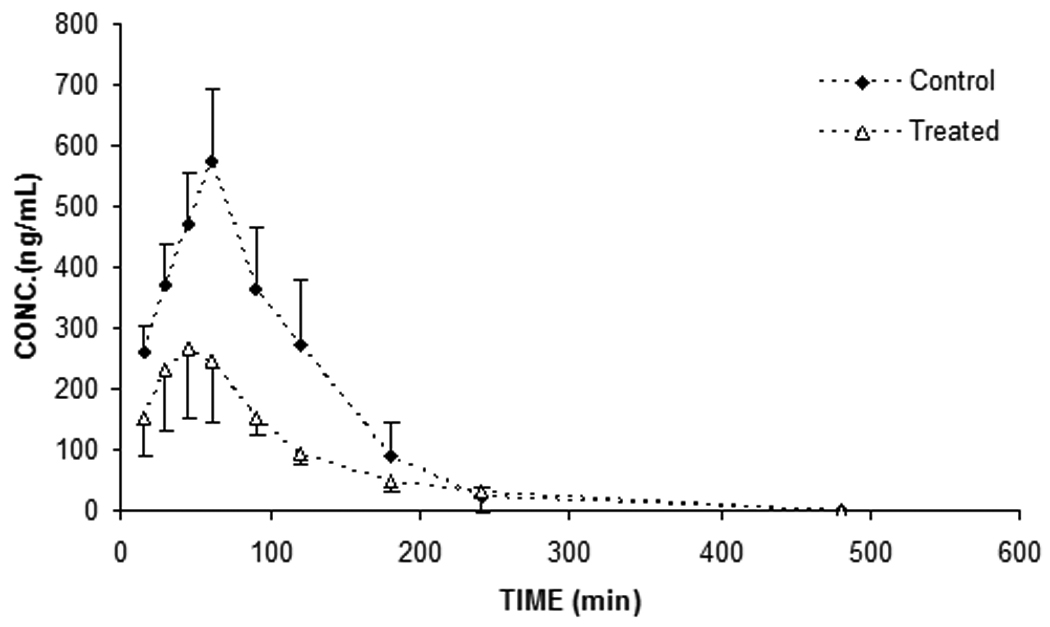
Plasma concentration-time profile after 6mg/kg oral dose of NVP upon short term (A) and (B) chronic (5 days) exposure to sutherlandia in rats. Each data point represents mean ± S.E. (n = 5), (*) and (**) represents significant difference from control (p < 0.05) and (p < 0.01) respectively.
Table. 1.
Pharmacokinetic parameters of NVP after short term and chronic oral administration of sutherlandia in rats
| NVP | PO (without sutherlandia) |
PO (with sutherlandia, short term) |
PO (after 5 days of sutherlandia, long term) |
|---|---|---|---|
| Dose (mg/kg) | 6 | 6 | 6 |
| Cmax (ng/mL) | 575.64±51.60 | 534.68±72.93 | 281.1±62.8** |
| AUC0-inf (min.ng/mL) | 60544.50±6579.32 | 62556.58±7996.58.55 | 33000.59±7168.55** |
| tmax (min) | 60 | 60 | 45 |
| λ_z(min−1) | 0.0213±0.0024 | 0.0171±0.0017 | 0.0153±0.0019 |
| Half-life (min) | 34.19±3.56 | 41.77±3.98 | 41.18±7.42 |
| MRT (min) | 87.26±4.85 | 98.13±4.3 | 108±15.87 |
represents significant difference from control (p < 0.01)
Fig. 2.
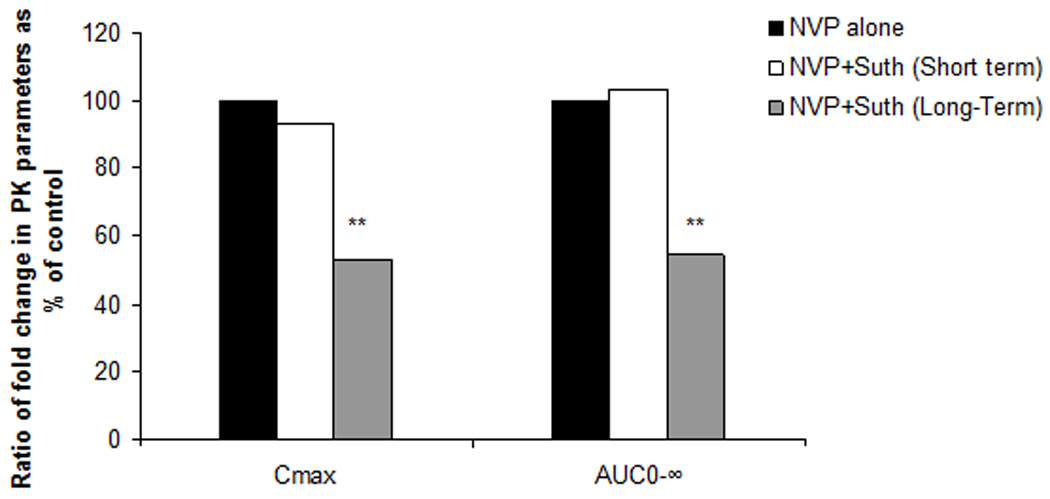
Ratio of the Cmax and AUC values of NVP as obtained in vehicle control (black bar), after short-term treatment (white bar) and long-term exposure (grey bar) to sutherlandia in rats. (*) represents significant difference from vehicle control (p < 0.01).
Quantitative RT-PCR analysis
Rat tissue does not express CYP3A4; hence mRNA expression levels of CYP3A2, a rat homologue of human CYP3A4 was quantitatively determined in the hepatic and intestinal tissues with and without chronic treatment with sutherlandia. A significant elevation in CYP3A2 mRNA expression levels was determined after 5 days oral administration of sutherlandia in rats (Fig. 3A). Quantitative analysis revealed an increase of 330% and 200% respectively (Fig. 3B) in the hepatic and intestinal rat CYP3A2 expression levels.
Fig. 3.
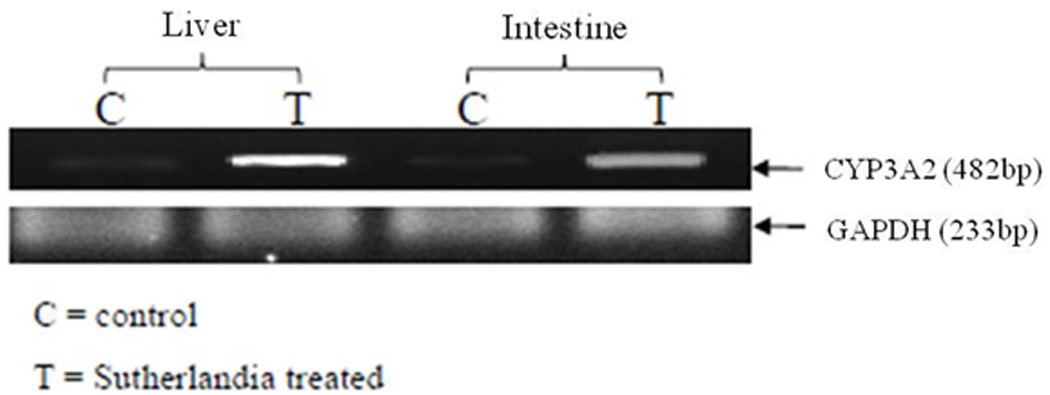
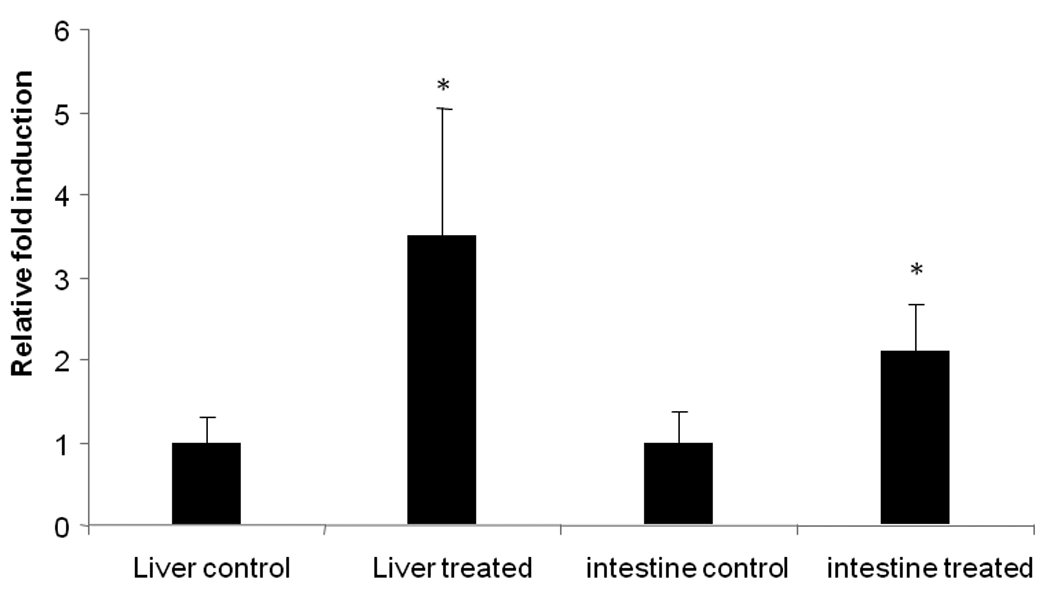
(A) PCR analysis: induction of CYP3A2 in rat hepatic and intestinal tissues with and without 5 day exposure to sutherlandia (12mg/kg). GAPDH was used as the house keeping gene. C and T represent control and treated group of rats respectively. (B) Quantitative PCR showing fold induction of CYP3A expression in rat liver and intestine with and without 5 day exposure of sutherlandia in rats. Each data point represents mean ± S.D. (n = 4). (*) represents significant difference from control (p < 0.05)
Modulation of CYP3A4 activity upon sutherlandia treatment in LS-180 cells
To ascertain any potential for a drug herb interaction upon sutherlandia co-administration in a clinical setting, human intestinal adenocarcinoma (LS-180) cells were selected as an in vitro model. Ninety-six hour post exposure, PCR analysis revealed a significant increase in the CYP3A4 mRNA expression in both rifampicin and sutherlandia treated groups (Fig. 4) compared to the medium control. Since, Rifampicin (25µM), a known inducer of CYP3A4, yielded an increase in CYP3A4 mRNA, LS-180 cells proved to be a good in vitro human model for studying CYP induction. To further, confirm the increase in functional activity of CYP3A4 post treatment, Vivid CYP3A4 assay was employed. The functional activity was measured in terms of rate of specific CYP3A4 metabolite formation. Results demonstrated a 2.0-fold and 1.8-fold increase in the functional activity of rifampicin and sutherlandia treated cells (Fig. 5). These results although cannot be directly compared with the in vivo oral absorption studies in rats, because the latter does not express CYP3A4 but CYP3A2. However, our results indicate a similar trend in increasing the metabolizing activity of the CYP3A isoforms in both the animal and cell culture models. To mimic, the short term co-administration experiment in rats, we also tested the inhibitory potential of sutherlandia on CYP3A4 activity using Vivid CYP3A4 inhibition assay. Sutherlandia was able to inhibit the CYP3A4 activity although at very high concentrations tested (Fig. 6). The MTT assay was performed to delineate whether the genetic and activity responses can be attributed to the cytotoxic effects of sutherlandia. It was observed that none of the tested concentrations had any significant cytotoxic effect on LS-180 cells (Data not shown).
Fig. 4.
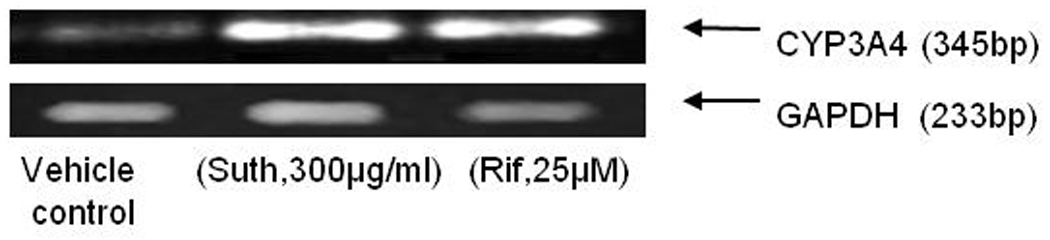
Induction of CYP3A4 in LS-180 cells after 96 h exposure to sutherlandia (300µg/mL) and rifampicin (25µM). GAPDH was used as the internal standard.
Fig. 5.
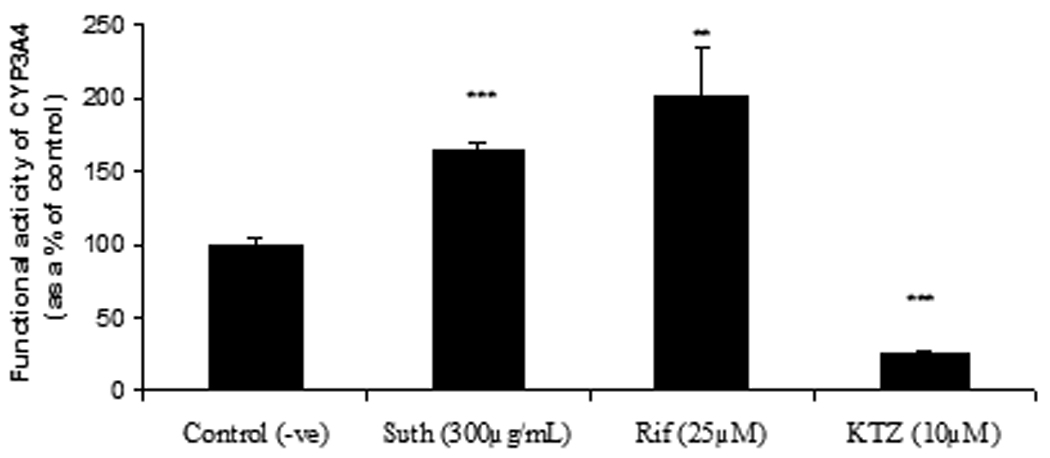
Vivid assay for determination of functional activity of induced CYP3A4 in LS-180 cells after 96 h exposure to sutherlandia (Suth, 300µg/mL), rifampicin (Rif, 25µM) and acute treatment with ketoconazole (KTZ, 10 µM; as a CYP3A4 inhibitor). Each data point represents mean ± S.D. (n = 6). (**) and (***) represents significant difference from control (p < 0.01) and (p < 0.001) respectively.
Fig. 6.
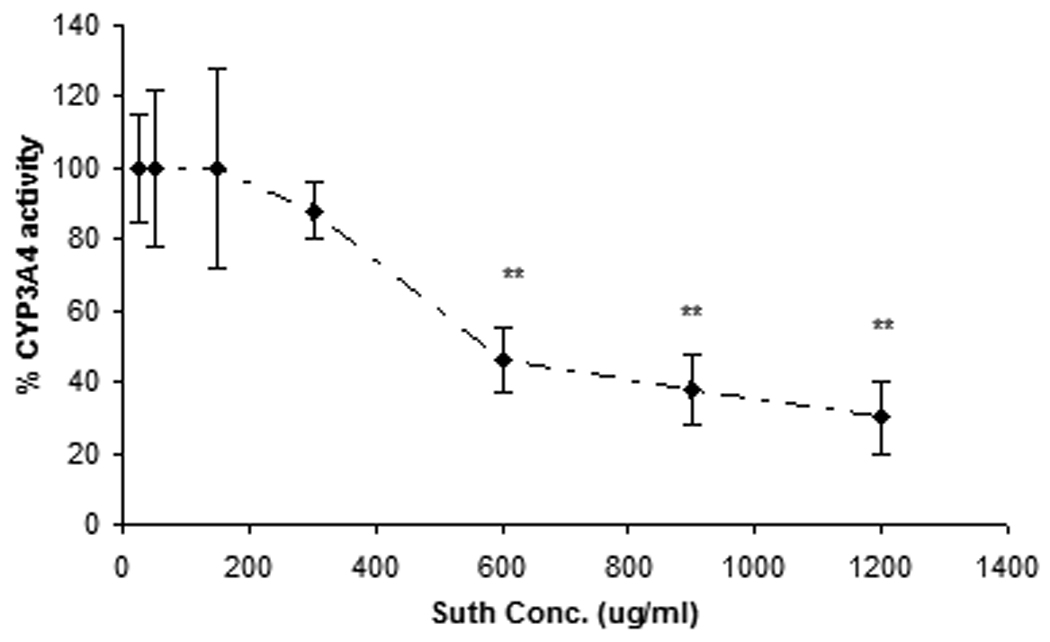
Vivid assay for determining the CYP3A4 inhibitory potency: Concentration-response curve shows the % of CYP3A4 activity in presence of increasing concentrations (25µg/mL–1200µg/mL) of sutherlandia. Each data point represents mean ± S.D. (n = 7), (**) represents significant difference from control (p < 0.01).
Discussion
Recently, many case reports have warranted about loss of therapeutic efficacy of prescribed medications with self administration of over the counter (OTC) herbal products. One of the most well documented facts involves St. John’s wort (SJW). Indeed, concomitant administration of SJW resulted in treatment failure with digoxin, antiretroviral drugs, cyclosporine and oral contraceptives (Hall et al., 2003; Johne et al., 1999; Karliova et al., 2000; Murphy et al., 2005; Piscitelli et al., 2000). These herb-drug interactions in most cases involve induction of efflux transporter P-gp and/or drug metabolizing enzyme CYP3A4. It has been reported that treatment with SJW extract for 14 days in humans resulted in 1.5- fold increase in the expression of duodenal CYP3A4 (Durr et al., 2000). Similar to SJW, sutherlandia is an OTC African herbal medicine known for its potential to reduce the HIV viral load and boost immune response and appetite in AIDS patients. Mills et al recently reported activation of PXR gene following chronic treatment with sutherlandia in an in vitro assay (Mills et al., 2005b). However, effect on functional activity of CYP3A homologues upon chronic treatment with sutherlandia remains to be elucidated. Till date, no work indicating pharmacokinetic interactions involving sutherlandia has been reported. This research article represents the first report demonstrating correlation between the elevated protein level expression and functional activity of CYP3A4 in LS-180 cells (CYP3A2 in rats) upon sutherlandia chronic exposure.
In our first objective, we demonstrated the induction of CYP3A2 gene expression in vivo in male Sprague Dawley rats by studying the effect of chronic treatment of sutherlandia using NVP as a substrate for rat CYP3A2. Plasma concentration of NVP did not change to a statistically significant level upon short term exposure to sutherlandia (Fig.1A). However, a significant decrease in the pharmacokinetic parameters such as AUC and Cmax for NVP was evident when administered after 5 days of chronic exposure in rats (Fig.1 B, Table.1). This indicates a probable induction of CYP3A2 in rats. In addition, quantitative RTPCR analysis also suggests significant elevation of intestinal and hepatic CYP3A2 mRNA (Fig. 3B). We postulate that reduction in plasma concentration of NVP is primarily due to enhanced metabolism by elevated levels of CYP3A2 over five day treatment of sutherlandia in rats resulting in enhanced intrinsic hepatic clearance of NVP.
Since rats may have a different mechanism of induction when compared to that of humans, we wanted to test if sutherlandia could also cause induction or inhibition of CYP3A4, human homologue of rodent CYP3A2. Human intestinal adenocarcinoma LS-180 cells were employed as an in vitro induction model. Recent reports have suggested this cell line to be a good in vitro model for predicting CYP3A4 mediated drug interactions (Gupta et al., 2008). Commonly ingested dose of sutherlandia is close to that of SJW (400mg b.i.d), but no reports exist stating the luminal concentrations achieved after oral dosing of sutherlandia in humans. Since luminal concentrations attained for SJW is reported to be approx 300µg/mL (Perloff et al., 2001; Tian et al., 2005), we selected the same concentration for sutherlandia to carry out in vitro induction experiments. Significant induction of CYP3A4 mRNA expression was observed with 96 h treatment of sutherlandia as shown by PCR analysis. Rifampicin (25µM), a known inducer of CYP3A4 also yielded similar results. Moreover, to prove the functional activity of induced CYP3A4 transcripts, VIVID® CYP3A4 assay was carried out. The assay is based on a simple principle that the VIVID® substrate (non florescent) releases a highly florescent metabolite upon oxidation specifically by CYP3A4 enzyme. Ninety six hour post exposure to sutherlandia (300µg/mL) in LS-180 cells raised metabolite formation to 173%. Positive control rifampicin also indicated a 200% increase in metabolite formation at the end of 15 min incubation relative to control (Fig. 5). Ketoconazole (10µM, known CYP3A4 inhibitor) reduced metabolite formation of VIVID® substrate to ≈5 fold relative to the control set of wells. These results suggest that LS-180 cells represent a suitable in vitro model to assess the role of herbal or therapeutic agents on CYP3A4 activity.
Since sutherlandia is taken as supplement for ailments such as diabetes and chronic depression along with HIV infection management, these pre-clinical results indicate that concomitant administration of sutherlandia along with prescription drugs, (CYP3A4 substrates) especially low therapeutic index drugs could possibly lead to therapeutic failure and clinically relevant drug-herb interactions. Due to the multi-component nature of sutherlandia, it is difficult to determine which active constituents might have caused this increase in mRNA expression of rat CYP3A2/human CYP3A4. We are currently carrying out studies to elucidate the effects of known active constituents i.e. L-canavanine, GABA and pinitol on CYP3A4 expression levels in vitro. Since these compounds are hydrophilic in nature, it is likely that there are other unknown components in the aqueous extract of sutherlandia that might be involved in rodent CYP3A induction. Recently Fu X et al (Fu et al., 2008; 2010) have characterized and isolated four flavanoid glycosides (sutherlandins A–D) and four cycloartanol glycosides (sutherlandiosides A–D) form aerial parts of sutherlandia. Due to the higher molecular weight and hydrophobic nature of these compounds, we suspect that these active constituents might be responsible for induction of CYP3A homologues. Moreover, dose dependent effect of sutherlandia and duration of exposure might increase CYP3A4 expression levels to a greater extent. In conclusion, this report describes that luminal and plasma concentration of sutherlandia achieved after chronic oral administration induced intestinal and hepatic CYP3A2 expression levels in rats and altered the pharmacokinetics of antiretroviral NVP. Furthermore, sutherlandia induces human CYP3A4 in LS-180 cells, thereby increasing its functional activity. Hence, clinical trials delineating the role of sutherlandia in causing potential drug-herb interactions with model CYP3A4 substrates are warranted.
Acknowledgments
We thank Dr. Sudharshan Hariharan (U.S. Food and Drug Administration, Silver Spring, MD) for his helpful scientific discussions.
This work was supported by the National Institutes of Health grants (R01EY009171-15, RO1AI071199); and a grant from University of Missouri-Columbia. Part of this work was presented at American Association of Pharmaceutical Scientist Annual Meeting and Exposition, Los Angeles, CA, USA, November 2009.
Abbreviations
- CYP
cytochrome P450
- MDR1
Multi-drug resistance transporter
- MRP
Multi-drug resistance associated protein
- P-gp
P glycoprotein
- BCRP
Breast cancer resistance protein
- sutherlandia
Sutherlandia frutescens
- AUC
area under the plasma concentration-time curve
- PXR
pregnane X receptor
- qRT-PCR
quantitative reverse transcriptase polymerase chain reaction
- Rif
Rifampicin
- KTZ
Ketoconazole
- MRT
mean residence time
- TBME
tertiary methyl butyl ether
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benet LZ, Cummins CL, Wu CY. Transporter-enzyme interactions: implications for predicting drug-drug interactions from in vitro data. Curr Drug Metab. 2003;4:393–398. doi: 10.2174/1389200033489389. [DOI] [PubMed] [Google Scholar]
- Chinkwo KA. Sutherlandia frutescens extracts can induce apoptosis in cultured carcinoma cells. J Ethnopharmacol. 2005;98:163–170. doi: 10.1016/j.jep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Durr D, Stieger B, Kullak-Ublick GA, Rentsch KM, Steinert HC, Meier PJ, Fattinger K. St John's Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther. 2000;68:598–604. doi: 10.1067/mcp.2000.112240. [DOI] [PubMed] [Google Scholar]
- Ernst E, Rand JI, Stevinson C. Complementary therapies for depression: an overview. Arch Gen Psychiatry. 1998;55:1026–1032. doi: 10.1001/archpsyc.55.11.1026. [DOI] [PubMed] [Google Scholar]
- Fernandes AC, Cromarty AD, Albrecht C, van Rensburg CE. The antioxidant potential of Sutherlandia frutescens. J Ethnopharmacol. 2004;95:1–5. doi: 10.1016/j.jep.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Fu X, Li XC, Smillie TJ, Carvalho P, Mabusela W, Syce J, Johnson Q, Folk W, Avery MA, Khan IA. Cycloartane glycosides from Sutherlandia frutescens. J Nat Prod. 2008;71:1749–1753. doi: 10.1021/np800328r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Li XC, Wang YH, Avula B, Smillie TJ, Mabusela W, Syce J, Johnson Q, Folk W, Khan IA. Flavonol glycosides from the south African medicinal plant Sutherlandia frutescens. Planta Med. 2010;76:178–181. doi: 10.1055/s-0029-1186030. [DOI] [PubMed] [Google Scholar]
- Gupta A, Mugundu GM, Desai PB, Thummel KE, Unadkat JD. Intestinal human colon adenocarcinoma cell line LS180 is an excellent model to study pregnane X receptor, but not constitutive androstane receptor, mediated CYP3A4 and multidrug resistance transporter 1 induction: studies with anti-human immunodeficiency virus protease inhibitors. Drug Metab Dispos. 2008;36:1172–1180. doi: 10.1124/dmd.107.018689. [DOI] [PubMed] [Google Scholar]
- Hall SD, Wang Z, Huang SM, Hamman MA, Vasavada N, Adigun AQ, Hilligoss JK, Miller M, Gorski JC. The interaction between St John's wort and an oral contraceptive. Clin Pharmacol Ther. 2003;74:525–535. doi: 10.1016/j.clpt.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Harnett SM, Oosthuizen V, van de Venter M. Anti-HIV activities of organic and aqueous extracts of Sutherlandia frutescens and Lobostemon trigonus. J Ethnopharmacol. 2005;96:113–119. doi: 10.1016/j.jep.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Hoffmann CJ, Charalambous S, Sim J, Ledwaba J, Schwikkard G, Chaisson RE, Fielding KL, Churchyard GJ, Morris L, Grant AD. Viremia, resuppression, and time to resistance in human immunodeficiency virus (HIV) subtype C during first-line antiretroviral therapy in South Africa. Clin Infect Dis. 2009;49:1928–1935. doi: 10.1086/648444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janneh O, Chandler B, Hartkoorn R, Kwan WS, Jenkinson C, Evans DJ, Owen A, Khoo SH. Intracellular accumulation of efavirenz and nevirapine is independent of P-glycoprotein activity in cultured CD4 T cells and primary human lymphocytes. J Antimicrob Chemother. 2009;64:1002–1007. doi: 10.1093/jac/dkp335. [DOI] [PubMed] [Google Scholar]
- Johne A, Brockmoller J, Bauer S, Maurer A, Langheinrich M, Roots I. Pharmacokinetic interaction of digoxin with an herbal extract from St John's wort (Hypericum perforatum) Clin Pharmacol Ther. 1999;66:338–345. doi: 10.1053/cp.1999.v66.a101944. [DOI] [PubMed] [Google Scholar]
- Karliova M, Treichel U, Malago M, Frilling A, Gerken G, Broelsch CE. Interaction of Hypericum perforatum (St. John's wort) with cyclosporin A metabolism in a patient after liver transplantation. J Hepatol. 2000;33:853–855. doi: 10.1016/s0168-8278(00)80321-9. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Laurito TL, Santagada V, Caliendo G, Oliveira CH, Barrientos-Astigarraga RE, De Nucci G. Nevirapine quantification in human plasma by high-performance liquid chromatography coupled to electrospray tandem mass spectrometry. Application to bioequivalence study. J Mass Spectrom. 2002;37:434–441. doi: 10.1002/jms.300. [DOI] [PubMed] [Google Scholar]
- Lavretsky H. Complementary and alternative medicine use for treatment and prevention of late-life mood and cognitive disorders. Aging health. 2009;5:61–78. doi: 10.2217/1745509X.5.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yang Y, Gange SJ, Weber K, Sharp GB, Wilson TE, Levine A, Robison E, Goparaju L, Ganhdi M, Merenstein D. Disclosure of complementary and alternative medicine use to health care providers among HIV-infected women. AIDS Patient Care STDS. 2009;23:965–971. doi: 10.1089/apc.2009.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenstein D, Yang Y, Schneider MF, Goparaju L, Weber K, Sharma A, Levine AM, Sharp GB, Gandhi M, Liu C. Association of complementary and alternative medicine use with highly active antiretroviral therapy initiation. Altern Ther Health Med. 2008;14:18–22. [PMC free article] [PubMed] [Google Scholar]
- Mills E, Cooper C, Seely D, Kanfer I. African herbal medicines in the treatment of HIV: Hypoxis and Sutherlandia. An overview of evidence and pharmacology. Nutr J. 2005a;4:19. doi: 10.1186/1475-2891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E, Foster BC, van Heeswijk R, Phillips E, Wilson K, Leonard B, Kosuge K, Kanfer I. Impact of African herbal medicines on antiretroviral metabolism. AIDS. 2005b;19:95–97. doi: 10.1097/00002030-200501030-00013. [DOI] [PubMed] [Google Scholar]
- Murphy PA, Kern SE, Stanczyk FZ, Westhoff CL. Interaction of St. John's Wort with oral contraceptives: effects on the pharmacokinetics of norethindrone and ethinyl estradiol, ovarian activity and breakthrough bleeding. Contraception. 2005;71:402–408. doi: 10.1016/j.contraception.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Ojewole JA. Analgesic, antiinflammatory and hypoglycemic effects of Sutherlandia frutescens R. BR. (variety Incana E. MEY.) [Fabaceae] shoot aqueous extract. Methods Find Exp Clin Pharmacol. 2004;26:409–416. [PubMed] [Google Scholar]
- Pal D, Mitra AK. MDR- and CYP3A4-mediated drug-herbal interactions. Life Sci. 2006;78:2131–2145. doi: 10.1016/j.lfs.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Paturi DK, Kwatra D, Ananthula HK, Pal D, Mitra AK. Identification and functional characterization of breast cancer resistance protein in human bronchial epithelial cells (Calu-3) Int J Pharm. 2010;384:32–38. doi: 10.1016/j.ijpharm.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perloff MD, von Moltke LL, Stormer E, Shader RI, Greenblatt DJ. Saint John's wort: an in vitro analysis of P-glycoprotein induction due to extended exposure. Br J Pharmacol. 2001;134:1601–1608. doi: 10.1038/sj.bjp.0704399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St John's wort. Lancet. 2000;355:547–548. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- Riska PS, Joseph DP, Dinallo RM, Davidson WC, Keirns JJ, Hattox SE. Biotransformation of nevirapine, a non-nucleoside HIV-1 reverse transcriptase inhibitor, in mice, rats, rabbits, dogs, monkeys, and chimpanzees. Drug Metab Dispos. 1999;27:1434–1447. [PubMed] [Google Scholar]
- Sia C. Spotlight on ethnomedicine: usability of Sutherlandia frutescens in the treatment of diabetes. Rev Diabet Stud. 2004;1:145–149. doi: 10.1900/RDS.2004.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- Tai J, Cheung S, Chan E, Hasman D. In vitro culture studies of Sutherlandia frutescens on human tumor cell lines. J Ethnopharmacol. 2004;93:9–19. doi: 10.1016/j.jep.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Tian R, Koyabu N, Morimoto S, Shoyama Y, Ohtani H, Sawada Y. Functional induction and de-induction of P-glycoprotein by St. John's wort and its ingredients in a human colon adenocarcinoma cell line. Drug Metab Dispos. 2005;33:547–554. doi: 10.1124/dmd.104.002485. [DOI] [PubMed] [Google Scholar]
- Trubetskoy O, Marks B, Zielinski T, Yueh MF, Raucy J. A simultaneous assessment of CYP3A4 metabolism and induction in the DPX-2 cell line. AAPS J. 2005;7:E6–E13. doi: 10.1208/aapsj070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen B, Chen Y, Fitch WL. Metabolic activation of nevirapine in human liver microsomes: dehydrogenation and inactivation of cytochrome P450 3A4. Drug Metab Dispos. 2009;37:1557–1562. doi: 10.1124/dmd.108.024851. [DOI] [PubMed] [Google Scholar]
- Wittwer CT, Herrmann MG, Gundry CN, Elenitoba-Johnson KS. Real-time multiplex PCR assays. Methods. 2001;25:430–442. doi: 10.1006/meth.2001.1265. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gong Y, Osiowy C, Minuk GY. Rapid detection of hepatitis B virus mutations using real-time PCR and melting curve analysis. Hepatology. 2002;36:723–728. doi: 10.1053/jhep.2002.35346. [DOI] [PubMed] [Google Scholar]


