Abstract
Purpose
Rat pups only void when the perigenital-bladder reflex is activated by the mother rat licking the perineum. Maternal separation causes bladder distention as well as stress responses and anxiety behaviors in adult rats. We determined if MS would change voiding reflex maturation in neonatal rats.
Materials and Methods
A total of 14 Sprague-Dawley rat pups were subjected to 6 hours of daily MS and 17 were subjected to 6 hours of MS with bladder emptying by perigenital stimulation at 3 hours on postnatal days 2 to 14. Age matched controls for the 2 groups remained with the mother. Spontaneous voiding in awake pups from 1 to 3 weeks was monitored in a metabolic cage and perigenital-bladder reflex latency was determined from 1 to 7 weeks. Cystometry was performed at 9 weeks with the rats under urethane anesthesia.
Results
Spontaneous voiding began at 3 weeks in all animals. The latency of the perigenital-bladder reflex at 3 weeks was shorter than the latency at 2 days in MS animals (3.3 vs 6.4 seconds, p < 0.01) but not in control or MSPG animals. MS animals maintained the perigenital-bladder reflex 2 weeks longer than control animals. The spontaneous voiding behavior of MSPG animals was similar to that in controls.
Conclusions
Intermittent bladder distention delays withdrawal of the spinal perigenital-bladder reflex but it does not affect maturation of the supraspinal bladder-bladder reflex that controls spontaneous voiding in older rats. This suggests that increased bladder afferent firing can selectively modulate spinal but not supraspinal mechanisms controlling postnatal changes in voiding function.
Keywords: bladder, urination, maternal behavior, reflex, rats, Sprague-Dawley
The neural mechanisms controlling bladder emptying undergo marked changes during the first 3 weeks of life in many mammals.1–6 After birth the rat pup cannot void spontaneously since voiding is controlled by the perigenital-bladder reflex, which is triggered by the mother licking the perigenital region of the pup.2,4,6 The perigenital-bladder reflex is controlled by spinal mechanisms and it is essential for voiding because separation of the rat pup from its mother during the first 3 postnatal weeks leads to complete urinary retention.7,8 After 3 weeks of life the supraspinal bladder-bladder reflex by which bladder filling and emptying is controlled by the pons emerges, leading to spontaneous voiding.9 Latency, or the duration of perigenital stimulation required to produce voiding, progressively lengthens as the perigenital-bladder reflex is down-regulated and it is eventually eliminated.4,6 Electrophysiological studies in spinal slice preparations suggest that down-regulation of the perigenital-bladder reflex depends on input from the brain to the spinal cord and is associated with a decrease in spinal connections to parasympathetic PGNs.10
We examined the influence of afferent input from the bladder on developmental changes in the perigenital-bladder and bladder-bladder reflexes in the neonatal rat. Increased afferent input from the bladder was induced by separating rat pups from their mother (MS) to cause urinary retention and bladder distention.4–6 MS is also a well characterized model of early childhood stress.11 Separation of rat pups from their mothers produces adult rats with increased anxiety behaviors due to increased corticotrophin releasing hormone levels.11 MS also induces visceral hyperalgesia in the rat colon.12 Rectal distention causes more pain in adult rats separated from their mothers during neonatal life than in control rats.12 Thus, MS could have multiple effects on lower urinary tract function, namely 1) increased afferent nerve firing from the bladder due to over distention could alter the developmental changes in bladder reflexes and 2) stress and increased corticotrophin releasing hormone levels might produce an irritative voiding disorder or hyperalgesia, as noted in the colon. By monitoring changes in the perigenital-bladder and bladder-bladder reflexes during the postnatal period we tested the hypothesis that increased afferent firing induced by bladder distention on days 2 to 14 of life would alter the subsequent disappearance of the neonatal perigenital-bladder reflex and the emergence of the mature voiding reflex, otherwise referred to as spontaneous voiding.
MATERIALS AND METHODS
A total of 14 Sprague-Dawley rat pups underwent bladder distention for 6 hours daily by MS on days 2 to 14 of life. MS for 3 hours was tested in preliminary experiments and it did not cause a significant increase in bladder volume. MS for 6 hours with bladder emptying at 3 hours (MSPG) in 17 rat pups on days 2 to 14 of life was used to examine the effect of separation induced stress without bladder distention. In each separation protocol the pups were placed as a group in a separate box from their mother from 9 a.m. to 3 p.m. daily. The corresponding control groups of 15 (MS control) and 12 (MSPG control) pups were left with the mother during this time. Upon the return of the mother to the pups she would immediately start grooming them and would empty their bladders.
Manual bladder emptying was accomplished by rapidly stroking the perigenital area for 60 seconds with a pipette tip.3,5 In the MSPG group the bladders were manually emptied at 3 hours of separation but not prior to the return to the mother at 6 hours. Voided urine volume and perigenital-bladder reflex latency were recorded. Shorter latency was interpreted as a stronger perigenital-bladder reflex. Initial latency and volume measurements at 2 days of life were done at an arbitrary time prior to maternal separation, so as to not affect the 6-hour separation period. Latency of the perigenital-bladder reflex was determined from 1 to 7 weeks of life in the MS and control groups, and from 1 to 3 weeks in the MSPG group. Animals in the MS and control groups were used for other experiments after weaning, so that not all animals were assessed at 7 weeks.
To look for early onset of the mature voiding reflex separated animals underwent weekly metabolic cage monitoring for 3 hours, followed by perigenital stimulation to determine bladder volume. Since we had previously noted no difference in voiding patterns after 3 hours of separation, we did not expect this period of monitoring to affect bladder function. Pups were placed in Nalgene® metabolic cages with hanging wire bottoms (3 to 4 per cage) and filter paper was placed under the wire rack to catch any urine that was voided. Pups normally huddle together to maintain warmth, and so they were placed as a group in the metabolic cage. Individually separating the pups would have added an additional stressor to the preparation. Since pups up to 3 weeks old depend on the mother for feeding, no food or water was provided during the monitoring period. Spontaneous voiding was evaluated by counting the number of spots on the filter paper. Spots were detected on the filter paper using ultraviolet light and those greater than 10 mm in diameter, corresponding to 10 μl urine, were counted as voids. Age matched controls for the MS group were not separated from the mother until 2 weeks of life, after which they underwent weekly 3-hour metabolic cage monitoring to assess spontaneous voiding. Spontaneous voiding in age matched controls for the MSPG group was evaluated at 3 weeks of life.
Cystometrograms were performed using urethane anesthesia at 9 weeks of life to determine if long-term bladder hyperactivity was induced by MS. Rats anesthetized with 2% to 4% halothane were injected with 1.5 mg/kg urethane subcutaneously and PE-50 tubing was implanted into the bladder dome. Continuous cystometry was performed starting 1 hour after implantation at 20 μl per minute with normal saline. Pressures were recorded using DataQ® and functional bladder capacity was calculated by multiplying the average duration of 5 to 7 voiding cycles by the rate of infusion. Comparisons of reflex latencies, bladder volumes and body weights between different groups of animals were performed using 1-way ANOVA, comparison of ratios (number of voids per animal) using the chi-square test and comparison of slopes using the F test.
RESULTS
Preliminary studies with 3 hours of maternal separation on postnatal day 4 did not detect a difference in bladder volume between 13 separated and 15 control pups (52 vs 31 μl, p = 0.09). Pups separated for 6 hours on postnatal day 5 had larger bladder volumes emptied by perigenital stimulation compared to volumes in control pups that were not separated (168 vs 57 μl, p < 0.01). Six-hour separation was used in all subsequent experiments.
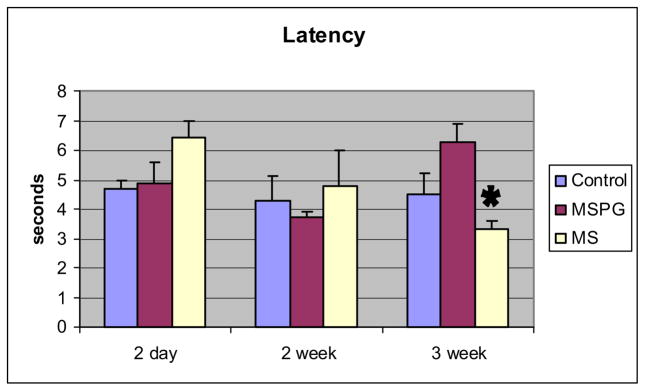
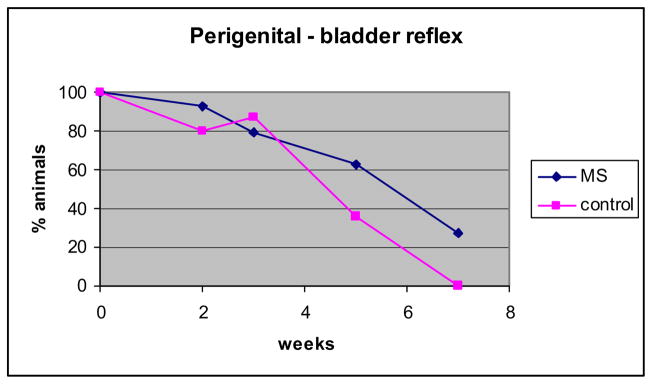
Mean latency ± SEM of the perigenital-bladder reflex was stable between ages 2 days and 3 weeks in the control (4.3 to 4.5 seconds, p = 0.7) and MSPG groups (4.9 to 6.3 seconds, p = 0.4) but it decreased significantly in the MS group (6.4 to 3.3 seconds, p < 0.01, fig. 1). A longer latency in controls compared to that in MS animals was also detected at 5 weeks (mean 43.8 ± 3.1 vs 20.3 ± 6.6 seconds, p = 0.03). The percent of animals showing a perigenital-bladder reflex was 80% to 100% at up to 3 weeks of life and then it decreased (fig. 2). The percent of animals in the MS group maintaining the perigenital-bladder reflex at 5 and 7 weeks was higher than in the control group but the curves were not significantly different (F test p = 0.25).
Fig. 1.
Changes in perigenital-bladder reflex latency between ages 2 days and 3 weeks in control, MSPG and MS animals. Mean latency ± SEM represents mean of 12 to 17 animals at each age. Asterisk indicates 2-day vs 3-week MS p = 2.6 × 10−4.
Fig. 2.
Percent of animals showing perigenital-bladder reflex from 2 days to 7 weeks. Slopes are not significantly different.
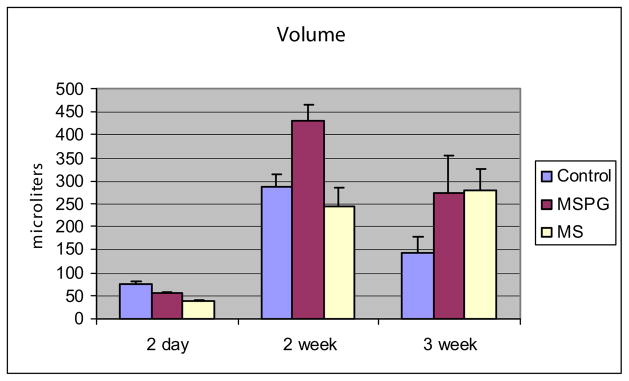
Spontaneous voiding was not detected at 1 week of life and it was rarely seen at 2 weeks of life. Spontaneous voiding was routinely detected in unanesthetized animals in a metabolism cage at age 3 weeks in control, MS and MSPG animals. Although there was a trend toward a decreased number of spontaneous voids in MS compared to control animals, no significant differences were noted in the total number of voids between the 2 groups (8 voids per 15 animals and 3 voids per 14 animals, respectively, chi-square 0.08). However, the volume of urine released by perigenital stimulation peaked at 2 weeks in the control and MSPG groups, and it remained high at 3 weeks in the MS group, suggesting that this group had less spontaneous voiding (fig. 3). There was no difference in the number of spontaneous voids between control and MSPG animals (13 voids per 12 animals and 18 voids per 17 animals, chi-square 0.77).
Fig. 3.
Changes in volume of urine eliminated via perigenital stimulation between ages 2 days and 3 weeks in control, MSPG and MS animals. Mean volume ± SEM represent mean of 12 to 17 animals at each age.
Cystometrograms performed at 9 weeks postnatally in 1 male and 2 female MS, and in 2 male and 2 female control animals revealed no difference in functional bladder capacity (mean 203 ± 31 vs 144 ± 25 μl, p = 0.19) and no evidence of detrusor hyperactivity (uninhibited nonvoiding contractions) during bladder filling. No cystometry or long-term assessment of voiding function was done in the MSPG group since voiding patterns were not changed at weaning.
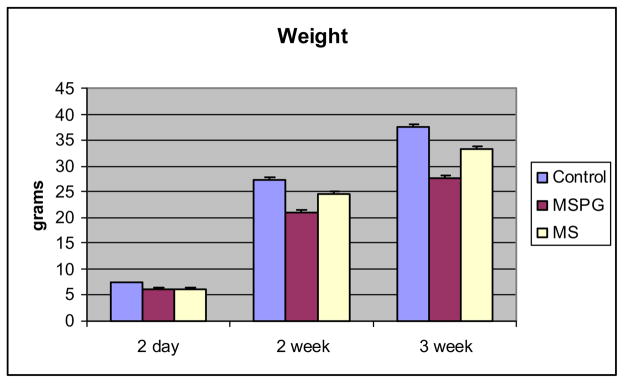
MS had no effect on somatic growth compared to that in control animals. The 3-week-to-2-day weight ratio was 5.1 in MS animals and 5.4 in control animals. MSPG resulted in delayed growth with a 3-week-to-2-day weight ratio of 4.4 (fig. 4).
Fig. 4.
Changes in body weight between ages 2 days and 3 weeks in control, MSPG and MS animals. Mean weight ± SEM represents mean of 12 to 17 animals at each age.
DISCUSSION
We observed that bladder distention can selectively delay down-regulation of the perigenital-bladder reflex without affecting the emergence of the bladder-bladder reflex. It appears that these 2 components are regulated independently rather than in a coordinated manner, as suggested by previous studies.10 Evidence for the persistence of the perigenital-bladder reflex in MS animals was indicated by the volume voided and changes in reflex latency. Previous studies in kittens have shown that the latency of this reflex progressively increases with age,3 which was also seen in the control group in this study (4.3 to 44 seconds from 2 days to 5 weeks). By comparison, in the MS group reflex latency decreased by almost 50% from 2 days to 3 weeks and it was more than 50% shorter than latency in control animals at 5 weeks. The shorter latency suggests that the amount of stimulation required to produce a coordinated voiding contraction is less than would be required in control situations. The decreased volume emptied in response to perigenital stimulation at 3 weeks in control and MSPG animals suggests that with the onset of spontaneous voiding the perigenital-bladder reflex becomes less efficient.
MS did not affect the emergence of the mature bladder-bladder reflex. Previous studies have shown that the entire circuit responsible for mature voiding is present at birth in rat pups but it is tonically inhibited by forebrain activity.6,14,15 Removal of inhibition by decerebration causes abrupt emergence of the bladder-bladder reflex,6 whereas spinal cord transection at the thoracic level blocks the emergence of the bladder-bladder reflex and down-regulation of the perigenital-bladder reflex.6,10 We expected a similar unmasking of the bladder-bladder reflex with MS. However, spontaneous voiding occurred at age 3 weeks in control and MS rats, suggesting that postnatal maturation of the bladder-bladder reflex depends more on brain activity16 than on bladder afferent activity.
Our data suggest that stress due to maternal separation did not have a great influence on the maturation of voiding reflexes. Maternal separation can have a long-term effect on pups by altering maternal care, which in turn alters glucocorticoid receptors in the offspring.13 A possible confounding factor would be if mother rats separated from their pups for a few hours daily were to perform less grooming and feeding after the pups were returned to them. This may have occurred in the MSPG group since pups grew less rapidly than in the control or MS group. However, even with this change in maternal care there was no difference in latency, volume emptied in response to perigenital stimulation or spontaneous voiding between the control and MSPG groups. Although it could be argued that the stress response may not be fully developed by 3 weeks of life, changes in glucocorticoid receptor methylation in response to maternal separation are found as early as days 1 to 6 of life.13 Thus, it appears that bladder distention, rather than neuroendocrine changes, has the major role in altering postnatal maturation of the perigenital-bladder reflex.
MS may affect the down-regulation of the perigenital-bladder reflex by increasing afferent input to the central nervous system from mechanoreceptors in the bladder. Electrophysiological data suggest that down-regulation of this reflex is associated with pruning of synaptic connections between interneurons and PGNs due to competition between developing brainstem inputs and preexisting interneuron-PGN connections.10 At many sites in the nervous system synaptic remodeling is activity dependent.16 Increased afferent activity in the spinal interneurons during MS induced bladder distention could maintain interneuron-PGN connections in the face of increasing brainstem signaling, which normally accompanies maturation of the supraspinal bladder-bladder reflex. The convergence of afferent input from the bladder on the same interneurons as somatic afferents from the perigenital region has been previously described in the sacral parasympathetic nucleus of the cat spinal cord.17
Two problems arise when attempting to extrapolate these findings to children. 1) Bladder distention that occurs due to maternal separation is low pressure, unlike the high pressure distention seen in posterior urethral valves and neuropathic bladder due to myelomeningocele. There is no obstructive component in this preparation, and so there is no gross morphological change to the bladder and no abnormality in compliance during cystometry. 2) Although infants have a perigenital-bladder reflex,18 they are born with a functional bladder-bladder reflex and show spontaneous bladder emptying. The major change that occurs in children is the acquisition of voluntary control over voiding and coordination of the bladder and external urinary sphincter. Since voiding mastery requires suppression of an involuntary reflex by voluntary control, it is possible that pediatric voiding dysfunction is actually a combination of immature and mature responses to the same stimulus. This may be a reason why children with voiding dysfunction have persistently positive bladder cooling tests, which suggests impaired inhibition of a sacral bladder reflex, similar to the perigenital-bladder reflex.19 Unmasking of the bladder cooling reflex and the perigenital-bladder reflex in spinal cord injured patients is likely due to synaptic remodeling in the spinal cord and the reemergence of neonatal reflex pathways.20 To our knowledge whether early urinary tract infections can cause long-term changes in the bladder function of children by increasing afferent input remains to be investigated. Understanding the mechanisms that control the maturation of voiding reflexes may provide new ways to treat pediatric voiding dysfunction by targeting neural rather than smooth muscle activity.
CONCLUSIONS
Intermittent, low pressure bladder distention delays withdrawal of the immature perigenital-bladder reflex but does not affect the maturation of the bladder-bladder reflex used for mature voiding. This suggests that the coordinated maturation of neonatal and mature voiding circuits can be altered by the afferent activity created by bladder stretch.
Acknowledgments
Supported by National Institutes of Health Grants K08 DK065759 (HYW), P01 HD039768 and R37 DK049430 (WCD).
Dr. Emeran Mayer, University of California-Los Angeles discussed maternal separation preparation with us.
Abbreviations and Acronyms
- MS
maternal separation
- MSPG
MS plus perigenital stimulation
- PGN
preganglionic neuron
References
- 1.Beach FA. Ontogeny of “coitus-related” reflexes in the female guinea pig. Proc Natl Acad Sci USA. 1966;56:526. doi: 10.1073/pnas.56.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baverstock P, Green B. Water recycling in lactation. Science. 1975;187:657. doi: 10.1126/science.1167701. [DOI] [PubMed] [Google Scholar]
- 3.de Groat WC, Douglas JW, Glass J, Simonds W, Weimer B, Werner P. Changes in somatovesical reflexes during postnatal development in the kitten. Brain Res. 1975;94:150. doi: 10.1016/0006-8993(75)90884-7. [DOI] [PubMed] [Google Scholar]
- 4.Maggi CA, Santicioli P, Meli A. Postnatal development of micturition reflex in rats. Am J Physiol. 1986;250:R926. doi: 10.1152/ajpregu.1986.250.5.R926. [DOI] [PubMed] [Google Scholar]
- 5.Thor KB, Blais DP, de Groat WC. Behavioral analysis of the postnatal development of micturition in kittens. Brain Res Brain Res. 1989;46:137. doi: 10.1016/0165-3806(89)90151-x. [DOI] [PubMed] [Google Scholar]
- 6.Kruse MN, De Groat WC. Micturition reflexes in decerebrate and spinalized neonatal rats. Am J Physiol. 1990;258:R1508. doi: 10.1152/ajpregu.1990.258.6.R1508. [DOI] [PubMed] [Google Scholar]
- 7.Kruse MN, de Groat WC. Spinal pathways mediate coordinated bladder/urethral sphincter activity during reflex micturition in decerebrate and spinalized neonatal rats. Neurosci Lett. 1993;152:141. doi: 10.1016/0304-3940(93)90503-d. [DOI] [PubMed] [Google Scholar]
- 8.Kruse MN, de Groat WC. Consequences of spinal cord injury during the neonatal period on micturition reflexes in the rat. Exp Neurol. 1994;125:87. doi: 10.1006/exnr.1994.1010. [DOI] [PubMed] [Google Scholar]
- 9.Mallory B, Steers WD, De Groat WC. Electrophysiological study of micturition reflexes in the rat. Am J Physiol. 1989;257:R410. doi: 10.1152/ajpregu.1989.257.2.R410. [DOI] [PubMed] [Google Scholar]
- 10.Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J Neurosci. 1997;17:8402. doi: 10.1523/JNEUROSCI.17-21-08402.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 12.Mayer EA, Collins SM. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122:2032. doi: 10.1053/gast.2002.33584. [DOI] [PubMed] [Google Scholar]
- 13.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 14.Sugaya K, Roppolo JR, Yoshimura N, Card JP, de Groat WC. The central neural pathways involved in micturition in the neonatal rat as revealed by the injection of pseudorabies virus into the urinary bladder. Neurosci Lett. 1997;223:197. doi: 10.1016/s0304-3940(97)13433-4. [DOI] [PubMed] [Google Scholar]
- 15.Sugaya K, De Groat WC. Micturition reflexes in the in vitro neonatal rat brain stem-spinal cord-bladder preparation. Am J Physiol. 1994;266:R658. doi: 10.1152/ajpregu.1994.266.3.R658. [DOI] [PubMed] [Google Scholar]
- 16.Penn AA, Shatz CJ. Brain waves and brain wiring the role of endogenous and sensory-driven neural activity in development. Pediatr Research. 1999;45:447. doi: 10.1203/00006450-199904010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Coonan EM, Downie JW, Du HJ. Sacral spinal cord neurons responsive to bladder pelvic and perineal inputs in cats. Neurosci Lett. 1999;260:137. doi: 10.1016/s0304-3940(98)00970-7. [DOI] [PubMed] [Google Scholar]
- 18.Boehm JJ, Haynes JL. Bacteriology of “midstream catch” urines. Studies in newborn infants. Am J Dis Child. 1966;111:366. doi: 10.1001/archpedi.1966.02090070064007. [DOI] [PubMed] [Google Scholar]
- 19.Gladh G, Mattsson S, Lindstrom S. Outcome of the bladder cooling test in children with nonneurogenic bladder problems. J Urol. 2004;172:1095. doi: 10.1097/01.ju.0000135617.02742.ad. [DOI] [PubMed] [Google Scholar]
- 20.de Groat WC. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Paraplegia. 1995;33:493. doi: 10.1038/sc.1995.109. [DOI] [PubMed] [Google Scholar]