The efficacy of lithium chloride in patients with low-grade neuroendocrine tumors was evaluated and it was found to be ineffective in obtaining radiographic responses.
Keywords: Neuroendocrine tumors, Lithium chloride, Glycogen synthase kinase-3β, Chemotherapy, Carcinoid tumors
Abstract
Background.
Low-grade neuroendocrine tumors (NETs) respond poorly to chemotherapy; effective, less toxic therapies are needed. Glycogen synthase kinase (GSK)-3β has been shown to regulate growth and hormone production in NETs. Use of lithium chloride in murine models suppressed carcinoid cell growth, reduced GSK-3β levels, and reduced expression of chromogranin A. This study assessed the efficacy of lithium chloride in patients with NETs.
Design.
Eligible patients had low-grade NETs. A single-arm, open-label phase II design was used. Lithium was dosed at 300 mg orally three times daily, titrated to serum levels of 0.8–1.0 mmol/L. The primary endpoint was objective tumor response by the Response Evaluation Criteria in Solid Tumors. Secondary endpoints included overall survival, progression-free survival, GSK-3β phosphorylation, and toxicity.
Results.
Fifteen patients were enrolled between October 3, 2007 and July 17, 2008, six men and nine women. The median age was 58 years. Patient diagnoses were carcinoid tumor for eight patients, islet cell tumor for five patients, and two unknown primary sites. Eastern Cooperative Oncology Group performance status scores were 0 or 1. Two patients came off study because of side effects. The median progression-free survival interval was 4.50 months. There were no radiographic responses. Because of an early stopping rule requiring at least one objective response in the first 13 evaluable patients, the study was closed to further accrual. Patients had pre- and post-therapy biopsies.
Conclusions.
Lithium chloride was ineffective at obtaining radiographic responses in our 13 patients who were treated as part of this study. Based on the pre- and post-treatment tumor biopsies, lithium did not potently inhibit GSK-3β at serum levels used to treat bipolar disorders.
Introduction
Low-grade neuroendocrine tumors (NETs) are rare, characteristically indolent tumors. When localized, resection can render patients disease free [1, 2]. Once they are metastatic, NETs are minimally responsive to chemotherapy. Combinations of cytotoxic chemotherapies have been tried with few positive results. A cytotoxic regimen including 5-fluorouracil (FU), doxorubicin, and streptozocin has been reported with an objective response rate in the range of 6%–69%, with the higher response rates of questionable statistical validity [3–6].
Biotherapy or targeted therapies have led to longer durations of stable disease, but most have shown objective response rates <20%. Combinations of interferon plus bevacizumab, interferon plus 5-FU, temozolomide plus thalidomide, topotecan, gemcitabine, bortezomib, or everolimus have been evaluated, with few response rates >20%, but many have led to longer durations of stable disease in the phase II setting [7–14]. Sunitinib showed a response rate of 16.7% in patients with pancreatic islet cell tumors in a phase II trial, and preliminary results of a phase III trial of sunitinib in pancreatic NET patients showed a progression free-survival (PFS) interval of 11.1 months, versus 5.5 months for placebo [15, 16]. Recently, a prospective, randomized, placebo-controlled phase III trial demonstrated a longer time to tumor progression (14.3 months versus 6.0 months) with octreotide, confirming antitumor activity and reaffirming somatostatin analogs as a key component of treatment of NETs [17].
Using metabolic pathways within NETs presents an attractive potential therapeutic target. In vitro analysis of cell lines of NETs demonstrated high basal levels of active, nonphosphorylated glycogen synthase kinase (GSK)-3β. Treatment of medullary thyroid and carcinoid cancer cells with lithium chloride (LiCl) resulted in significant growth reduction [18, 19]. Lithium is a known GSK-3β inhibitor whose safety has been established as a result of its use in the treatment of bipolar disorder [20]. In order to determine whether modulating this pathway has potential activity in NET patients, we developed a mouse model testing the role of GSK-3β.
Preclinical Data
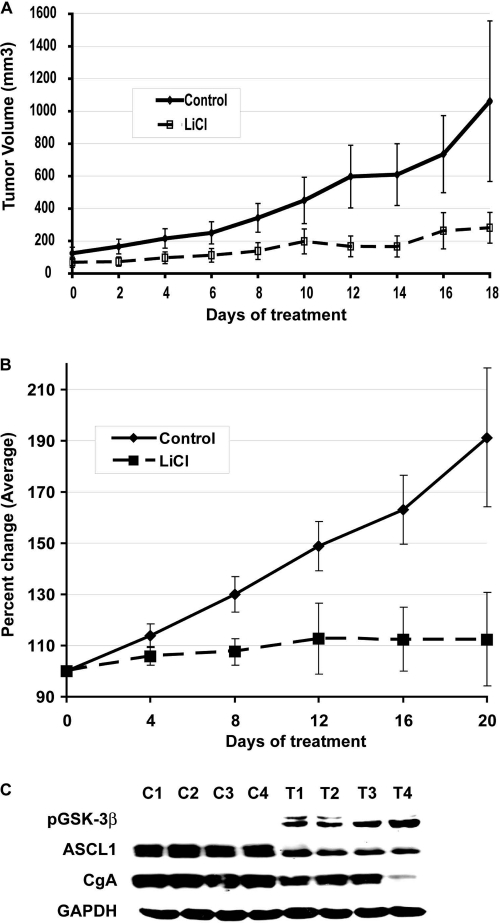
In our lab (H.C.), athymic immunocompromised Nu/Nu mice were injected s.c. with BON and H727 carcinoid cancer cells and treated with either saline or LiCl i.p. Tumor cell lines were received from the American Type Culture Collection (H727) and Drs. Mark Evers and Courtney Townsend from the University of Texas, Galveston (BON). Cell lines were verified and used within 6 months of receipt. Tumor response was assessed every 2 days using a caliper measurement. At the end of the 18- to 20-day-long experiments, the mice were sacrificed and the tumors were resected and weighed. The serum levels of lithium in treated animals were in the range of 0.2–1.0 mmol/L. We monitored the mice for signs of lithium toxicity, such as weight loss or behavioral changes, and observed none. As shown in Figure 1A, tumors in the control group steadily increased in volume over the 18-day period. Tumors in the LiCl group, in contrast, hardly grew at all. The difference in mean tumor volume between the two groups was large and statistically significant (p < .005). The relative tumor volume reduction was 54% in the treated group at the end of the experiment. Lithium treatment of mice with H727 bronchopulmonary carcinoid tumors also suppressed tumor growth (Fig. 1B). Analysis of tumor lysates from lithium-treated mice confirmed induction of phosphorylated GSK-3β proteins and a significant reduction in NET markers such as achaete-scute complex-like 1 and chromogranin A (Fig. 1C).
Figure 1.
Lithium inhibits the growth of carcinoid tumors in vivo. An s.c. xenograft mouse model was used for BON (A) and H727 (B) cells. After palpable tumors developed, i.p. injections of lithium chloride (LiCl) (340 mg/kg body weight) were administered for up to 20 days, the tumors were measured, and the tumor volume was calculated as described in methods every 2 days. As a control, an equal volume of saline was injected into control mice. (A): The growth of the LiCl-treated tumors was slower than that of the BON tumors in saline-injected mice. The lower tumor volume for the LiCl-treated BON tumors started after the second treatment with LiCl, and this difference was steady until the 12th day of treatment. At day 14, the tumor volume was 54% lower, and this persisted until the end of the experiment except for a slight increase in tumor volume on day 18. (B): There was a significant decrease in tumor growth in lithium-treated H727 tumors. Data presented as calculated average percent change in tumor volume. (C): Western analysis of H727 tumor lysates. Lithium treatment decreased the neuroendocrine markers achaete-scute complex-like 1 (ASCL1) and chromogranin A (CgA). Treatment also increased levels of phosphorylated glycogen synthase kinase (GSK)-3β proteins. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control.
Based on these data, and the demonstrable safety of lithium in humans at desired serum levels, we performed a single-institution phase II study in patients with low-grade NETs.
Materials and Methods
Study Objectives
The study was a single-arm, open-label, phase II design sponsored by the National Cancer Institute (NCI) (R21CA117117). The primary objective was to evaluate the response rate of patients with low-grade NETs treated with LiCl. Secondary objectives included an evaluation of PFS, overall survival (OS), and the safety profile of LiCl. To evaluate the ability of lithium to phosphorylate GSK-3β, a correlative analysis was performed on tumor samples using Western blot analysis with antibodies against GSK-3β, phosphorylated GSK-3β, and β-catenin. The study was approved by the University of Wisconsin institutional review board.
Patients
Patients were eligible if they were aged >18 years, with an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0–2, and were able to provide informed consent for the study. A pathologically confirmed metastatic low-grade NET was required. Grade was determined by a single pathologist based on morphology and immunohistochemistry to ensure eligibility for enrollment was met. Patients with higher-grade NETs were excluded in order to receive systemic chemotherapy consistent with good clinical practice. Prior systemic therapy for the NET was allowed, but no concurrent chemotherapy or radiotherapy (for the NET or other malignancy) was allowed. Laboratory exclusion criteria included an absolute neutrophil count <1,000 cells/μL, a platelet count <75,000 cells/μL, total bilirubin >2.0× the institutional upper limit of normal (ULN), aspartate aminotransferase >3× ULN or >5× ULN if liver metastases were present, serum creatinine >ULN, and serum sodium within normal limits. Patients on other neuroleptics or patients taking LiCl for another indication were not allowed in the study. Pregnant or lactating women were excluded from the study, and women of childbearing potential and sexually active males were required to agree to hormonal or barrier methods of contraception.
Treatment
After registration and signed informed consent, patients were treated with LiCl at 300 mg by mouth three times daily for a 28-day cycle. Lithium levels were checked, and the dose was titrated to a serum lithium level of 0.8–1.2 (units), levels safely used to manage bipolar disorders. Tumor burden was reassessed every 8 weeks with computed tomography or magnetic resonance imaging. If progressive disease was seen, treatment was stopped. In the setting of stable disease or objective response, treatment was continued.
Study Assessments
Patients were assessed for objective response by the Response Evaluation Criteria in Solid Tumors (RECIST) after every two cycles [21]. At each (monthly) clinic visit, pill counts were confirmed using a pill diary, and toxicities were graded as part of the clinic visit. At the conclusion of the treatment, patients underwent tumor biopsies, and their tumors were assessed for levels of GSK-3β using the same method as those performed in the preclinical mouse model outlined above.
Safety
Toxicity was graded using the NCI Common Terminology Criteria, version 3.0. Dose modifications for toxicity were performed based on the worst toxicity observed during the treatment course.
Statistical Analysis
A Simon two-stage optimal design was used, with the study stopping early if no objective responses were seen in the first 13 patients. If objective responses were seen, accrual would continue to a total of 33 patients to detect a response probability of 20% with 85% power. Data were analyzed using SAS software (SAS Institute, Inc., Cary, NC).
The PFS interval was calculated from the date of study enrollment to documented disease progression by the RECIST or death. PFS and OS times were summarized using point estimates of the median time to progression/time to death, and associated 90% confidence intervals. The data are presented graphically using Kaplan–Meier plots.
Correlates
The analysis of GSK-3β pathway components in NETs was considered exploratory. GSK-3β and phosphorylated GSK-3β protein levels were measured by Western blotting for each measurement time point. Mean percentage changes between pre-and post-treatment levels were recorded. Changes between pre- and post-treatment levels were evaluated using nonparametric Wilcoxon signed rank tests and paired t-tests.
Results
Fifteen patients were enrolled between October 3, 2007 and July 17, 2008. The median patient age was 58 years, with a range of 47–74 years. Nine women and six men were enrolled. All patients had an ECOG PS score of 0 or 1. Eight patients had carcinoid tumors, five patients had islet cell tumors, and the other two were not specified NETs.
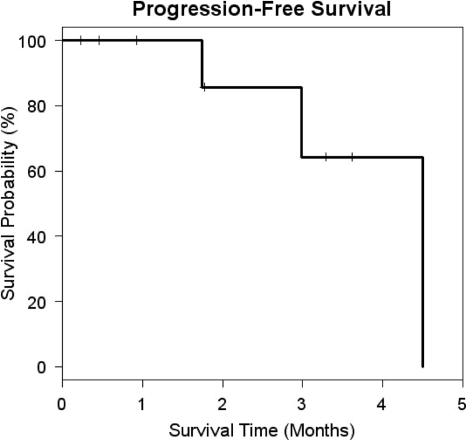
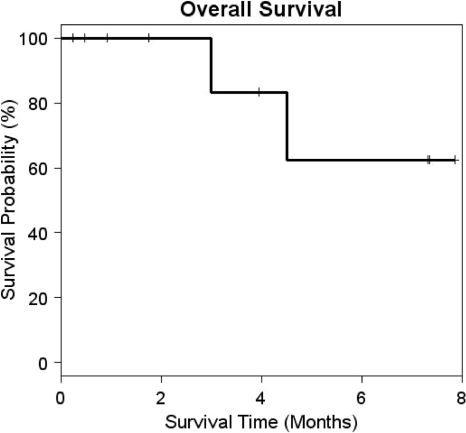
No objective responses by the RECIST were seen. Stable disease was seen in eight patients. The median PFS interval was 4.50 months (Fig. 2). The median OS time had not yet been reached (Fig. 3). Based on the protocol design, the study was stopped at 15 patients because of a lack of objective responses. Two patients were enrolled in the study prior to the assessments of the first 13 patients. Their data for toxicity and response were recorded because they received the investigational agent.
Figure 2.
Progression-free survival. The median progression-free survival interval was 4.50 months (95% confidence interval, 2.99–4.50 months).
Figure 3.
Overall survival. The median overall survival duration was not reached.
Only one grade 3 toxicity was identified, which manifested as a tremor. The most common grade 2 toxicities at least possibly related to the study drug were anorexia (four patients), fatigue (two patients), dehydration, dizziness, and abdominal discomfort (one patient each).
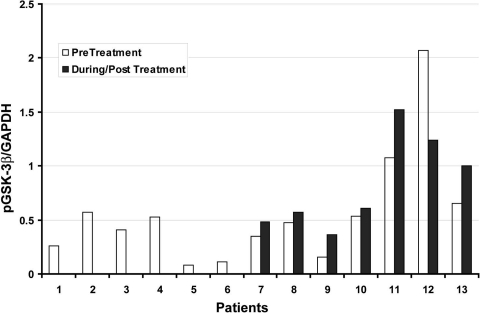
Pretreatment tumor biopsies were obtained from 13 patients. Seven patients had post treatment biopsies that were adequate for analysis of phosphorylated protein. There was not a consistent change in GSK-3β phosphorylation to the degree that was seen in the murine tumor model (Fig. 4). Two patients (Fig. 4, sample #11 and #13) had small increases in GSK-3β phosphorylation, which correlated with a best response of stable disease.
Figure 4.
Phosphorylated GSK before and after treatment.
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; pGSK-3β, phosphorylated glycogen synthase kinase-3β.
Discussion
Preclinical data in a murine model suggested that alteration in the GSK-3β pathway could potentially have antitumor effects in patients with low-grade gastrointestinal NETs. In treated patients, no objective responses were seen, and the trial was stopped early because of a lack of demonstrable efficacy. However, eight patients did have stable disease. Few grade 3 side effects were caused by LiCl. Based on the results of this trial, we do not believe that LiCl will reduce tumor volume in patients with low-grade NETs.
It is possible that altering the GSK-3β pathway may have efficacy in patients with NETs, but the doses of LiCl prescribed do not phosphorylate and inhibit GSK-3β. Treating with a higher dose of lithium is not advisable, given that all patients had lithium levels monitored and had their dose adjusted to maintain a lithium level at just under the level at which lithium toxicities become a major concern. A more potent GSK-3β phosphorylator may be worth exploring, given the preclinical data showing an effect on NET growth.
Finding an animal model for low-grade NETs is challenging, because cell lines typically behave more aggressively than in situ tumors. It is certainly possible that the GSK-3β phosphorylation signal demonstrated in our model did not translate into patients because of this discrepancy. Finding more precise animal models for low-grade NET is an avenue for future research.
This study includes the systematic application of preclinical data from a tumor model to clinical practice with humans. All eligible patients had post-treatment biopsies to measure treatment effect through a method developed through our animal model. Analyzing the biopsy tissue provided a valuable biomarker of lithium's effect, or lack thereof, on phosphorylation of GSK-3β in human NET tissue, thus informing us that interfering with GSK-3β with a more robust inhibitor may be warranted.
Shortcomings of this study include a small sample size. However, it is statistically improbable that enrolling more patients would have shown greater effectiveness, and thus the study was stopped appropriately according to predefined stopping rules.
In spite of some recent advances, there remains an unmet need for effective pharmacotherapy for metastatic NETs. Future studies using sound preclinical data and validated biomarkers are necessary to advance the treatment of this disease.
Acknowledgments
This study was funded by NCI grant R21CA117117, a Research Scholars Grant from the American Cancer Society (H.C.), National Institutes of Health grants DK063015, DK064735, DK066169, and CA109053 (H.C.), the George H.A. Clowes, Jr., Memorial Research Career Development Award of the American College of Surgeons (H.C.), a grant from the Vilas Foundation (H.C.), the Robert Draper Technology Innovation Award (M.K.), and research grants from the Carcinoid Cancer Foundation (H.C. and M.K.). This research was also supported in part by grant P30 CA014520 from the National Cancer Institute.
Author Contributions
Conception/Design: Muthusamy Kunnimalaiyaan, Herbert Chen, Kyle D. Holen
Provision of study material or patients: Muthusamy Kunnimalaiyaan, Herbert Chen, Kyle D. Holen, Li Ning, Mary Ndiaye, Noelle K. LoConte, William R. Schelman, Daniel L. Mulkerin
Collection and/or assembly of data: Sam J. Lubner, Muthusamy Kunnimalaiyaan, Herbert Chen, Kyle D. Holen, Li Ning, Mary Ndiaye, Noelle K. LoConte, William R. Schelman, Daniel L. Mulkerin
Data analysis and interpretation: Sam J. Lubner, Muthusamy Kunnimalaiyaan, Herbert Chen, Kyle D. Holen, Li Ning, Mary Ndiaye
Manuscript writing: Sam J. Lubner, Muthusamy Kunnimalaiyaan, Herbert Chen, Kyle D. Holen, Noelle K. LoConte, William R. Schelman, Daniel L. Mulkerin
Final approval of manuscript: Sam J. Lubner, Muthusamy Kunnimalaiyaan, Herbert Chen, Kyle D. Holen, Li Ning, Mary Ndiaye, Noelle K. LoConte, William R. Schelman, Daniel L. Mulkerin
References
- 1.Musunuru S, Chen H, Rajpal S, et al. Metastatic neuroendocrine hepatic tumors: Resection improves survival. Arch Surg. 2006;141:1000–1004. doi: 10.1001/archsurg.141.10.1000. discussion 1005. [DOI] [PubMed] [Google Scholar]
- 2.Sippel RS, Chen H. Carcinoid tumors. Surg Oncol Clin N Am. 2006;15:463–478. doi: 10.1016/j.soc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Cheng PN, Saltz LB. Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer. 1999;86:944–948. [PubMed] [Google Scholar]
- 4.Gonzalez MA, Biswas S, Clifton L, et al. Treatment of neuroendocrine tumours with infusional 5-fluorouracil, folinic acid and streptozocin. Br J Cancer. 2003;89:455–456. doi: 10.1038/sj.bjc.6601167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762–4771. doi: 10.1200/JCO.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, streptozocin-fluorouracil, or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 7.Ansell SM, Mahoney MR, Green EM, et al. Topotecan in patients with advanced neuroendocrine tumors: A phase II study with significant hematologic toxicity. Am J Clin Oncol. 2004;27:232–235. doi: 10.1097/01.coc.0000054535.19808.f4. [DOI] [PubMed] [Google Scholar]
- 8.Frank M, Klose KJ, Wied M, et al. Combination therapy with octreotide and alpha-interferon: Effect on tumor growth in metastatic endocrine gastroenteropancreatic tumors. Am J Gastroenterol. 1999;94:1381–1387. doi: 10.1111/j.1572-0241.1999.01090.x. [DOI] [PubMed] [Google Scholar]
- 9.Kulke MH, Stuart K, Enzinger PC, et al. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol. 2006;24:401–406. doi: 10.1200/JCO.2005.03.6046. [DOI] [PubMed] [Google Scholar]
- 10.Kunz P, Kuo T, Kaiser H, et al. A phase II study of capecitabine, oxaliplatin, and bevacizumab for metastatic or unresectable neuroendocrine tumors: Preliminary results. Presented at the American Society of Clinical Oncology 2008 Annual Meeting; May 30–June 3, 2008; Chicago, Illinois. [Google Scholar]
- 11.Pinchot SN, Holen K, Sippel RS, et al. Carcinoid tumors. The Oncologist. 2008;13:1255–1269. doi: 10.1634/theoncologist.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saltz L, Kemeny N, Schwartz G, Kelsen D. A phase II trial of alpha interferon and 5-fluorouracil in patients with advanced carcinoid and islet cell tumors. Cancer. 1994;74:958–961. doi: 10.1002/1097-0142(19940801)74:3<958::aid-cncr2820740326>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Shah MH, Young D, Kindler HL, et al. Phase II study of the proteasome inhibitor bortezomib (PS-341) in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2004;10:6111–6118. doi: 10.1158/1078-0432.CCR-04-0422. [DOI] [PubMed] [Google Scholar]
- 14.Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: Results of a phase II study. J Clin Oncol. 2008;26:4311–4318. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulke MH, Lenz HJ, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26:3403–3410. doi: 10.1200/JCO.2007.15.9020. [DOI] [PubMed] [Google Scholar]
- 16.Raymond E, Raoul J, Niccoli P, et al. Phase III, randomized, double-blind trial of sunitinib versus placebo in patients with progressive, well-differentiated pancreatic islet cell tumours. Presented at the 11th World Congress on Gastrointestinal Cancer; June 24–27, 2009; Barcelona, Spain. [Google Scholar]
- 17.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 18.Greenblatt DY, Ndiaye M, Chen H, et al. Lithium inhibits carcinoid cell growth in vitro. Am J Transl Res. 2010;2:248–253. [PMC free article] [PubMed] [Google Scholar]
- 19.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, et al. Inactivation of glycogen synthase kinase-3β, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther. 2007;6:1151–1158. doi: 10.1158/1535-7163.MCT-06-0665. [DOI] [PubMed] [Google Scholar]
- 20.Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]