Abstract
The plant hormone abscisic acid (ABA) mediates seed dormancy, controls seedling development and triggers tolerance to abiotic stresses, including drought. Core ABA signaling components consist of a recently identified group of ABA receptor proteins of the PYRABACTIN RESISTANCE (PYR)/REGULATORY COMPONENT OF ABA RECEPTOR (RCAR) family that act as negative regulators of members of the PROTEIN PHOSPHATASE 2C (PP2C) family. Inhibition of PP2C activity enables activation of SNF1-RELATED KINASE 2 (SnRK2) protein kinases, which target downstream components, including transcription factors, ion channels and NADPH oxidases. These and other components form a complex ABA signaling network. Here, an in depth analysis of the evolution of components in this ABA signaling network shows that (i) PYR/RCAR ABA receptor and ABF-type transcription factor families arose during land colonization of plants and are not found in algae and other species, (ii) ABA biosynthesis enzymes have evolved to plant- and fungal-specific forms, leading to different ABA synthesis pathways, (iii) existing stress signaling components, including PP2C phosphatases and SnRK kinases, were adapted for novel roles in this plant-specific network to respond to water limitation. In addition, evolutionarily conserved secondary structures in the PYR/RCAR ABA receptor family are visualized.
Introduction
Abscisic acid (ABA) is a stress-related signaling molecule reported in all kingdoms of life except in Archaea. Although well known and best studied in higher plants, in particular in Arabidopsis thaliana, there is evidence that the hormone is synthesized in plant-associated bacteria, plant pathogenic fungi, certain cyanobacteria, algae, lichens, protozoa, sponges and apparently even in human granulocytes [1–3]. In Arabidopsis and probably in all Embryophyta, the main abiotic factor leading to formation of ABA and thus triggering of signaling events is any form of limited cellular water availability. High ABA levels lead to preservation of seed dormancy [4], inhibition of germination and lateral root formation [5] and reduction of water transpiration through stomatal pores [6,7]. In lower photosynthetic species such as algae, cyanobacteria and lichen, drought or salt stress is also a factor that induces ABA synthesis but knowledge on signaling pathways is extremely limited [3]. Factors affecting ABA levels in non-photosynthetic organisms described so far are heat (Axinella polypoides, Homo sapiens [1]) and nutrient limitation (Apicomplexa [2]).
Since the discovery of ABA in the 1960s [8] and the first identification of plant ABA pathway mutants in the 1980s [9], tremendous progress has been made in identifying mechanisms and genes involved in ABA metabolism, transport and signal transduction (reviewed in [10–12]).
Here we aim to place the various aspects of the plant ABA signaling network in an evolutionary context. In particular we try to shed light on the appearance of ABA signaling as part of diverse adaptations necessary to cope with environmental factors typical for the terrestrial habitat. For a more general detailed overview of ABA signaling in plants the reader may also consider other recent reviews [7,11–13].
Comprehensive Overview of Core Components and Modulators of ABA Signaling
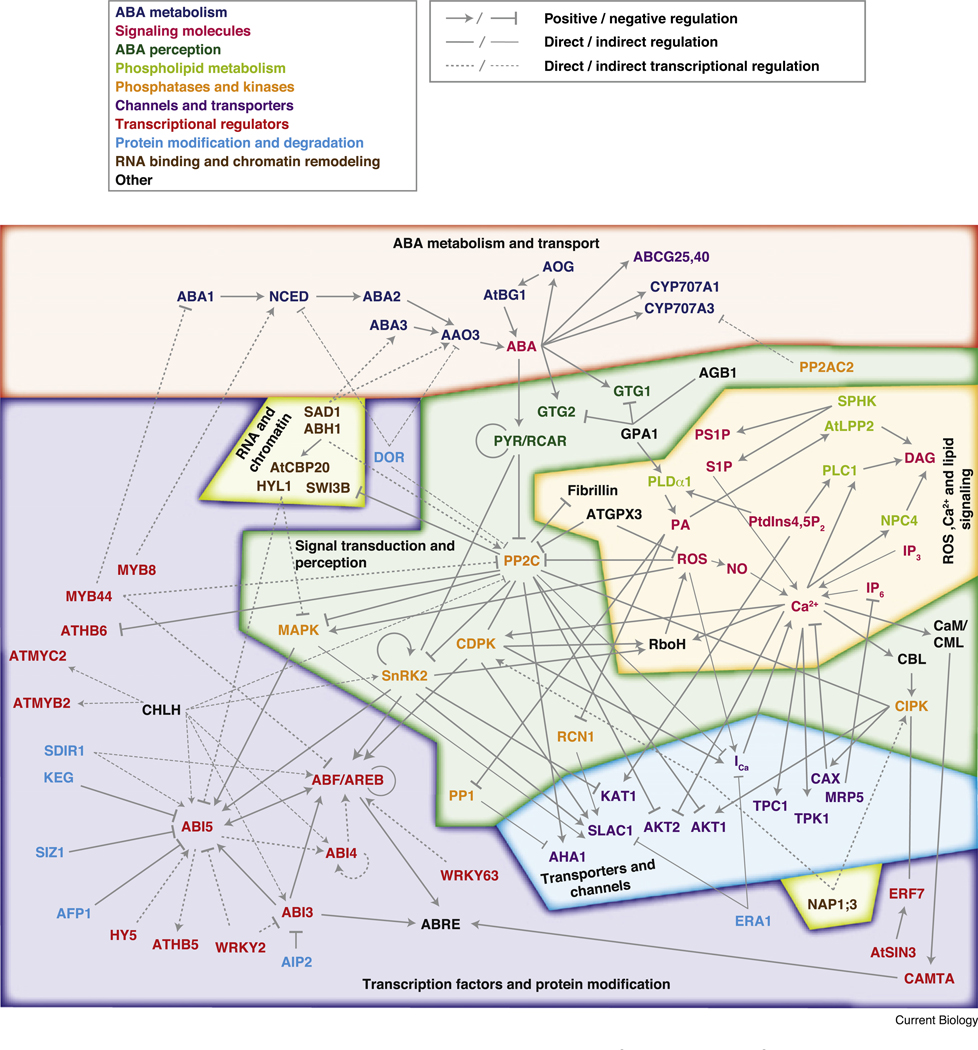
ABA signaling can be divided into three different layers: (i) ABA metabolism and transport, (ii) ABA perception and signal transduction and (iii) ABA signal response and modulation (Figure 1). The onset of ABA signaling begins with ABA synthesis [10] and its long-distance transport [14,15]. ABA perception and signal transduction consists of so-called core signaling components, including PYRABACTIN RESISTANCE (PYR)/REGULATORY COMPONENT OF ABA RECEPTOR (RCAR) ABA receptors [16,17], group APROTEIN PHOSPHATASE 2Cs (PP2Cs) [18] and members of the SNF1-RELATED PROTEIN PROTEIN KINASE 2 (SnRK2) group of kinases [19–21]. PYR/RCAR–PP2C complex formation leads to inhibition of PP2C activity [16,17,22], thereby allowing activation of SnRK2s which target ion channels, NADPH oxidases and ABF/AREB/ABI5 type basic/region leucine zipper (bZIP) transcription factors (Figure 1) [23–26]. The complexity of ABA signaling in Arabidopsis is reflected by the number of PYR/RCARs (Figures 2 and 3) [16,17,27], 6–9 PP2Cs and 3 SnRK2s, which function in ABA signaling (see Supplemental Table S1 published with this article online for gene lists and respective references).
Figure 1. The ABA signaling network derived and inferred from curated literature listed in Supplemental Table S1.
The network is divided into six main functional categories: ABA metabolism and transport (red); perception and signal transduction (dark green); ROS, Ca2+ and lipid signaling (orange); transporters and channels (blue); transcription factors and protein modification (purple); and RNA processing and chromatin remodeling (light green). ABA signaling nodes are given by their protein or molecule names and colored according to their role in ABA metabolism (dark blue); ABA perception (dark green); signaling molecules (magenta); phospholipid metabolism (light green); phosphatases and kinases (orange); channels and transporters (purple); transcriptional regulators (red); protein modification and degradation (light blue); RNA binding and chromatin remodeling (brown) and others (black). For more detailed information about the ABA signaling components please refer to the text and Supplemental Table S1 and references therein. Connections represent positive (arrow) and negative (block) regulation or currently unknown (line). Regulations are direct (bold line), indirect (faint line) or transcriptional (dashed line).
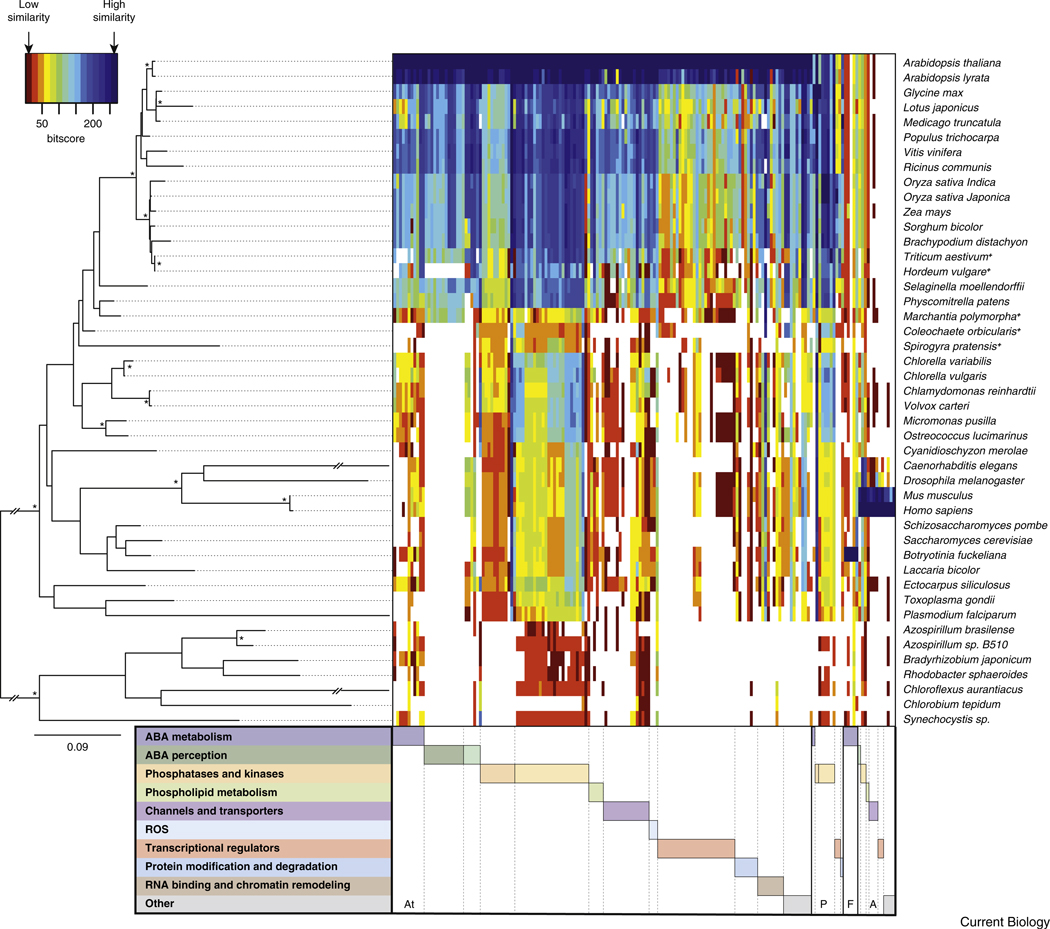
Figure 2. Similarity heatmap of proteins involved in ABA metabolism and signaling mechanisms.
An interactive version of this figure displaying details of all proteins investigated is provided as Supplemental Figure S1. The color key (top left) represents the similarity to the closest match and ranges from dark red (low similarity) to blue (high similarity). White areas represent no hit below the e-value threshold (<10 E −10) applied. Note the absence or low similarity of many ABA signaling proteins at the transition from algae to land plants. The coloring in the heatmap is scaled column-wise based on bit values from protein blasts against genome (all) and nucleotide/EST (+) databases listed in Supplemental Table S3. Homologs of Arabidopsis (At), other plant (P), fungal (F) and animal (A) proteins are ordered according to their functional category as in Figure 1. Tools used for data analyses are listed in Supplemental Table S4. The displayed guiding tree (left) used for ordering the organisms was generated with 16S/18S rRNA sequences. Nodes labeled with an asterisk have bootstrap support greater than 95%. Scale bar indicates 0.09 substitutions per site.
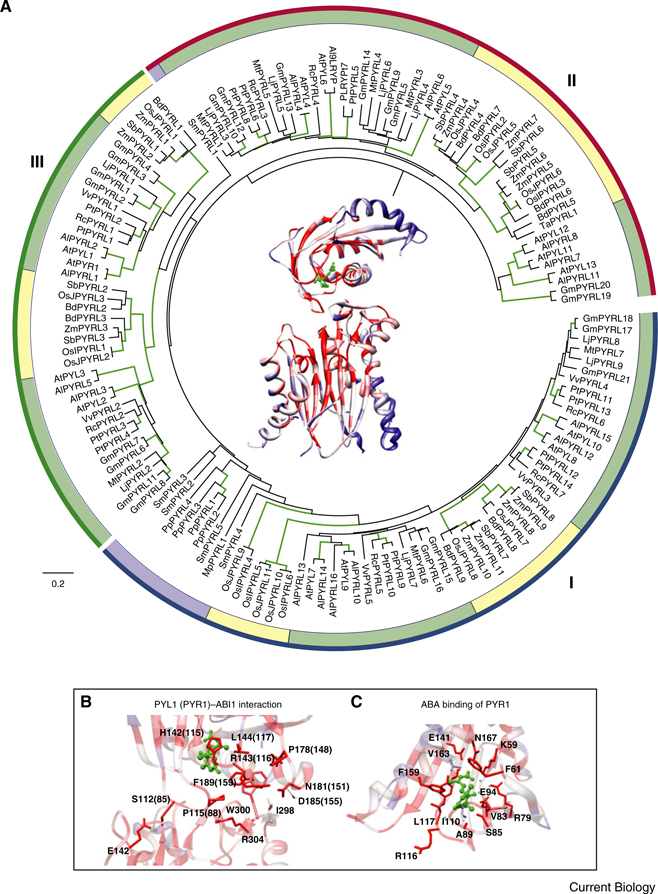
Figure 3. Conservation of land plant PYR/RCAR ABA receptors.
(A, center) Structure of PYL1 (upper part) in complex with ABI1 (lower part) (PDB: 3KDJ [75]) colored according to percent conservation in an alignment of 23 ABI1 and 149 PYR/RCAR plant homologs. The color of secondary structures changes gradually from blue (amino acid conservation ≤2%) via white (~50%) to dark red (100%). The bound ABA molecule is colored green. A maximum likelihood phylogenetic tree of PYR/RCAR homologs (PYRL) in sequenced plant genomes encircles the central PYL1–ABI1 structure. The phylogenetic tree is divided into three (I, II, III) clades according to [16] with Arabidopsis PYR/RCARs labeled red. A putative protein from Marchantia polymorpha (liverwort, MpPYRL1) probably representing the earliest identified homolog at the transition to land plants is labeled blue. Green, yellow and purple bars indicate PYRL proteins in dicots, monocots and lower plants, respectively. The underlying protein alignment and phylogenetic tree was evaluated using programs and web resources listed in Supplemental Tables S3 and S4. Branches with bootstrap confidence ≥95% are highlighted in green (100 bootstraps). The tree was rooted using Mesorhizobium loti mlr1698 as an out-group [68]. Scale bar indicates 0.2 substitutions per site. Accessions of Arabidopsis PYR/RCARs and their homologs are given in Supplemental Table S2. (B) Detailed view of the molecular contacts between PYL1 (upper part) and ABI1 (lower part) (PDB: 3KDJ [75]). Relevant amino acids and positions are given either for PYL1 or for PYR1 (parentheses). (C) Conservation of the PYR1 ABA binding pocket (PDB: 3K3K [73]). Coloring scheme in panel B and C is as described for panel A. Molecular graphics images were produced using tools described in Supplemental Table S4.
Several small molecules act as intracellular messengers and transmit specific aspects of ABA signaling. For example, ABA causes the production of reactive oxygen species (ROS) that down-regulate the activity of the PP2C phosphatases [28] and activate Ca2+-permeable channels (ICa) [29–31]. In addition, H2O2 also activates mitogen-activated protein kinases (MAPKs). MAPKs are inhibited by PP2Cs [18,32], phosphorylate ABI5 [33] and regulate S-type anion channel activity [34].
Phosphatidic acid, synthesized by phospholipase D [35], binds and inhibits protein phosphatase 1 (PP1) and PP2A phosphatases, which function in ABA and light signaling and interactions among these stimuli [36–38]. Additional enzymes involved in phospholipid metabolism also play a role in ABA signaling [39] (Figure 1).
Changes in cellular Ca2+ levels activate Ca2+-DEPENDENT PROTEIN KINASES (CDPKs) [40] and CALCINEURIN B-LIKE PROTEIN (CBL) INTERACTING PROTEIN KINASES (CIPKs), the latter mediated through interaction with CBLs (reviewed in [41]). CIPKs (SnRK3s) appear to be involved in plant ion homeostasis and abiotic stress tolerance by regulating H+, Na+, Ca2+ and NO3− transporters and K+ channels and interacting with transcription factors [41,42]. In addition, CIPKs physically interact with PP2Cs [43] but it still needs to be elucidated if CIPKs and PP2Cs can (de)phosphorylate each other or if both act together as a signaling module regulating target proteins such as K+ channels (reviewed in [41]). CDPKs function in ABA-induced stomatal closing, and anion-channel and Ca2+-channel activation [44] and fulfill a dual function, as they directly phosphorylate PP2Cs and targets of SnRK2s, e.g. SLAC1, NADPH oxidases and ABFs [45–48]. The functional consequence of PP2C phosphorylation by CDPKs still needs to be clarified. Ca2+ in addition may function in parallel to ABA-induced transcriptional activation through calmodulin-binding transcription activators (CAMTAs) that can bind to ABA-regulated cis-acting elements (ABREs) [49].
ABA signaling affects developmental processes through the B3-type transcription factor ABI3 [50]. ABI3 recognizes ABA-responsive elements (ABREs) [51] and interacts with ABI5 [52]. ABI4, an AP2/ERF transcription factor, integrates ABA and sugar signaling [53,54] and controls ABI5 expression [55]. In contrast to ABF/AREB/ABI5, ABI4 and ABI3, which function early in the ABA signaling cascade, additional transcription factors (Figures 1 and 2, Supplemental Figure S1, Supplemental Table S1) may act as convergence points in the crosstalk between ABA and other plant hormone signaling networks, light signaling, plant defense and abiotic stress responses or developmental cues. They modulate the expression of ABA synthesis genes, PP2Cs and ABF/AREB/ABI5 or bind to ABRE elements (Figure 1). Thus, further work is needed to understand the architecture of the large transcriptional network in ABA signaling.
Post-translational protein modifications such as farnesylation [56,57], sumoylation [58] and ubiquitination [59] modulate ABA signaling, in some cases by targeting transcriptional regulators such as ABI3 and ABI5 (Figure 1). Furthermore, mRNA-binding proteins regulate transcript abundance of ABA synthesis genes and ABA signaling transducers [60,61]. Finally, chromatin remodeling factors could be targeted by PP2Cs [62].
Together, all of these ABA signaling components form a complex network that integrates and transduces ABA-mediated signals (Figure 1, Supplemental Table S1) that requires further characterization.
Key Components in ABA Signaling are Plant Specific
Overall sequence comparison of ABA signaling components as depicted in Figure 2 shows remarkable land-plant-specific conservation (see Supplemental Table S1 for a detailed list of proteins). Note that an interactive electronic version of Figure 2 displaying detailed information of all proteins is provided in Supplemental Figure S1. In particular, analyses show that ABA perception by PYR/RCAR family proteins and ABA-dependent transcriptional regulation first occurred in land plants (Figure 2, Supplemental Figure S1). Therefore, one can assume that within the plant kingdom ABA signaling evolved to its current stage as a consequence of the high selective pressure exerted by the temporal absence of water, for example, in coastal areas [63]. It is tempting to speculate that ABA signaling emerged as a more flexible, energy efficient way to produce osmo- and drought-protectant solutes than the continuous high production of these components observed in small ancient desiccation-tolerant plant species [63].
Phylum-Specific ABA Biosynthesis Pathways
Two related ABA metabolism pathways have evolved in land plants and fungi (Figure 2, Supplemental Figure S1). The plastid-specific indirect pathway, which is characteristic of land plants, starts with the rate-limiting conversion of 9′-cis neo/violaxanthin to xanthoxin by 9′-cis epoxycarotenoid dioxigenases (NCED) [10]. Based on the presence of NCED homologs, 9′-cis neo/violaxanthin and other evidence [64], the moss Physcomitrella patens is currently the most ancient fully sequenced land plant containing this indirect ABA synthesis pathway. The indirect pathway probably evolved during land colonization [3,64] (Figure 2).
The direct cytosolic ABA biosynthesis pathway described for fungal pathogens (Botryotinia fuckeliana, Botrytis cinerea) [65] starts with farnesyldiphosphate, which is also an intermediate of the cis neo/violaxanthin biosynthesis pathway (for details see [3,10]). The near ubiquitous presence of the isoprenoid pathway [66] renders farnesyldiphosphate the core precursor of ABA biosynthesis in plants and fungi and possibly in other ABA-producing organisms (bacteria and algae [3], protozoa [2], sponges and human [1]).
The degradation of ABA is catalyzed by members of the ancient cytochrome P450 superfamily (AtCYP707A; reviewed in [10,67]). Their role in hormone degradation and in particular the degradation of ABA emerged probably in a later phase of the land transition since the algae Chlamydomonas reinhardtii and the moss Physcomitrella do not encode orthologs of AtCYP707A [67] (Figure 2 and Supplemental Figure S1).
ABA Perception by PYR/RCAR Family Proteins
In vascular plants, structural and molecular evidence shows that members of the PYR/RCAR family play a central role in ABA sensing (Figure 3) [16,17]. The members of this family belong to the Bet V I-like superfamiliy. Comparative analyses of different structures and sequences show that Bet V I-like proteins are present in all kingdoms of life (reviewed in [68]). Interestingly, this superfamily contains previously known hormone-binding proteins like PR10 (brassinosteroids [69]) and the cytokinin-specific binding protein CBP [70].
Overlaying PYR/RCAR protein structures with an alignment of 149 PYR/RCAR homologs used in the phylogenetic tree (Figure 3A) shows overall low conservation in the amino-terminal α1 helix of PYR/RCAR proteins (blue; <2%) and a 50% (white) or higher conservation (red; up to 100%) in the β-sheets and helices α2 and 3 (Figure 3A, center). ABA and PP2C binding residues, except for PYR1I110 and PYR1V163 (Figure 3C) and PYL1D185 (PYR1D155) (Figure 3B), are highly conserved in plant homologs [71–75]. Interestingly, key amino acids PYR1K59 and PYR1R116, crucial for ABA and PP2C binding [73] (Figure 3B,C), are found in most homologs. Overlaying the ABI1 structure with an alignment of homologs from 23 different plant species listed in Figure 2 shows conservation in the catalytic center. However, PYR/RCAR-interacting residues, including ABI1W300, which is most important for the PYR/RCAR–ABA– ABI1 interaction [71,72,75], are not conserved in algae (Figure 3B).
Based on current genomic and functional data, AtPYR/RCAR-related proteins are found only in land plants (Figure 2, Supplemental Figure S1). The earliest emergence of potential PYR/RCAR homologs can be observed at the evolutionary stage of Marchantia polymorpha (liverwort; MpPYRL1; Figure 3, Supplemental Table S2), a species assumed to represent the basal lineage of the land plant phylum [63]. AtPYR/RCARs and their homologs in other plant species can be grouped into three clades (I, II, III) [16] (Figure 3). In clades I–III, mono- and dicotyledonous-specific subclades are present. Clade III is subdivided into two subfamilies containing either AtPYR1/PLY1 or AtPYL2/3 (Figure 3A). So far, ABA binding and in vivo functions were described for members of all three clades (clade I: AtPYL8/9; clade II: AtPYL5; clade III: AtPYR1 and AtPYL1/2 [16,17,22,27,71–73,75]).
The Role of Protein Phosphatases
PP2Cs are the best characterized protein phosphatases involved in ABA signaling. In eukaryotes PP2Cs inhibit stress-activated protein kinase cascades by dephosphorylating MAPKs and receptor-like kinases (reviewed in [18]). In Arabidopsis, which contains 10 groups and a total of 76 PP2Cs, only members of group A have been implicated in ABA signaling [18]. These are ABI1, ABI2, HAB1 (P2C–HA) and PP2CA (AHG3), which have been shown to directly interact with PYR/RCAR ABA receptors [16,17,22,27]. HAB2, AHG1 and the recently identified highly ABA-induced HAI1, HAI2 (AIP1) and HAI3 also belong to the PP2C group A [18,21].
The variety of PP2C targets (Figure 1) may reflect a negative regulatory role of PP2Cs at different layers of ABA signaling. Recently, MpABI1 from Marchantia polymorpha (liverwort), has been identified as a negative regulator of ABA signaling [76]. Liverworts do not produce seeds, lateral roots and stomatal structures found in higher plants (for details on stomata evolution see [63] and references therein). However, ABA still plays a role in stress tolerance in this organism. Thus, MpABI1 seems so far the most ancient, characterized PP2C involved in ABA signaling. It is tempting to speculate that MpPYRL1/ABI1 play a similar role in ABA signaling as described for their Arabidopsis homologs. In the next higher lineage of land plants, i.e. in the moss Physcomitrella, ectopic expression of the dominant abi1–1 resulted in ABA hyposensitive phenotypes; e.g. decreased ABA-induced freezing tolerance and enhanced osmotic stress sensitivity [77]. Similarly, disruption of PpABI1A caused ABA hypersensitive phenotypes [77]. Overall, a total of 51 PP2C genes are encoded in the Physcomitrella genome, a number that is 1.5 times smaller than that in Arabidopsis [18,77]. Physcomitrella lacks group B and J members [77] and the group A of ABA-regulated PP2Cs contains only two genes compared to nine genes in Arabidopsis, suggesting that higher land plants increased the number of group A PP2Cs during evolution.
Roles of Protein Kinases
Protein kinases that function in ABA signaling belong to the SnRK2, SnRK3 (CIPK), CDPK (reviewed in [12,41]) and MAPK families [34,78]. With the exception of MAPKs [78], CDPK, SnRK2 and SnRK3 subfamilies of the SNF-1-like clade are mainly found in plants [13,40,41] (Supplemental Figure S1).
In Arabidopsis the SnRK2 subfamily consists of 10 members that have been categorized into three different subclasses (I, II and III) [79]. With the exception of SnRK2.9, all SnRK2s are activated by osmotic and salt stress [80]. Only SnRK2.2/3/6/7/8 are activated by ABA [19,80,81]. Among them, SnRK2.2/3/6, members of the subclass III, exhibit the strongest ABA activation [80]. The SnRK2 subfamily is conserved in land plants and their role in ABA signaling and osmotic stress responses have also been shown for members from pea, barley, maize and rice [79,82–84]. Similarly to Arabidopsis, rice harbors 10 and maize harbors 11 SnRK2 genes [79,84]. These three species each encode 3 proteins that belong to the SnRK2.2/3/6 group. The rice and maize genes group together with SnRK2.6 (OST1), while AtSnRK2.2 and AtSnRK2.3 form a separate subgroup [84]. SnRK2s have also been identified in the (club) mosses Selaginella moellendorffii and Physcomitrella [13]. SnRK2s in algae, including Chlamydomonas, were classified as distinct in their sequences from higher plant SnRK2s [13]. SnRK2s appear to be land plant specific, with subclass III being the most ancient and subclass I the most recent form [13]. The carboxy-terminal region of subclass III SnRKs is necessary for the interaction with group A PP2Cs [20]. Sequence analyses of SnRK2.2/2.6 homologs from algae reveal that these kinases lack the PP2C–binding region or that they differ significantly from their Arabidopsis orthologs, supporting the hypothesis that SnRK2-mediated ABA signaling evolved in land plants.
On the basis of phenotypic analyses of loss-of-function mutants and the ability of CIPKs to interact with PP2Cs, CIPK1/3/8/14/15/20/23/24 were implicated in ABA signaling [41,43]. The CIPK gene family is also found in algae but not in Chlamydomonas. Protozoans (unicellular eukaryotes: Naegleria gruberi and Trichomonas vaginalis) harbor single members of CIPK proteins [41]. One region in CIPKs has been identified to mediate interaction with the PP2C ABI2 and designated as the PPI motif [43]. The PPI motif seemed to emerge in algae, as it is found in Chlorella variabilis but not in Ostreococcus lucimarinus, suggesting that the CIPK–PP2C interaction might have already existed in at least some algae.
CDPKs have been identified in land plants, algae and protozoans, such as Plasmodium falciparum, but not in yeast and nematodes [40] (Supplemental Figure S1). Thus, CDPKs might exist only in plants and protozoans [40]. The CDPKs that are involved in ABA signaling are CPK3/4/6/11/32 [44,45,47]. CPK4 and CPK11 are closely related genes and their function may partially overlap because both phosphorylate the transcription factors ABF1 and ABF4 [47]. CPK3/6 and CPK23 regulate Ca2+-permeable channels and S-type anion channel currents [44,48]. Recently, it has been shown that ABI1 can be phosphorylated by CPK23 in vitro and that the presence of ABI1 stimulated CPK23 autophosphorylation [48]. These in vitro findings suggest that CDPKs target core ABA signaling components. Whether the CPK23–ABI1 interaction also exists in planta needs to be analyzed. Research in potato showed that StCDPK5 positively regulated ROS production by phosphorylating NADPH oxidases in response to pathogens [46]. NADPH oxidases function in ABA signal transduction [30] and are also targeted by the OST1 kinase [26].
MAPKs are abundant in all eukaryotes (Figure 2, Supplemental Figure S1). MAPKs phosphorylate a wide range of target proteins, including other kinases and/or transcription factors [78]. Diverse stimuli, including pathogens, abiotic stress, H2O2, ABA and phytohormones activate MAPKs (reviewed in [78]). A role for MAPKs in ABA signaling has been shown for MPK1/2/3/6/9/12 [34,78]. Interestingly, MPK6 activity is inhibited by physical interaction with the PP2C ABI1 [32]. In contrast to CIPKs, which may directly interact with the active center of ABI2 [43], MPK6 interacted with the amino terminus of ABI1 (aa 1–93) [32], suggesting a different mechanism for interaction. A negative regulation of MAPK pathways by PP2Cs is well conserved in yeast and mammals and a MAPK interaction motif in group A PP2Cs has been identified at least in HAB2 and AtPP2CA but not in ABI1 (reviewed in [18]).
Taken together, protein kinases except SnRK2s existed before the land colonization by plants. In addition, none of these protein kinase families function specifically in ABA signaling. Even the strongly ABA-activated kinase SnRK2.6 (OST1) could be activated by osmotic stress in an ABA-independent manner [20]. Considering the related phosphorylation preferences of SnRKs and CDPKs [85], one can speculate that ABA-specific signaling may have evolved from overlapping stress signaling functionality.
Membrane Proteins
Functions in ABA signaling of transmembrane G-protein coupled receptors and associated heterotrimeric GTP-binding proteins have been found for Arabidopsis and animals [86,87] (Figure 1). These advances in plasma membrane-associated ABA signaling have been reviewed and presented in detail elsewhere and the reader is referred to these articles [1,11,86–88] (Figure 1 and Supplemental Figure S1; see also genes listed in Supplemental Table S1).
K+ fluxes across cell membranes, which regulate stomatal aperture, can be mediated by voltage-dependent K+ channels [89]. These channels share topological similarities with Shaker-type channels in Drosophila melanogaster and mammals [90]. The domain architecture of plant K+ channels, however, differs from their animal counterparts. Cytosolic Ca2+, PP2C phosphatases, SnRK2s and CIPKs regulate K+ channel activity [25,91–93]. Plant shaker channels are conserved among mono- and dicots [94]. While Physcomitrella contains one homolog that does not belong to any of the plant Shaker channel groups [94], this family of proteins is absent in unicellular green algae such as Chlamydomonas [90] (Figure 2, Supplemental Figure S1).
Voltage-dependent anion channels that mediate ABA-induced stomatal closure have been defined as slow-(S-type; SLAC) and rapid-activated (R-type; QUAC) channels [91,95]. SLAC1 functions in stomatal closure in response to diverse stimuli, including ABA, Ca2+, CO2, ROS and ozone [96,97]. SLAC1 is a distant homolog of bacterial (TehA) and fungal (Mae1) C4-dicarboxylate transporters [96,97]. In contrast to TehA and Mae1, SLAC1 contains an amino-terminal extension that is required for the phosphorylation-dependent channel activation [24,48,98,99]. The Arabidopsis genome encodes four SLAC1 homologs [90,96,97]. Two of them were able to functionally complement slac1, suggesting functional conservation [96]. Close homologs were also identified in rice, poplar and the unicellular green algae Chlamydomonas [90,97] (Figure 2, Supplemental Figure S1).
Due to the reduced R-type anion channel activity in Atalmt12 in response to malate, AtALMT12 (QUAC1) was suggested to represent an R-type channel [100]. Atalmt12 exhibited reduced ABA-, Ca2+-, CO2- and dark-induced stomatal closure [100,101]. First identified in wheat [102], aluminium-activated malate transporters (ALMT) share a typical architecture consisting of the uncharacterized protein family five domain (UPF0005) and 5–7 transmembrane domains [103]. Although the UPF0005 domain is found in viruses and all kingdoms of life, the ALMT architecture is found only in land plants and not in the unicellular green algae Chlamydomonas [90,103] (Figure 2, Supplemental Figure S1).
NADPH oxidases (NOX), which produce extracellular ROS, are widely distributed in eukaryotic organisms [104] (Figure 2, Supplemental Figure S1). During evolution, NOX diverged to five subtypes differing in their amino-terminal extension [104]. The prototypical plant-specific subtype designated as respiratory burst oxidase homolog (Rboh) contains two amino-terminally located Ca2+-binding EF hands [104]. Specific NOX subtypes in animals (NOX5 and DUOX) harbor four EF hand domains or two EF hand domains and a peroxidase-like domain [104]. Recently, the structural characterization of rice OsRbohB revealed a four EF-hand fold similar to calcineurin B and therefore overall a NOX5 architecture [105]. Rbohs are activated by Ca2+ and phosphorylation [104,106] and function in ABA-induced activation of Ca2+ channels and induction of stomatal closure [30]. One OST1-dependent phosphorylation site modulating RbohF activity (S174) [26] is conserved in all ten Arabidopsis Rbohs and in RbohF orthologs from land plants, including the moss Physcomitrella.
ATP-binding cassette (ABC) proteins constitute a large, diverse and ubiquitous superfamily (Figure 2, Supplemental Figure S1) with more than 120 members each in Arabidopsis and rice [107]. ABC proteins transport hormones, lipids, metals, secondary metabolites and xenobiotics [107]. ABA transporters belong to the G subfamily (WBC and PDR subfamilies). AtABCG25 exports ABA from vascular tissues [15] whereas AtABCG40 was reported to import ABA into guard cells [14].
Taken together, ion channels and transporters have served as a powerful platform and as rapid signaling targets to identify genes and mechanisms that mediate early rapid ABA signal transduction [7].
Transcriptional Regulators
Many ABA-induced genes contain a conserved ABRE in their promoter regions (reviewed in [108]). Transcription factors identified as being able to bind ABREs were designated as ABF/AREBs (reviewed in [108]). In Arabidopsis these transcriptional activators belong to the group A subfamily of bZIP transcription factors [109]. Another group A bZIP transcription factor that was identified in a forward genetic screen for ABA-resistant germination is ABI5 [110]. The bZIP transcription factors regulate different processes in plants, including pathogen defense, light and stress signaling and are conserved in eukaryotes [109,111]. Compared to the 75 members in Arabidopsis, the human genome harbors 56 genes, Drosophila 27 and yeast 14 (reviewed in [111]). These transcription factors seem to have emerged in protozoans (unicellular eukaryotes). The protozoan Giardia lamblia contains just one bZIP transcription factor. However, another protozoan, Dictyostelium discoideum, harbors 19 members (reviewed in [111]). Our search for ABF/AREB/ABI5 orthologs in non-land plant species did not result in any identified protein with an ABF/AREB-type protein architecture (Figure 2, Supplemental Figure S1), underlining the lineage-specific evolution of bZIP proteins in plants and animals. Interestingly, we could identify ABF/AREBs in Selaginella, but not in Physcomitrella and Marchantia polymorpha, suggesting that ABF/AREBs evolved in mosses. ABRE cis-acting elements are also plant specific [111]. The high diversity of these transcription factors is reflected by sequence differences between ABF/AREB/ABI5 orthologs from Arabidopsis and other di- or monocotyledonous species (Figure 2, Supplemental Figure S1). However, the regulation of ABF/AREBs by SnRK2s and their function in ABA signaling is conserved in rice, wheat and barley [112–114].
APETALA2 (AP2)/ethylene responsive factor (ERF) domain transcription factors belong to a large plant-specific multi-gene family and have been divided into four subfamilies (AP2, DREB, ERF and RAV) [115]. The AP2/ERF gene family size radiated along with land colonization and harbors 147 members in Arabidopsis, compared to 13–14 in unicellular green algae (Chlorella sp. and Chlamydomonas) and 57 in the clubmoss Selaginella (reviewed in [115]). ABI4 is a single member of the A-3 subgroup of the DREB subfamily [115] and provides a link between ABA and sugar signaling [53,54]. Single ABI4 orthologs from maize and rice have been characterized, showing high homology to Arabidopsis ABI4 in the AP2 domain but high diversity outside this domain [116,117] (Figure 2, Supplemental Figure S1). ZmABI4 from maize could only partially rescue the Atabi4-1 phenotype [116].
B3 domain transcription factors control embryo maturation and the transition to dormancy and are mainly involved in hormone signaling pathways [118,119]. Five major classes of genes contain the B3 domain, including the ABI3/Viviparous1 (VP1) family [119]. The ABI3 family in Arabidopsis consists of ABI3, FUS3 and LEC2, and these proteins have partially overlapping roles in mid-to-late embryo development (reviewed in [118]). B3 domain transcription factors emerged in algae (Chlamydomonas, Volvox carteri) with a single member that is more similar to the ABI3 group [119] (Figure 2, Supplemental Figure S1). Compared to the other B3 domain transcription factor families, the ABI3 family is relatively small with five members in the moss Physcomitrella and rice and two in poplar [119]. The evolutionary role of ABI3 in ABA signaling is reflected by its physical interaction with ABI5 [52]. The Physcomitrella PpABI3 is capable of interacting with wheat HvABI5, although to a lesser extent than AtABI3, suggesting conservation of this interaction [120]. PpABI3 mediates ABA-dependent dessication tolerance in Physcomitrella [121] but did not fully complement the Atabi3–6 mutant, suggesting functional diversification [120]. This is also supported by differential ABA dependencies of the transcriptional activation of reporter gene constructs by different ABI3 orthologs [51,120].
Transcription factor families involved in ABA signaling have been identified in land plants, algae and other eukaryotic kingdoms (Figure 2, Supplemental Figure S1). While CAMTAs, which can bind to ABA-responsive elements [49], also exist in animals, the ABF/AREB subfamily of bZIP transcription factors is specific for land plants (Figure 2, Supplemental Figure S1). Note that ABF/AREBs are direct targets of core ABA signaling components (Figure 1). Plant transcription factor families radiated during the evolution of higher plants [115], suggesting a co-evolution of these transcription factors with land colonization by plants.
Perspectives
The presence of ABA biosynthesis in various prokaryotes and eukaryotes as well as its role in stress-related signaling indicates that ABA is a very ancient molecule. ABA signaling in its current form today is the result of divergent evolution originating from an ancient repertoire of proteins, chemical ABA precursors and other small signaling molecules present in early unicellular eukaryotes. Evolutionary processes as part of dynamic adaptations lead to the occupation of novel ecological niches and to the development of specific signaling mechanisms.
The PYR/RCAR–PP2C–dependent ABA signal transduction network found in Arabidopsis is one example of land plant specific adaption (Figures 1 and 2). In contrast to ethylene and auxin signaling components, which partially exist in algae [122,123], sequence similarities suggest that the ABA signaling network described for Arabidopsis is conserved only in land plants (Figures 2 and 3, Supplemental Figure S1). Genome sequences and molecular data from species adapted to special environments, such as the salt cress Thellungiella halophila, and from more ancient species like liverworts (Marchantia polymorpha) are needed to identify species-specific adaptations in an evolutionary context.
Supplementary Material
Acknowledgements
We thank Shintaro Munemasa for critical discussions. We apologize to the authors whose relevant contributions and original articles could not be cited or discussed due to space limitations. This research was supported by NIH (GM060396-ES010337) and NSF (MCB0918220) and the Division of Chemical, Geo and Biosciences at the Office of Energy Biosciences of the Department of Energy (DE-FG02-03ER15449) grants (J.I.S.), by a SNF fellowship (to F.H.) and by a Feodor Lynen-fellowship from the Alexander von Humboldt-Foundation (to R.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information
Supplemental Information includes one figure and four tables and can be found with this article online at doi:10.1016/j.cub.2011.03.015.
References
- 1.Bruzzone S, Moreschi I, Usai C, Guida L, Damonte G, Salis A, Scarfi S, Millo E, De Flora A, Zocchi E. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger. Proc. Natl. Acad. Sci. USA. 2007;104:5759–5764. doi: 10.1073/pnas.0609379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagamune K, Xiong L, Chini E, Sibley LD. Plants, endo-symbionts and parasites: Abscisic acid and calcium signaling. Commun. Integr. Biol. 2008;1:62–65. doi: 10.4161/cib.1.1.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartung W. The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen. Funct. Plant Biol. 2010;37:806–812. [Google Scholar]
- 4.Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 2008;59:387–415. doi: 10.1146/annurev.arplant.59.032607.092740. [DOI] [PubMed] [Google Scholar]
- 5.Xiong L, Wang RG, Mao G, Koczan JM. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol. 2006;142:1065–1074. doi: 10.1104/pp.106.084632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hetherington AM. Guard cell signaling. Cell. 2001;107:711–714. doi: 10.1016/s0092-8674(01)00606-7. [DOI] [PubMed] [Google Scholar]
- 7.Kim TH, Bohmer M, Hu HH, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milborrow BV. The chemistry and physiology of abscisic-acid. Annu. Rev. Plant Physiol. 1974;25:259–307. [Google Scholar]
- 9.Koornneef M, Jorna ML, Derswan D, Karssen CM. The isolation of abscisic-acid (aba) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (l) heynh. Theor. Appl. Genet. 1982;61:385–393. doi: 10.1007/BF00272861. [DOI] [PubMed] [Google Scholar]
- 10.Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 11.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant Biol. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- 12.Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev. 2010;24:1695–1708. doi: 10.1101/gad.1953910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umezawa T, Nakashima K, Miyakawa T, Kuromori T, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K. Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant Cell Physiol. 2010;51:1821–1839. doi: 10.1093/pcp/pcq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang J, Hwang JU, Lee M, Kim YY, Assmann SM, Martinoia E, Lee Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA. 2010;107:2355–2360. doi: 10.1073/pnas.0909222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuromori T, Miyaji T, Yabuuchi H, Shimizu H, Sugimoto E, Kamiya A, Moriyama Y, Shinozaki K. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc. Natl. Acad. Sci. USA. 2010;107:2361–2366. doi: 10.1073/pnas.0912516107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- 17.Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schweighofer A, Hirt H, Meskiene I. Plant PP2C phosphatases: emerging functions in stress signaling. Trends Plant Sci. 2004;9:236–243. doi: 10.1016/j.tplants.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Mustilli A-C, Merlot S, Vavasseur A, Fenzi F, Giraudat J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell. 2002;14:3089–3099. doi: 10.1105/tpc.007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K. The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J. Biol. Chem. 2006;281:5310–5318. doi: 10.1074/jbc.M509820200. [DOI] [PubMed] [Google Scholar]
- 21.Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2009;106:17588–17593. doi: 10.1073/pnas.0907095106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santiago J, Rodrigues A, Saez A, Rubio S, Antoni R, Dupeux F, Park SY, Marquez JA, Cutler SR, Rodriguez PL. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. Plant J. 2009;60:575–588. doi: 10.1111/j.1365-313X.2009.03981.x. [DOI] [PubMed] [Google Scholar]
- 23.Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc. Natl. Acad. Sci. USA. 2006;103:1988–1993. doi: 10.1073/pnas.0505667103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KAS, et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. USA. 2009;106:21425–21430. doi: 10.1073/pnas.0912021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato A, Sato Y, Fukao Y, Fujiwara M, Umezawa T, Shinozaki K, Hibi T, Taniguchi M, Miyake H, Goto DB, et al. Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem. J. 2009;424:439–448. doi: 10.1042/BJ20091221. [DOI] [PubMed] [Google Scholar]
- 26.Sirichandra C, Gu D, Hu HC, Davanture M, Lee S, Djaoui M, Valot B, Zivy M, Leung J, Merlot S, et al. Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 2009;583:2982–2986. doi: 10.1016/j.febslet.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura N, Sarkeshik A, Nito K, Park SY, Wang A, Carvalho PC, Lee S, Caddell DF, Cutler SR, Chory J, et al. PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C–interacting proteins in Arabidopsis. Plant J. 2010;61:290–299. doi: 10.1111/j.1365-313X.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meinhard M, Grill E. Hydrogen peroxide is a regulator of ABI1, a protein phosphatase 2C from Arabidopsis. FEBS Lett. 2001;508:443–446. doi: 10.1016/s0014-5793(01)03106-4. [DOI] [PubMed] [Google Scholar]
- 29.Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 30.Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. NADPH oxidase AtRbohD and AtRbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell. 2006;18:2749–2766. doi: 10.1105/tpc.106.044230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leung J, Orfanidi S, Chefdor F, Meszaros T, Bolte S, Mizoguchi T, Shinozaki K, Giraudat J, Bogre L. Antagonistic interaction between MAP kinase and protein phosphatase 2C in stress recovery. Plant Sci. 2006;171:596–606. [Google Scholar]
- 33.Lu C, Han MH, Guevara-Garcia A, Fedoroff NV. Mitogen-activated protein kinase signaling in postgermination arrest of development by abscisic acid. Proc. Natl. Acad. Sci. USA. 2002;99:15812–15817. doi: 10.1073/pnas.242607499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jammes F, Song C, Shin D, Munemasa S, Takeda K, Gu D, Cho D, Lee S, Giordo R, Sritubtim S, et al. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. USA. 2009;106:20520–20525. doi: 10.1073/pnas.0907205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacob T, Ritchie S, Assmann SM, Gilroy S. Abscisic acid signal transduction in guard cells is mediated by phospholipase D activity. Proc. Natl. Acad. Sci. USA. 1999;96:12192–12197. doi: 10.1073/pnas.96.21.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwak JM, Moon JH, Murata Y, Kuchitsu K, Leonhardt N, DeLong A, Schroeder JI. Disruption of a guard cell-expressed protein phosphatase 2A regulatory subunit, RCN1, confers abscisic acid insensitivity in Arabidopsis. Plant Cell. 2002;14:2849–2861. doi: 10.1105/tpc.003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takemiya A, Shimazaki K. Phosphatidic acid inhibits blue light-induced stomatal opening via inhibition of protein phosphatase 1. Plant Physiol. 2010;153:1555–1562. doi: 10.1104/pp.110.155689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tseng TS, Briggs WR. The Arabidopsis rcn1–1 mutation impairs dephosphorylation of Phot2, resulting in enhanced blue light responses. Plant Cell. 2010;22:392–402. doi: 10.1105/tpc.109.066423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunt L, Mills LN, Pical C, Leckie CP, Aitken FL, Kopka J, Mueller-Roeber B, McAinsh MR, Hetherington AM, Gray JE. Phospholipase C is required for the control of stomatal aperture by ABA. Plant J. 2003;34:47–55. doi: 10.1046/j.1365-313x.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- 40.Harmon AC, Gribskov M, Harper JF. CDPKs - a kinase for every Ca2+ signal? Trends Plant Sci. 2000;5:154–159. doi: 10.1016/s1360-1385(00)01577-6. [DOI] [PubMed] [Google Scholar]
- 41.Kudla J, Batistič O, Hashimoto K. Calcium signals: the lead currency of plant information processing. Plant Cell. 2010;22:541–563. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song CP, Agarwal M, Ohta M, Guo Y, Halfter U, Wang P, Zhu JK. Role of an Arabidopsis AP2/EREBP-type transcriptional repressor in abscisic acid and drought stress responses. Plant Cell. 2005;17:2384–2396. doi: 10.1105/tpc.105.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohta M, Guo Y, Halfter U, Zhu JK. A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2. Proc. Natl. Acad. Sci. USA. 2003;100:11771–11776. doi: 10.1073/pnas.2034853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mori IC, Murata Y, Yang Y, Munemasa S, Wang YF, Andreoli S, Tir-iac H, Alonso JM, Harper JF, Ecker JR, et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion-and Ca2+-permeable channels and stomatal closure. PLoS Biol. 2006;4:e327. doi: 10.1371/journal.pbio.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi HI, Park HJ, Park JH, Kim S, Im MY, Seo HH, Kim YW, Hwang I, Kim SY. Arabidopsis calcium-dependent protein kinase AtCPK32 interacts with ABF4, a transcriptional regulator of abscisic acid-responsive gene expression, and modulates its activity. Plant Physiol. 2005;139:1750–1761. doi: 10.1104/pp.105.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shima-moto K, Doke N, Yoshioka H. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell. 2007;19:1065–1080. doi: 10.1105/tpc.106.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu SY, Yu XC, Wang XJ, Zhao R, Li Y, Fan RC, Shang Y, Du SY, Wang XF, Wu FQ, et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell. 2007;19:3019–3036. doi: 10.1105/tpc.107.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, Liese A, Wellmann C, Al-Rasheid KA, Grill E, et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. USA. 2010;107:8023–8028. doi: 10.1073/pnas.0912030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaplan B, Davydov O, Knight H, Galon Y, Knight MR, Fluhr R, Fromm H. Rapid transcriptome changes induced by cytosolic Ca2+ transients reveal ABRE-related sequences as Ca2+-responsive cis elements in Arabidopsis. Plant Cell. 2006;18:2733–2748. doi: 10.1105/tpc.106.042713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM. Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell. 1992;4:1251–1261. doi: 10.1105/tpc.4.10.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ezcurra I, Wycliffe P, Nehlin L, Ellerstrom M, Rask L. Transactivation of the Brassica napus napin promoter by ABI3 requires interaction of the conserved B2 and B3 domains of ABI3 with different cis-elements: B2 mediates activation through an ABRE, whereas B3 interacts with an RY/G-box. Plant J. 2000;24:57–66. doi: 10.1046/j.1365-313x.2000.00857.x. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura S, Lynch TJ, Finkelstein RR. Physical interactions between ABA response loci of Arabidopsis. Plant J. 2001;26:627–635. doi: 10.1046/j.1365-313x.2001.01069.x. [DOI] [PubMed] [Google Scholar]
- 53.Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM. The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell. 1998;10:1043–1054. doi: 10.1105/tpc.10.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rook F, Corke F, Card R, Munz G, Smith C, Bevan MW. Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J. 2001;26:421–433. doi: 10.1046/j.1365-313x.2001.2641043.x. [DOI] [PubMed] [Google Scholar]
- 55.Bossi F, Cordoba E, Dupre P, Mendoza MS, Roman CS, Leon P. The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J. 2009;59:359–374. doi: 10.1111/j.1365-313X.2009.03877.x. [DOI] [PubMed] [Google Scholar]
- 56.Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P. A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science. 1996;273:1239–1241. doi: 10.1126/science.273.5279.1239. [DOI] [PubMed] [Google Scholar]
- 57.Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science. 1998;282:287–290. doi: 10.1126/science.282.5387.287. [DOI] [PubMed] [Google Scholar]
- 58.Miura K, Lee J, Jin JB, Yoo CY, Miura T, Hasegawa PM. Sumoylation of ABI5 by the Arabidopsis SUMO E3 ligase SIZ1 negatively regulates abscisic acid signaling. Proc. Natl. Acad. Sci. USA. 2009;106:5418–5423. doi: 10.1073/pnas.0811088106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopez-Molina L, Mongrand S, Kinoshita N, Chua NH. AFP is a novel negative regulator of ABA signaling that promotes ABI5 protein degradation. Genes Dev. 2003;17:410–418. doi: 10.1101/gad.1055803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hugouvieux V, Kwak JM, Schroeder JI. An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell. 2001;106:477–487. doi: 10.1016/s0092-8674(01)00460-3. [DOI] [PubMed] [Google Scholar]
- 61.Xiong L, Gong Z, Rock CD, Subramanian S, Guo Y, Xu W, Galbraith D, Zhu JK. Modulation of abscisic acid signal transduction and biosynthesis by an Sm-like protein in Arabidopsis. Dev. Cell. 2001;1:771–781. doi: 10.1016/s1534-5807(01)00087-9. [DOI] [PubMed] [Google Scholar]
- 62.Saez A, Rodrigues A, Santiago J, Rubio S, Rodriguez PL. HAB1-SWI3B interaction reveals a link between abscisic acid signaling and putative SWI/SNF chromatin-remodeling complexes in Arabidopsis. Plant Cell. 2008;20:2972–2988. doi: 10.1105/tpc.107.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pittermann J. The evolution of water transport in plants: an integrated approach. Geobiology. 2010;8:112–139. doi: 10.1111/j.1472-4669.2010.00232.x. [DOI] [PubMed] [Google Scholar]
- 64.Takaichi S, Mirauro M. Distribution and geometric isomerism of neoxanthin in oxygenic phototrophs: 9′-cis, a sole molecular form. Plant Cell Physiol. 1998;39:968–977. [Google Scholar]
- 65.Siewers V, Kokkelink L, Smedsgaard J, Tudzynski P. Identification of an abscisic acid gene cluster in the grey mold Botrytis cinerea. Appl. Environ. Microbiol. 2006;72:4619–4626. doi: 10.1128/AEM.02919-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vandermoten S, Haubruge É, Cusson M. New insights into short-chain prenyltransferases: structural features, evolutionary history and potential for selective inhibition. Cell. Mol. Life Sci. 2009;66:3685–3695. doi: 10.1007/s00018-009-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mizutani M, Ohta D. Diversification of P450 genes during land plant evolution. Annu. Rev. Plant Biol. 2010;61:291–315. doi: 10.1146/annurev-arplant-042809-112305. [DOI] [PubMed] [Google Scholar]
- 68.Radauer C, Lackner P, Breiteneder H. The Bet v 1 fold: an ancient, versatile scaffold for binding of large, hydrophobic ligands. BMC Evol. Biol. 2008;8:286. doi: 10.1186/1471-2148-8-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Markovic-Housley Z, Degano M, Lamba D, von Roepenack-Lahaye E, Clemens S, Susani M, Ferreira Ft, Scheiner O, Breiteneder H. Crystal structure of a hypoallergenic isoform of the major birch pollen allergen Bet v 1 and its likely biological function as a plant steroid carrier. J. Mol. Biol. 2003;325:123–133. doi: 10.1016/s0022-2836(02)01197-x. [DOI] [PubMed] [Google Scholar]
- 70.Fujimoto Y, Nagata R, Fukasawa H, Yano K, Azuma M, Iida A, Sugimoto S, Shudo K, Hashimoto Y. Purification and cDNA cloning of cytokinin-specific binding protein from mung bean (Vigna radiata) Eur. J. Biochem. 1998;258:794–802. doi: 10.1046/j.1432-1327.1998.2580794.x. [DOI] [PubMed] [Google Scholar]
- 71.Melcher K, Ng LM, Zhou XE, Soon FF, Xu Y, Suino-Powell KM, Park SY, Weiner JJ, Fujii H, Chinnusamy V, et al. A gate-latch-lock mechanism for hormone signalling by abscisic acid receptors. Nature. 2009;462:602–608. doi: 10.1038/nature08613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miyazono K, Miyakawa T, Sawano Y, Kubota K, Kang HJ, Asano A, Miyauchi Y, Takahashi M, Zhi Y, Fujita Y, et al. Structural basis of abscisic acid signalling. Nature. 2009;462:609–614. doi: 10.1038/nature08583. [DOI] [PubMed] [Google Scholar]
- 73.Nishimura N, Hitomi K, Arvai AS, Rambo RP, Hitomi C, Cutler SR, Schroeder JI, Getzoff ED. Structural mechanism of abscisic acid binding and signaling by dimeric PYR1. Science. 2009;326:1373–1379. doi: 10.1126/science.1181829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santiago J, Dupeux F, Round A, Antoni R, Park SY, Jamin M, Cutler SR, Rodriguez PL, Marquez JA. The abscisic acid receptor PYR1 in complex with abscisic acid. Nature. 2009;462:665–668. doi: 10.1038/nature08591. [DOI] [PubMed] [Google Scholar]
- 75.Yin P, Fan H, Hao Q, Yuan X, Wu D, Pang Y, Yan C, Li W, Wang J, Yan N. Structural insights into the mechanism of abscisic acid signaling by PYL proteins. Nat. Struct. Mol. Biol. 2009;16:1230–1236. doi: 10.1038/nsmb.1730. [DOI] [PubMed] [Google Scholar]
- 76.Tougane K, Komatsu K, Bhyan SB, Sakata Y, Ishizaki K, Yamato KT, Kohchi T, Takezawa D. Evolutionarily conserved regulatory mechanisms of abscisic acid signaling in land plants: characterization of ABSCISIC ACID INSENSITIVE1-like type 2C protein phosphatase in the liverwort Marchantia polymorpha. Plant Physiol. 2010;152:1529–1543. doi: 10.1104/pp.110.153387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Komatsu K, Nishikawa Y, Ohtsuka T, Taji T, Quatrano RS, Tanaka S, Sakata Y. Functional analyses of the ABI1-related protein phosphatase type 2C reveal evolutionarily conserved regulation of abscisic acid signaling between Arabidopsis and the moss Physcomitrella patens. Plant Mol. Biol. 2009;70:327–340. doi: 10.1007/s11103-009-9476-z. [DOI] [PubMed] [Google Scholar]
- 78.Colcombet J, Hirt H. Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem. J. 2008;413:217–226. doi: 10.1042/BJ20080625. [DOI] [PubMed] [Google Scholar]
- 79.Kobayashi Y, Yamamoto S, Minami H, Kagaya Y, Hattori T. Differential activation of the rice sucrose nonfermenting 1-related protein kinase 2 family by hyperosmotic stress and abscisic acid. Plant Cell. 2004;16:1163–1177. doi: 10.1105/tpc.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boudsocq M, Barbier-Brygoo H, Lauriere C. Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyper-osmotic and saline stresses in Arabidopsis thaliana. J. Biol. Chem. 2004;279:41758–41766. doi: 10.1074/jbc.M405259200. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K. ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol. 2002;43:1473–1483. doi: 10.1093/pcp/pcf188. [DOI] [PubMed] [Google Scholar]
- 82.Li J, Wang XQ, Watson MB, Assmann SM. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science. 2000;287:300–303. doi: 10.1126/science.287.5451.300. [DOI] [PubMed] [Google Scholar]
- 83.Shen Q, Gomez-Cadenas A, Zhang P, Walker-Simmons MK, Sheen J, Ho TH. Dissection of abscisic acid signal transduction pathways in barley aleurone layers. Plant Mol. Biol. 2001;47:437–448. doi: 10.1023/a:1011667312754. [DOI] [PubMed] [Google Scholar]
- 84.Huai J, Wang M, He J, Zheng J, Dong Z, Lv H, Zhao J, Wang G. Cloning and characterization of the SnRK2 gene family from Zea mays. Plant Cell Rep. 2008;27:1861–1868. doi: 10.1007/s00299-008-0608-8. [DOI] [PubMed] [Google Scholar]
- 85.Vlad F, Turk BE, Peynot P, Leung J, Merlot S. A versatile strategy to define the phosphorylation preferences of plant protein kinases and screen for putative substrates. Plant J. 2008;55:104–117. doi: 10.1111/j.1365-313X.2008.03488.x. [DOI] [PubMed] [Google Scholar]
- 86.Pandey S, Nelson DC, Assmann SM. Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell. 2009;136:136–148. doi: 10.1016/j.cell.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 87.Sturla L, Fresia C, Guida L, Bruzzone S, Scarfi S, Usai C, Fruscione F, Magnone M, Millo E, Basile G, et al. LANCL2 is necessary for abscisic acid binding and signaling in human granulocytes and in rat insulinoma cells. J. Biol. Chem. 2009;284:28045–28057. doi: 10.1074/jbc.M109.035329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Assmann SM. Plant G proteins, phytohormones, and plasticity: three questions and a speculation. Sci. STKE. 2004:re20. doi: 10.1126/stke.2642004re20. [DOI] [PubMed] [Google Scholar]
- 89.Schroeder JI, Raschke K, Neher E. Voltage dependence of K+ channels in guard-cell protoplasts. Proc. Natl. Acad. Sci. USA. 1987;84:4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ward JM, Maser P, Schroeder JI. Plant ion channels: gene families, physiology, and functional genomics analyses. Annu. Rev. Physiol. 2009;71:59–82. doi: 10.1146/annurev.physiol.010908.163204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schroeder JI, Hagiwara S. Cytosolic calcium regulates ion channels in the plasma-membrane of Vicia faba guard cells. Nature. 1989;338:427–430. [Google Scholar]
- 92.Cherel I, Michard E, Platet N, Mouline K, Alcon C, Sentenac H, Thibaud JB. Physical and functional interaction of the Arabidopsis K+ channel AKT2 and phosphatase AtPP2CA. Plant Cell. 2002;14:1133–1146. doi: 10.1105/tpc.000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell. 2006;125:1347–1360. doi: 10.1016/j.cell.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 94.Pilot G, Pratelli R, Gaymard F, Meyer Y, Sentenac H. Five-group distribution of the Shaker-like K+ channel family in higher plants. J. Mol. Evol. 2003;56:418–434. doi: 10.1007/s00239-002-2413-2. [DOI] [PubMed] [Google Scholar]
- 95.Keller BU, Hedrich R, Raschke K. Voltage-dependent anion channels in the plasma-membrane of guard-cells. Nature. 1989;341:450–453. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Ya-mada M, Uchimiya H, Hashimoto M, Iba K. CO2 regulator SLAC1 and its homologs are essential for anion homeostasis in plant cells. Nature. 2008;452:483–486. doi: 10.1038/nature06720. [DOI] [PubMed] [Google Scholar]
- 97.Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmaki A, Brosche M, Moldau H, Desikan R, et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452:487–491. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee SC, Lan W, Buchanan BB, Luan S. A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc. Natl. Acad. Sci. USA. 2009;106:21419–21424. doi: 10.1073/pnas.0910601106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vahisalu T, Puzorjova I, Brosche M, Valk E, Lepiku M, Moldau H, Pechter P, Wang YS, Lindgren O, Salojarvi J, et al. Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J. 2010;62:442–453. doi: 10.1111/j.1365-313X.2010.04159.x. [DOI] [PubMed] [Google Scholar]
- 100.Meyer S, Mumm P, Imes D, Endler A, Weder B, Al-Rasheid KA, Geiger D, Marten I, Martinoia E, Hedrich R. AtALMT12 representsan R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 2010;63:1054–1062. doi: 10.1111/j.1365-313X.2010.04302.x. [DOI] [PubMed] [Google Scholar]
- 101.Sasaki T, Mori IC, Furuichi T, Munemasa S, Toyooka K, Matsuoka K, Murata Y, Yamamoto Y. Closing plant stomata requires a homolog of an aluminum-activated malate transporter. Plant Cell Physiol. 2010;51:354–365. doi: 10.1093/pcp/pcq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004;37:645–653. doi: 10.1111/j.1365-313x.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- 103.Delhaize E, Gruber BD, Ryan PR. The roles of organic anion permeases in aluminium resistance and mineral nutrition. FEBS Lett. 2007;581:2255–2262. doi: 10.1016/j.febslet.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 104.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 105.Oda T, Hashimoto H, Kuwabara N, Akashi S, Hayashi K, Kojima C, Wong HL, Kawasaki T, Shimamoto K, Sato M, et al. Structure of the N-terminal regulatory domain of a plant NADPH oxidase and its functional implications. J. Biol. Chem. 2010;285:1435–1445. doi: 10.1074/jbc.M109.058909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319:1241–1244. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- 107.Verrier PJ, Bird D, Burla B, Dassa E, Forestier C, Geisler M, Klein M, Kolukisaoglu Ü, Lee Y, Martinoia E, et al. Plant ABC proteins -a unified nomenclature and updated inventory. Trends Plant Sci. 2008;13:151–159. doi: 10.1016/j.tplants.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 108.Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular ressponses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- 109.Jakoby M, Weisshaar B, Droge-Laser W, Vicente-Carbajosa J, Tiedemann J, Kroj T, Parcy F. bZIP transcription factors in Arabidopsis. Trends Plant Sci. 2002;7:106–111. doi: 10.1016/s1360-1385(01)02223-3. [DOI] [PubMed] [Google Scholar]
- 110.Finkelstein RR, Lynch TJ. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell. 2000;12:599–610. doi: 10.1105/tpc.12.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deppmann CD, Alvania RS, Taparowsky EJ. Cross-species annotation of basic leucine zipper factor interactions: Insight into the evolution of closed interaction networks. Mol. Biol. Evol. 2006;23:1480–1492. doi: 10.1093/molbev/msl022. [DOI] [PubMed] [Google Scholar]
- 112.Johnson RR, Wagner RL, Verhey SD, Walker-Simmons MK. The abscisic acid-responsive kinase PKABA1 interacts with a seed-specific abscisic acid response element-binding factor, TaABF, and phosphorylates TaABF peptide sequences. Plant Physiol. 2002;130:837–846. doi: 10.1104/pp.001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Casaretto J, Ho TH. The transcription factors HvABI5 and HvVP1 are required for the abscisic acid induction of gene expression in barley aleurone cells. Plant Cell. 2003;15:271–284. doi: 10.1105/tpc.007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kobayashi Y, Murata M, Minami H, Yamamoto S, Kagaya Y, Hobo T, Yamamoto A, Hattori T. Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J. 2005;44:939–949. doi: 10.1111/j.1365-313X.2005.02583.x. [DOI] [PubMed] [Google Scholar]
- 115.Dietz KJ, Vogel MO, Viehhauser A. AP2/EREBP transcription factors are part of gene regulatory networks and integrate metabolic, hormonal and environmental signals in stress acclimation and retrograde signalling. Protoplasma. 2010;245:3–14. doi: 10.1007/s00709-010-0142-8. [DOI] [PubMed] [Google Scholar]
- 116.Niu X, Helentjaris T, Bate NJ. Maize ABI4 binds coupling element1 in abscisic acid and sugar response genes. Plant Cell. 2002;14:2565–2575. doi: 10.1105/tpc.003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matsukura S, Mizoi J, Yoshida T, Todaka D, Ito Y, Maruyama K, Shinozaki K, Yamaguchi-Shinozaki K. Comprehensive analysis of rice DREB2-type genes that encode transcription factors involved in the expression of abiotic stress-responsive genes. Mol. Genet. Genomics. 2010;283:185–196. doi: 10.1007/s00438-009-0506-y. [DOI] [PubMed] [Google Scholar]
- 118.Suzuki M, McCarty DR. Functional symmetry of the B3 network controlling seed development. Curr. Opin. Plant Biol. 2008;11:548–553. doi: 10.1016/j.pbi.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 119.Romanel EA, Schrago CG, Counago RM, Russo CA, Alves-Ferreira M. Evolution of the B3 DNA binding superfamily: new insights into REM family gene diversification. PLoS One. 2009;4:e5791. doi: 10.1371/journal.pone.0005791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marella HH, Sakata Y, Quatrano RS. Characterization and functional analysis of ABSCISIC ACID INSENSITIVE3-like genes from Physcomitrella patens. Plant J. 2006;46:1032–1044. doi: 10.1111/j.1365-313X.2006.02764.x. [DOI] [PubMed] [Google Scholar]
- 121.Khandelwal A, Cho SH, Marella H, Sakata Y, Perroud PF, Pan A, Quatrano RS. Role of ABA and ABI3 in desiccation tolerance. Science. 2010;327:546. doi: 10.1126/science.1183672. [DOI] [PubMed] [Google Scholar]
- 122.De Smet I, Zhang H, Inze D, Beeckman T. A novel role for abscisic acid emerges from underground. Trends Plant Sci. 2006;11:434–439. doi: 10.1016/j.tplants.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 123.Timme RE, Delwiche CF. Uncovering the evolutionary origin of plant molecular processes: comparison of Coleochaete (Coleochaetales) and Spirogyra (Zygnematales) transcriptomes. BMC Plant Biol. 2010;10:96. doi: 10.1186/1471-2229-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.