Abstract
While it is well established that CD8+ T cells generated in the absence of CD4+ T cells mediate defective recall responses, the mechanism by which CD4+ T cells confer help in the generation of CD8+ T-cell responses remains poorly understood. To determine whether CD4+ T-cell-derived IL-21 is an important regulator of CD8+ T-cell responses in help-dependent and -independent viral infections, we examined these responses in the IL-21Rα−/− mouse model. We show that IL-21 has a role in primary CD8+ T-cell responses and in recall CD8+ T-cell responses in help-dependent viral infections. This effect is due to a direct action of IL-21 in enhancing the proliferation of virus-specific CD8+ T cells and reducing their TRAIL expression. These findings indicate that IL-21 is an important mediator of CD4+ T-cell help to CD8+ T cells.
Keywords: Anti-viral immunity, CD8+ T cells, IL-21, Memory cells, T-cell help
Introduction
CD8+ T cells that develop in the absence of CD4+ T-cell help can be compromised in their ability to generate primary immune responses and are unable to generate long-lived memory cells [1–3]. Multiple pathways have been implicated in the defective secondary expansion of unhelped CD8+ T cells, including interactions between CD4+ T cells, dendritic cells and CD8+ T cells, and CD4+ T-cell production of cytokines [2, 4]. However, the precise mechanism employed by CD4+ T cells to provide help in the generation of CD8+ T-cell memory remains unknown.
The relative importance of CD4+ T-cell help differs in the setting of different types of antigen exposure. CD4+ T cells play a particularly important role in promoting primary CD8+ T-cell responses to non-inflammatory antigens, while primary CD8+ T-cell responses to other antigens, such as those encoded by lymphocytic choriomeningitis virus (LCMV), vaccinia virus, or Listeria monocytogenes, are relatively help-independent [3, 5, 6]. CD8+ T-cell recall responses to all antigens are dependent on CD4+ T-cell help, as are CD8+ T-cell responses during chronic infection [3, 6, 7].
How cytokines contribute to T-cell memory development has been delineated by examining primary and secondary immune responses in common gamma chain (γC) family receptor-deficient mice [4, 8, 9]. These data highlight the importance of γC cytokines in the differentiation of robust CD8+ T-cell memory responses. The most recently identified member of the γC family of molecules is IL-21, a cytokine that is closely related to IL-2 and IL-15 [10]. IL-21 is produced by NKT cells and activated CD4+ T cells [11]. Activated IL-21-producing CD4+ T cells regulate the responses generated by other immune cells [12–16]. Emerging data suggest that IL-21 may also have effects on the generation of CD8+ T-cell effector function [11].
Three studies have evaluated the role of IL-21 in systemic LCMV infections. These studies have shown that IL-21-deficient and IL-21Rα-deficient mice were more susceptible than WT mice to chronic LCMV infections and generated reduced CD8+ T-cell responses to LCMV, while these mice were able to generate normal primary responses during an acute LCMV infection [17–19]. Secondary CD8+ T-cell responses were also unaltered to the strain of LCMV that initiates an acute infection [19]. These authors conclude that IL-21 plays a role in chronic infections and CD8+ T-cell exhaustion. However, it is also possible that the differences between CD8+ T-cell responses to acute and chronic strains of LCMV reflect the relative help dependence of these infections.
In the current study, we examined primary and secondary CD8+ T-cell responses in the IL-21Rα−/− mouse model to replication-incompetent recombinant adenovirus (rAd), a help-dependent virus, and replication-competent recombinant vaccinia virus (rVac), a relatively help-independent virus. We show that IL-21 has a critical and unique role in the development of both primary and secondary CD8+ T-cell responses in response to these viruses. This effect is due to the direct action of IL-21 on the proliferation and survival of antigen-specific CD8+ T cells mediated by the downregulation of TRAIL.
Results
Kinetics of primary responses to virally encoded antigens in IL-21Rα−/− mice
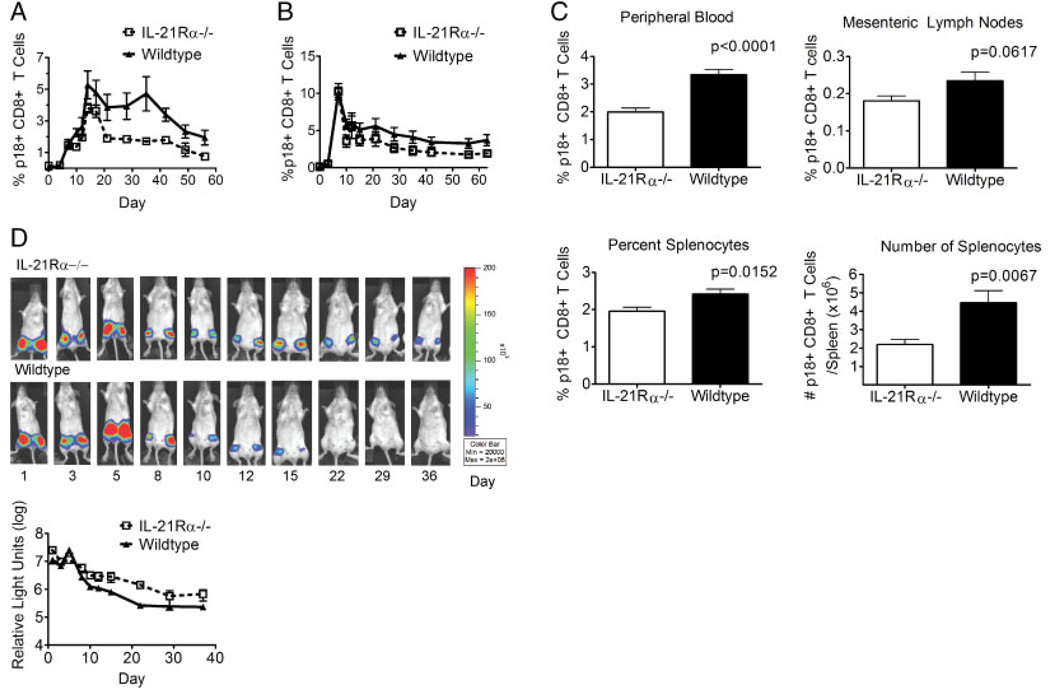
We first sought to examine the influence of IL-21 signaling on the kinetics of an antigen-specific CD8+ T-cell response to a virally encoded antigen. IL-21Rα−/− and WT mice were inoculated with recombinant replication-incompetent adenovirus expressing HIV-1 env (rAd-gp140) and peripheral blood was monitored by staining with the H-2Dd/p18 tetramer. The peripheral blood CD8+ T-cell tetramer responses in the WT and knockout groups were indistinguishable through day 7 following inoculation (Fig. 1A). Beginning on day 10, however, the percent of p18-specific CD8+ T cells was reduced in the knockout mice as compared to the WT mice (Fig. 1A). However, the most profound difference between these mice was seen after the contraction of the response (days 21–56). These differences were statistically significant at days 10 and 35–56 (Fig. 1A). While the magnitude of the immune response seen in the IL-21Rα-deficient mice was different than that seen in WT mice, the kinetics of the responses appeared the same. These data indicate that IL-21Rα knockout mice are compromised in their ability to generate a primary virus-specific CD8+ T-cell response, resulting in a lower percentage of tetramer-positive CD8+ T cells at the memory phase time points.
Figure 1.
IL-21Rα−/− mice generate reduced antigen-specific primary CD8+ T-cell responses to virally encoded antigens. (A) WT and IL-21Rα−/− mice were inoculated with rAd-gp140 and p18-specific CD8+ T cells in the peripheral blood of individual mice were quantitated with an H-2Dd/p18 tetramer. Data are presented as the percentages of CD8+ T cells that bind tetramer and represent the means of six mice per group ± SE and are representative of eight experiments. (B) WT and IL-21Rα−/− mice were inoculated with rVac-gp160 and p18-specific CD8+ T cells in the peripheral blood of individual mice were quantitated with an H-2Dd/p18 tetramer. Data are presented as the percentages of CD8+ T cells that bind tetramer and represent the means of six mice per group ± SE and are representative of two experiments. (C) WT and IL-21Rα−/− mice were inoculated with rAd-gp140 and sacrificed 44 days post-inoculation. Lymphocytes were isolated from peripheral blood, mesenteric lymph nodes and spleen, and p18-specific CD8+ T cells of individual mice were quantitated with an H-2Dd/p18 tetramer. Splenocytes from individual mice were counted to allow enumeration of the number of tetramer-positive cells per mouse. Data represent the means ± SE of 11 mice per group and are representative of three experiments. (D) In vivo expression of the luciferase protein from a recombinant adenovirus (rAd). WT and IL-21Rα−/− mice were inoculated with rAd-luciferase. The levels of luciferase expression were measured over time in the inoculated mice using IVIS. Upper panel: Representative images of luciferase expression in the mice following priming inoculation. Lower panel: The mean values of the amount of luciferase expressed by groups of four mice ± SE following inoculation and are representative of two experiments. The Mann–Whitney test was used for statistical comparisons.
Intraperitoneal inoculation of a replication-competent vaccinia virus expressing the HIV-1 env (rVac-gp160) was then done to evaluate the role of IL-21 in the immune response to a helper-independent pathogen [3, 6]. In the vaccinia virus inoculated mice, the tetramer responses in the WT and knockout mice were of similar magnitude through day 7 (peak) following inoculation (Fig. 1B). Starting on day 10 and continuing throughout the rest of the study, the percent of p18-specific CD8+ T cells was statistically significantly smaller in the knockout mice than in the WT mice, although the differences between groups were not as large as those seen in the groups injected with rAd-gp140 (Fig. 1B). As in the rAd studies, the kinetics of the responses appeared the same in these two mouse strains and demonstrate a reduction in the percentage of tetramer-positive CD8+ T cells at the memory phase time points, late after primary infection. These data confirm that IL-21Rα knockout mice are compromised in their ability to generate a primary virus-specific CD8+ T-cell response, whether the virus is replication-competent and relatively CD4-help independent or replication-defective and CD4 help dependent.
One potential explanation for the reduced tetramer percentages seen in the peripheral blood of IL-21Rα−/− mice may be the trafficking of tetramer-positive cells out of the peripheral blood to other lymphoid organs or the representation of these data as percentages rather than absolute number. To assess these possibilities, IL-21Rα−/− and WT mice inoculated with rAd-gp140 were sacrificed at day 44 following inoculation and p18 tetramer cells were examined in the peripheral blood as well as the mesenteric lymph nodes and spleen. IL-21Rα−/− mice had statistically significantly lower tetramer percentages in peripheral blood and spleen, and statistically significantly fewer tetramer-positive cells in the spleen (Fig. 1C). These knockout mice also had lower tetramer percentages in mesenteric lymph node, although this difference did not reach the level of statistical significance (Fig. 1C). These data indicate that IL-21Rα−/− mice develop smaller tetramer responses in secondary lymphoid organs and that the peripheral blood findings are not due to trafficking of cells into secondary lymphoid organs; rather, they reflect a general reduction in the memory phase of the response. These data indicate that IL-21Rα−/− mice developed smaller tetramer responses overall and are not a consequence of expressing the data as percent tetramer-positive cells.
CD8+ T-cell responses generated in response to viral infections contribute to the clearance of the infecting virus. We wanted to determine whether the different magnitude antigen-specific immune responses resulted in differential antigen clearance. To address this possibility, IL-21Rα−/− and WT mice were inoculated intramuscularly with rAd expressing luciferase and then imaged using in vivo bioluminescence imaging (IVIS) to monitor the expression of luciferase over time. In this experiment, IL-21Rα−/− and WT mice showed similar levels of antigen expression through day 10 following injection (Fig. 1D). Following day 10, WT mice rapidly cleared the rAd-luciferase; IL-21Rα−/− mice had considerably less clearance of rAd-luciferase. This divergence of antigen clearance correlates temporally with the emergence of the antigen-specific CD8+ T-cell response. These data suggest that the diminished tetramer responses in IL-21Rα−/− mice have functional consequences.
Profile of CD8+ T-cell subpopulations in the primary immune response to virally encoded antigens
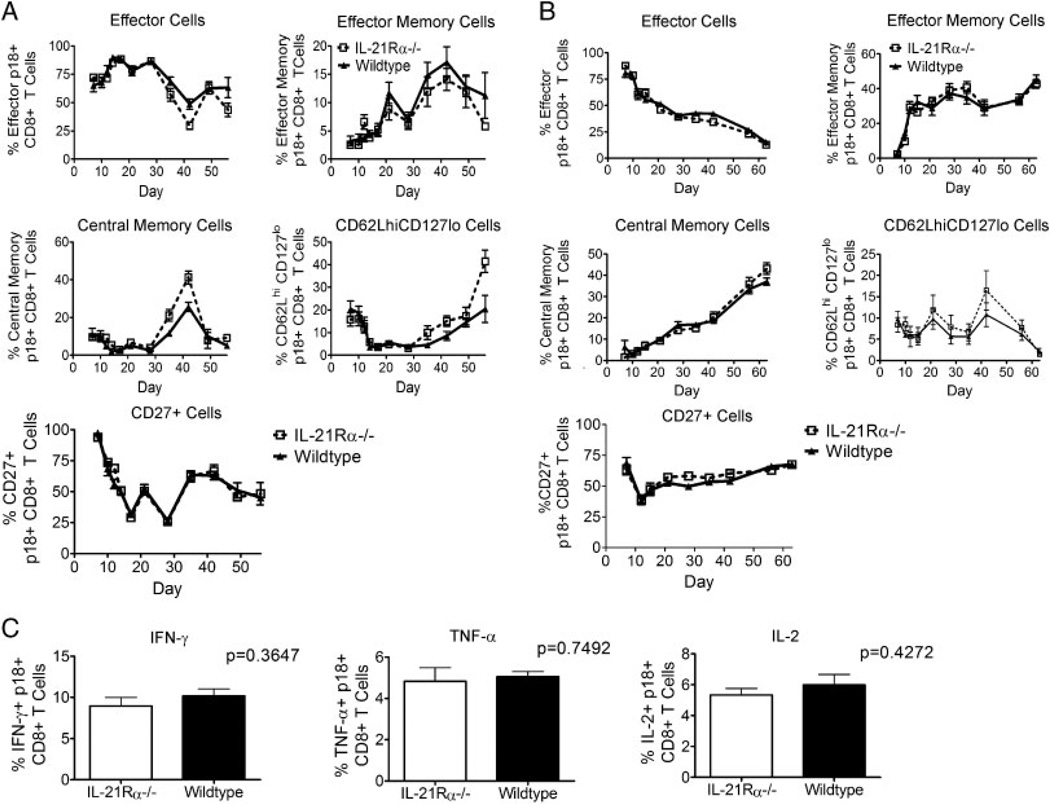
It was next important to determine whether a deficiency in IL-21Rα signaling was responsible for the functionally different tetramer-positive CD8+ T cells in these mouse strains. We therefore first examined the quality of the CD8+ T-cell immune responses in the IL-21Rα−/− mice by staining tetramer-positive cells from knockout and WT mice with antibodies against CD62L and CD127 to determine whether IL-21 preferentially induces antigen-specific CD8+ T-cell subpopulations. No differences were seen in central memory (CD62LhiCD127hi), effector memory (CD62LloCD127hi), or effector (CD62LloCD127lo) CD8+ T-cell populations in these mice (Fig. 2A). At day 35 and thereafter, the CD62LhiCD127lo tetramer-positive CD8+ T-cell population was greater in the knockout than in the WT mice, largely due to the differences in CD62L staining (Fig. 2A). Tetramer-positive cells were also stained with antibodies against the T-cell co-stimulatory molecule CD27 to analyze memory populations. No differences in the percent CD27-positive tetramer-positive CD8+ T cells were seen when comparing the two groups of mice. Similar data with CD127 and CD62L-defined memory subsets and CD27 expression were generated in mice inoculated with rVac-gp160 (Fig. 2B). These data suggest that IL-21Rα−/− mice may generate a memory response similar to that generated by WT mice and that there was not a preferential reduction in any specific memory CD8+ T-cell subset in these mice.
Figure 2.
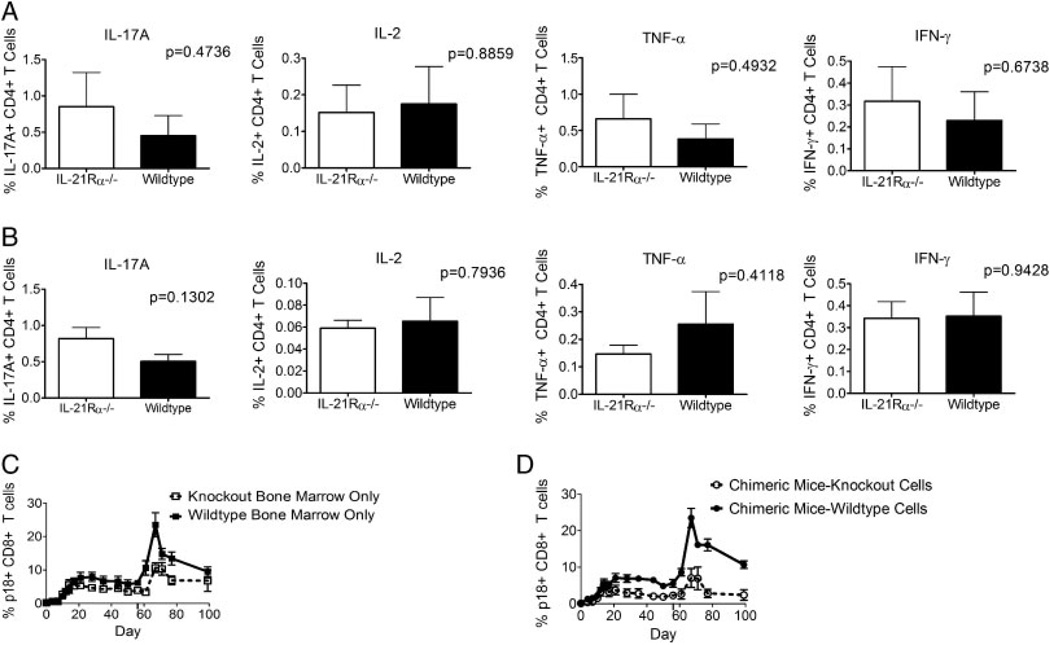
IL-21Rα−/− mice make similar memory phenotype responses and produce similar cytokines as wildtype mice during primary responses. (A) WT and IL-21Rα−/− mice were inoculated with rAd-gp140 and p18-specific CD8+ T cells in the peripheral blood of individual mice were divided into effector (CD62Llo CD127lo), effector memory (CD62Llo CD127hi), central memory (CD62LhiCD127hi), and CD62LhiCD127locell subsets. Percentage of CD27+ p18-specific CD8+ T cells in peripheral blood of individual mice was also measured. Data represent the means of six mice per group ± SE and are representative of eight experiments. (B) WT and IL-21Rα−/− mice were inoculated with rVac-gp160 and p18-specific CD8+ T cells in the peripheral blood of individual mice were divided into effector, effector memory, central memory, and CD62LhiCD127locell subsets. Percentage of CD27+ p18-specific CD8+ T cells in peripheral blood of individual mice was also measured. Data represent the means of six mice per group ± SE and are representative of two experiments. (C) WT and IL-21Rα−/− mice were inoculated with rAd-gp140 and sacrificed 44 days post-inoculation. Splenocytes were subjected to intracellular cytokine staining. Data are presented as the percentages of tetramer-positive CD8+ T cells staining positively for IFN-γ, TNF-α, or IL-2 and represent the means ± SE of 11 mice per group and are representative of three experiments. The Mann–Whitney test was used for statistical comparisons.
The quality of anti-viral CD8+ T cells from IL-21Rα−/− and WT mice was also assessed by looking at the ability of these cells to produce cytokines. IL-21Rα−/− and WT mice were inoculated with rAd-gp140 and sacrificed at day 44 following inoculation, and splenocytes from these animals were then assessed for IFN-γ, TNF-α, and IL-2 intracellular expression in response to p18 peptide stimulation. No differences were seen in the ability of the tetramer-positive cells to produce these cytokines (Fig. 2C). These data indicate that the IL-21Rα−/− and WT mice generate very similar CD8+ T-cell effector functions. These data, like the cell-surface phenotype data, suggest that IL-21Rα−/− mice generate CD8+ cell responses that are qualitatively very similar to those of WT mice.
Kinetics of secondary CD8+ T-cell responses to virally encoded antigens in IL-21Rα−/− mice
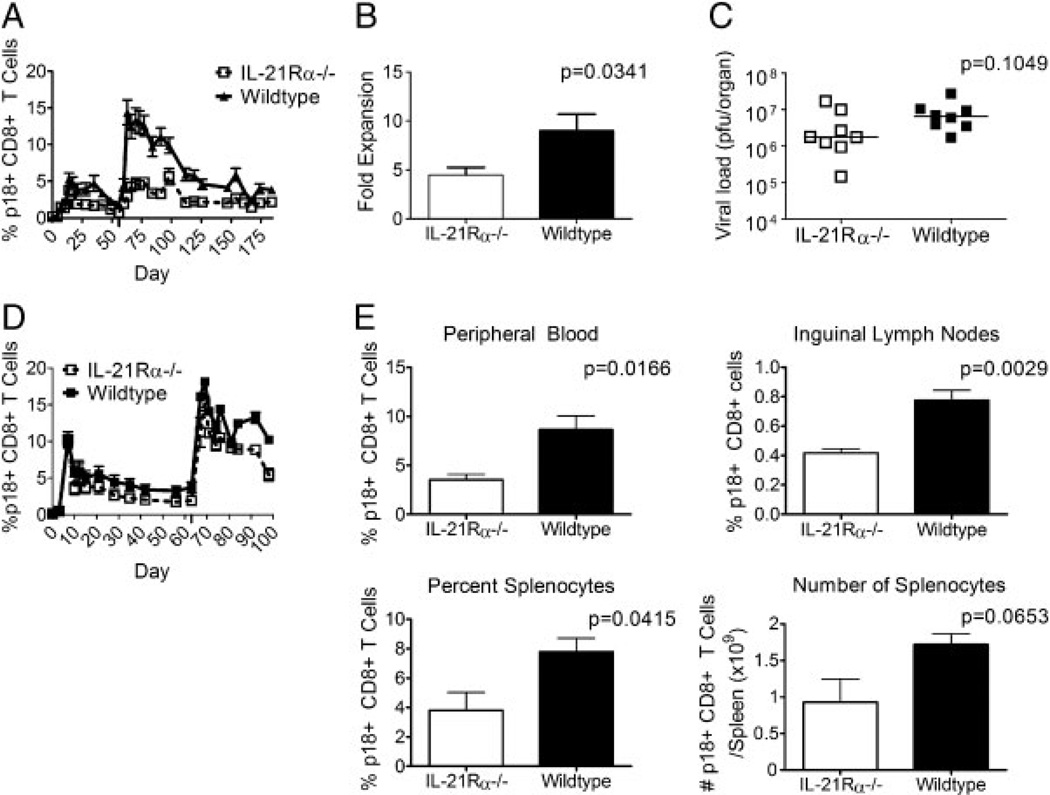
Given that the cell-surface phenotyping and intracellular cytokine staining data suggested that IL-21Rα−/− mice generated memory responses that are similar but smaller than those generated by WT mice, we were interested in determining the ability of these mice to generate secondary CD8+ T-cell responses to viral antigens. rAd-gp140-inoculated IL-21Rα−/− and WT mice were re-inoculated with rAd-gp140 on day 56 following primary inoculation, and tetramer responses in the peripheral blood were monitored. The WT mice generated normal secondary responses to the rAd-gp140 infection, while the IL-21Rα−/− mice generated greatly diminished responses, statistically significantly lower than the WT mice at all time points tested (Fig. 3A). In fact, the IL-21Rα−/− mice injected with rAd-gp140 generated secondary tetramer-positive CD8+ T-cell responses identical in magnitude to their primary responses (Fig. 3A). To control for the per-cell expansion capacity of the tetramer-positive cells, the fold expansion of peak secondary response/response at the memory time point at day of boost was calculated. While the WT tetramer-positive cells expanded nine-fold, knockout tetramer-positive cells expanded only 4.4-fold over their responses at the day of the second rAd-gp140 inoculation (p = 0.0341, Fig. 3B). These data indicate that the reduced secondary responses in IL-21R-deficient mice were not solely due to differences in the numbers of tetramer-positive cells present at the time of the second rAd-gp140 inoculation, but actually reflect a difference in the ability of these cells to undergo secondary expansion. These data indicate that although IL-21Rα−/− knockout mice make memory responses similar to those of WT mice as determined by cell-surface phenotype and cytokine production, and these mice generate greatly compromised secondary virus-specific CD8+ T-cell responses.
Figure 3.
IL-21Rα−/− mice generate reduced antigen-specific secondary CD8+ T-cell responses to virally encoded antigens. (A) WT and IL-21Rα−/− mice were inoculated with rAd-gp140 and then re-inoculated with rAd-gp140 eight weeks later and p18-specific CD8+ T cells were quantitated with an H-2Dd/p18 tetramer. Data are presented as the percentages of CD8+ T cells that bind tetramer and represent the means of six mice per group ± SE and are representative of four experiments. (B) The fold increase of cells undergoing secondary expansion after secondary rAd-gp140 inoculation was calculated by dividing the tetramer percentage for each mouse at the peak time point by the tetramer percentage for the same mouse at the day of boost. Data represent the means ± SE of six mice per group and are representative of four experiments. (C) CD8+ T cells from rAd-gp140 primed WT or IL-21Rα−/− mice were transferred into Nude/SCID mice and then reconstituted mice were challenged intranasally with rVac-gp160. Plaque forming units in the ovaries of these mice were quantitated 6 days after challenge. (D) WT and IL-21Rα−/− mice were inoculated with rVac-gp160 and then re-inoculated with rVac-gp160 nine weeks later. p18-specific CD8+ T cells in the peripheral blood of individual mice were quantitated with an H-2Dd/p18 tetramer. Data are presented as the percentages of CD8+ T cells that bind tetramer and represent the means of six mice per group ± SE and are representative of two experiments. (E) WT and IL-21Rα−/− mice were inoculated with rAd-gp140 and then re-inoculated with rAd-gp140 eight weeks later and then sacrificed 21 days post-secondary inoculation. Lymphocytes were isolated from peripheral blood, inguinal lymph nodes, and spleen, and p18-specific CD8+ T cells of individual mice were quantitated with an H-2Dd/p18 tetramer. Splenocytes from individual mice were counted to allow enumeration of the number of tetramer-positive cells per mouse. Data represent the mean ± SE of four mice per group and are representative of three experiments. The Mann–Whitney test was used for statistical comparisons.
In addition, we examined the protective capacity of the virus-specific CD8+ T cells. IL-21Rα−/− and WT mice were inoculated with rAd-gp140. Three months following inoculation, mice were sacrificed and 1 × 106 CD8+ T cells were transferred to Nude/SCID mice. Recipient mice were then challenged intranasally with 10LD50 rVac-gp160 and 6 days later vaccinia plaques in the ovaries of these mice were enumerated. There was no statistically significant difference in the plaque forming units found in ovaries of the Nude/SCID mice reconstituted with either IL-21Rα−/− or WT cells, suggesting that these two cell populations were comparably protective on a per-cell basis. The difference in these cell populations was therefore primarily determined by their ability to expand (Fig. 3C).
To confirm that IL-21Rα knockout mice make reduced secondary responses to recombinant virally encoded antigens, we measured tetramer responses to a prime and homologous challenge using rVac-gp160 as the infecting virus. In this experiment, rVac-gp160 inoculated IL-21Rα−/− and WT mice were re-inoculated with rVac-gp160 at day 63 following the first rVac-gp160 inoculation, and tetramer responses in the peripheral blood were monitored. As with the rAd-gp140 inoculated mice, the WT mice generated normal secondary responses following the second rVac-gp160 inoculation, while the IL-21Rα−/− mice generated greatly diminished responses that were statistically significantly lower than those generated by the WT mice at all time points tested (Fig. 3D). While the rVac-gp160 data were not as dramatic as the rAd-gp140 data, these findings confirmed that although IL-21Rα knockout mice make memory responses that are qualitatively similar to those made by WT mice as assayed by cell-surface phenotype and cytokine production, these knockout mice generate greatly compromised secondary virus-specific CD8+ T-cell responses. These data are particularly interesting in light of the difference in replicative capacity and help-dependence of these two pathogens, and suggest that IL-21 may be more important in a CD4-help-dependent response and may function as part of that CD4 help.
It was possible that the apparent reduced tetramer responses after a second recombinant virus inoculation in IL-21Rα−/− mice simply were a consequence of trafficking of tetramer positive cells out of the peripheral blood. To address this possibility, rAd-gp140 inoculated IL-21Rα−/− and WT mice were inoculated a second time with rAd-gp140 intramuscularly and then sacrificed 21 days later. Tetramer responses were determined in the peripheral blood, inguinal lymph nodes, and spleens of these mice. IL-21Rα−/− mice had statistically significantly lower tetramer percentages in peripheral blood and inguinal lymph nodes than did WT mice (Fig. 3E). The number and percentage of tetramer-positive cells in the spleen were also lower in the IL-21Rα−/− mice, although this difference did not reach statistical significance (Fig. 3E). These data demonstrate that although IL-21Rα−/− mice make memory responses similar in quality to WT mice as assayed by cell-surface phenotype and cytokine production, these mice generate greatly compromised secondary virus-specific CD8+ T-cell responses.
Kinetics and profile of CD8+ T cells that respond to virally encoded antigens in IL-15Rα−/− mice
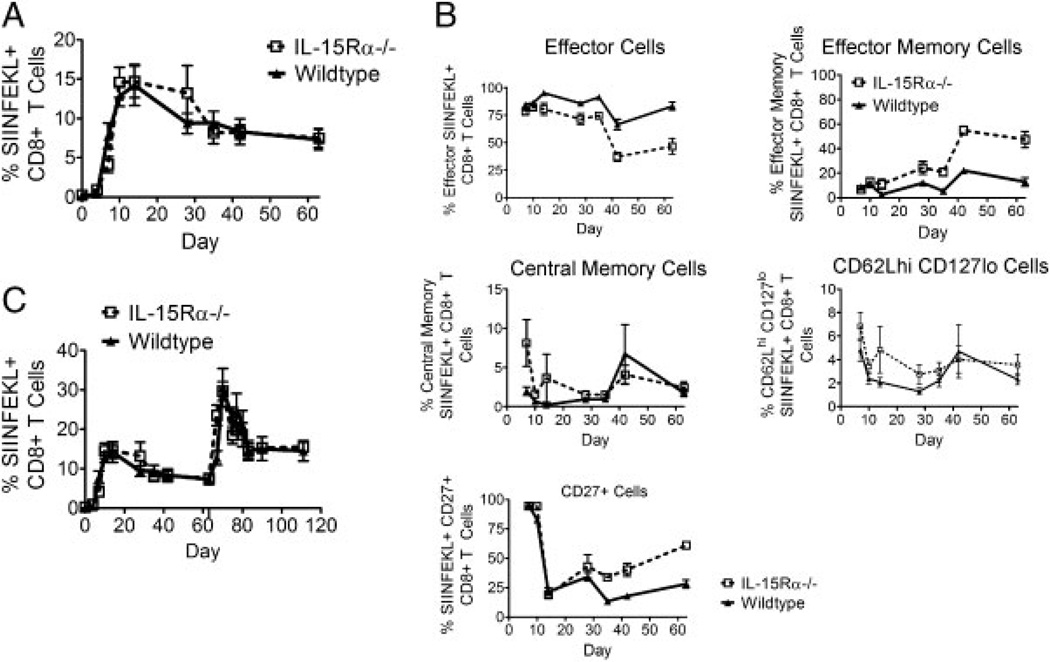
Others have suggested that IL-21 functions mainly to augment the activity of IL-15 on CD8+ T cells [10, 20–22]. We therefore next wanted to characterize the CD8+ T-cell response to virus infection in IL-15Rα−/− mice to determine how it differs from that of IL-21Rα−/− mice. IL-15Rα−/− and WT mice were inoculated with recombinant replication-incompetent adenovirus expressing oval-bumin (rAd-Ova) and peripheral blood was monitored with a SIINFEKL/H-2Kb tetramer at multiple time points after inoculation. Consistent with what was previously demonstrated in this knockout strain of mice, tetramer responses in the WT and knockout groups of mice were only slightly different until day 7 following infection and were then indistinguishable for the remainder of the primary response (Fig. 4A). This immune response to virus infection differed from that seen in IL-21Rα−/− mice.
Figure 4.
IL-15Rα−/− mice generate WT antigen-specific primary and secondary CD8+ T-cell responses to virally encoded antigens yet more knockout cells have a memory phenotype. (A) WT and IL-15Rα−/− mice were inoculated with rAd-Ova; SIINFEKL-specific CD8+ T cells in the peripheral blood of individual mice were quantitated with an H-2Kb/SIINFEKL tetramer. Data are presented as the percentages of CD8+ T cells that bind tetramer and represent the means of five mice per group ± SE and are representative of two experiments. (B) WT and IL-15Rα−/− mice were inoculated with rAd-Ova and SIINFEKL-specific CD8+ T cells in the peripheral blood of individual mice were divided into effector, effector memory, central memory, and CD62LhiCD127locell subsets. Percentage of CD27+ p18-specific CD8+ T cells in peripheral blood of individual mice was also measured. Data represent the means of five mice per group ± SE and are representative of two experiments. (C) WT and IL-15Rα−/− mice were inoculated with rAd-Ova and then re-inoculated with rAd-Ova nine weeks later and responses were quantitated with an H-2Kb/SIINFEKL tetramer. Data are presented as the percentages of CD8+ T cells that bind tetramer and represent the means of five mice per group ± SE and are representative of two experiments. The Mann–Whitney test was used for statistical comparisons.
We also characterized the evolution of CD8+ T-cell subpopulations in IL-15Rα−/− mice following rAd inoculation. We did this by staining tetramer-positive CD8+ cells from knockout and WT mice with antibodies against CD62L and CD127. Fewer IL-15Rα−/− tetramer-positive CD8+ T cells had an effector phenotype and more had an effector memory phenotype than WT tetramer-positive CD8+ T cells (Fig. 4B). This relative distribution of CD8+ T-cell subpopulations is quite different than that seen in IL-21Rα−/− mice infected with rAd-gp140 as shown in Fig. 2 and suggests that IL-15 and IL-21 drive very different CD8+ T-cell programming patterns.
We next inoculated IL-15Rα−/− and WT mice with rAd-Ova 63 days following the first inoculation of rAd-Ova and analyzed tetramer-positive CD8+ T cells to characterize the role of IL-15 in secondary expansion of CD8+ T cells. The tetramer responses in the WT and IL-15Rα knockout groups were indistinguishable at all time points tested, consistent with what was previously observed in IL-15Rα−/− mice [9] and distinct from that seen in IL-21Rα−/− mice (Fig. 4C). These data are surprising, however, in light of the cell-surface phenotype of the primary responding CD8+ T cells, which suggested that there was an increase in both effector and effector memory cells in the infected IL-15Rα−/− mice. These data therefore indicate that phenotypic differences may not always correlate with the capacity to generate a secondary response and also suggest that IL-21 and IL-15 have unique functions in the development of a CD8+ T-cell response.
Defective cellular immune responses in IL-21Rα−/− mice are due to deficiencies in CD8+ T cells
IL-21 is required for the generation of the inflammatory Th17 subset and the T follicular helper subset of CD4+ T cells [13–16]. Since CD4+ T cells play a crucial role in the generation of functional CD8+ memory T cells, it is possible that defects in help from CD4+ T cells in IL-21Rα−/− mice result in defective signals to antigen-specific CD8+ T cells as they expand in response to viral infection. To assess this potential mechanism to explain the abnormalities in CD8+ T cells in IL-21Rα−/− mice, cytokine production by CD4+ T cells was examined in these mice. IL-21Rα−/− and WT mice were inoculated intramuscularly with rAd-gp140 and then sacrificed 44 days later; intracellular production of IL-17A, IFN-γ, TNF-α, and IL-2 in response to pooled HIV Env peptides was evaluated in splenocytes of these animals. No differences were seen between splenocytes of the mouse strains in the ability of the CD4+ T cells to produce these cytokines (Fig. 5A). These data indicate that antigen-stimulated CD4+ T cells generate similar cytokines in IL-21Rα−/− and WT mice and that deficient CD4+ T-cell responses do not explain the defective secondary CD8+ T-cell responses in these mice.
Figure 5.
Defective secondary responses in IL-21Rα−/− mice are due to abnormalities in CD8+ T cells (A) WT and IL-21Rα−/− mice were inoculated with rAd-gp140 and sacrificed 44 days post-inoculation. Splenocytes were subjected to intracellular cytokine staining. Data are presented as the percentages of CD4+ T cells staining positively for IL-17A, IL-2, TNF-α, or IFN-γ and represent the means ± SE of 11 mice per group and are representative of three experiments. (B) WT and IL-21Rα−/− mice were inoculated with rAd-gp140 and then re-inoculated with rAd-gp140 eight weeks later and then sacrificed 21 days post-secondary inoculation. Splenocytes were subjected to intracellular cytokine staining. Data are presented as the percentages of CD4+ T cells staining positively for IL-17A, IL-2, TNF-α, and IFN-γ and represent the means ± SE of four mice per group and are representative of three experiments. (C) Irradiated mice were reconstituted with either WT Thy1.1+ or IL-21Rα−/− Thy 1.2+ bone marrow cells and then inoculated with rAd-gp140 six weeks later. These mice were then re-inoculated with rAd-gp140 eight weeks later and p18-specific CD8+ T cells were quantitated with an H-2Dd/p18 tetramer. Data are presented as the percentages of Thy1.1+ or Thy 1.2+ CD8+ T cells that bind tetramer and represent the means of six mice per group ± SE and are representative of two experiments. (D) Irradiated mice were reconstituted with WT Thy1.1+ and IL-21Rα−/− Thy 1.2+ bone marrow cells and then inoculated with rAd-gp140 six weeks later. These mice were then re-inoculated with rAd-gp140 eight weeks later and p18-specific CD8+ T cells were quantitated with an H-2Dd/p18 tetramer. Data are presented as the percentages of Thy1.1+ or Thy1.2+ CD8+ T cells that bind tetramer and represent the means of six mice per group ± SE and are representative of two experiments. The Mann–Whitney test was used for statistical comparisons.
Cytokine production by CD4+ T cells during a secondary immune response was also evaluated by inoculating IL-21Rα−/− and WT mice twice with rAd-gp140 intramuscularly and then sacrificing the mice on day 21 following the second inoculation. IL-17A, IFN-γ, TNF-α, and IL-2 production was assessed in splenocytes of these mice following stimulation with pooled HIV Env peptides. Similar profiles of cytokine production by CD4+ T cells were seen in both mouse strains (Fig. 5B). These findings provide further evidence that deficient CD4+ T-cell responses do not explain the defective secondary CD8+ T-cell responses in these knockout mice.
We then sought to determine whether CD8+ T cells from IL-21Rα−/− mice are deficient in their ability to generate antiviral responses because they are intrinsically defective or because they interact with other cells that do not function normally because of a lack of IL-21 signaling. We generated mixed bone marrow chimeric mice in which irradiated mice (Thy1.1+) were reconstituted with T-cell-depleted bone marrow from WT (Thy1.1+) and IL-21Rα−/− donors (Thy1.2+). These chimeric mice allow us to track the development of WT and IL-21R knockout CD8+ T-cell responses in the environment of the same other cell types.
Groups of mice reconstituted either with IL-21Rα−/− only, WT only, or a mixture of IL-21Rα−/− and WT bone marrow were inoculated with rAd-gp140 intramuscularly and peripheral blood was monitored by p18 tetramer staining. Mice were then inoculated intramuscularly a second time with rAd-gp140 56 days following the first inoculation and peripheral blood tetramer responses were monitored. The kinetics of the tetramer responses in the group of mice reconstituted with only WT bone marrow were similar to those in WT mice following the first and second inoculation of rAd-gp140 (Fig. 5C). The kinetics of the tetramer responses in the group reconstituted with only knockout bone marrow were similar to those in knockout mice following both the first and second inoculation of rAd-gp140 (Fig. 5C). In the chimeric mice, the Thy1.1+ WT cells generated primary and secondary responses that were similar to WT responses, while the Thy1.2+ knockout cells generated deficient primary and secondary responses (Fig. 5D). The Thy1.1+ cells in the chimeric mice generated responses that were indistinguishable from the Thy1.1+ cells from the WT-only reconstituted mice, while the Thy1.2+ cells in the chimeric mice generated responses that were indistinguishable from the Thy1.2+ cells from the knockout-only reconstituted mice. We conclude from these experiments that IL-21Rα−/− mice generate deficient primary and secondary CD8+ T-cell responses to virally encoded antigens due to endogenous defects in CD8+ T cells rather than defective or inadequate help from CD4+ T cells.
IL-21Rα−/− cells proliferate poorly and express more TRAIL than WT cells
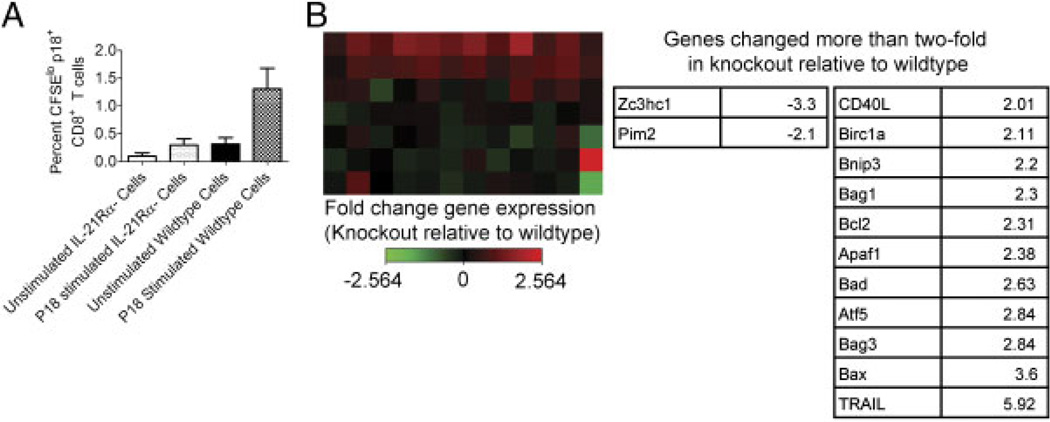
Having shown that IL-21Rα−/− mice generate deficient primary and secondary CD8+ T-cell responses to virally encoded antigens due to endogenous defects in CD8+ T-cell signaling, we were interested in determining the effects of the IL-21 signal on CD8+ T cells. Since we and others have previously shown that IL-21 can promote T-cell proliferation [10–11, 22–25], we reasoned that a defective proliferative capacity of IL-21Rα−/− tetramer-positive CD8+ T cells could explain diminished primary or secondary CD8+ T-cell responses. To examine the proliferative capacity of the IL-21Rα−/− tetramer-positive CD8+ cells, we examined the ability of these cells to divide by measuring CFSE dilution. IL-21Rα−/− and WT mice were inoculated with rAd-gp140 and sacrificed 21 days later. CD8+ T cells were isolated from spleens and labeled with CFSE. These labeled CD8+ T cells were then cultured with p18 Env peptide or left unstimulated for 7 days and the percentages of CFSElo tetramer-positive CD8+ T lymphocytes were monitored (Fig. 6A). A comparable percentage of unstimulated IL-21Rα−/− and WT tetramer-positive CD8+ T cells underwent cell division (p = 0.1082). When cells were stimulated with peptide antigen, however, significantly more WT cells underwent cell division than did IL-21Rα−/− cells (p = 0.0235). These findings indicate that IL-21Rα−/− cells do not proliferate as well as WT cells upon exposure to antigen.
Figure 6.
Mechanism underlying deficient IL-21Rα−/− CD8+ T-cell responses. WT and IL-21Rα−/− mice were inoculated with rAd-gp140 and sacrificed 21 days post-inoculation. Lymphocytes were isolated from spleens and CD8+ T cells were enriched by negative selection. (A) CD8+ T cells were labeled with CFSE and placed in culture either unstimulated or stimulated with p18 peptide for seven days. Data are presented as the percentages of CD8+ T cells that have divided and represent the means ± SE of four mice per group. This study is representative of two experiments. The Mann–Whitney test was used for statistical comparisons. (B) WT and IL-21Rα−/− CD8+ T cells were stimulated with p18 peptide for 4 h, followed by RNA extraction. RNA was then subjected to RT-PCR-based array to measure the expression of 84 apoptosis-related genes. A heat map showing the relative fold increase in gene expression in knockout cells relative to WT cells is shown, and genes that are up- or downregulated more than two-fold in knockout cells relative to WT cells is shown in the table. Data represent RNA extracted from four mice per group.
Another potential mechanism that might explain defective IL-21Rα−/− CD8+ T-cell primary and secondary responses is a defect in the survival capacity of these cells. CD4+ T-cell help allows CD8+ T cells to survive after secondary stimulation instead of undergoing activation-induced cell death [2]. We have previously demonstrated that IL-21 regulates a balance between CD8+ T-cell proliferation and survival in vitro [23]. The large differences between the plateau phase of the CD8+ T-cell responses in the vaccinated WT and IL-21Rα−/− mice suggested that there may have been differential survival of this cell population between the groups of vaccinated animals. Because of our previous findings that IL-21 may be involved in regulating CD8+ T-cell survival and the well-defined role for CD4+ T-cell help in regulating AICD, we also investigated the expression of genes related to apoptosis and survival in Env epitope-specific CD8+ T cells from WT and IL-21Rα−/− mice. IL-21Rα−/− and WT mice were inoculated with rAd-gp140 and sacrificed 21 days later. CD8+ T cells were isolated from spleens and stimulated with the HIV-1 Env epitope peptide p18. These stimulated cells were then subjected to RT-PCR array analysis, and the expression of 84 apoptosis-related genes was examined. The fold change in expression of each gene in knockout CD8+ T cells was compared to that in WT CD8+ T cells: 2 pro-survival genes were downregulated more than two-fold and 11 genes (including both pro-survival and pro-apoptosis genes) were induced more than two-fold. These genes are listed in Fig. 6B. Interestingly, the gene with the largest fold difference between the re-stimulated knockout and WT CD8+ T cells was TRAIL, which was induced 5.92-fold in knockout cells over WT cells. These data suggest that IL-21 induces CD8+ T-cell proliferation and perhaps survival via downregulation of TRAIL, leading to the generation of robust primary and secondary CD8+ T-cell responses in response to viral infection.
Discussion
In these experiments, we have shown that IL-21 has important roles in both primary and secondary CD8+ T-cell responses. IL-21Rα−/− mice developed diminished primary tetramer responses following inoculation with recombinant viruses that were most apparent after the peak of the response. Interestingly, although no differences were observed in the cell surface molecules or cytokines expressed by these cells that usually predict a functional memory response, these mice generated compromised secondary virus-specific CD8+ T-cell responses. Our experiments with mixed bone marrow chimeric mice suggest that IL-21Rα−/− mice generate deficient primary and secondary responses to virally encoded antigens due to defective signaling of the IL-21 receptor in the CD8+ T cells themselves rather than poor help from other cell types. Further, we showed that IL-21 plays this important role in CD8+ T-cell responses by enhancing CD8+ T-cell proliferation and by downregulating expression of TRAIL.
The differences between CD8+ T-cell responses in the rAd-gp140-inoculated and the rVac-gp160-inoculated mice likely reflect the relative dependency of those responses on CD4+ T cells. The CD8+ T-cell responses in IL-21Rα−/− mice were more deficient in rAd-gp140 than in rVac-gp160 inoculated mice. This persistent viral replication may stimulate other anti-viral responses that may compensate for the loss of IL-21 and help to stimulate CD8+ T-cell memory responses. In fact, the immune response to vaccinia has been characterized as “helper-independent” as this virus induces an inflammatory response, while replication-incompetent adenovirus has been characterized as “helper-dependent” [3, 6].
The findings in the current study and those in the studies by Frohlich et al. [19], Yi et al. [18], and Elsaesser et al. [17], may appear conflicting. The studies by these other groups showed that a deficiency in IL-21 signaling resulted in a modest deficiency in primary CD8+ T-cell responses generated following an acute LCMV infection, but a more dramatically diminished CD8+ T-cell response following inoculation with an LCMV virus that established a chronic infection [17–19]. Frohlich et al. [19] actually reported no defect in primary CD8+ T-cell responses generated following acute influenza or vaccinia virus infections, and no defect in secondary CD8+ T-cell responses to the acute LCMV virus. It is likely that the differences between the findings in acute and chronic LCMV infections as well as the differences between findings in our studies and these LCMV experiments reflect the relative help dependence of the responses under investigation. The primary immune response to an acute LCMV infection is relatively help independent, while CD8+ T-cell responses to chronic infections are relatively dependent on CD4+ T-cell help [3, 5–7]. The data shown in our study and the other studies are actually consistent. In all of the viral infections examined, only minor differences were seen in the CD8+ T-cell responses between WT and IL-21Rα-deficient mice at early time points after infection, while more dramatic differences were seen in these responses by day 30 [17–19].
Our data are consistent with other studies suggesting that IL-21 has a role in CD8+ T-cell responses as well. Analysis of IL-21Rα−/− mice showed that they had normal numbers of CD8+ T cells but a reduced primary response to a vaccinia-encoded antigen on day 5 following vaccinia virus inoculation [22]. In this study, the kinetics of the CD8+ T-cell response, the phenotypic profile of the responding cell population, and the ability of these mice to mount a secondary CD8+ T-cell response were not examined. Other investigators showed that transgenic mice that over-express IL-21 have decreased CD127 expression on CD8+ T cells and an accumulation of CD8+ memory T cells (CD62L+, Ly6C+, CD122+). These cells proliferate and accumulate, but do not differ in their survival from the CD8+ T cells of WT mice [26].
One surprising finding in the current study is that in both the IL-21Rα−/− and IL-15Rα−/− mice, the percentages of tetramer-positive cells expressing cell-surface molecules associated with memory responses were not predictive of the capacity of these cells to generate secondary responses. At day 35 following inoculation with rAd-gp140 and throughout the later period of infection, the IL-21Rα-deficient tetramer-positive CD8+ T cells were comprised of an increase in the subpopulation CD62LhiCD127lo cells. The significance of this subpopulation of CD8+ T cells is unclear; these cells have been described either as naïve cells or as transitional memory cells. It is possible that characteristics of these or other memory CD8+ T cells that are predictive of their ability to generate secondary responses could be uncovered by examining CD8+ T-cell expression of other molecules, such as PD-1, CD43, CD44, or KLRG-1. However, other groups have shown that associations between cell-surface phenotype, ability to produce cytokines, ability to degranulate, and memory capability are not as clear as once thought [27–29].
One potential mechanism underlying differences in immunologic memory generation is differences in antigen persistence. Some groups have demonstrated an association between reduced antigen persistence and increased memory responses or persistent antigen and decreased memory responses [28, 30–32]. IL-21Rα−/− mice clear antigen more slowly than WT mice and have an associated decrease in memory responses. This increase in antigen persistence may explain the phenotypic idiosyncrasies of the memory CD8+ T cells that we observed in the IL-21Rα−/− mice. However, arguing against this explanation, we see the same phenotypic profile of CD8+ T cells in mice inoculated with replication incompetent rAd-gp140 and mice inoculated with replication competent rVac-gp160, vectors with dramatically different kinetics of transgene expression.
The current data expand our understanding of the role of γC cytokines in CD8+ T-cell responses. Previous studies have suggested that IL-21 has little effect on CD8+ T cells alone and mainly functions as a redundant cytokine in conjunction with IL-15 or to augment the effects of IL-15 on CD8+ T cells [10, 20–22]. Our studies suggest that IL-21 and IL-15 may, in fact, have opposing roles in CD8+ T-cell development. We demonstrated that IL-21Rα-deficient mice generate defective secondary CD8+ T-cell populations that have a normal memory phenotype, while IL-15Rα-deficient mice generate normal secondary CD8+ T-cell populations that have a skewing toward an effector memory phenotype. Interestingly, the phenotype of these cells in the IL-21Rα−/− mice is similar to that seen in IL-2Rα−/− bone marrow chimeric mice [4]. These findings were reminiscent of earlier reports suggesting that IL-2 functions as the help that CD4+ T cells provide to CD8+ T cells [3, 6]. IL2 and IL21 are closely linked genes (180 kb apart in humans and 95 kb apart in mice), have similar intron and exon structures, and may have arisen though gene duplication [10, 33]. Furthermore, IL-21Rα also has high sequence homology to IL-2Rβ [10]. Therefore, it is not surprising that IL-2 and IL-21 may have similar roles in CD8+ T-cell responses, each providing a form of help to CD8+ T cells.
Consistent with previous studies, the current data indicate that IL-21 can promote CD8+ T-cell proliferation [10–11, 19, 22–25]. These data suggest a potential mechanism for the decreased primary and secondary CD8+ T-cell responses seen in IL-21Rα−/− mice. Previous studies have indicated that CD8+ T cells deprived of CD4+ T-cell help undergo poor secondary expansion and upregulate the apoptosis-inducing ligand TRAIL, which leads to their apoptosis via AICD [2]. The current data also indicate that IL-21Rα−/− CD8+ T cells express more TRAIL than do WT cells, suggesting that these cells may undergo increased AICD. These data further suggest that a lack of IL-21 signaling may be part of the deficiency seen in the CD4+ T-cell help-deficient CD8+ T cells.
In recent years, a number of factors required for the generation, homeostatic turnover, and long-term survival of memory CD8+ T cells have been identified, including cytokines such as IL-2, IL-7, and IL-15, as well as other forms of help provided by CD4+ T cells. Here, we show that the CD4+ T-cell-produced cytokine IL-21 is important for in the generation of primary and secondary CD8+ T-cell responses, augmenting CD8+ T-cell proliferation and downregulating TRAIL expression.
Materials and methods
Mice and infection
Six- to twelve-week-old BALB/c (Thy1.2+), BALB/c Thy1.1+, IL-15Rα−/−, and WT B6 littermate control mice were purchased from Jackson Laboratories. A breeding pair of IL-21Rα−/− mice were provided by M. Grusby, Harvard School of Public Health, and then bred at the Beth Israel Deaconess Medical Center (BIDMC) facility. All mice were maintained under specific-pathogen-free conditions and research on mice was approved by the BIDMC Institutional Animal Care and Use Committee. Mice in each experiment were age and sex matched. Groups of mice were immunized either intraperitoneally with 2 × 107 pfu of replication-competent NYCBH strain vaccinia virus expressing HIV-1 B10 (rVac-gp160) (provided by D. Panicali, Therion Biologics Corporation), intramuscularly with 2 × 107 particles of recombinant replication-incompetent E1-deleted adenovirus serotype 5 expressing chicken ovalbumin (rAd-Ova) (obtained from the University of Iowa Gene Transfer Vector Core), or intramuscularly with 2 × 107 particles of recombinant replication-incompetent E1-deleted E3-inactivated adenovirus serotype 5 expressing HIV-1 HxB2 env (rAd-gp140) (provided by G. Nabel, Vaccine Research Center, National Institutes of Health). The injection volume was always 100 µL; 50 µL was delivered into each quadriceps muscle for intramuscular injections. To measure luciferase expression following immunization, mice were immunized in intramuscularly with 1 × 109 particles recombinant replication-incompetent E1-deleted E3-inactivated adenovirus serotype 5 expressing firefly luciferase (rAd-Luc) (provided by D. Barouch, BIDMC).
Bioimaging of luciferase protein expression
Bioimaging of vectors expressing firefly luciferase was done at the Longwood Small Animal Imaging Facility at BIDMC using the In Vivo Imaging System 50 (IVIS-50) distributed by Xenogen. Mice were anesthetized with isofluorane and injected intraperitoneally with 100 µL of an isotonic salt solution containing 30 mg/mL d-luciferin (Xenogen). Eleven minutes after luciferin injection, photonic emissions were measured using an IVIS-50 charge-coupled-device camera using a 5-min exposure. Luciferase quantification was done using Living Image software to identify and measure regions of interest.
Phenotypic T lymphocyte analyses
Flow cytometric staining of peripheral blood cells, splenocytes, or other lymphocyte populations was performed and gated as previously described [30]. Tetrameric H-2Dd complexes folded with the gp120 p18 epitope peptide (RGPGRAFVTI) were prepared as previously described [34] and conjugated to PE or APC. H-2Kb/SIINFEKL tetramer, a reagent specific for the ovalbumin epitope peptide SIINFEKL presented by H-2Kb was purchased from Beckman Coulter.
Intracellular cytokine staining
Splenocytes were obtained from individual mice, and peripheral blood mononuclear cells were isolated using Lympholyte-M. Intracellular cytokine staining was performed as previously described [30].
Adoptive transfer and intranasal vaccinia challenge
Three months after rAd-gp140 immunization, mice were sacrificed, and CD8+ T cells were purified from spleens. 1 × 106 purified CD8+ T cells from WT or IL-21Rα−/− mice were adoptively transferred into Nude/SCID mice. The tetramer percentage for splenocytes from each genotype was equivalent at 0.1% of total CD8+ cells. Recipient mice were then challenged intranasally with 10 LD50 of rVac-gp160 on the same day as adoptive transfer. Mice were sacrificed 6 days post-challenge and pfu in ovaries were enumerated.
Generation of mixed bone marrow chimeras
Bone marrow preparations from femur and tibia were filtered and washed with PBS-2% FBS and red blood cells were lysed. The cells were resuspended at 107/mL and then incubated with 2 mg/mL each of anti-CD4 (GK1.5), anti-CD8 (53.6.72), and anti-Thy1 (30H12) for 20 min. T cells were then depleted by incubation with Low-Tox-M Rabbit complement (Cedarlane) for 30 min. 5 × 106 T-cell-depleted bone marrow cells from each of the indicated donors were injected intravenously in 500 µL volume into lethally irradiated BALB/c Thy1.1+ hosts (950 rad). Mice were infected with rAd-gp140 six weeks post-transplant.
CFSE labeling and in vitro stimulation
Splenocytes were then enriched for CD8+ T cells via AUTOMACS separation after staining with the Miltenyi CD8+ T-cell isolation kit following the manufacturer’s protocol. CD8+ T-cell populations were 85–95% pure following enrichment. The cells were then labeled with CFSE and resuspended at 2 × 106 cells/mL in supplemented RPMI medium and 1g/mL p18 peptide for 7 days at 37°C.
RT-PCR array analysis
Splenocytes were teased into single cell suspensions and enriched for CD8+ T cells as described above. CD8+ T-cell populations were then stimulated with 2 µg/mL p18 peptide for 4 h at 37°C. RNA was then extracted using the Qiagen RNeasy Minikit according to the manufacturer’s protocol. Gene expression was examined using the SABiosciences RT2 Profiler Mouse Apoptosis PCR Array system. One microgram of total RNA from CD8+ T cells from each mouse was reverse transcribed using the SABiosciences RT2 First Strand Kit, and then RT-PCR was performed for each RNA sample using the SABiosciences RT2 SYBR Green/ROX qPCR Master Mix and RT2 Profiler Mouse Apoptosis PCR Array Reagents. Ct values were then uploaded to the SABiosciences PCR Array Data Analysis Web Portal for analysis.
Statistical analysis
Statistical tests were performed using the Mann–Whitney test and a p value of <0.05 was considered significant. Tests were done with Graph Pad Prism Software.
Acknowledgements
Bioluminescence imaging was performed at the Longwood Small Animal Imaging Facility. Thomas Rogers assisted with intramuscular inoculations. This work was supported by Novo-Nordisk and the Center for HIV/AIDS Vaccine Immunology (NIH Grant AI-067854).
Abbreviations
- γC
Common gamma chain (CD132)
- LCMV
lymphocytic choriomeningitis virus
- rAd
recombinant adenovirus
- rVac
recombinant vaccinia virus
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Sun JC, Williams MA, Bevan MJ. CD4+T cells are required for the maintenance, not programming, of memory CD8+T cells after acute infection. Nat. Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ. CD4+T-cell help controls CD8+T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 3.Bevan MJ. Helping the CD8(+) T-cell response. Nat. Rev. Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 4.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingstone AM, Wilson EB, Ontiveros F, Wang JC. Unravelling the mechanisms of help for CD8+T cell responses. Immunol. Res. 2009;45:209–217. doi: 10.1007/s12026-009-8102-0. [DOI] [PubMed] [Google Scholar]
- 6.Harty JT, Badovinac VP. Shaping and reshaping CD8+T-cell memory. Nat. Rev. Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 7.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 9.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 11.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 13.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 14.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A Fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 20.Bolesta E, Kowalczyk A, Wierzbicki A, Eppolito C, Kaneko Y, Takiguchi M, Stamatatos L, et al. Increased level and longevity of protective immune responses induced by DNA vaccine expressing the HIV-1 Env glycoprotein when combined with IL-21 and IL-15 gene delivery. J. Immunol. 2006;177:177–191. doi: 10.4049/jimmunol.177.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alves NL, Arosa FA, van Lier RA. IL-21 sustains CD28 expression on IL-15-activated human naive CD8+T cells. J. Immunol. 2005;175:755–762. doi: 10.4049/jimmunol.175.2.755. [DOI] [PubMed] [Google Scholar]
- 22.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE. Synergy of IL-21 and IL-15 in regulating CD8+T cell expansion and function. J. Exp. Med. 2005;201:139. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker BR, Parvani JG, Meyer D, Hey AS, Skak K, Letvin NL. IL-21 induces apoptosis of antigen-specific CD8+T lymphocytes. J. Immunol. 2007;179:3596–3603. doi: 10.4049/jimmunol.179.6.3596. [DOI] [PubMed] [Google Scholar]
- 24.Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. 2007;109:4135–4142. doi: 10.1182/blood-2006-10-054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559. doi: 10.1016/s1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 26.Allard EL, Hardy MP, Leignadier J, Marquis M, Rooney J, Lehoux D, Labrecque N. Overexpression of IL-21 promotes massive CD8+ memory T cell accumulation. Eur. J. Immunol. 2007;37:3069–3077. doi: 10.1002/eji.200637017. [DOI] [PubMed] [Google Scholar]
- 27.Jackson SS, Schmitz JE, Kuroda MJ, McKay PF, Sumida SM, Martin KL, Yu F, et al. Evaluation of CD62L expression as a marker for vaccine-elicited memory cytotoxic T lymphocytes. Immunology. 2005;116:443–453. doi: 10.1111/j.1365-2567.2005.02243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+T-cell memory. Immunol. Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 30.Hovav AH, Panas MW, Osuna CE, Cayabyab MJ, Autissier P, Letvin NL. The impact of a boosting immunogen on the differentiation of secondary memory CD8+T cells. J. Virol. 2007;81:12793–12802. doi: 10.1128/JVI.01519-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Faassen H, Saldanha M, Gilbertson D, Dudani R, Krishnan L, Sad S. Reducing the stimulation of CD8+T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset. J. Immunol. 2005;174:5341–5350. doi: 10.4049/jimmunol.174.9.5341. [DOI] [PubMed] [Google Scholar]
- 32.Badovinac VP, Porter BB, Harty JT. CD8+T cell contraction is controlled by early inflammation. Nat. Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 33.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 34.Staats HF, Bradney CP, Gwinn WM, Jackson SS, Sempowski GD, Liao HX, Letvin NL, Haynes BF. Cytokine requirements for induction of systemic and mucosal CTL after nasal immunization. J. Immunol. 2001;167:5386–5394. doi: 10.4049/jimmunol.167.9.5386. [DOI] [PubMed] [Google Scholar]