Abstract
The cases of 7 adult dogs with generalized seizures managed by surgical excision and radiation therapy for frontal lobe meningiomas were reviewed. The neurological examination was unremarkable in 6 of the 7 dogs. Five dogs were operated on using a bilateral transfrontal sinus approach and 2 using a unilateral sinotemporal approach to the frontal lobe. One dog was euthanized 14 d after surgery; radiation therapy was initiated 3 wk after surgery in the remaining 6 dogs. Long-term follow-up consisted of neurological examination and magnetic resonance imaging (MRI) and/or computed tomography (CT) scan after radiation therapy. The mean survival time for dogs that had surgery and radiation therapy was 18 mo after surgery. Frontal lobe meningiomas have been associated with poor prognosis. However, the surgical approaches used in these cases, combined with radiation therapy, allow a survival rate for frontal lobe meningiomas similar to that for meningiomas located over the cerebral convexities.
Résumé
Traitement chirurgical et radiothérapie de méningiomes du lobe frontal chez 7 chiens. Les cas de 7 chiens adultes avec des crises d’épilepsie généralisées gérées par ablation chirurgicale et radiothérapie pour des méningiomes du lobe frontal ont été examinés. L’examen neurologique était normal chez 6 des 7 chiens. Cinq chiens ont été opérés à l’aide d’une approche par le sinus transfrontal bilatéral et 2 à l’aide d’une approche sinotemporale unilatérale du lobe frontal. Un chien a été euthanasié 14 jours après la chirurgie; la radiothérapie a été entamée 3 semaines après la chirurgie chez les autres 6 chiens. Le suivi à long terme se composait d’un examen neurologique et d’une IRM et/ou d’un tomodensitogramme après la radiothérapie. La durée moyenne de survie pour les chiens qui avaient subi la chirurgie et la radiothérapie était de 18 mois après la chirurgie. Les méningiomes du lobe frontal ont été associés à un pronostic sombre. Cette étude documente que les approches chirurgicales utilisées pour ces cas, combinées à une radiothérapie, permettent un taux de survie pour les méningiomes du lobe frontal semblable à celui des méningiomes situés sur les convexités cérébrales.
(Traduit par Isabelle Vallières)
Introduction
Meningiomas are usually solitary tumors that are believed to arise from meningothelial cells of the arachnoid membrane and pia mater. In dogs, intracranial meningiomas account for 30% to 45% of all primary intracranial tumors (1,2). They are usually located over the cerebral convexities and below the brain stem (3). Within these localizations, meningiomas that arise over the frontal lobe of the brain are fairly frequent, with an incidence of 26% to 35% in dogs (4,5).
Reported median survival time for dogs with brain meningiomas that receive only medical treatment is 2.5 mo (4). Although external-beam radiation therapy as a primary treatment modality for meningiomas has been suggested for humans (6), surgical removal of operable intracranial meningiomas combined with radiation therapy seems to be the treatment of choice for dogs (7,8). However, before Glass et al (9) presented a mean survival time of 5.3 mo in 5 dogs with frontal meningioma, surgical approach to the frontal lobe held a poor prognosis, due to insufficient exposure and post-operative complications (10).
Materials and methods
Seven adult dogs were presented to our veterinary teaching hospital for seizure activity (Table 1). Neurological examination was carried out in each dog by a European College of Veterinary Neurology diplomate or a trained resident. The procedures performed included a complete blood (cell) count (CBC), a serum biochemical profile, thoracic radiographs, and abdominal ultrasound. Magnetic resonance imaging (MRI) (0.2 Tesla permanent magnet, Signa Profile; General Electric Medical Systems, General Electric Company, Paris, France) was used in 3 dogs. T1- and T2-weighted spin-echo, fluid attenuated inversion recovery (FLAIR) and T1-WI after intravenous administration of gadolinium-chelate contrast agent (Dotarem, Guerbet, Roissy-Charles de Gaulle, France) images were obtained. A computed tomography (CT) scan (CGR 12000 or General Electric CT Pace-or helicoid scanner-HISPEED Cte/plus; General Electric Medical System) was performed in 4 dogs. Contiguous axial sections (4 mm) were acquired before and after injection of iodinated contrast medium (2 mL/kg) (Telebrix 35; Guerbet). Each dog was placed on phenobarbital (Gardenal; Sanofi-Aventis, Maisons-Alfort, France), 2.5 mg/kg body weight (BW) q12h and prednisone therapy (Megasolone; Mérial, Lyon, France), 0.25 mg/kg BW, q12h before surgery to limit seizure occurrence and peritumoral inflammation and/or edema.
Table 1.
Clinical signs associated with 7 dogs subjected to surgery for general seizures
| Case number/Signalment | Clinical signs | Duration of neurological signs prior to admission | Imaging |
|---|---|---|---|
| 1. Labrador M 11 y | General seizures | 2 mo | MRI |
| 2. Polish shepherd M 11 y | General seizures | 1 mo | CT |
| 3. Bichon F 13 y | General seizures | 1 mo | MRI |
| 4. German shepherd F 11 y | General seizures, unilateral amaurosis, ataxia | 2 mo | CT |
| 5. Golden retriever M 8 y | General seizures | 6 mo | MRI |
| 6. Crossbreed FN 13 y | General seizures | 2 mo | CT |
| 7. Pyrenees shepherd M 12 y | General seizures | 10 mo | CT |
M — male; F — female; FN — female neutered; MRI — magnetic resonance imaging; CT — computed tomography.
Surgical technique
All dogs were operated on by the same surgeon (PM), and anesthetic protocols were similar for all dogs. Dogs were premedicated with morphine chlorhydrate (Morphine; Lavoisier, Chaix et du Marais, La Chaussée St-Victor, France), 0.1 mg/kg BW and midazolam (Hypnovel; Roche, Neuilly-Sur-Seine, France), 0.2 mg/kg BW. Anesthesia was induced with propofol (Rapinovet; Mallinckrodt Veterinary, Levallois-Perret, France), 4 mg/kg BW and diazepam (Valium; Roche, Neuilly-Sur-Seine, France), 0.2 mg/kg BW. Dogs were intubated and maintained on oxygen and isofluothane (Forene; Zeneca Pharma, Cergy, France) using a mechanical ventilator controlling capnography and oxymetry. Cephalexine (Rilexine; Virbac, Carros, France), 30 mg/kg BW, was administered at the beginning and the end of surgery in all dogs. Five dogs were operated on using a bilateral transfrontal sinus approach to the frontal lobe and olfactory bulb, and the remaining 2 using a unilateral sino temporal approach (Table 2).
Table 2.
Surgical approaches and outcomes for 7 dogs treated for general seizures
| Case | Surgical approach | Measurement/height | Histological diagnosis | Outcome | Imaging follow-up |
|---|---|---|---|---|---|
| 1 | Unilateral sinotemporal | 14 × 9 × 11 mm | Meningioma/transitional | Complete resolution of seizures without phenobarbital. Euthanized 34 mo after surgery/with RT | MRI 2, 8, 12 and 26 mo after surgery |
| 2 | Unilateral sinotemporal | 10 × 9 × 12 mm | Meningioma/transitional | Two episodes of seizures. Alive 13 mo after surgery/with RT+ phenobarbital | CT scan 3 and 12 mo after surgery |
| 3 | Bilateral transinusal | 3 × 8 × 8 mm | Meningioma/granular cells | Complete resolution of seizures without phenobarbital. Death at 17 mo after surgery/with RT | CT scan 3 and 12 months, and MRI 16 and 17 mo after surgery |
| 4 | Bilateral transinusal | 11 × 8 × 12 mm | Meningioma/transitional | Complete resolution of seizures without phenobarbital. Euthanized 25 mo after surgery/with RT | CT scan 3 and 12 mo after surgery |
| 5 | Bilateral transinusal | 25 × 17 × 10 mm | Meningioma/meningothelial | Resolution of seizures. Euthanized 14 d after surgery/without RT | |
| 6 | Bilateral transinusal | 8 × 15 × 13 mm | Meningioma/psammomatous | Resolution of seizures under phenobarbital. Death at 4 mo after surgery/with RT due to domestic accident | CT scan 1 mo after surgery |
| 7 | Bilateral transinusal | 16 × 12 × 10 mm | Meningioma/transitional | Two episodes of general seizures, and one partial when phenobarbital decreased. Alive 15 mo after surgery/with RT+phenobarbital | MRI 3 wk, 2 and 4 and 10 mo after surgery |
RT — radiation therapy.
MRI — magnetic resonance imaging.
CT — computed tomography.
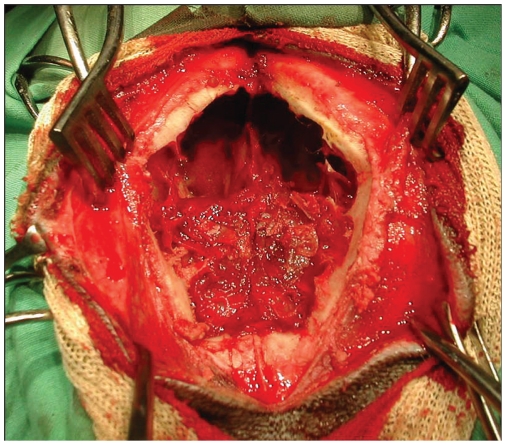
A diamond-shaped bone flap was removed in the bilateral transinusal approach. The flap extended rostrolaterally to the orbit and rostromedially to the junction of the nasal bones. The oscillating bone saw was beveled from 30° to 45° in order to facilitate replacement and tight closure of the bone flap at the end of surgery. The inner part of the frontal sinus bone was resected using a high speed burr, allowing access to the dura mater which was incised. Stay sutures were placed on the duramater and inside the tumor in order to mobilize the neoplasia. A blunt dissection was made between the tumor and the cerebrum using a bipolar coagulation with the aim of complete removal of the mass. The dissection was stopped as soon as the cerebrum appeared free of tumor tissue under microscopic control (Figure 1). The diamond-shaped bone plate was repositioned in 4 cases. In case number 3, a polymethyl methacrylate prosthesis was put in place to cover the bony defect created by the surgical technique, because the bone plate was damaged during surgery.
Figure 1.
Frontal view of dog 4 at the bilateral transinusal approach.
The surgical approach for the unilateral sinotemporal procedure was similar to the one discussed. A half-diamond shaped bone flap extended from the temporal bone to the frontal bone. The bone plate removed was not repositioned in these cases, as the bony defect was smaller, and the cosmetic appearance after closure of the skin over the defect was satisfactory.
Histological diagnosis of meningioma was confirmed by examination of tissue samples obtained at surgery. Tissues were fixed in 10% neutral buffered formalin and routinely processed for examination by light microscopy, with a 4-μm-thick section stained with hematoxylin-eosin-safran. Slide examinations were performed by one pathologist (ER) and classified following the World Health Organization (WHO) classification for domestic animals. Radiation therapy was initiated 3 wk after surgery in 6 dogs. Each dog was irradiated by using a 5 MeV linear accelerator (Orion megavoltage; Varian Medical Systems, Buc, France). Dose distribution was determined by a software program (Eclipse 3D treatment-planning software; Varian Medical Systems) and was based on initial CT findings. The planning target volume included the tumor and a 10-mm margin. All dogs received a total dose of 36 Gy given in 3 Gy fractions for 4 wk on an alternate-day schedule.
Long-term follow-up was made by neurological examination and CT or magnetic resonance imaging (MRI) scans. Dog number 1 had MRI at 2, 8, 12, and 26 mo after surgery. Dog number 2 had a CT scan at 3 and 12 mo after surgery. Dog number 3 had CT scan at 3 and 12 mo, and MRI at 16 and 17 mo after surgery. Dog number 4 had CT scan at 3 and 12 mo after surgery. Dog number 6 had CT scan 1 mo after surgery. Dog number 7 had MRI 3, 2, 4, and 10 mo after surgery.
Results
Seven adult dogs (from 7 to 13 y old; 3 females and 4 males) with seizure activity of 1 to 10 mo duration were presented to our Veterinary Teaching Hospital from March 2006 to January 2008. Neurological examination was unremarkable in 6 dogs. One dog displayed abnormal behavior, circling to the right and unilateral left amaurosis (Table 1).
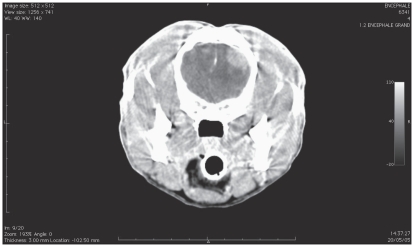
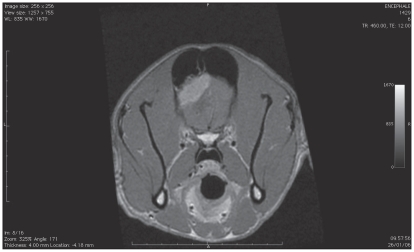
The results of CBC, abdominal ultrasound, and thoracic radiographs were unremarkable in all dogs. Renal parameters, glycemia, total protein, albumin, and liver enzyme activity were within normal ranges. The MRI images performed in 3 dogs implicated the frontal lobe, extra-axial appearing hyper/isointense on T2WI, isointense on T1WI and contrast enhancement on T1WI +gadolinium lesions. A diffuse peritumoral edema was recorded in these dogs (Figures 2, 3). The CT scan imaging in 4 dogs demonstrated the appearance of extra-axial, non-uniform contrast enhancement lesions, located in the frontal lobes.
Figure 2.
Computed tomography scan after contrast injection of dog 2 before surgery.
Figure 3.
Magnetic resonance imaging of dog 5 before surgery; T1WI after gadolinium.
During the first 24 h after surgery, the dogs were on intensive care. A urinary catheter was placed in 3 of 4 dogs that were unable to express their bladder. All dogs received postoperative morphine chlorhydrate (Morphine; Lavoisier) at 0.2 mg/kg body weight (BW) q8h for 24/48 h, prednisone (Megasolone; Mérial) at 0.25 mg/kg BW q12h, phenobarbital (Gardenal; Sanofi-Aventis) at 2.5 mg/kg q12h and antacids. Immediate post-operative modified Glasgow scoring was between 13 and 18 in all dogs except for dog 5, which was 12. No dogs developed seizure activity during hospitalization.
The dogs were hospitalized between 1 and 14 d. Five dogs were able to walk normally 24 h after surgery and leave the hospital 2 to 5 d after surgery. One dog manifested a nonambulatory bilateral hind limb upper motor neuron paresis after surgery. This paresis improved progressively for 4 mo without specific treatment. Fifteen months after surgery, a slight propioceptive deficit (PD) was still present. Dog number 5 did not recover from surgery. After 14 d in a comatose condition, due to the poor prognosis, the owners decided to euthanize the dog.
All meningiomas were classified by the pathologist as grade I: 4 transitional types, 1 psammomatous type, 1 meningothelial type, and 1 granular cell type. Six dogs received radiation therapy and were followed from 4 to 34 mo. Except for dog number 7 that presented a bilateral posterior PD, all dogs had no detectable abnormalities on neurologic examination at each follow-up appointment. The MRI and/or CT scans were not consistent with mass recurrence. Moreover, neither nasal infection nor fistulae were recorded in any case. Three dogs were weaned from their antiepileptic treatment and the other 3 dogs had their seizures controlled under phenobarbital.
The mean survival time for the 6 dogs that underwent radiation therapy was 18 mo (from 4 to 34 mo). Of these 6 dogs, dog number 7 is still alive 15 mo after surgery and dog number 2 was lost after a follow-up of 13 mo after surgery. Dogs numbers 1 and 4 were euthanized by their local veterinarian after developing cluster seizures 34 and 25 mo after surgery. Unfortunately, these dogs did not receive any treatment and no pathological diagnosis was undertaken. Dog number 6 died 4 mo after surgery due to a domestic accident. Dog number 3 was operated on by the local veterinarian for a rectal fistula 17 mo after intracranial surgery. The dog developed a status epilepticus after the procedure. Due to the poor control of the seizures, the dog was referred to our hospital 5 d later. Magnetic resonance images were not consistent with tumor relapse; however, a hyperintense T2WI and T1WI with mild contrast enhancement at the level of the right caudatus nucleus, internal capsula, and piriform lobe was recorded. These images were compatible with an ischemic lesion, and in spite of a 3-day induced coma, the dog died.
Discussion
The current WHO classification of human meningiomas divides meningiomas into grades I, II (atypical), and III (anaplastic or malignant) with 14 types, based on histomorphological features (11). In dogs and cats, meningiomas are usually grade I, and 9 variants are defined: meningothelial, fibroblastic, transitional, psammomatous, angioblastic, papillary, granular cell, myxoid, or anaplastic (12,13).
Results of intracranial cancer surgery are decisively related to the nature of the tumor and its localization (14). The histological nature of the cases we describe does not differ from other studies (5,13,15,16) since grade I meningiomas prevailed, and the transitional variant was the most common type. However, the successful frontal approach has not often been described before.
Despite the fact that meningiomas in dogs and cats are usually benign (16), invasion of the brain in type I meningiomas results in a prognosis equivalent to that of an atypical meningioma, even if the tumor appears otherwise benign (14). As meningiomas in dogs are usually grade I, incomplete resection, due to their particular anatomic location, may explain why frontal meningiomas used to have a poor prognosis. Excision of olfactory bulb tumors in dogs is limited by the anatomy of the area, extent of the tumor and exposure provided by the surgical approach (10).
Transfrontal craniotomy in veterinary medicine was described in dogs in 1972 by Parker and Cunningham (17) and in 1982 by DeWet et al (18). These approaches combine a rostro-tentorial approach with a transfrontal approach. In 1987 Kostolich and Dulisch (10) described a proximal transfrontal sinus approach and destruction of the cribriform plate. A good exposition of the tumor, necessary to allow a broad excision, was limited with these techniques. Moreover, post-operative complications, such as general seizures, and post-operative surgical infection were recorded (10). In 2000, Glass et al (9) proposed a modified bilateral transfrontal sinus approach in 5 dogs that provided increased access to the canine olfactory bulbs and frontal lobes without major complications, with a post-operative survival time between 4 and 9 mo.
In our cohort, the unilateral sinotemporal approach and the bilateral transfrontal sinus approach allowed for a wide access to the frontal lobe that in our opinion is the key to its success. This wider approach allowed us gentle manipulation of the brain, avoiding excessive trauma to the frontal lobe. Moreover, a perfect monitoring of the vascular structures, such as the sagital dorsal sinus was possible. Prevention of excessive bleeding leads to reduced hematoma formation that could lead to seizure activity by compression of the frontal lobe. General seizures that were the main deleterious complication in the older approaches (9,10,18) were not recorded during our postoperative period, and 3 of 6 dogs were weaned from antiepileptic therapy within 4 to 5 mo after surgery.
Two complications were recorded at the short-term follow-up. Dog 5 remained at a modified Glasgow score of 12, for 14 d, and dog 7 had hind limb paresis. This latter dog displayed an important increase of arterial and IC pressure during surgery (Cushing’s response). Magnetic resonance imaging of the brain was conducted 3 wk after surgery, and no signs of tumor relapse or edema were recorded. However, since no imaging of the spinal cord was done, a complication related to the surgery (lesion of the fronto-temporal cortex), a fibro cartilaginous embolism, or a herniated disc could not be excluded. Nevertheless, the dog recovered over time without specific treatment, and is still alive 15 mo after surgery. We observed that dogs 5 and 6 had the largest tumors, suggesting that larger tumors may be more difficult to manage, and that an enlarged approach is important.
Axlund et al (7) documented a median survival time of 16.5 mo, as opposed to 5 mo for surgery alone, for intracranial meningiomas. This difference is almost the same between our results (18 mo) and the results of Glass et al (9) in 2000 (5.3 mo). Radiation necrosis is a major complication related to radiation therapy of brain masses in dogs (19), and leads to rapid neurological deterioration. The radiation tolerance of normal tissues depends on total dose, dose per fraction, total time of exposure, volume, radiation quality, and adjunctive therapies (20). No neurological deterioration was recorded after radiation therapy in our series. However, as histological examination was not performed after death in any dog, late onset secondary effects of radiation therapy were not evaluated.
To our knowledge, our series represents the first report of surgery combined with radiation therapy in canine frontal meningiomas with a mean survival time of 18 mo. This result is comparable to that published by Axlund et al (7) in 2002. As a consequence, we suggest that the unilateral sinotemporal approach, and the bilateral transfrontal sinus approach with radiation therapy for frontal meningiomas in dogs, may lead to a survival time at least comparable to that for meningiomas located over the cerebral convexities. Therefore meningiomas located in the frontal lobes are not necessarily associated with a poor prognosis. Moreover, our results suggest, as indicated by Commins et al (14) that tumor size, leading to a critical surgical approach, may contribute to poor prognosis. More studies are needed to evaluate whether tumor size, duration of clinical signs before surgery, and the histopathological variety are related to the prognosis. CVJ
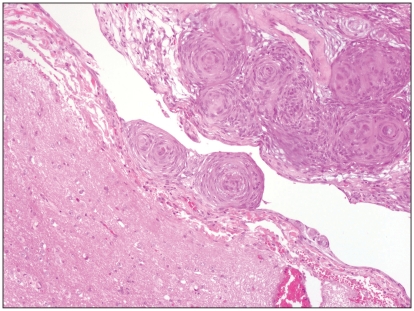
Figure 4.
A well-delimited transitional meningioma in dog 4. Hematoxylin-eosin-safran 10×.
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Heidner GL, Kornegay JN, Page RL, et al. Analysis of survival in a retrospective study of 86 dogs with brain tumors. J Vet Int Med. 1991;5:219–26. doi: 10.1111/j.1939-1676.1991.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 2.Snyder JM, Shofer FS, Winkle JV, et al. Canine intracranial primary neoplasia: 173 cases (1986–2003) J Vet Intern Med. 2006;20:669–675. doi: 10.1892/0891-6640(2006)20[669:cipnc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Adamo PF, Forrest L, Dubielzig R. Canine and feline meningiomas: Diagnosis, treatment and prognosis. Compend Contin Educ Pract Vet. 2004;26:951–964. [Google Scholar]
- 4.Foster ES, Carrillo JM, Patnaik AK. Clinical signs of tumors affecting the rostral cerebrum in 43 dogs. J Vet Int Med. 1988;2:71–74. doi: 10.1111/j.1939-1676.1988.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 5.Patnaik AK, Lieberman PH, Erlandson RA, et al. Paranasal meningioma in the dog: A clinicopathologic study of ten cases. Vet Path. 1986;23:369–373. doi: 10.1177/030098588602300403. [DOI] [PubMed] [Google Scholar]
- 6.Rogers L, Mehta M. Role of radiation therapy in treating intracranial meningiomas. Neurosurg Focus. 2007;23:E4. doi: 10.3171/FOC-07/10/E4. [DOI] [PubMed] [Google Scholar]
- 7.Axlund TW, McGlasson ML, Smith AN. Surgery alone or in combination with radiation therapy for treatment of intracranial Meningiomas in dogs: 31 cases (1989–2002) J Am Vet Med Assoc. 2002;221:1597–1600. doi: 10.2460/javma.2002.221.1597. [DOI] [PubMed] [Google Scholar]
- 8.Lester NV, Hopkins AL, Bova FJ, et al. Radiosurgery using a stereotactic headframe system for irradiation of brain tumors in dogs. J Am Vet Med Assoc. 2001;219:1562–1567. doi: 10.2460/javma.2001.219.1562. [DOI] [PubMed] [Google Scholar]
- 9.Glass EN, Kapatkin A, Vite C, et al. A modified bilateral transfrontal sinus approach to the canine frontal lobe and olfactory bulb: Surgical technique and five cases. J Am Hosp Assoc. 2000;36:43–50. doi: 10.5326/15473317-36-1-43. [DOI] [PubMed] [Google Scholar]
- 10.Kostolich M, Dulisch ML. Surgical approach to the canine olfactory bulb for meningioma removal. Vet Surg. 1987;16:273–277. doi: 10.1111/j.1532-950x.1987.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 11.Perry A, Louis DN, Scheithauer BW, et al. Meningiomas. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. World Health Organization Classification of Tumours of the Central Nervous System. 4th ed. Lyon, France: IARC; 2007. pp. 64–172. [Google Scholar]
- 12.Adamo PF, Cantile C, Steinberg H. Evaluation of progesterone and estrogen receptor expression in 15 meningiomas of dogs and cats. Am J Vet Res. 2003;64:1310–1318. doi: 10.2460/ajvr.2003.64.1310. [DOI] [PubMed] [Google Scholar]
- 13.Montoliu P, Añor S, Vidal E, Pumarola M. Histological and immunohistochemical study of 30 cases of canine meningioma. J Comp Pathol. 2006;135:200–207. doi: 10.1016/j.jcpa.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Commins DL, Atkinson MD, Burnett ME. Review of meningioma histopathology. Neurosurg Focus. 2007;23:E3. doi: 10.3171/FOC-07/10/E3. [DOI] [PubMed] [Google Scholar]
- 15.Greco JJ, Aiken SA, Berg JM, et al. Evaluation of intracranial meningioma resection with surgical aspirator in dogs: 17 cases (1996–2004) J Am Vet Med Assoc. 2006;229:394–400. doi: 10.2460/javma.229.3.394. [DOI] [PubMed] [Google Scholar]
- 16.Sturges BK, Dickinson PJ, Bollen AW, et al. Magnetic resonance imaging and histological classification of intracranial meningioma in 112 dogs. J Vet Intern Med. 2008;22:586–595. doi: 10.1111/j.1939-1676.2008.00042.x. [DOI] [PubMed] [Google Scholar]
- 17.Parker AJ, Cunnigham JG. Transfrontal craniotomy in the dog. Vet Rec. 1972;90:622–624. doi: 10.1136/vr.90.22.622. [DOI] [PubMed] [Google Scholar]
- 18.DeWet PD, Ali II, Peterson DN. Surgical approach to the rostral cranial fossa by radical trasfrontal craniotomy in the dog. J S Afr Vet Assoc. 1982;53:40–41. [PubMed] [Google Scholar]
- 19.Brearley MJ, Jeffery ND, Phillips SM, et al. Hypofractionated radiation therapy of brain masses in dogs: A restrospective analysis of survival of 83 cases (1991–1996) J Vet Int Med. 1999;13:408–412. doi: 10.1892/0891-6640(1999)013<0408:hrtobm>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 20.New P. Radiation injury to the nervous system. Curr Poin Neurol. 2001;14:725–734. doi: 10.1097/00019052-200112000-00008. [DOI] [PubMed] [Google Scholar]