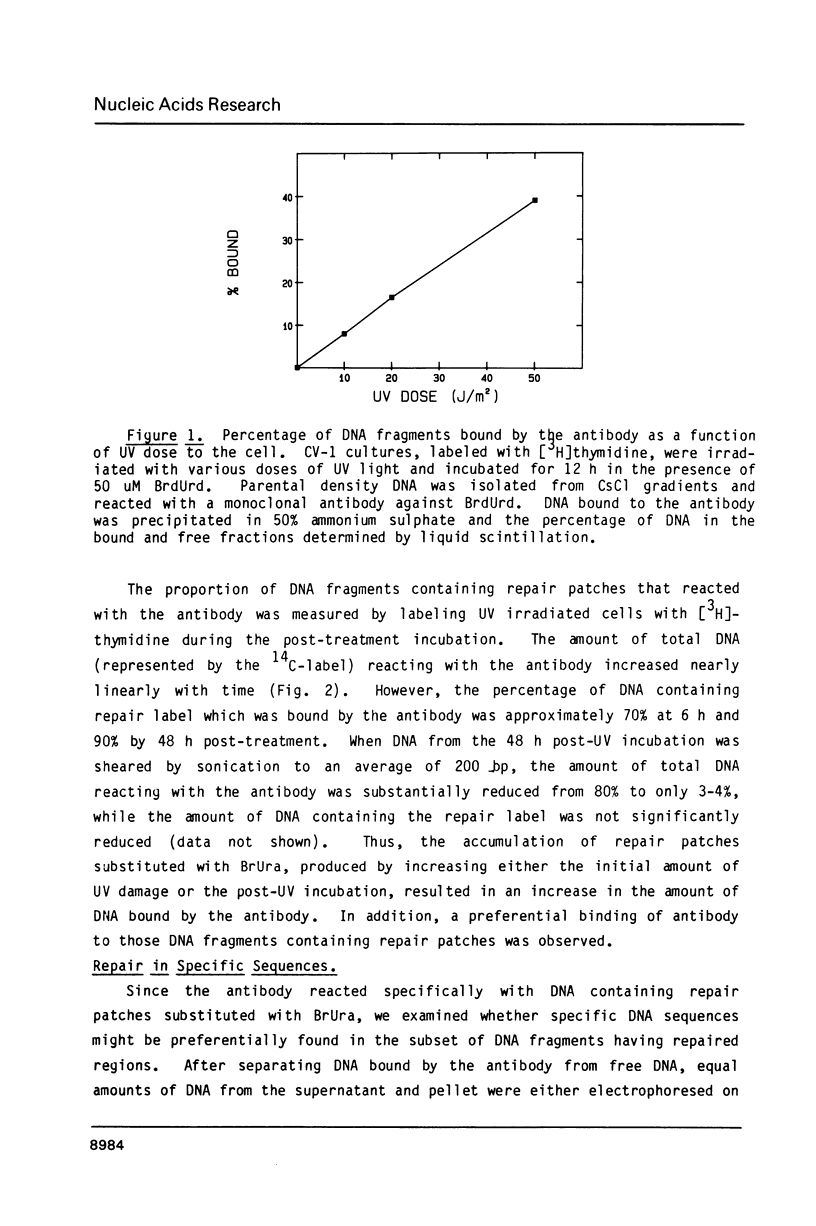
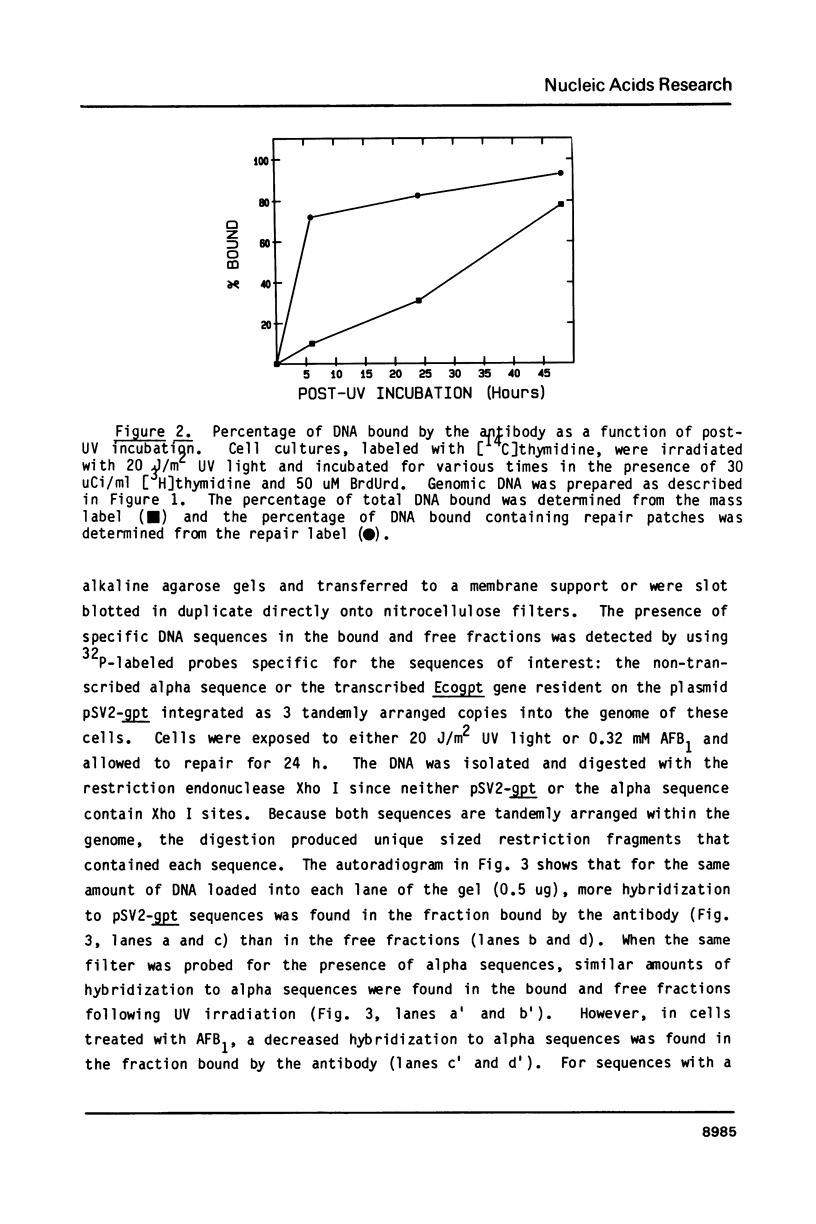
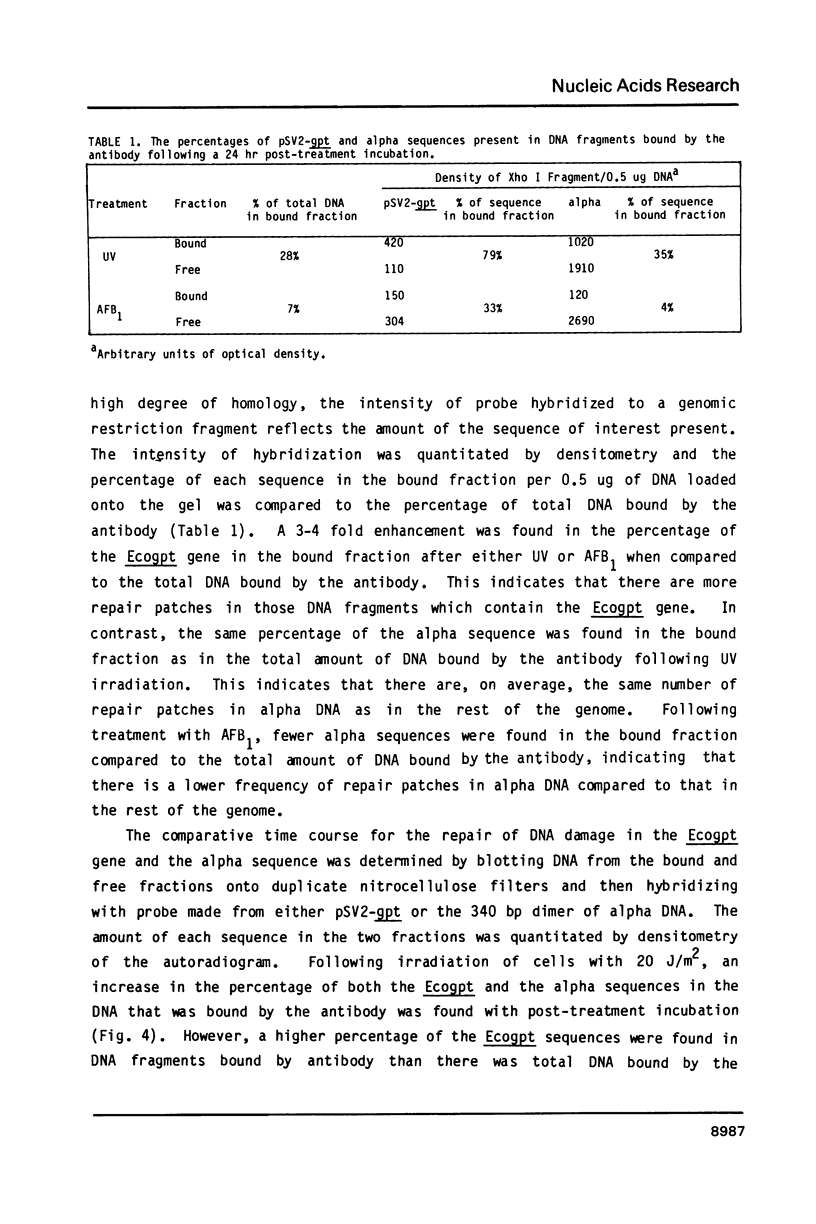
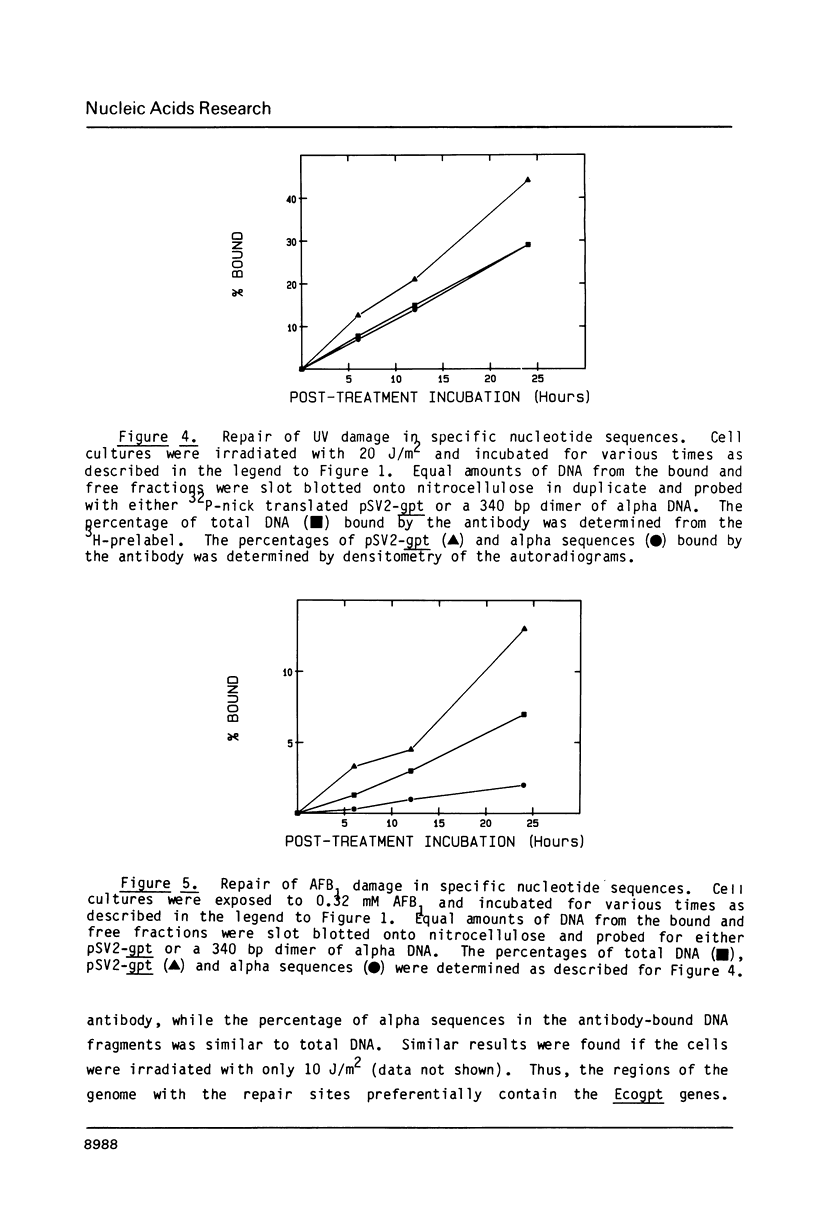
Abstract
An immunological method was developed that isolates DNA fragments containing bromouracil in repair patches from unrepaired DNA using a monoclonal antibody that recognizes bromouracil. Cultured monkey cells were exposed to either UV light or the activated carcinogen aflatoxin B1 and excision repair of damage in DNA fragments containing the integrated and transcribed E. coli gpt gene was compared to that in the genome overall. A more rapid repair, of both UV and AFB1 damage was observed in the DNA fragments containing the E. coli gpt genes. The more efficient repair of UV damage was not due to a difference in the initial level of pyrimidine dimers as determined with a specific UV endonuclease. Consistent with previous observations using different methodology, repair of UV damage in the alpha sequences was found to occur at the same rate as that in the genome overall, while repair of AFB1 damage was deficient in alpha DNA. The preferential repair of damage in the gpt gene may be related to the functional state of the sequence and/or to alterations produced in the chromatin conformation by the integration of plasmid sequences carrying the gene.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., Lakmaker F., Feltkamp T. E. Immunology of DNA. I. The influence of reaction conditions on the Farr assay as used for the detection of anti-ds DNA. J Immunol Methods. 1976;10(1):27–37. doi: 10.1016/0022-1759(76)90004-1. [DOI] [PubMed] [Google Scholar]
- Arrand J. E., Murray A. M. Benzpyrene groups bind preferentially to the DNA of active chromatin in human lung cells. Nucleic Acids Res. 1982 Mar 11;10(5):1547–1555. doi: 10.1093/nar/10.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey G. S., Nixon J. E., Hendricks J. D., Sinnhuber R. O., Van Holde K. E. Carcinogen aflatoxin B1 is located preferentially in internucleosomal deoxyribonucleic acid following exposure in vivo in rainbow trout. Biochemistry. 1980 Dec 9;19(25):5836–5842. doi: 10.1021/bi00566a027. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Hanawalt P. C. Survival of UV-irradiated mammalian cells correlates with efficient DNA repair in an essential gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3830–3833. doi: 10.1073/pnas.83.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Brutlag D. L. Molecular arrangement and evolution of heterochromatic DNA. Annu Rev Genet. 1980;14:121–144. doi: 10.1146/annurev.ge.14.120180.001005. [DOI] [PubMed] [Google Scholar]
- Cech T., Pardue M. L. Cross-linking of DNA with trimethylpsoralen is a probe for chromatin structure. Cell. 1977 Jul;11(3):631–640. doi: 10.1016/0092-8674(77)90080-0. [DOI] [PubMed] [Google Scholar]
- Cohn S. M., Lieberman M. W. The distribution of DNA excision-repair sites in human diploid fibroblasts following ultraviolet irradiation. J Biol Chem. 1984 Oct 25;259(20):12463–12469. [PubMed] [Google Scholar]
- Cohn S. M., Lieberman M. W. The use of antibodies to 5-bromo-2'-deoxyuridine for the isolation of DNA sequences containing excision-repair sites. J Biol Chem. 1984 Oct 25;259(20):12456–12462. [PubMed] [Google Scholar]
- Davies R. L., Fuhrer-Krusi S., Kucherlapati R. S. Modulation of transfected gene expression mediated by changes in chromatin structure. Cell. 1982 Dec;31(3 Pt 2):521–529. doi: 10.1016/0092-8674(82)90308-7. [DOI] [PubMed] [Google Scholar]
- Garel A., Zolan M., Axel R. Genes transcribed at diverse rates have a similar conformation in chromatin. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4867–4871. doi: 10.1073/pnas.74.11.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Irvin T. R., Wogan G. N. Quantitation of aflatoxin B1 adduction within the ribosomal RNA gene sequences of rat liver DNA. Proc Natl Acad Sci U S A. 1984 Feb;81(3):664–668. doi: 10.1073/pnas.81.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M., Cerutti P. A. Excision of N-acetoxy-2-acetylaminofluorene-induced DNA adducts from chromatin fractions of human fibroblasts. Cancer Res. 1980 Nov;40(11):4313–4319. [PubMed] [Google Scholar]
- Leadon S. A., Cerutti P. A. A rapid and mild procedure for the isolation of DNA from mammalian cells. Anal Biochem. 1982 Mar 1;120(2):282–288. doi: 10.1016/0003-2697(82)90349-9. [DOI] [PubMed] [Google Scholar]
- Leadon S. A., Hanawalt P. C. Ultraviolet irradiation of monkey cells enhances the repair of DNA adducts in alpha DNA. Carcinogenesis. 1984 Nov;5(11):1505–1510. doi: 10.1093/carcin/5.11.1505. [DOI] [PubMed] [Google Scholar]
- Leadon S. A., Tyrrell R. M., Cerutti P. A. Excision repair of aflatoxin B1-DNA adducts in human fibroblasts. Cancer Res. 1981 Dec;41(12 Pt 1):5125–5129. [PubMed] [Google Scholar]
- Madhani H. D., Bohr V. A., Hanawalt P. C. Differential DNA repair in transcriptionally active and inactive proto-oncogenes: c-abl and c-mos. Cell. 1986 May 9;45(3):417–423. doi: 10.1016/0092-8674(86)90327-2. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y., Sekiguchi M. Physical association of pyrimidine dimer DNA glycosylase and apurinic/apyrimidinic DNA endonuclease essential for repair of ultraviolet-damaged DNA. Proc Natl Acad Sci U S A. 1981 May;78(5):2742–2746. doi: 10.1073/pnas.78.5.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose K., Nikaido O. Transcriptionally active and inactive genes are similarly modified by chemical carcinogens or X-ray in normal human fibroblasts. Biochim Biophys Acta. 1984 Apr 5;781(3):273–278. doi: 10.1016/0167-4781(84)90093-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thayer R. E., Singer M. F., McCutchan T. F. Sequence relationships between single repeat units of highly reiterated African Green monkey DNA. Nucleic Acids Res. 1981 Jan 10;9(1):169–181. doi: 10.1093/nar/9.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. A dominant role for DNA secondary structure in forming hypersensitive structures in chromatin. Cell. 1983 Apr;32(4):1191–1203. doi: 10.1016/0092-8674(83)90302-1. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wieshahn G. P., Hyde J. E., Hearst J. E. The photoaddition of trimethylpsoralen to Drosophila melanogaster nuclei: a probe for chromatin substructure. Biochemistry. 1977 Mar 8;16(5):925–932. doi: 10.1021/bi00624a018. [DOI] [PubMed] [Google Scholar]
- Yu F. L. Preferential binding of aflatoxin B1 to the transcriptionally active regions of rat liver nucleolar chromatin in vivo and in vitro. Carcinogenesis. 1983;4(7):889–893. doi: 10.1093/carcin/4.7.889. [DOI] [PubMed] [Google Scholar]
- Zolan M. E., Cortopassi G. A., Smith C. A., Hanawalt P. C. Deficient repair of chemical adducts in alpha DNA of monkey cells. Cell. 1982 Mar;28(3):613–619. doi: 10.1016/0092-8674(82)90216-1. [DOI] [PubMed] [Google Scholar]
- Zolan M. E., Smith C. A., Calvin N. M., Hanawalt P. C. Rearrangement of mammalian chromatin structure following excision repair. Nature. 1982 Sep 30;299(5882):462–464. doi: 10.1038/299462a0. [DOI] [PubMed] [Google Scholar]
- Zolan M. E., Smith C. A., Hanawalt P. C. Formation and repair of furocoumarin adducts in alpha deoxyribonucleic acid and bulk deoxyribonucleic acid of monkey cells. Biochemistry. 1984 Jan 3;23(1):63–69. doi: 10.1021/bi00296a010. [DOI] [PubMed] [Google Scholar]
- van Zeeland A. A., Smith C. A., Hanawalt P. C. Sensitive determination of pyrimidine dimers in DNA of UV-irradiated mammalian cells. Introduction of T4 endonuclease V into frozen and thawed cells. Mutat Res. 1981 Jun;82(1):173–189. doi: 10.1016/0027-5107(81)90148-2. [DOI] [PubMed] [Google Scholar]




