Abstract
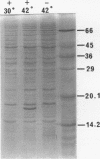
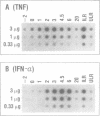
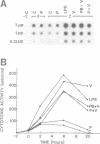
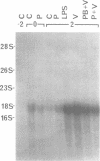
Sendai virus induces human peripheral blood leukocytes to produce high levels of tumor necrosis factor (TNF) mRNA. TNF mRNA can represent as much as 0.6% of the total mRNA. Kinetic studies indicate that the level of TNF mRNA peaks about 2 hours before that of IFN-alpha mRNA produced in the same system. Although the peak levels of TNF and IFN-alpha mRNA were similar, TNF in the culture supernatants was at a 200 fold lower level than IFN-alpha. Cloning and sequence analysis of TNF cDNA isolated from peripheral blood leukocytes RNA showed that normal human cells in response to Sendai virus produce TNF identical to that previously isolated and cloned from tumor-derived cell lines. A bacterial expression system was used to produce the cloned TNF at a maximum level of 2 X 10(6) units per ml of culture.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Holtmann H., Toker L., Hahn T., Wallach D. Tumor necrosis factor induction by Sendai virus. J Immunol. 1986 Apr 15;136(8):2938–2942. [PubMed] [Google Scholar]
- Aggarwal B. B., Kohr W. J., Hass P. E., Moffat B., Spencer S. A., Henzel W. J., Bringman T. S., Nedwin G. E., Goeddel D. V., Harkins R. N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- Armstrong C. A., Klostergaard J., Granger G. A. Isolation and initial characterization of tumoricidal monokine(s) from the human monocytic leukemia cell line THP-1. J Natl Cancer Inst. 1985 Jan;74(1):1–9. [PubMed] [Google Scholar]
- Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Cantell K., Hirvonen S., Kauppinen H. L., Myllylä G. Production of interferon in human leukocytes from normal donors with the use of Sendai virus. Methods Enzymol. 1981;78(Pt A):29–38. doi: 10.1016/0076-6879(81)78094-7. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. R., McKinnon K. P., Koren H. S. Lipopolysaccharide (LPS) stimulates fresh human monocytes to lyse actinomycin D-treated WEHI-164 target cells via increased secretion of a monokine similar to tumor necrosis factor. J Immunol. 1985 Dec;135(6):3978–3987. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collins T., Lapierre L. A., Fiers W., Strominger J. L., Pober J. S. Recombinant human tumor necrosis factor increases mRNA levels and surface expression of HLA-A,B antigens in vascular endothelial cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1986 Jan;83(2):446–450. doi: 10.1073/pnas.83.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hafeman D. G., Lucas Z. J. Polymorphonuclear leukocyte-mediated, antibody-dependent, cellular cytotoxicity against tumor cells: dependence on oxygen and the respiratory burst. J Immunol. 1979 Jul;123(1):55–62. [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Haranaka K., Satomi N., Sakurai A., Kunii O. Role of lipid A in the production of tumor necrosis factor and differences in antitumor activity between tumor necrosis factor and lipopolysaccharide. Tohoku J Exp Med. 1984 Dec;144(4):385–396. doi: 10.1620/tjem.144.385. [DOI] [PubMed] [Google Scholar]
- Jones J. F. Interactions between human neutrophils and vaccinia virus: induction of oxidative metabolism and virus inactivation. Pediatr Res. 1982 Jul;16(7):525–529. doi: 10.1203/00006450-198207000-00005. [DOI] [PubMed] [Google Scholar]
- Kelker H. C., Oppenheim J. D., Stone-Wolff D., Henriksen-DeStefano D., Aggarwal B. B., Stevenson H. C., Vilcek J. Characterization of human tumor necrosis factor produced by peripheral blood monocytes and its separation from lymphotoxin. Int J Cancer. 1985 Jul 15;36(1):69–73. doi: 10.1002/ijc.2910360112. [DOI] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marmenout A., Fransen L., Tavernier J., Van der Heyden J., Tizard R., Kawashima E., Shaw A., Johnson M. J., Semon D., Müller R. Molecular cloning and expression of human tumor necrosis factor and comparison with mouse tumor necrosis factor. Eur J Biochem. 1985 Nov 4;152(3):515–522. doi: 10.1111/j.1432-1033.1985.tb09226.x. [DOI] [PubMed] [Google Scholar]
- Matthews N. Production of an anti-tumour cytotoxin by human monocytes: comparison of endotoxin, interferons and other agents as inducers. Br J Cancer. 1982 Apr;45(4):615–617. doi: 10.1038/bjc.1982.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986 Mar 1;163(3):740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Sullivan S. A., Williamson B. D., Carswell E. A., Old L. J. Purification and characterization of a human tumor necrosis factor from the LuKII cell line. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6637–6641. doi: 10.1073/pnas.82.19.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M., Rubinstein S., Familletti P. C., Miller R. S., Waldman A. A., Pestka S. Human leukocyte interferon: production, purification to homogeneity, and initial characterization. Proc Natl Acad Sci U S A. 1979 Feb;76(2):640–644. doi: 10.1073/pnas.76.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby M. R., Aggarwal B. B., Rinderknecht E., Svedersky L. P., Finkle B. S., Palladino M. A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985 Sep;135(3):2069–2073. [PubMed] [Google Scholar]
- Shirai T., Yamaguchi H., Ito H., Todd C. W., Wallace R. B. Cloning and expression in Escherichia coli of the gene for human tumour necrosis factor. 1985 Feb 28-Mar 6Nature. 313(6005):803–806. doi: 10.1038/313803a0. [DOI] [PubMed] [Google Scholar]
- Silberstein D. S., David J. R. Tumor necrosis factor enhances eosinophil toxicity to Schistosoma mansoni larvae. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1055–1059. doi: 10.1073/pnas.83.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto M., Yip Y. K., Vilcek J. Interferon-gamma enhances expression of cellular receptors for tumor necrosis factor. J Immunol. 1986 Apr 1;136(7):2441–2444. [PubMed] [Google Scholar]
- Vilcek J., Palombella V. J., Henriksen-DeStefano D., Swenson C., Feinman R., Hirai M., Tsujimoto M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J Exp Med. 1986 Mar 1;163(3):632–643. doi: 10.1084/jem.163.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. M., Creasey A. A., Ladner M. B., Lin L. S., Strickler J., Van Arsdell J. N., Yamamoto R., Mark D. F. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]