SUMMARY
Although the urinary bladder urothelium has classically been thought of as a passive barrier to ions and solutes, a number of novel properties have been recently attributed to urothelial cells. Studies have revealed that the urothelium is involved in sensory mechanisms (i.e. the ability to express a number of sensor molecules or respond to thermal, mechanical and chemical stimuli) and can release chemical mediators. Localization of afferent nerves next to the urothelium suggests that urothelial cells could be targets for neurotransmitters released from bladder nerves or that chemicals released by urothelial cells could alter afferent nerve excitability. Taken together, these and other findings highlighted in this article suggest a sensory function for the urothelium. Elucidation of mechanisms that influence urothelial function might provide insights into the pathology of bladder dysfunction.
Keywords: barrier function, neuron-like properties, sensor function, transducer function
INTRODUCTION TO THE ANATOMY AND BARRIER FUNCTION OF THE UROTHELIUM
The urothelium is the epithelial lining of the urinary tract between the renal pelvis and the urinary bladder. Urothelium is composed of at least three layers: a basal cell layer attached to a basement membrane, an intermediate layer, and a superficial or apical layer composed of large hexagonal cells (diameters of 25–250 μm) known as ‘umbrella cells’.1,2 The umbrella cells are interconnected by tight junctions and are covered on their apical surface by crystalline proteins called uroplakins which assemble into hexagonal plaques.1–4 Uroplakins and other urothelial cellular differentiation markers, such as cyto keratin 20, are not expressed in the stratified epithelium of the urethra.5 In some species, the umbrella cells and perhaps also the intermediate cells have projections to the basement membrane.1,6,7 The barrier function of the urothelium is dependent on several features of the umbrella cell layer: these features include tight-junction complexes that reduce the movement of ions and solutes between cells, and specialized lipid molecules and uroplakin proteins in the apical membrane, which reduce the permeability of the cells to small molecules (e.g. water, urea, protons).1,2,8,9 The apical surface of the urothelium is also covered with a sulfated poly saccharide glycosaminoglycan layer that is thought to act as a nonspecific anti-adherence factor and as a defense mechanism against infection.5,10
RESPONSE OF THE UROTHELIUM TO INJURY
Basal cells, which are thought to be precursors for other cell types, normally exhibit a low (3–6 months) turnover rate, which is in fact the slowest turnover of any mammalian epithelial cells. It has been shown that neither urine-derived factors nor cyclic mechanical changes contribute to the maintenance of urothelial differentiation; however, accelerated proliferation can occur in pathologic conditions.11 For example, when protamine sulfate was used to damage selectively only the umbrella cell layer, it was shown that the urothelium rapidly undergoes both functional and structural changes in order to restore the barrier. The initiation of urothelial proliferation is thought to involve upregulation of growth factors. such as fibroblast growth factor and nerve growth factor.12,13
Although the urothelium maintains a tight barrier to ion and solute flux, a number of local factors (e.g. tissue pH, mechanical or chemical trauma, and bacterial infection) can modulate the barrier function of the urothelium.7,14 Other conditions, such as interstitial cystitis or spinal cord injury can also change the urothelial barrier.15,16 When the barrier is compromised, water, urea and toxic substances can pass into the underlying tissue (neural and/or muscle layers), which results in symptoms of urgency, frequency and pain during bladder filling and voiding. In some pathologic conditions, disruption of the urothelial barrier is associated with ultrastructural changes and alterations in the levels of chemical mediators such as nitric oxide (NO) and ATP which can alter epithelial function and/or integrity.15,16 Disruption of urothelial barrier integrity has also been linked to the expression of substances such as antiproliferative factor, which has been detected in the urine of patients with interstitial cystitis, and has been shown to be secreted by bladder epithelial cells obtained from these patients. Antiproliferative factor can inhibit epithelial proliferation, which adversely affects barrier function.17,18
Urinary tract infections with uropathogenic Escherichia coli (UPEC) are initiated by bacterial adherence to uroplakin proteins on the apical surface of umbrella cells.14,19 Adherence is followed by bacterial invasion of the bladder wall. Internalization of UPEC in the umbrella cells and formation of intracellular colonies (biofilm-like pods) of UPEC in umbrella cells has been implicated in the mechanism of chronic urinary tract infection.19
Disruption of urothelial function by distant pathologic conditions is thought to be mediated by neural or hormonal mechanisms. For example, spinal-cord transection in rats leads to a rapid alteration in the urothelial barrier, including ultrastructural changes and increased permeability.15 These changes were prevented by administration of a ganglionic blocking agent before spinal-cord transection, which indicated that efferent autonomic pathways are involved in the acute effects of spinal-cord injury on bladder urothelium. Other types of urothelial–neural interactions are also likely; various stimuli have been reported to induce urothelial cells to release chemical mediators that can, in turn, modulate the activity of afferent nerves.1,20,21 These findings have raised the possibility that the urothelium could have a role in sensory mechanisms in the urinary tract.
In summary, modification of the urothelium and/or loss of epithelial integrity in a number of pathologic conditions can result in the passage of toxic and irritating urinary constituents through the urothelium, or release of neuroactive substances from the urothelium, which leads to changes in the properties of sensory nerves and sensory symptoms such as urinary frequency and urgency. Chemical communication between the nervous system and urothelial cells might, therefore, have an important role in the generation of urinary bladder dysfunction.
INNERVATION OF THE UROTHELIUM
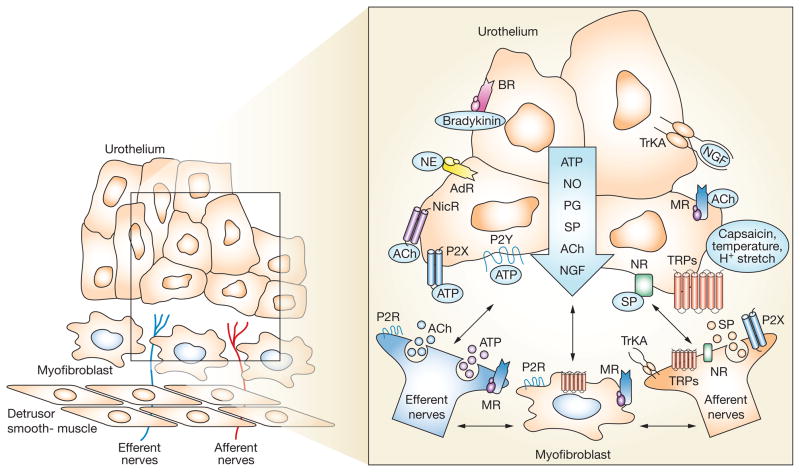
Studies have shown that both afferent and autonomic efferent nerves are located in close proximity to the urothelium (Figure 1).20–24 Peptide-immunoreactive and TRPV1-immunoreactive nerve fibers that are presumed to be afferent nerves are localized throughout the urinary bladder musculature and in a plexus that lies beneath, and extends into, the urothelium.20,21 Markers for cholinergic and adrenergic nerves have also been detected in these same regions. The anatomic substrate for bidirectional urothelial–neural communication exists in the urinary bladder. In addition, a layer of cells with morphologic properties similar to those of myofibroblasts has been detected in the sub urothelial space of the bladder in both humans and animals (Figure 1).25–27 These cells (which also make close contact with nerves) can release and be activated by ATP and, therefore, could act as an intermediary in urothelium–afferent nerve interactions.
Figure 1.
Hypothetical model that depicts possible interactions between bladder afferent and efferent nerves, urothelial cells, smooth muscle and myofibroblasts. Stimulation of receptors and channels on urothelial cells can release mediators that target bladder nerves and other cell types; urothelial cells can also be targets for neurotransmitters released from nerves or other cell types. Urothelial cells can be activated by either autocrine (i.e. autoregulation) or paracrine (release from nearby nerves or other cells) mechanisms. Abbreviations: ACh, acetylcholine; AdR, adrenergic receptor; BR, bradykinin receptor; H+, proton; MR, muscarinic receptor; NE, norepinephrine; NGF, nerve growth factor; NR, neurokinin receptor; NicR, nicotinic receptor; NO, nitric oxide; P2R, purinergic 2 receptor unidentified subtype; P2X and P2Y, purinergic receptors; PG, prostaglandin; SP, substance P; Trk-A, receptor tyrosine kinase A, high affinity receptor for nerve growth factor; TRPs, transient receptor potential channels.
SENSORY ROLES OF THE UROTHELIUM
Transducer role of the urothelium
While the urothelium has been historically viewed primarily as a barrier, it is increasingly recognized to be a responsive structure that is capable of detecting physiological and chemical stimuli, and of releasing a number of signaling molecules (Figure 1). Several substances are released from urothelial cells following physical and chemical stimulation: ATP; NO; substance P; acetylcholine, adenosine, antiproliferative factor, cytokines, prostanoids and various trophic factors. Data accumulated over several years indicate that urothelial cells display a number of properties similar to those of sensory neurons such as nociceptors and mechanoreceptors (Figure 1), and that both types of cells use diverse signal-transduction mechanisms to detect physiological stimuli.
Signaling role of the urothelium
Examples of neuronal ‘sensor molecules’ (i.e. receptors and ion channels) that have been identified in the urothelium include receptors for bradykinin, neurotrophins (Trk-A and p75), purines (P2X and P2Y), norepinephrine (α and β), acetylcholine (nicotinic and muscarinic receptors), protease-activated receptors, amiloride-sensitive and mechanosensitive Na+ channels, and a number of transient receptor potential (TRP) channels (TRPV1, TRPV2, TRPV4, TRPM8) (Table 1).28–38
Table 1.
Comparison of sensor properties of urinary bladder urothelial cells and primary afferent nerves.
| Sensor function or stimuli | Urothelial sensor molecules | Primary afferent sensor molecules |
|---|---|---|
| ATP | Purinergic receptors (P2X and P2Y) | Purinergic receptors (P2X and P2Y) |
| Capsaicin and resiniferatoxin | TRPV1 | TRPV1 |
| Heat | TRPV1, TRPV2, TRPV4 | TRPV1, TRPV2, TRPV3, TRPV4 |
| Cold | TRPM8, TRPA1 | TRPM8, TRPA1 |
| H+ | TRPV1 | TRPV1, ASIC, DRASIC |
| Osmolarity | TRPV4 | TRPV4 |
| Bradykinin | B1 and B2 bradykinin receptors | B1 and B2 bradykinin receptors |
| Acetylcholine | Nicotinic and muscarinic receptors | Nicotinic and muscarinic receptors |
| Norepinephrine | Adrenergic receptors (α and β subtypes) | Adrenergic receptors (α and β subtypes) |
| Nerve growth factor | p75 and Trk-A | p75 and Trk-A |
| Mechanosensitivity | Amilioride-sensitive Na+ channels | Amilioride-sensitive Na+ channels |
Abbreviations: ASIC, acid-sensing ion channel; DRASIC, dorsal root acid-sensing ion channel; H+, protons; Na+, sodium ion; p75, low affinity pan-neurotrophic receptor; Trk-A, receptor tyrosine kinase A, high affinity receptor for nerve growth factor; TRPA, transient receptor potential ankyrin-type channel; TRPV, transient receptor potential vanilloid-type channel; TRPM, transient receptor potential melastatin-type channel.
ROLE OF DIFFERENT RECEPTORS AND SUBSTANCES IN UROTHELIAL FUNCTION
ATP and purinergic receptors
Since the first report (in 1997) of distension-evoked ATP release from bladder urothelium, evidence that supports a role for urothelial-derived ATP release in both autocrine and paracrine signaling in the bladder has emerged.33 P2X and P2Y purinergic receptor subtypes are expressed in cells (urothelium, nerves and myofibroblasts) that are located at or near the luminal surface of the bladder, which suggests that ATP has an important role in chemical communication in the bladder. Basolateral ATP release from urothelial cells following either chemical or mechanical stimuli21,29,32 might signal to adjacent urothelial cells as well as to other cell types (myofibroblasts or bladder nerves) within the submucosa (Figure 1). In addition, the close proximity of urothelial cells to myofibroblasts and bladder nerves, the sensitivity of these cells to ATP (indicated by an ATP-induced increase in intracellular Ca2+ in afferent neurons, urothelial cells and myofibroblasts), and the evidence for cell–cell coupling mediated by gap junctions provide support for a number of physical and chemical interactions that could influence bladder function.25,39 Activation of P2Y or P2X receptors by ATP release during bladder distension could, therefore, have a role in autocrine and paracrine signaling throughout the urothelium. This hypothesis is supported by a study published in 2005, which found that urothelial-derived ATP release in the urinary bladder purportedly acts as a trigger for exocytosis—in part via autocrine activation of urothelial purinergic (P2X and P2Y) receptors.40
Involvement of ATP in bladder dysfunction
By activation of a population of suburothelial bladder afferent nerves that express P2X3 or P2X2 receptors, ATP released from urothelial cells during bladder distension could trigger sensations of fullness and pain, or induce reflex changes in bladder activity.30 This concept receives support from studies in P2X3 null mice which exhibited urinary bladder hyporeflexia.41 By contrast, clinical studies showed that patients who exhibited neurogenic detrusor over-activity have increased P2X3 immuno reactive suburothelial innervation.42 Taken together, these data suggest that purinergic receptors and neural–epithelial interactions are essential for normal bladder function.
In the last 5 years, various studies have shown that pathology often results in augmented ATP release from the urothelium, which can cause painful sensations by excitation of purinergic (P2X) receptors on sensory fibers.29,30,43 There is speculation that this type of noncholinergic mechanism could have a role in a number of bladder pathologies (e.g. idiopathic detrusor instability, interstitial cystitis and bladder-outflow obstruction), as well as in the aging bladder.44,45 In 2001, it has been shown in sensory neurons that ATP can potentiate the response of vanilloids (by lowering the threshold for protons, capsaicin and heat).46 This novel mechanism represents a means by which large amounts of ATP released from damaged or sensitized cells, in response to injury or inflammation, are thought to trigger the sensation of pain. These findings have clinical significance; they indicate that alterations to afferent nerves or epithelial cells in pelvic viscera might contribute to the sensory abnormalities observed in a number of pelvic disorders, such as interstitial cystitis—a chronic clinical disease characterized by urgency, frequency and bladder pain upon filling.47–49 Studies of a comparable disease in cats (i.e. feline interstitial cystitis) revealed an augmented stretch-evoked release of urothelium-derived ATP and changes in P2X and P2Y receptor profiles in urothelial cells.29,50,51 This observation is consistent with similar findings in patients with interstitial cystitis.43 These results suggest that urothelial sensor molecules exhibit plasticity in pathologic conditions, and might contribute to bladder pain syndromes.
Capsaicin and TRPV1
One example of a urothelial sensor molecule is transient receptor potential cation channel subfamily V member 1 (TRPV1), which is known to have a prominent role in nociception and in urinary bladder function (Figure 2). It is well established that painful sensations induced by capsaicin, the spicy substance in chili peppers, are caused by stimulation of TRPV1.52,53 This ion-channel protein is also activated by moderate heat, protons and lipid metabolites such as anandamide (an endogenous ligand of both cannabinoid and vanilloid receptors). TRPV1 is expressed throughout the afferent limb of the micturition reflex pathway, including in urinary bladder unmyelinated (C-fiber) nerves that detect bladder distension or the presence of irritant chemicals.54,55 In the urinary bladder, one of the more remarkable findings is that TRPV1 is not only expressed by afferent nerves in close contact with urothelial cells, but it is also expressed by urothelial cells (Figure 2).20,21 Activation of urothelial TRPV1 receptors with capsaicin or resiniferatoxin increases levels of intracellular Ca2+ and evokes neurotransmitter (NO or ATP) release in cultured cells. As noted in sensory neurons, these responses are enhanced by low pH, blocked by TRPV1 antagonists, and eliminated in TRPV1-null mice. In neurons, TRPV1 is thought to integrate or amplify the response to various stimuli and, therefore, has an essential role in the development of inflammation-induced hyperalgesia. It seems likely that urothelial TRPV1 might participate in a similar manner in the detection of irritant stimuli following bladder inflammation or infection.
Figure 2.
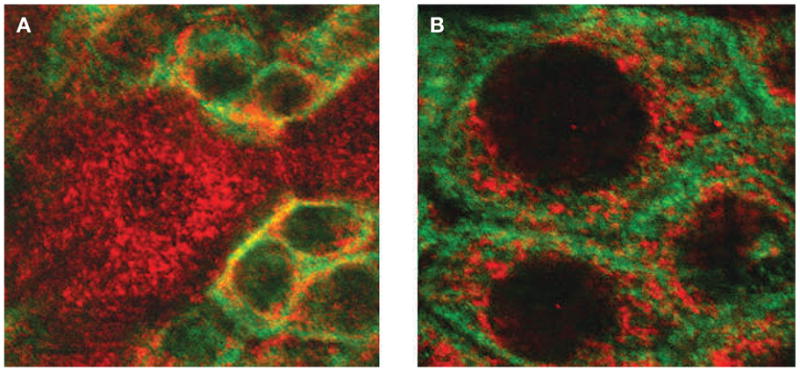
Expression of transient receptor potential cation channel subfamily V member 1 (TRPV1) in urothelial cells of the rat urinary bladder. (A) Confocal image of bladder urothelium in bladder whole mounts that have been immunofluorescently labeled for TRPV1 (cyanine 3, red) and cytokeratin 17 (fluorescein, green), a marker for basal urothelial cells. Scale bar 15 μm. (B) Enlarged image of basal cells depicting TRPV1 (cyanine 3, red) and cytokeratin (fluorescein, green) immunoreactivity. Scale bar 5 μm.
Although TRPV1-null mice are anatomically normal, they exhibit a number of alterations in bladder function, including a reduction of in vitro, stretch-evoked ATP release and membrane capacitance. Urothelial cells cultured from TRPV1-null mice also show a decrease in hypotonic-evoked ATP release.21 These findings demonstrate that the functional significance of TRPV1 in the bladder extends beyond an involvement in pain sensation to include participation in normal voiding functions. TRPV1 is essential for mechanically evoked purinergic signaling by the urothelium.
TRPV1 Involvement in hypersensitivity disorders
Intravesical instillation of vanilloids (capsaicin or resiniferatoxin) improves urodynamic parameters in patients with detrusor overactivity. This procedure has been reported to reduce bladder pain in some patients with hypersensitivity disorders, presumably by desensitization of bladder nerves.54,55 This treatment could also target TRPV1 on urothelial cells; persistent activation might lead to receptor desensitization or depletion of urothelial transmitters. Bladder biopsies taken from patients with neurogenic detrusor overactivity showed increased TRPV1 expression in both bladder nerves and the urothelium.8 In these patients, intravesical treatment with resiniferatoxin reduced TRPV1 immunoreactivity in both suburothelial afferent nerve cells and urothelial cells. Parallel changes in TRPV1 expression in both nerves and urothelial cells further support a neuron-like role for the urothelium, and indicate a possible role for TRPV1 in the pathophysiology of the neurogenic bladder.
The role of acetylcholine and muscarinic receptors in the overactive bladder
Patients with overactive bladder are typically treated with muscarinic-receptor antagonists.56,57 These agents prevent the stimulation of postjunctional muscarinic receptors by acetylcholine released from bladder efferent nerves and result in increased bladder capacity. While these agents also target muscarinic receptors on the bladder smooth muscle, evidence that the urothelium expresses the full complement of muscarinic receptors (M1–M5) has sparked an interest in finding out more about the role of the urothelium in the overactive bladder.31,58,59
Since antimuscarinic agents effectively enhance the storage phase of micturition, when parasympathetic nerves are silent, it is postulated that the release of acetylcholine from the urothelium might contribute to detrusor overactivity.60 In addition, release of acetylcholine from nearby bladder efferent nerves could activate urothelial muscarinic receptors. This activation, in turn, could lead to the release of mediators such as ATP that alter bladder sensation by stimulating nearby sensory afferent nerves.29 In addition, distension-evoked release of acetylcholine could target muscarinic receptors on smooth muscle and other cell types, as well as on bladder nerves. Accordingly, targeting muscarinic receptors activated by acetylcholine released from the urothelium and/or other urothelial-release mechanisms may prove to be an effective therapy. In this regard, the use of botulinum toxin has been investigated for treating a number of bladder disorders including neurogenic detrusor overactivity, detrusor-sphincter dyssynergia as well as interstitial cystitis.61–63 The inhibition of muscle contraction is due to the ability to block release of a number of transmitters (ACh; ATP) from bladder nerves by blocking exocytosis.64 However, besides targeting bladder nerves, botulinum toxins have recently been shown to prevent the release of transmitters from bladder urothelium.65
Role of adrenergic receptors in bladder outlet obstruction
The urothelium expresses both α and β subtypes of adrenergic receptors, which can trigger the release of a number of mediators including ATP and NO.28,66 It is possible that neurally released norepinephrine might have an effect on the urothelium, and might influence bladder function, because of the close proximity of adrenergic nerves that innervate blood vessels in the suburothelial region.22,23,67 Patients with a variety of lower urinary tract disorders including overactive bladder, benign prostatic hyperplasia and even interstitial cystitis—are often treated with selective α1-adrenergic antagonists.68,69 Studies in mice that lack the α1D adrenergic receptor have indicated that there is an important role for these receptors in the regulation of bladder function.70 The α1D adrenergic receptor is expressed within the bladder urothelium, and it seems that tonic activation of urothelial α1D receptors by catecholamines might be involved in bladder sensory mechanisms.71 Catecholamines could be released from nerves adjacent to the urothelium or from nerves that innervate nearby blood vessels, as the suburothelial region receives a rich blood supply. It has been shown that stimulation of both α-adrenergic and β-adrenergic receptors on urothelial cells evokes the release of NO.28,66 It has also been shown that cats diagnosed with feline interstitial cystitis exhibit an increase in norepinephrine content in the urinary bladder, compared with healthy cats.72 In addition, urothelial cells from cats with feline interstitial cystitis exhibit an augmented release of mediators including ATP and NO, which is similar to that observed in human interstitial cystitis.29,73 It is not known whether catecholamines are involved in the increased release of these mediators, or whether a tonic activation of the urothelium via circulating or other sources of catecholamines has a role in the sensory abnormalities of feline interstitial cystitis.
Nitric oxide
NO is thought to be involved in many functions of the lower urinary tract, ranging from inhibition of neurotransmission in the urethra to modulation of bladder afferent nerves and bladder reflex pathways in the spinal cord.74 The localization of neuronal nitric oxide synthase (NOS) and/or NADPH diaphorase (a marker for neuronal NOS) in efferent and afferent nerve fibers and the expression of multiple NOS isoforms (neuronal, endothelial, and inducible) within the urothelium indicate that NO has a role in the micturition reflex pathway.28,74 The release of NO from the urothelium or other cells is thought to either facilitate or inhibit the activity of bladder afferent nerves. For example, a reduction in NO levels adjacent to the urothelium (caused by intravesical administration of oxyhemoglobin) results in bladder hyperactivity, which suggests that there is an inhibitory role for NO in the control of bladder function.75 Injury or chronic inflammation has been shown to alter the expression of NOS, which raises the possibility that the neurotransmitter function of NO is plastic and can be altered by chronic pathologic conditions.76 For example, intravesical administration of NO donors suppresses the bladder hyperactivity in animals treated with cyclophosphamide.77,78 In addition, it has been reported that NO levels are decreased in patients with interstitial cystitis, a clinical syndrome that is characterized by bladder pain, urinary urgency and frequency.79 Alterations in NO levels could, therefore, affect the excitability of sensory fibers in the urinary bladder. NO might also have a role in epithelial function,80 as a change in NO production or synthesis in intestinal epithelial cells can increase their permeability to hydrophilic macromolecules. This mechanism might contribute to the loss of membrane barrier integrity in many disease states.
CONCLUSION AND FUTURE DIRECTIONS
Research during the last few years has markedly changed how we view the function of the urothelium. This tissue not only acts as a highly efficient barrier, but also exhibits properties similar to those of nociceptive and mechanoceptive afferent neurons. Activation of urothelial cells by chemical, thermal or mechanical stimuli can evoke the release of various mediators or neurotransmitters that influence neural activity and ultimately bladder function. These observations open new exciting avenues for research in urothelial biology—including pharmacologic interventions that aim to target urothelial receptor and ion channel expression, or neurotransmitter release mechanisms—could lead to new strategies for the clinical management of bladder disorders.
KEY POINTS.
The urothelium, a specialized lining of the urinary tract, has historically been viewed as a passive barrier to ions and solutes
There is evidence that the urothelium responds to both physiological and chemical stimuli and can release a number of signaling molecules
Release of chemical mediators from urothelial cells indicates that these cells have specialized sensory and signaling properties that could allow reciprocal communication with neighboring urothelial cells, as well as afferent and efferent nerves or other cells (i.e. myofibroblasts and immune or inflammatory cells) within the bladder wall
Various types of transient receptor potential channels including TRPV1 are expressed in the urothelium as well as in bladder afferent nerves
Results from TRPV1-null mice demonstrate that TRPV1 receptors are essential for normal mechanically evoked purinergic signaling by the urothelium, and indicate that the function of these receptors extends beyond pain sensation to include participation in normal bladder function
Acknowledgments
This work was supported by NIH grants to L Birder (RO1 DK54824 and RO1 DK57284).
Footnotes
Competing interests
The authors have declared associations with the following companies/organizations: Abbott Laboratories, Allergan, Astellas Pharma, Boehringer Ingleheim, Dynogen Pharmaceuticals, Eli Lilly, Hydra Biosciences, Johnson & Johnson, Lilly ICOS LLC, Novartis AG, Omeros, Pfizer, Roche Palo Alto LLC, and Sanofi Aventis. See the article online for full details of the relationship.
Contributor Information
Lori A Birder, Department of Medicine, Renal-Electrolyte Division, and also holds a secondary appointment in the Department of Pharmacology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
William C de Groat, Department of Pharmacology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
References
- 1.Apodaca G. The uroepithelium: not just a passive barrier. Traffic. 2004;5:117–128. doi: 10.1046/j.1600-0854.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 2.Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol. 2000;278:F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- 3.Wang E, et al. Hydrostatic pressure-regulated ion transport in bladder uroepithelium. Am J Physiol Renal Physiol. 2003;285:F651–F663. doi: 10.1152/ajprenal.00403.2002. [DOI] [PubMed] [Google Scholar]
- 4.Tammela T, et al. Urothelial permeability of the isolated whole bladder. Neurourol Urodyn. 1993;12:39–47. doi: 10.1002/nau.1930120106. [DOI] [PubMed] [Google Scholar]
- 5.Romih R, et al. Differentiation of epithelial cells in the urinary tract. Cell Tissue Res. 2005;320:259–268. doi: 10.1007/s00441-004-1005-4. [DOI] [PubMed] [Google Scholar]
- 6.Martin BF. Cell replacement and differentiation in transitional epithelium: a histological and autoradiographic study of the guinea-pig bladder and ureter. J Anatomy. 1972;112:433–455. [PMC free article] [PubMed] [Google Scholar]
- 7.Hicks M. The mammalian urinary bladder: an accommodating organ. Biol Rev. 1975;50:215–246. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 8.Brady CM, et al. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int. 2004;93:770–776. doi: 10.1111/j.1464-410X.2003.04722.x. [DOI] [PubMed] [Google Scholar]
- 9.Acharya P, et al. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol. 2004;287:F305–F318. doi: 10.1152/ajprenal.00341.2003. [DOI] [PubMed] [Google Scholar]
- 10.Parsons CL, et al. Antibacterial activity of bladder surface mucin duplicated by exogenous glycosaminoglycan (heparin) Infect Immun. 1979;24:552–557. doi: 10.1128/iai.24.2.552-557.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavelle J, et al. Bladder permeability barrier: recovery from selective injury of surface epithelial cells. Am J Physiol Renal Physiol. 2002;283:F242–F253. doi: 10.1152/ajprenal.00307.2001. [DOI] [PubMed] [Google Scholar]
- 12.Bassuk JA, et al. Induction of urothelial cell proliferation by fibroblast growth factor-7 in RAG1-deficient mice. Adv Exp Med Biol. 2003;539:623–633. doi: 10.1007/978-1-4419-8889-8_40. [DOI] [PubMed] [Google Scholar]
- 13.de Boer WI, et al. Functions of fibroblast and transforming growth factors in primary organ-like cultures of normal human urothelium. Lab Invest. 1996;75:147–156. [PubMed] [Google Scholar]
- 14.Anderson G, et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 15.Apodaca G, et al. Disruption of bladder epithelium barrier function after spinal cord injury. Am J Physiol Renal Physiol. 2003;284:F966–F976. doi: 10.1152/ajprenal.00359.2002. [DOI] [PubMed] [Google Scholar]
- 16.Truschel ST, et al. Involvement of nitric oxide (NO) in bladder afferent and urothelial abnormalities following chronic spinal cord injury [abstract #842.9] Soc Neurosci Abstr Program 2001 [Google Scholar]
- 17.Keay SK, et al. An antiproliferative factor from interstitial cystitis patients is a Frizzled 8 protein-related sialoglycopeptide. Proc Natl Acad Sci USA. 2004;101:11803–11808. doi: 10.1073/pnas.0404509101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conrads TP, et al. CKAP4/p63 is a receptor for the frizzled-8 protein-related antiproliferative factor from interstitial cystitis patients. J Biol Chem. doi: 10.1074/jbc.M604581200. in press. [DOI] [PubMed] [Google Scholar]
- 19.Schilling J, Hultgren S. Recent advances into the pathogenesis of recurrent urinary tract infections: the bladder as a reservoir for uropathogenic Escherichia coli. Int J Antimicrob Agents. 2002;19:457–460. doi: 10.1016/s0924-8579(02)00098-5. [DOI] [PubMed] [Google Scholar]
- 20.Birder L, et al. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birder L, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 22.Dickson A, et al. Peptidergic sensory and parasympathetic fiber sprouting in the mucosa of the rat urinary bladder in a chronic model of cyclophosphamide-induced cystitis. Neuroscience. 2006;139:671–685. doi: 10.1016/j.neuroscience.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 23.Jen PY, et al. Immunohistochemical localization of neuromarkers and neuropeptides in human fetal and neonatal urinary bladder. Br J Pharmacol. 1995;75:230–235. doi: 10.1111/j.1464-410x.1995.tb07317.x. [DOI] [PubMed] [Google Scholar]
- 24.Kunze A, et al. Quantitative immunohistochemical study of the innervation of the guinea-pig lower urinary tract. Br J Urol. 2006;98:424–429. doi: 10.1111/j.1464-410X.2006.06235.x. [DOI] [PubMed] [Google Scholar]
- 25.Sui GP, et al. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int. 2002;90:118–129. doi: 10.1046/j.1464-410x.2002.02834.x. [DOI] [PubMed] [Google Scholar]
- 26.Wiseman OJ, et al. The ultrastructure of bladder lamina propria nerves in healthy subjects and patients with detrusor hyperreflexia. J Urol. 2002;168:2040–2045. doi: 10.1016/S0022-5347(05)64291-7. [DOI] [PubMed] [Google Scholar]
- 27.Brading AF, McCloskey KD. Mechanisms of disease: specialized interstitial cells of the urinary tract—an assessment of current knowledge. Nat Clin Pract Urol. 2005;2:546–554. doi: 10.1038/ncpuro0340. [DOI] [PubMed] [Google Scholar]
- 28.Birder L, et al. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci. 2002;22:8063–8070. doi: 10.1523/JNEUROSCI.22-18-08063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birder L, et al. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol. 2003;285:F423–F429. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- 30.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 31.Chess-Williams R. Muscarinic receptors of the urinary bladder: detrusor, urothelial and prejunctional. Auton Autacoid Pharmacol. 2002;22:133–145. doi: 10.1046/j.1474-8673.2002.00258.x. [DOI] [PubMed] [Google Scholar]
- 32.Chopra B, et al. Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J Physiol. 2005;562:859–871. doi: 10.1113/jphysiol.2004.071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferguson DR, et al. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beckel J, et al. Expression of functional nicotinic acetylcholine receptors in rat bladder epithelial cells. Am J Physiol. 2006;290:F103–F110. doi: 10.1152/ajprenal.00098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petruska JC, Mendell LM. The many functions of nerve growth factor: multiple actions on nociceptors. Neurosci Lett. 2004;361:168–171. doi: 10.1016/j.neulet.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Wolf-Johnston AS, et al. Increased NGF and TRPV1 expression in urinary bladder and sensory neurons from cats with feline interstitial cystitis [abstract 608.4] Soc Neurosci Abstr Program 2003 [Google Scholar]
- 37.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2005;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 38.Carattino MD, et al. Mutations in the pore region modify epithelial sodium channel gating by shear stress. J Biol Chem. 2005;280:4393–4401. doi: 10.1074/jbc.M413123200. [DOI] [PubMed] [Google Scholar]
- 39.Kanai AJ, et al. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol. 2006 doi: 10.1152/ajprenal.00229.2006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang E, et al. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest. 2005;115:2412–2422. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cockayne DA, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 42.Brady CM, et al. P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur Urol. 2004;46:247–253. doi: 10.1016/j.eururo.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 43.Sun Y, et al. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol. 2001;166:1951–1956. [PubMed] [Google Scholar]
- 44.Burnstock G. Purinergic signaling. Br J Pharmacol. 2006;147 (Suppl 1):S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersson KE, Hedlund P. Pharmacologic perspective on the physiology of the lower urinary tract. Urology. 2002;60:13–20. doi: 10.1016/s0090-4295(02)01786-7. [DOI] [PubMed] [Google Scholar]
- 46.Tominaga M, et al. Potentiation of capsaicin receptor activation by metabotropic ATP receptors: a possible mechanism for ATP-evoked pain and hypersensitivity. Proc Natl Acad Sci USA. 2001;29:820–825. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nickel JC. Interstitial cystitis—an elusive clinical target? J Urol. 2003;170:816–817. doi: 10.1097/01.ju.0000081996.84687.ac. [DOI] [PubMed] [Google Scholar]
- 48.Parsons CL, et al. The role of urinary potassium in the pathogenesis and diagnosis of interstitial cystitis. J Urol. 1998;159:1862–1867. doi: 10.1016/S0022-5347(01)63178-1. [DOI] [PubMed] [Google Scholar]
- 49.Gillenwater JY, Wein AJ. Summary of the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases workshop on interstitial cystitis, National Institutes of Health, Bethesda, Maryland, August 28–29, 1987. J Urol. 1998;140:203–206. doi: 10.1016/s0022-5347(17)41529-1. [DOI] [PubMed] [Google Scholar]
- 50.Birder L, et al. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287:F1084–F1091. doi: 10.1152/ajprenal.00118.2004. [DOI] [PubMed] [Google Scholar]
- 51.Wellner MC, Isenberg G. Stretch effects on whole-cell currents of guinea pig urinary bladder myocytes. J Physiol. 1994;480:439–448. doi: 10.1113/jphysiol.1994.sp020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caterina MJ. The vanilloid receptor: a molecular gateway to the pain pathway. Ann Rev Neurosci. 2001;24:602–607. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 53.Caterina MJ, et al. The capsaicin receptor: a heat activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 54.Maggi CA. Therapeutic potential of capsaicin-like molecules: studies in animals and humans. Life Sci. 1992;51:1777–1781. doi: 10.1016/0024-3205(92)90047-s. [DOI] [PubMed] [Google Scholar]
- 55.Chancellor MB, de Groat WC. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J Urol. 1999;162:3–11. doi: 10.1097/00005392-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Staskin DR, MacDiarmid SA. Using anticholinergics to treat overactive bladder: the issue of treatment tolerability. Am J Med. 2006;119:9–15. doi: 10.1016/j.amjmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Pathak AS, Aboseif SR. Overactive bladder: drug therapy versus nerve stimulation. Nat Clin Pract Urol. 2005;2:310–311. doi: 10.1038/ncpuro0227. [DOI] [PubMed] [Google Scholar]
- 58.Abrams P, et al. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148:565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hegde SS. Muscarinic receptors in the bladder: from basic research to therapeutics. Br J Pharmacol. 2006;147 (Suppl 2):S80–S87. doi: 10.1038/sj.bjp.0706560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshida M, et al. Non-neuronal cholinergic system in human bladder urothelium. Urology. 2006;67:425–430. doi: 10.1016/j.urology.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 61.Tiwari A, Naruganahalli KS. Current and emerging investigational medical therapies for the treatment of overactive bladder. Expert Opin Investig Drugs. 2006;15:1017–1037. doi: 10.1517/13543784.15.9.1017. [DOI] [PubMed] [Google Scholar]
- 62.Chancellor MB. Urgency, botulinum toxin and how botulinum toxin can help urgency. J Urol. 2005;174:818. doi: 10.1097/01.ju.0000175099.01082.d0. [DOI] [PubMed] [Google Scholar]
- 63.Toft BR, Nordling J. Recent developments of intravesical therapy of painful bladder syndrome/interstitial cystitis: a review. Current Opin Urol. 2006;16:268–272. doi: 10.1097/01.mou.0000232048.81965.16. [DOI] [PubMed] [Google Scholar]
- 64.Apostolidis A, et al. Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol. 2006;49:644–650. doi: 10.1016/j.eururo.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 65.Smith CP, et al. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Neurochem Int. 2005;47:291–297. doi: 10.1016/j.neuint.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 66.Birder L, et al. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol Renal Physiol. 1998;275:F226–F229. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- 67.Inoue T, Gabella G. A vascular network closely linked to the epithelium of the urinary bladder of the rat. Cell Tissue Res. 1991;263:137–143. doi: 10.1007/BF00318409. [DOI] [PubMed] [Google Scholar]
- 68.Toh KL, Ng CK. Urodynamic studies in the evaluation of young men presenting with lower urinary tract symptoms. Int J Urol. 2006;13:520–523. doi: 10.1111/j.1442-2042.2006.01347.x. [DOI] [PubMed] [Google Scholar]
- 69.Yassin A, et al. Alpha-adrenoceptors are a common demoninator in the pathophysiology of erectile function and BPH/LUTS—implications for clinical practice. Andrologia. 2006;38:1–12. doi: 10.1111/j.1439-0272.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- 70.Chan Q, et al. Function of the lower urinary tract in mice lacking alpha1D-adrenoceptor. J Urol. 2005;174:370–374. doi: 10.1097/01.ju.0000161210.17365.cc. [DOI] [PubMed] [Google Scholar]
- 71.Ishihama H, et al. Activation of alpha1D adrenergic receptors in the rat urothelium facilitates the micturition reflex. J Urol. 2006;175:358–364. doi: 10.1016/S0022-5347(05)00016-9. [DOI] [PubMed] [Google Scholar]
- 72.Buffington CA, et al. Norepinephrine content and adrenoceptor function in the bladder of cats with feline interstitial cystitis. J Urol. 2002;167:1876–1880. [PubMed] [Google Scholar]
- 73.Birder L, et al. Altered inducible nitric oxide synthase expression and nitric oxide production in urinary bladder from cats with feline interstitial cystitis. J Urol. 2005;173:625–629. doi: 10.1097/01.ju.0000145900.22849.1d. [DOI] [PubMed] [Google Scholar]
- 74.Andersson KE, Persson K. Nitric oxide synthase and the lower urinary tract: possible implications for physiology and pathophysiology. Scand J Urol Nephrol Suppl. 1995;175:43–53. [PubMed] [Google Scholar]
- 75.Pandita RK, et al. Intravesical oxyhemoglobin initiates bladder overactivity in conscious, normal rats. J Urol. 2000;164:545–550. [PubMed] [Google Scholar]
- 76.de Groat WC, et al. Modification of urinary bladder function after spinal cord injury. Adv Neurol. 1997;72:347–364. [PubMed] [Google Scholar]
- 77.Ozawa H, et al. Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J Urol. 1999;162:2211–2216. doi: 10.1016/S0022-5347(05)68161-X. [DOI] [PubMed] [Google Scholar]
- 78.Korkmaz A, et al. Peroxynitrite may be involved in bladder damage caused by cyclophosphamide in rats. J Urol. 2005;173:1793–1796. doi: 10.1097/01.ju.0000154344.80669.e3. [DOI] [PubMed] [Google Scholar]
- 79.Hosseini A, et al. Nitric oxide as an objective marker for evaluation of treatment response in patients with classic interstitial cystitis. J Urol. 2004;172:2261–2265. doi: 10.1097/01.ju.0000144761.69398.be. [DOI] [PubMed] [Google Scholar]
- 80.Kolios G, et al. Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunology. 2004;113:427–437. doi: 10.1111/j.1365-2567.2004.01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]