SUMMARY
Botulinum toxins can effectively and selectively disrupt and modulate neurotransmission in striated muscle. Recently, urologists have become interested in the use of these toxins in patients with detrusor overactivity and other urological disorders. In both striated and smooth muscle, botulinum toxin A (BTX-A) is internalized by presynaptic neurons after binding to an extracellular receptor (ganglioside and presumably synaptic vesicle protein 2C). In the neuronal cytosol, BTX-A disrupts fusion of the acetylcholine-containing vesicle with the neuronal wall by cleaving the SNAP-25 protein in the synaptic fusion complex. The net effect is selective paralysis of the low-grade contractions of the unstable detrusor, while still allowing high-grade contraction that initiates micturition. Additionally, BTX-A seems to have effects on afferent nerve activity by modulating the release of ATP in the urothelium, blocking the release of substance P, calcitonin gene-related peptide and glutamate from afferent nerves, and reducing levels of nerve growth factor. These effects on sensory feedback loops might not only help to explain the mechanism of BTX-A in relieving symptoms of overactive bladder, but also suggest a potential role for BTX-A in the relief of hyperalgesia associated with lower urinary tract disorders.
Keywords: botulinum toxin A, detrusor, mechanism of action, muscle, overactive bladder
INTRODUCTION
Botulinum toxins are produced by the anaerobic, Gram-positive organism Clostridium botulinum. Of the seven different strains (serotypes A through G), only two (serotypes A and B) are commercially available. All serotypes block transmission at neuromuscular junctions to varying degrees, but the effects of botulinum neurotoxin type A (BTX-A) are the most prolonged; this serotype has been the most extensively studied, mainly in striated muscle.1–3 BTX-A has received regulatory approval for use in several disorders characterized by excessive muscle contractility or tonus in striated muscle (e.g. cervical dystonia, blepharospasm, hemifacial spasm, and limb spasticity) or overactive secretion of sweat (e.g. hyperhidrosis). More recently, researchers have discovered that BTX-A is effective in disorders such as overactive bladder and esophageal spasm, which suggests that its effects in smooth muscle may be similar to those in striated muscle.4 In addition, the ability to obtain bladder biopsy specimens during intradetrusor administration has led to new understanding of how BTX-A acts on smooth muscle. BTX-A is currently being used in clinical trials for urological disorders in the USA and the European Union; therefore, it is important to understand the mechanism of how BTX-A works, and to investigate any differences that might exist when it is applied to different tissue types.
A step-wise mechanism of action of BTX-A was first suggested by Simpson in 1979, and involves the toxin binding to, and internalization within, the presynaptic membrane of cholinergic neurons, followed by translocation into the neuronal cytosol and then inhibition of acetylcholine release.5 This Review explores the mechanisms of BTX-A activity, its structure and its effects on both striated and smooth muscles.
MECHANISM OF ACTION
Internalization and action at the neuromuscular junction
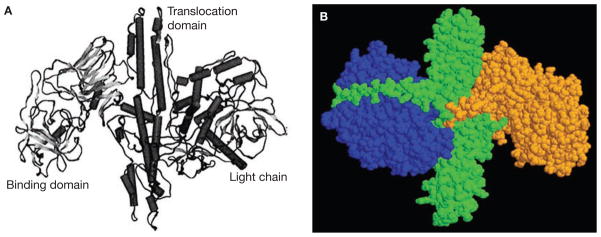
BTX-A is initially synthesized as an inactive, single chain of 1,285 amino acids.6 Activation occurs when the single chain is cleaved by an endogenous clostridial protease,7,8 resulting in a polypeptide that contains a 50 kDa light chain and a 100 kDa heavy chain covalently linked by a single disulphide bond.9 BTX-A is folded into three functional domains that directly affect the toxin’s action on target cells (Figure 1A,B).6,8,10,11 The light chain contains a zinc-dependent endopeptidase catalytic domain, while the heavy chain contains a translocation domain at its N-terminal end and a binding domain at its C-terminus.9,12 The functional domains work together to inhibit signal transmission at the neuromuscular junction in three discrete stages: binding and internalization, translocation, and exocytosis inhibition.
Figure 1.
Structure of BTX-A. (A) 3D crystalline structure of BTX-A. (B) Alternative 3D structural view of BTX-A. The light chain is shown in blue. The remainder of the molecule forms the heavy chain, its C-terminus shown in orange and its N-terminus shown in green. Abbreviation: BTX-A, botulinum toxin A. Permission obtained from Bentham Science Publishers © Aoki KR et al. (2004) Curr Med Chem 11: 3085–3092 (part A) and from Annual Reviews © Simpson LL (2004) Annu Rev Pharmacol Toxicol 44: 167–193 (part B).
Binding and internalization
BTX-A attaches to ectoacceptors on the cholinergic nerve terminal in muscle via its heavy-chain binding domain.13–16 Studies on cultured hippocampal neurons from the rat or mouse phrenic nerve of the hemidiaphragm have shown that BTX-A recognizes and enters the ganglioside during neurotransmitter exocytosis when more-active receptors are exposed, exploiting the secretory vesicle recycling pathway.17,18 Thus, endocytosis of BTX-A is enhanced by synaptic activity.17,19 Once internalization has occurred, the BTX-A activity remains within the vicinity of the nerve terminal.
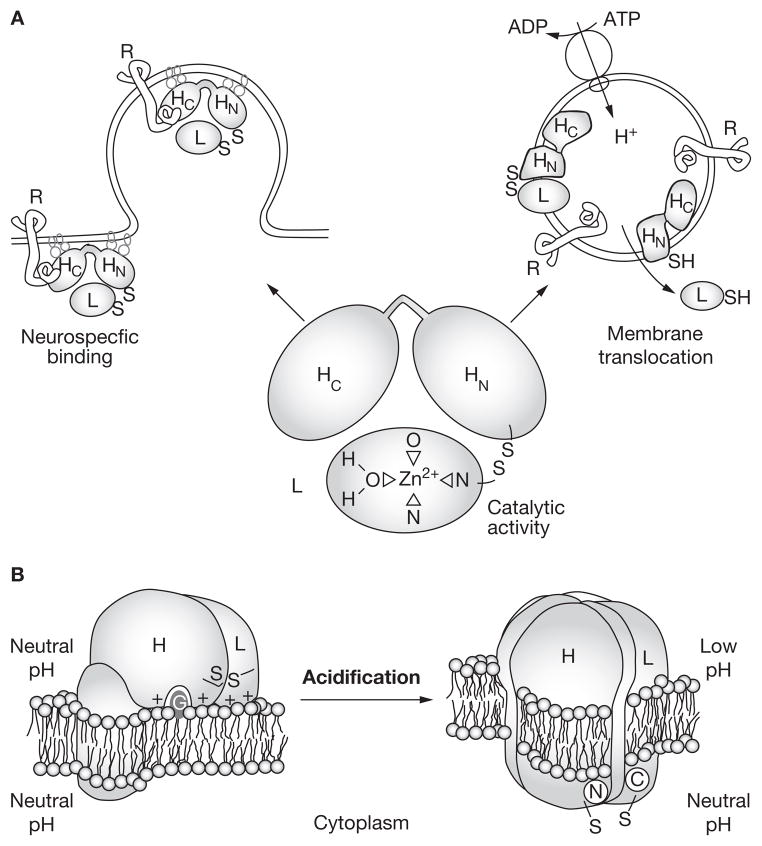
Translocation
Once internalized and sequestered within a vesicle, BTX-A undergoes a pH-dependent conformational change that causes the dissociation of the heavy and light chains and increases the toxin’s hydrophobicity.20–22 The heavy chain is essential in the pH-dependent creation of ion channels or pores in the vesicle wall (Figure 2)20,23–25 and distributes the light chain to the pore,26,27 but only the light chain undergoes translocation into the cytosol. Although the pores seem to be too small to allow light-chain passage,6,28 possible mechanisms by which this may be overcome include the light chain changing shape at low pH,10,21,26,29–31 and the light chain divesting its zinc atom (subsequently reacquiring zinc from the cytosol to regain its protease properties).30,31
Figure 2.
Internalization and translocation of BTX-A. (A) BTX-A binds to specific receptors on the neuronal surface via the heavy chain C-terminal binding domain, and enters the neuron by endocytosis. Once within the vesicle, BTX-A undergoes a pH-dependent conformational change that results in dissociation of the light and heavy chains. The catalytic light chain is translocated to the neuron’s cytosol through pores that are formed by the heavy chain N-terminal translocation domain. (B) The BTX-A heavy chain undergoes various conformational changes upon acidification of the vesicle interior, which allows the formation of pores through which the light chain can pass into the cytosol. Abbreviations: BTX-A, botulinum toxin A; H, heavy chain; L, light chain; R, receptor. Permission obtained from Elsevier © Schiavo G et al. (1990) J Physiol (Paris) 84: 180–187.
Exocytosis inhibition
Under normal physiological conditions, action potentials within motor neurons cause acetylcholine-containing synaptic vesicles to fuse with the presynaptic membrane where they release the neurotransmitter into the neuromuscular junction (Figure 3A–C).32 Vesicle fusion is mediated by a set of SNARE (soluble N-ethylmaleimide-sensitive fusion attachment protein receptor) proteins, two of which (syntaxin and SNAP-25 [synaptosome-associated protein 25 kDa]) are anchored in the wall of the presynaptic membrane, and the third (synaptobrevin) is associated with the vesicle (Figure 3A).32 Liberated acetylcholine then diffuses across the synaptic cleft and binds to receptors on the surface of muscle cells, triggering contraction. There are other soluble proteins that are involved in exocytosis, but, owing to space constraints, this Review will focus on SNARE proteins.33,34
Figure 3.
Inhibition of acetylcholine exocytosis by BTX-A. (A,B) Release of acetylcholine at the neuromuscular junction is mediated by the assembly of a synaptic fusion complex that allows the membrane of the acetylcholine-containing synaptic vesicle to fuse with the neuronal cell membrane. The synaptic fusion complex is a set of SNARE proteins, which includes synaptobrevin, SNAP-25 and syntaxin. (C) After membrane fusion, acetylcholine is released into the synaptic cleft and then bound by receptors on the muscle cell. (D) BTX-A binds to the neuronal cell membrane at the nerve terminus and enters the neuron by endocytosis. (E,F) The light chain of BTX-A cleaves specific sites on the SNAP-25 protein, preventing complete assembly of the synaptic fusion complex and thereby blocking acetylcholine release. Without acetylcholine release, the muscle is unable to contract. Abbreviations: BTX-A, botulinum toxin A; SNAP-25, synaptosome-associated protein 25 kDa; SNARE, soluble N-ethylmaleimide-sensitive fusion attachment protein receptor. Permission obtained from Elsevier © Smith CP and Chancellor MB (2004) J Urol 171: 2128–2137.
In nerve terminals affected by BTX, the light chain cleaves specific peptide bonds in the synaptic fusion complex, preventing exocytosis of the acetylcholine vesicle at the nerve terminal, and therefore inhibiting neurotransmission and muscle contraction (Figure 3D–F).32 Each BTX serotype cleaves a distinct protein site; BTX-A cleaves SNAP-25.19,35–37 BTX-E also cleaves SNAP-25, whereas serotypes B, D, F and G cleave synaptobrevin, and BTX-C primarily cleaves syntaxin, although it also has the ability to cleave SNAP-25.8 The cleavage of SNAP-25 by BTX-A is extremely specific because BTX-A recognizes a highly conserved, nine-residue binding motif that occurs four times within the SNAP-25 molecule.38–41
The differences in SNARE-binding profiles between the BTX serotypes may explain the different amount of time it takes for each protein to inhibit acetylcholine exocytosis.42,43 Alternatively, the intraneuronal localization may affect the duration of inhibition.39 BTX-A has the longest duration of activity when it is used for the treatment of dystonias, inducing clinical effects on neuromuscular activity for longer than 4 months, compared with about 2 months for BTX-B and less than 4 weeks for BTX-E.44,45 Cell-culture studies that calculated the inhibitory half-life of BTX-A provided further evidence for a prolonged effect of BTX-A; the inhibitory half-life of BTX-A was more than 31 days, compared with 10 days for BTX-B, 2 days for BTX-F, and 19–20 h for BTX-E.42 Recovery of cholinergic neurotransmission is dependent on removal of the BTX protease and restoration of intact SNARE proteins.19,42,46
ACTION ON STRIATED VERSUS SMOOTH MUSCLE
None of the clinically available clostridial neurotoxins cause death of neurons or myocytes, or changes in other cellular constituents. Thus, BTX serotypes are not toxic to tissue; however, in muscle they act as biochemical neuromodulators, temporarily inactivating cholinergic transmission at the neuromuscular junction.
In striated muscle, BTX-A administration interferes with signaling between α motor neurons and extrafusal muscle fibers, as well as signaling between γ motor neurons and the intrafusal fibers of muscle spindles.47–49 During the initial weeks after injection, muscles treated with BTX-A show some variability in the size of muscle fibers, and demonstrate intra-cellular changes consistent with denervation (i.e. central mitochondria dispersed toward the periphery).50,51 These effects are temporary, and BTX-A does not, therefore, produce persistent changes in the muscle fiber after recovery from paralysis.50–52 BTX-A can produce beneficial gross muscular changes, such as reversing hypertrophy in dystonic muscle groups.53
Physiological changes induced by BTX-A include a reduction in resting membrane potentials and fibrillation potentials, and the elimination of extrajunctional acetylcholinesterase activity.54 There is also evidence forncpuro_2007_279f3.eps subsequent compensatory nerve sprouting and the creation of extrajunctional synapses.55–58 When exocytosis at the parent terminal eventually recovers, the nerves retract and endplate functioning returns to normal.55,59
BTX-A administration has a similar effect on neuroeffector transmission in both smooth and striated muscle. When BTX-A is administered to the bladder wall there is an increase in bladder capacity, with a reduction in incontinence episodes and symptoms of urgency. If high doses of BTX-A are used, near-complete neuromuscular blockade of the detrusor results in impaired voiding and/or urinary retention.4,60–63 However, there is no compelling evidence at this time that BTX-A acts on the detrusor smooth muscle.
CELLULAR ACTION TO CLINICAL EFFECT
In healthy bladders, the smooth muscle bundles of the detrusor are not well coupled electrically; therefore, the detrusor’s smooth muscle requires dense innervation. During the filling of a healthy bladder, parasympathetic excitatory neurons are silent and the detrusor is relatively quiescent, allowing storage of urine at a low pressure. Micturition is induced by activation of the parasympathetic nerves and release of acetylcholine, which stimulates postjunctional M3 muscarinic receptors to induce a large-amplitude destrusor contraction. Acetylcholine also stimulates prejunctional M1 muscarinic receptors on post-ganglionic parasympathetic nerve terminals to facilitate its own release, and thereby amplify excitatory input to the detrusor.64
In overactive bladders, there is increased coupling of the smooth muscle bundles of the detrusor, which leads to greater nerve excitability in response to low-grade efferent stimuli.65 Bladder strips from animals with spinal cord injuries show an upregulation of presynaptic muscarinic facilitatory mechanisms in cholinergic nerve terminals.66 This upregulation in the cholinergic nerve terminals may also contribute to the raised contractile response to low-grade stimulation, and suggests that such increased synaptic activity would render the neurons associated with the smooth muscle of the detrusor highly susceptible to BTX-A binding and internalization.
Effects on acetylcholine and ATP release
In animal bladder models, BTX-A inhibited the release of acetylcholine in response to high-grade, but not low-grade, stimulation.63 ATP has also been implicated as a neurotransmitter in the generation of unstable contractions in idiopathic detrusor overactivity.67–69 Animal studies of bladder strips from guinea pigs70 and rats71 indicate that BTX-A inhibits the release of both acetylcholine and ATP, providing a rationale for its possible use in treating patients with idiopathic detrusor overactivity.
There is increasing evidence that the urothelium acts as a mechanosensor, releasing ATP that activates purinergic receptors in subepithelial neurons; these in turn convey information to the central nervous system to activate micturition.72,73 In conditions of increased sensory nerve transmission after chronic inflammation and spinal cord injury,74 increased release of ATP from the urothelium might activate the ATP receptor P2X3 in epithelial and subepithelial layers to increase afferent nerve activity, which would account for the higher frequency of bladder contractions that are reported in both human and animal models of spinal cord injury.74
A study in 2004 showed that BTX-A inhibits ATP release from the urothelial but not the serosal side of the bladder, suggesting that BTX-A inhibits transmitter release not only from efferent nerve endings but from sensory nerve terminals or urothelium as well.74 The findings indicate that the release of ATP is, at least partly, vesicular. Although BTX-A action at the neuromuscular junction is SNAP-25-dependent, this may not be the case with urothelial ATP release, since SNAP-25 has not been found in the urothelium. A plausible explanation for the clinical efficacy of BTX-A in the treatment of human neurogenic bladder dysfunction is that it impairs urothelial ATP release after spinal cord injury.
Effect on P2X3 and capsaicin-sensitive TRPV1 sensory receptors
Extracellular ATP is implicated in several sensory processes that range from pain response to regulation of organ motility. The ATP receptor P2X3 is almost exclusively expressed in sensory neurons, and there is accumulating evidence that this receptor has a specific role in nociception. Activation of P2X3 by ATP leads to a much stronger nociceptive effect in inflamed tissue compared with normal tissue.75,76
The TRPV1 receptor (transient receptor potential channel, vanilloid family member 1) is believed to function as an integrator of noxious stimuli, such as acids, heat, pollutants with a negative electronic charge, and endogenous proinflammatory substances.77 Specifically, TRPV1 has a key role in the perception of peripheral thermal and inflammatory pain.78 Recent findings indicate that BTX-A blocks TRPV1 membrane translocation induced by protein kinase C, which suggests that activity-dependent delivery of channels to the neuronal surface may contribute to the build-up and maintenance of thermal inflammatory hyperalgesia in peripheral nociceptor terminals.79,80
Successful BTX-A treatment for overactive bladder is associated with substantially reduced levels of TRPV1 and/or P2X3 expression in suburothelial nerve fibers.81 These changes may indicate a direct effect of BTX-A on the afferent innervation of the bladder, and/or may be secondary to the action of BTX-A on the efferent innervation of the detrusor.81 The progressive reduction in suburothelial sensory receptor levels suggests either a cascade mechanism of action involving complex inhibition at a local and central level, or a prolonged inhibitory effect of the cleaved product of SNAP-25.72 A 2007 study showed the possible persistence of a SNAP-25 cleavage product in biopsy specimens of detrusor muscle injected with BTX-A from patients with myelomeningocele who did not respond adequately to BTX-A treatment.82
Effects on calcitonin gene-related peptide and substance P release
Sensory axons in the bladder contain both calcitonin gene-related peptide (CGRP) and substance P. These neuropeptides are released from nociceptive sensory endings in response to noxious stimuli, and function as inflammatory response mediators.83 Substance P acts on mast cells to induce degranulation, resulting in release of histamine and cytokines, which directly sensitizes or excites nociceptors.83 Together with bradykinin and prostaglandins, substance P and CGRP also cause migration of leukocytes to the site of injury and clotting responses.84,85
Substance P and CGRP levels may have a role in the pathophysiology of overactive bladder; both neuropeptides have been reported at high concentrations in biopsy samples from the bladders of women with overactive bladder.86 Preclinical models have shown that BTX-A blocks the release of CGRP, substance P and glutamate from afferent nerve terminals.84,87 The proposed effect of BTX-A on sensory pathways is supported by data from preclinical models of bladder pain performed in rats, in which intravesical application of BTX-A significantly reduced pain responses and inhibited CGRP release from afferent nerve terminals.87,88 These findings may help to explain the mechanism of action of BTX-A in overactive bladder, as well as suggesting that BTX-A might have clinical use in disorders such as interstitial cystitis and sensory urgency.
Inhibition of nerve growth factor release and receptor transport
Nerve growth factor (NGF) is a signaling protein that is produced in the smooth muscle of the urinary tract and urothelium of the bladder. In both animals and humans, bladder production of NGF increases in response to disorders such as spinal cord injury, denervation, inflammation, distension and hypertrophy.89 Raised NGF levels have been reported to trigger bladder overactivity, such as that seen in men with benign prostatic hyperplasia, women with interstitial cystitis, and patients with idiopathic detrusor overactivity. Intravesical BTX-A injection lowers the NGF content in the bladder tissue of patients with neurogenic detrusor overactivity; however, it is unknown whether reduced bladder NGF results from decreased production, reduced uptake or a combination of both.90
Sensory effects of BTX-A: evidence from nonurological medical specialties
Neurologists have long recognized a reduction in pain in patients after intramuscular injection of BTX-A; this benefit is often reported before there is evidence of muscle relaxation, and pain relief often exceeds and outlasts the reduction in spasm, suggesting a direct analgesic effect.91–93
Changes in afferent activity may influence pain through both direct sensory effects and via the reorganization of the central nervous system, owing to prolonged, reduced muscle spindle feedback into the central nervous system caused by inhibition of acetylcholine release from γ motor neurons.92
Histological effects of BTX-A injection
Only one study, by Haferkamp and colleagues,94 has examined ultrastructural changes in overactive human detrusor tissue after BTX-A injection. They collected 30 biopsy specimens from 24 patients with neurogenic overactive bladder, before and 3 months after BTX-A injection and during the wearing-off phase of the toxin’s efficacy. The researchers reported no significant changes in muscle cell fascicles, intercellular collagen content or muscle-cell degeneration when they compared biopsy samples taken before and after BTX-A administration, although these results cannot be extrapolated to the possible structural effects of repeat injections. Unlike in striated muscle, axonal sprouting in detrusor smooth muscle was limited after BTX-A administration. The results of an immunohistochemical study also suggested no significant axonal sprouting in the suburothelium of successfully treated patients.81
A single retrospective study has investigated the histopathological changes in excised human neurogenic overactive bladder tissue that might have been associated with intradetrusor BTX-A injection.95 Full-thickness specimens from bladders previously treated with one or more injections of BTX-A showed significantly less fibrosis, but no differences in inflammation and edema compared with untreated bladders. There were no significant differences between specimens from responders and nonresponders to BTX-A with regard to inflammation, edema and fibrosis caused by BTX-A, although there was a trend towards reduced fibrosis and edema in responders. Treated bladders were injected with a mean of 1.5 ± 0.8 injections, and the mean time between the last injection and surgery was 6.8 ± 2.8 months.95
The action of BTX-A seems to be reversible, long-lasting, and does not induce any enduring pathological changes; therefore, it has theoretical longevity in terms of its clinical usefulness in urological dysfunction. Regular BTX-A blockade of striated muscle activity over a 12-year period was shown to be clinically safe and effective in a study by Mejia and colleagues;96 however, the long-term effects at a cellular level cannot be determined because biopsy was not performed. Although most long-term results are positive, more data are needed from both smooth and striatal muscle, as there is still much to learn about the effects of long-term exposure to BTX-A.
CONCLUSIONS
BTX-A is effective for treating various disorders that involve pathological neuromuscular activity. Advances have been made in our understanding of how BTX-A works at a molecular level, yet questions remain. Further research is needed to characterize the protein receptors to which BTX-A binds, to expand our understanding of light-chain translocation within the motor neuron, to establish the process by which the light chain interacts with SNAP-25, and to explore the toxin’s long-term effects on smooth muscle cells. Although there has been speculation that diffusion or proteolysis of the toxin’s light chain eventually occurs, the mechanism for the durability as well as loss of BTX-A action remains to be determined. In addition, much of the evidence for the mechanism of action of BTX-A is based on animal tissue (nonprimate mammalian), which raises the possibility of differences in how BTX-A will affect humans; therefore, further study using specimens from humans and/or other primates would be beneficial.
BTX-A acts by binding to the nerve endings within muscles, blocking the release of acetylcholine, and probably other neurotransmitters, to modulate muscle contraction and reduce the sensitization of sensory nerve endings. There is increasing evidence for an additional, direct effect on the sensory (bladder) pathways and subsequent modulation of the efferent overactivity or hyperexcitability involved in the pathophysiology of the detrusor and other muscle dysfunction. BTX-A has a low potential for migration to surrounding and distant tissues, so selective injection permits specific paralysis of the overactive detrusor muscle. Therapy with BTX-A does not only help alleviate muscle spasticity, but, in view of its proposed anti-nociceptive properties and its effects on sensory feedback loops, could also provide substantial relief of hyperalgesia associated with various lower urinary tract disorders.
KEY POINTS.
There has been recent interest in the potential use of botulinum toxins, particularly botulinum toxin A (BTX-A), in patients with detrusor overactivity and other urological disorders
After internalization of BTX-A into the cytosol of presynaptic neurons, it disrupts fusion of the acetylcholine-containing vesicle with the neuronal wall by cleaving the SNAP-25 protein in the synaptic fusion complex
In detrusor overactivity, the effect of BTX-A results in selective paralysis of the low-grade contractions of the unstable detrusor, while still allowing the high-grade contractions that initiate micturition
BTX-A also seems to affect afferent nerve activity by modulating the release of ATP in the urothelium, blocking the release of substance P, calcitonin gene-related peptide and glutamate from afferent nerves, and reducing the levels of nerve growth factor
In view of its effects on sensory feedback loops, BTX-A could have a potential role in relieving hyperalgesia associated with lower urinary tract disorders, in addition to its reported beneficial effects on symptoms of overactive bladder
Acknowledgments
Natalie Avenell-Mills provided research and editorial assistance for the development of the manuscript, with support from Allergan, Inc.
Footnotes
Competing interests
The authors have declared associations with the following company/organization: Allergan, Inc. See the article online for full details of the relationships.
Contributor Information
Michael B Chancellor, Neurourology Program in the Department of Urology at the William Beaumont Hospital in Royal Oak, MI, USA.
Clare J Fowler, Uroneurology at the National Hospital of Neurology & Neurosurgery in Queen Square, London, UK.
Apostolos Apostolidis, Neurourology at the 2nd Department of Urology at the Aristotle University of Thessaloniki, Greece.
William C de Groat, Pharmacology at the University of Pittsburgh School of Medicine in Pittsburgh, PA, USA.
Christopher P Smith, Scott Department of Urology, Baylor College of Medicine, Houston, TX, USA.
George T Somogyi, Scott Department of Urology Neurourology Laboratory and a Professor in the Scott Department of Urology at the Baylor College of Medicine, Houston, TX, USA.
K Roger Aoki, Biological Sciences Department at Allergan, Inc.
References
- 1.Billante CR, et al. Comparison of neuromuscular blockade and recovery with botulinum toxins A and F. Muscle Nerve. 2002;26:395–403. doi: 10.1002/mus.10213. [DOI] [PubMed] [Google Scholar]
- 2.Jurasinski CV, et al. Correlation of cleavage of SNAP-25 with muscle function in a rat model of Botulinum neurotoxin type A induced paralysis. Toxicon. 2001;39:1309–1315. doi: 10.1016/s0041-0101(01)00082-4. [DOI] [PubMed] [Google Scholar]
- 3.Shen J, et al. How muscles recover from paresis and atrophy after intramuscular injection of botulinum toxin A: study in juvenile rats. J Orthop Res. 2006;24:1128–1135. doi: 10.1002/jor.20131. [DOI] [PubMed] [Google Scholar]
- 4.Schurch B, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200. doi: 10.1097/01.ju.0000162035.73977.1c. [DOI] [PubMed] [Google Scholar]
- 5.Simpson LL. The action of botulinal toxin. Rev Infect Dis. 1979;1:656–662. doi: 10.1093/clinids/1.4.656. [DOI] [PubMed] [Google Scholar]
- 6.Lacy DB, et al. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat Struct Biol. 1998;5:898–902. doi: 10.1038/2338. [DOI] [PubMed] [Google Scholar]
- 7.Aoki KR, Guyer B. Botulinum toxin type A and other botulinum toxin serotypes: a comparative review of biochemical and pharmacological actions. Eur J Neurol. 2001;8 (suppl 5):21–29. doi: 10.1046/j.1468-1331.2001.00035.x. [DOI] [PubMed] [Google Scholar]
- 8.Simpson LL. Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol. 2004;44:167–193. doi: 10.1146/annurev.pharmtox.44.101802.121554. [DOI] [PubMed] [Google Scholar]
- 9.Kozaki S, et al. Immunological characterization of papain-induced fragments of Clostridium botulinum type A neurotoxin and interaction of the fragments with brain synaptosomes. Infect Immun. 1989;57:2634–2639. doi: 10.1128/iai.57.9.2634-2639.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki KR. Botulinum toxin: a successful therapeutic protein. Curr Med Chem. 2004;11:3085–3092. doi: 10.2174/0929867043363802. [DOI] [PubMed] [Google Scholar]
- 11.Segelke B, et al. Crystal structure of Clostridium botulinum neurotoxin protease in a product-bound state: evidence for noncanonical zinc protease activity. Proc Natl Acad Sci U S A. 2004;101:6888–6893. doi: 10.1073/pnas.0400584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bandyopadhyay S, et al. Role of the heavy and light chains of botulinum neurotoxin in neuromuscular paralysis. J Biol Chem. 1987;262:2660–2663. [PubMed] [Google Scholar]
- 13.Black JD, Dolly JO. Interaction of 125I-labeled botulinum neurotoxins with nerve terminals. II. Autoradiographic evidence for its uptake into motor nerves by acceptor-mediated endocytosis. J Cell Biol. 1986;103:535–544. doi: 10.1083/jcb.103.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolly JO, et al. Acceptors for botulinum neurotoxin reside on motor nerve terminals and mediate its internalization. Nature. 1984;307:457–460. doi: 10.1038/307457a0. [DOI] [PubMed] [Google Scholar]
- 15.Black JD, Dolly JO. Selective location of acceptors for botulinum neurotoxin A in the central and peripheral nervous systems. Neuroscience. 1987;23:767–779. doi: 10.1016/0306-4522(87)90094-7. [DOI] [PubMed] [Google Scholar]
- 16.Williams RS, et al. Radioiodination of botulinum neurotoxin type A with retention of biological activity and its binding to brain synaptosomes. Eur J Biochem. 1983;131:437–445. doi: 10.1111/j.1432-1033.1983.tb07282.x. [DOI] [PubMed] [Google Scholar]
- 17.Dong M, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312:592–596. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- 18.Mahrhold S, et al. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 2006;580:2011–2014. doi: 10.1016/j.febslet.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 19.Keller JE, Neale EA. The role of the synaptic protein SNAP-25 in the potency of botulinum neurotoxin type A. J Biol Chem. 2001;276:13476–13482. doi: 10.1074/jbc.M010992200. [DOI] [PubMed] [Google Scholar]
- 20.Blaustein RO, et al. The N-terminal half of the heavy chain of botulinum type A neurotoxin forms channels in planar phospholipid bilayers. FEBS Lett. 1987;226:115–120. doi: 10.1016/0014-5793(87)80562-8. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Singh BR. Spectroscopic analysis of pH-induced changes in the molecular features of type A botulinum neurotoxin light chain. Biochemistry. 2000;39:6466–6474. doi: 10.1021/bi992729u. [DOI] [PubMed] [Google Scholar]
- 22.Montecucco C, et al. Bacterial protein toxins penetrate cells via a four-step mechanism. FEBS Lett. 1994;346:92–98. doi: 10.1016/0014-5793(94)00449-8. [DOI] [PubMed] [Google Scholar]
- 23.Kalandakanond S, Coffield JA. Cleavage of SNAP-25 by botulinum toxin type A requires receptor-mediated endocytosis, pH-dependent translocation, and zinc. J Pharmacol Exp Ther. 2001;296:980–986. [PubMed] [Google Scholar]
- 24.Schiavo G, et al. Membrane interactions of tetanus and botulinum neurotoxins: a photolabelling study with photoactivatable phospholipids. J Physiol (Paris) 1990;84:180–187. [PubMed] [Google Scholar]
- 25.Schmid MF, et al. Direct visualization of botulinum neurotoxin-induced channels in phospholipid vesicles. Nature. 1993;364:827–830. doi: 10.1038/364827a0. [DOI] [PubMed] [Google Scholar]
- 26.Koriazova LK, Montal M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat Struct Biol. 2003;10:13–18. doi: 10.1038/nsb879. [DOI] [PubMed] [Google Scholar]
- 27.Hoch DH, et al. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers: relevance to translocation of proteins across membranes. Proc Natl Acad Sci U S A. 1985;82:1692–1696. doi: 10.1073/pnas.82.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheridan RE. Gating and permeability of ion channels produced by botulinum toxin types A and E in PC12 cell membranes. Toxicon. 1998;36:703–717. doi: 10.1016/s0041-0101(97)00131-1. [DOI] [PubMed] [Google Scholar]
- 29.Fu FN, et al. Spectroscopic analysis of low pH and lipid-induced structural changes in type A botulinum neurotoxin relevant to membrane channel formation and translocation. Biophys Chem. 2002;99:17–29. doi: 10.1016/s0301-4622(02)00135-7. [DOI] [PubMed] [Google Scholar]
- 30.Simpson LL, et al. Chelation of zinc antagonizes the neuromuscular blocking properties of the seven serotypes of botulinum neurotoxin as well as tetanus toxin. J Pharmacol Exp Ther. 1993;267:720–727. [PubMed] [Google Scholar]
- 31.Simpson LL, et al. The role of zinc binding in the biological activity of botulinum toxin. J Biol Chem. 2001;276:27034–27041. doi: 10.1074/jbc.M102172200. [DOI] [PubMed] [Google Scholar]
- 32.Smith CP, Chancellor MB. Emerging role of botulinum toxin in the management of voiding dysfunction. J Urol. 2004;171:2128–2137. doi: 10.1097/01.ju.0000127725.48479.89. [DOI] [PubMed] [Google Scholar]
- 33.Martin TF. The molecular machinery for fast and slow neurosecretion. Curr Opin Neurobiol. 1994;4:626–632. doi: 10.1016/0959-4388(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 34.Schiavo G, et al. Tetanus and botulinum neurotoxins are zinc proteases specific for components of the neuroexocytosis apparatus. Ann N Y Acad Sci. 1994;710:65–75. doi: 10.1111/j.1749-6632.1994.tb26614.x. [DOI] [PubMed] [Google Scholar]
- 35.Bittner MA, et al. Isolated light chains of botulinum neurotoxins inhibit exocytosis. Studies in digitonin-permeabilized chromaffin cells. J Biol Chem. 1989;264:10354–10360. [PubMed] [Google Scholar]
- 36.Dolly JO, et al. Insights into the extended duration of neuroparalysis by botulinum neurotoxin A relative to the other shorter-acting serotypes: differences between motor nerve terminals and cultured neurons. In: Brin MF, et al., editors. Scientific and Therapeutic Aspects of Botulinum Toxin. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 91–102. [Google Scholar]
- 37.O’Sullivan GA, et al. Rescue of exocytosis in botulinum toxin A-poisoned chromaffin cells by expression of cleavage-resistant SNAP-25. Identification of the minimal essential C-terminal residues. J Biol Chem. 1999;274:36897–36904. doi: 10.1074/jbc.274.52.36897. [DOI] [PubMed] [Google Scholar]
- 38.Turton K, et al. Botulinum and tetanus neurotoxins: structure, function and therapeutic utility. Trends Biochem Sci. 2002;27:552–558. doi: 10.1016/s0968-0004(02)02177-1. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Salas E, et al. Plasma membrane localization signals in the light chain of botulinum neurotoxin. Proc Natl Acad Sci U S A. 2004;101:3208–3213. doi: 10.1073/pnas.0400229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Washbourne P, et al. Botulinum neurotoxin types A and E require the SNARE motif in SNAP-25 for proteolysis. FEBS Lett. 1997;418:1–5. doi: 10.1016/s0014-5793(97)01328-8. [DOI] [PubMed] [Google Scholar]
- 41.Breidenbach MA, Brunger AT. New insights into clostridial neurotoxin-SNARE interactions. Trends Mol Med. 2005;11:377–381. doi: 10.1016/j.molmed.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Foran PG, et al. Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J Biol Chem. 2003;278:1363–1371. doi: 10.1074/jbc.M209821200. [DOI] [PubMed] [Google Scholar]
- 43.Meunier FA, et al. Dynamics of motor nerve terminal remodeling unveiled using SNARE-cleaving botulinum toxins: the extent and duration are dictated by the sites of SNAP-25 truncation. Mol Cell Neurosci. 2003;22:454–466. doi: 10.1016/s1044-7431(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 44.Eleopra R, et al. Different time courses of recovery after poisoning with botulinum neurotoxin serotypes A and E in humans. Neurosci Lett. 1998;256:135–138. doi: 10.1016/s0304-3940(98)00775-7. [DOI] [PubMed] [Google Scholar]
- 45.Sloop RR, et al. Human response to botulinum toxin injection: type B compared with type A. Neurology. 1997;49:189–194. doi: 10.1212/wnl.49.1.189. [DOI] [PubMed] [Google Scholar]
- 46.Keller JE, et al. Persistence of botulinum neurotoxin action in cultured spinal cord cells. FEBS Lett. 1999;456:137–142. doi: 10.1016/s0014-5793(99)00948-5. [DOI] [PubMed] [Google Scholar]
- 47.Filippi GM, et al. Botulinum A toxin effects on rat jaw muscle spindles. Acta Otolaryngol. 1993;113:400–404. doi: 10.3109/00016489309135834. [DOI] [PubMed] [Google Scholar]
- 48.Rosales RL, et al. Extrafusal and intrafusal muscle effects in experimental botulinum toxin-A injection. Muscle Nerve. 1996;19:488–496. doi: 10.1002/(SICI)1097-4598(199604)19:4<488::AID-MUS9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 49.Modugno N, et al. Botulinum toxin restores presynaptic inhibition of group Ia afferents in patients with essential tremor. Muscle Nerve. 1998;21:1701–1705. doi: 10.1002/(sici)1097-4598(199812)21:12<1701::aid-mus12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 50.Borodic GE, Ferrante R. Effects of repeated botulinum toxin injections on orbicularis oculi muscle. J Clin Neuroophthalmol. 1992;12:121–127. doi: 10.3109/01658109209058127. [DOI] [PubMed] [Google Scholar]
- 51.Spencer RF, McNeer KW. Botulinum toxin paralysis of adult monkey extraocular muscle. Structural alterations in orbital, singly innervated muscle fibers. Arch Ophthalmol. 1987;105:1703–1711. doi: 10.1001/archopht.1987.01060120101035. [DOI] [PubMed] [Google Scholar]
- 52.Harris CP, et al. Histologic features of human orbicularis oculi treated with botulinum A toxin. Arch Ophthalmol. 1991;109:393–395. doi: 10.1001/archopht.1991.01080030095046. [DOI] [PubMed] [Google Scholar]
- 53.Borodic GE, et al. Botulinum A toxin for the treatment of adult-onset spasmodic torticollis. Plast Reconstr Surg. 1991;87:285–289. doi: 10.1097/00006534-199102000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Thesleff S, et al. Trophic interrelations at the neuromuscular junction as revealed by the use of botulinal neurotoxins. J Physiol (Paris) 1990;84:167–173. [PubMed] [Google Scholar]
- 55.de Paiva A, et al. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci U S A. 1999;96:3200–3205. doi: 10.1073/pnas.96.6.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alderson K, et al. Botulinum-induced alteration of nerve-muscle interactions in the human orbicularis oculi following treatment for blepharospasm. Neurology. 1991;41:1800–1805. doi: 10.1212/wnl.41.11.1800. [DOI] [PubMed] [Google Scholar]
- 57.Juzans P, et al. Nerve terminal sprouting in botulinum type-A treated mouse levator auris longus muscle. Neuromuscul Disord. 1996;6:177–185. doi: 10.1016/0960-8966(96)00041-7. [DOI] [PubMed] [Google Scholar]
- 58.Angaut-Petit D, et al. Terminal sprouting in mouse neuromuscular junctions poisoned with botulinum type A toxin: morphological and electrophysiological features. Neuroscience. 1990;37:799–808. doi: 10.1016/0306-4522(90)90109-h. [DOI] [PubMed] [Google Scholar]
- 59.Van Putten MJ, et al. In vivo analysis of end-plate noise of human extensor digitorum brevis muscle after intramuscularly injected botulinum toxin type A. Muscle Nerve. 2002;26:784–790. doi: 10.1002/mus.10274. [DOI] [PubMed] [Google Scholar]
- 60.Chancellor MB, et al. Successful use of bladder botulinum toxin injection to treat refractory overactive bladder [abstract DP50]. 98th Annual Meeting of the American Urological Association; Chicago, IL, USA. 2003. [Google Scholar]
- 61.Popat R, et al. A comparison between the response of patients with idiopathic detrusor overactivity and neurogenic detrusor overactivity to the first intradetrusor injection of botulinum-A toxin. J Urol. 2005;174:984–989. doi: 10.1097/01.ju.0000169480.43557.31. [DOI] [PubMed] [Google Scholar]
- 62.Smith CP, et al. Single-institution experience in 110 patients with botulinum toxin A injection into bladder or urethra. Urology. 2005;65:37–41. doi: 10.1016/j.urology.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 63.Smith CP, et al. Effect of botulinum toxin A on the autonomic nervous system of the rat lower urinary tract. J Urol. 2003;169:1896–1900. doi: 10.1097/01.ju.0000049202.56189.54. [DOI] [PubMed] [Google Scholar]
- 64.Somogyi GT, et al. M1 muscarinic receptor-induced facilitation of ACh and noradrenaline release in the rat bladder is mediated by protein kinase C. J Physiol. 1996;496 (Pt 1):245–254. doi: 10.1113/jphysiol.1996.sp021681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chu FM, Dmochowski R. Pathophysiology of overactive bladder. Am J Med. 2006;119:3–8. doi: 10.1016/j.amjmed.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 66.Somogyi GT, et al. Frequency dependence of muscarinic facilitation of transmitter release in urinary bladder strips from neurally intact or chronic spinal cord transected rats. Br J Pharmacol. 1998;125:241–246. doi: 10.1038/sj.bjp.0702041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bayliss M, et al. A quantitative study of atropine-resistant contractile responses in human detrusor smooth muscle, from stable, unstable and obstructed bladders. J Urol. 1999;162:1833–1839. [PubMed] [Google Scholar]
- 68.O’Reilly BA, et al. P2X receptors and their role in female idiopathic detrusor instability. J Urol. 2002;167:157–164. [PubMed] [Google Scholar]
- 69.Nishiguchi J, et al. Detrusor overactivity induced by intravesical application of adenosine 5′-triphosphate under different delivery conditions in rats. Urology. 2005;66:1332–1337. doi: 10.1016/j.urology.2005.06.099. [DOI] [PubMed] [Google Scholar]
- 70.MacKenzie I, et al. The effects of purified botulinum neurotoxin type A on cholinergic, adrenergic and non-adrenergic, atropine-resistant autonomic neuromuscular transmission. Neuroscience. 1982;7:997–1006. doi: 10.1016/0306-4522(82)90056-2. [DOI] [PubMed] [Google Scholar]
- 71.Smith CP, et al. Effect of stimulation intensity and botulinum toxin isoform on rat bladder strip contractions. Brain Res Bull. 2003;61:165–171. doi: 10.1016/s0361-9230(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 72.Apostolidis A, et al. Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol. 2006;49:644–650. doi: 10.1016/j.eururo.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 73.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84:935–986. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 74.Khera M, et al. Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem Int. 2004;45:987–993. doi: 10.1016/j.neuint.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Ford AP, et al. Purinoceptors as therapeutic targets for lower urinary tract dysfunction. Br J Pharmacol. 2006;147 (suppl 2):S132–S143. doi: 10.1038/sj.bjp.0706637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paukert M, et al. Inflammatory mediators potentiate ATP-gated channels through the P2X(3) subunit. J Biol Chem. 2001;276:21077–21082. doi: 10.1074/jbc.M101465200. [DOI] [PubMed] [Google Scholar]
- 77.Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur J Biochem. 2004;271:1814–1819. doi: 10.1111/j.1432-1033.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- 78.Morenilla-Palao C, et al. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem. 2004;279:25665–25672. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- 79.Davis JB, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 80.Charrua A, et al. Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol. 2007;177:1537–1541. doi: 10.1016/j.juro.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 81.Apostolidis A, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005;174:977–982. doi: 10.1097/01.ju.0000169481.42259.54. [DOI] [PubMed] [Google Scholar]
- 82.Schulte-Baukloh H, et al. Persistence of the synaptosomal-associated protein-25 cleavage product after intradetrusor botulinum toxin A injections in patients with myelomeningocele showing an inadequate response to treatment. BJU Int. 2007;100:1075–1080. doi: 10.1111/j.1464-410X.2007.07137.x. [DOI] [PubMed] [Google Scholar]
- 83.Basbaum AI, Jessell TM. The perception of pain. In: Kandel ER, et al., editors. Principles of Neural Science. 4. New York: McGraw Hill; 2000. pp. 472–491. [Google Scholar]
- 84.Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005;26:785–793. doi: 10.1016/j.neuro.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 85.Zubrzycka M, Janecka A. Substance P: transmitter of nociception (Minireview) Endocr Regul. 2000;34:195–201. [PubMed] [Google Scholar]
- 86.Smet PJ, et al. Distribution and colocalization of calcitonin gene-related peptide, tachykinins, and vasoactive intestinal peptide in normal and idiopathic unstable human urinary bladder. Lab Invest. 1997;77:37–49. [PubMed] [Google Scholar]
- 87.Chuang YC, et al. Intravesical botulinum toxin A administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol. 2004;172:1529–1532. doi: 10.1097/01.ju.0000137844.77524.97. [DOI] [PubMed] [Google Scholar]
- 88.Rapp DE, et al. Botulinum toxin type A inhibits calcitonin gene-related peptide release from isolated rat bladder. J Urol. 2006;175:1138–1142. doi: 10.1016/S0022-5347(05)00322-8. [DOI] [PubMed] [Google Scholar]
- 89.Steers WD, Tuttle JB. Mechanisms of Disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol. 2006;3:101–110. doi: 10.1038/ncpuro0408. [DOI] [PubMed] [Google Scholar]
- 90.Giannantoni A, et al. Botulinum-A toxin injections into the detrusor muscle decrease nerve growth factor bladder tissue levels in patients with neurogenic detrusor overactivity. J Urol. 2006;175:2341–2344. doi: 10.1016/S0022-5347(06)00258-8. [DOI] [PubMed] [Google Scholar]
- 91.Relja M, Telarovic S. Botulinum toxin type-A and pain responsiveness in cervical dystonia: a dose response study. Mov Disord. 2005;20:P77. [Google Scholar]
- 92.Foster KA. The analgesic potential of clostridial neurotoxin derivatives. Expert Opin Investig Drugs. 2004;13:1437–1443. doi: 10.1517/13543784.13.11.1437. [DOI] [PubMed] [Google Scholar]
- 93.Tarsy D, First ER. Painful cervical dystonia: clinical features and response to treatment with botulinum toxin. Mov Disord. 1999;14:1043–1045. doi: 10.1002/1531-8257(199911)14:6<1043::aid-mds1026>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 94.Haferkamp A, et al. Lack of ultrastructural detrusor changes following endoscopic injection of botulinum toxin type A in overactive neurogenic bladder. Eur Urol. 2004;46:784–791. doi: 10.1016/j.eururo.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 95.Comperat E, et al. Histologic features in the urinary bladder wall affected from neurogenic overactivity—a comparison of inflammation, oedema and fibrosis with and without injection of botulinum toxin type A. Eur Urol. 2006;50:1058–1064. doi: 10.1016/j.eururo.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 96.Mejia NI, et al. Long-term botulinum toxin efficacy, safety, and immunogenicity. Mov Disord. 2005;20:592–597. doi: 10.1002/mds.20376. [DOI] [PubMed] [Google Scholar]