Abstract
Background:
There is a lack of reliable hepatoprotective drugs in modern medicine to prevent and treat drug-induced liver damage. Leaves of Sacred/Holy Basil, i.e. Green Tulsi (Ocimum sanctum), belonging to family Lamiaceae are used traditionally for their hepatoprotective effect. We wanted to evaluate the hepatoprotective activity of Ocimum sanctum and observe whether synergistic hepatoprotection exists with silymarin.
Materials and Methods:
Albino rats (150–200 g) were divided into five groups. Groups A and B were normal and experimental controls, respectively. Groups C, D and E received the alcoholic extract of Ocimum Sanctum leaves (OSE) 200 mg/kg BW/day, silymarin 100 mg/kg BW/day and OSE 100 mg/kg BW/day + silymarin 50 mg/kg BW/day p.o., respectively, for 10 days. Hepatotoxicity was induced in Groups B, C, D and E on the eighth day with paracetamol 2 g/kg BW/day. The hepatoprotective effect was evaluated by performing an assay of the serum proteins, albumin globulin ratio, alkaline phosphatase, transaminases and liver histopathology. The assay results were presented as mean and standard error of mean (SEM) for each group. The study group was compared with the control group by one-way ANOVA, followed by Bonferoni's test. A P-value of <0.01 was considered significant.
Results:
In groups C, D and E, liver enzymes and albumin globulin ratio were significantly (P < 0.01) closer to normal than in group B. Reduction in sinusoidal congestion, cloudy swelling and fatty changes and regenerative areas of the liver were observed on histopathological examination in groups C, D and E, whereas group B showed only hepatic necrosis.
Conclusion:
The Ocimum sanctum alcoholic leaf extract shows significant hepatoprotective activity and synergism with silymarin.
Keywords: Hepatoprotective, hepatotoxicity, Ocimum sanctum, paracetamol, silymarin
INTRODUCTION
The liver performs the normal metabolic homeostasis of the body as well as biotransformation, detoxification and excretion of many endogenous and exogenous compounds, including pharmaceutical and environmental chemicals. Drug-induced hepatotoxicity is a major cause of iatrogenic diseases, accounting for one in 600 to one in 3500 of all hospital admissions.[1
Traditional medicine is the sum total of knowledge, skills and practices based on the theories, beliefs and experiences indigenous to different cultures that are used to maintain health as well as to prevent, diagnose, improve or treat physical and mental illnesses. Herbal treatments are the most popular form of traditional medicine. Herbal medicines include herbs, herbal materials, herbal preparations and finished herbal products that contain parts of plants or other plant materials as active ingredients.[2] However, no scientific data regarding the identity and effectiveness of these herbal products were available, except in the treatise of Ayurveda and Unani medicine.[3] The World Health Organization (WHO) has laid emphasis on promoting the use of traditional medicine for health care.[2] Hence, we see afocus on research on traditional and herbal medicine, especially in developing countries, with individual as well as collaborative efforts by national research organizations.[4]
There is an acute necessity of reliable hepatoprotective drugs in modern medical practice.
Plants and natural products have been used traditionally worldwide for the prevention and treatment of liver disease. Scientific research has supported the claims of the medicinal efficacy of several of these herbal compounds, as evidenced from the voluminous work on their hepatoprotective potentials.[5] More than 700 mono- and polyherbal formulations from over a hundred different plants are available for use.[6]
Sacred or Holy Basil, i.e. Green Tulsi (Ocimum sanctum), is a well known medicinal plant, which grows wild as well as in households and temples in India. It has been traditionally regarded as possessing rejuvenating, tonic and vitalizing properties that contribute to longevity and a healthy life.[7] Leaves of Ocimum sanctum possess expectorant, diaphoretic, antiseptic, spasmolytic, stimulant and anticatarrhal properties and are used as cold and cough remedies, for fever, pain,[8] gastrointestinal disorders (like dyspepsia, vomiting), worm infestations, skin diseases, snakebite and scorpion sting.[9]
Significant hepatoprotective activity of Ocimum sanctum was reported earlier against paracetamol, carbon tetrachloride and anti-tubercular drug-induced hepatoxicity in albino rats.[9–11] We wanted to build on these findings in relation to paracetamol-induced hepatotoxicity and observe for a synergistic or an additive effect with the combination of Ocimum sanctum and a standard hepatoprotectant.
Thus, the aim of our study was to:
Evaluate the hepatoprotective activity of Tulsi (Ocimum sanctum) leaves on paracetamol-induced hepatotoxicity in albino rats as compared with silymarin.
Evaluate whether the combination of Tulsi (Ocimum sanctum) and silymarin had a synergistic or an additive hepatoprotective activity.
MATERIALS AND METHODS
Type of Study
Prospective and interventional.
Place of Study
Department of Pharmacology, with technical support from the departments of Pathology and Biochemistry, Assam Medical College and Hospital.
Period of Study
1st January–31st December, 2005.
Ethical Committee Clearance
Ethical clearance was taken from the animal ethics committee of the institute (634/02/A/CPCSEA, dated 19/05/2002).
Experimental Animals
Healthy albino rats (Rattus norvegicus) of Wistar strain (both male and female), weighing 100–200 g each (obtained from Central Animal House, Assam Medical College, Dibrugarh) were given the standard diet with water ad libitum during the entire period of the experiment as per the recommendation of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) for laboratory animal facilities.[12]
Drugs
All drug suspensions were prepared for the different groups with 3% (W/V) aqueous suspension of gum acacia as vehicle.
Test drug
Ocimum sanctum alcoholic leaf extract (OSE). This was prepared as follows:
One kilogram of fresh Ocimum sanctum leaves, identified by Ms. Belinda Lahon, PhD in Botany, University of North Bengal, was collected and washed thoroughly with cold water, dried in the shade at room temperature and, thereafter, crushed in an electrical mixer-grinder. Hundred grams of this air-dried powder of the leaves was soaked in 90% ethyl alcohol and was allowed to stand for 15 min in a tightly covered container. The soaked powder was then transferred to a percolator, where it was firmly packed in and allowed to macerate for 24 h at room temperature, followed by slow percolation. The procedure was repeated over the next 24 h, with sufficient amounts of 90% alcohol until no further extraction was possible. Alcohol was evaporated to a soft extract and the residue was transferred to a vacuum desiccator, thus, obtaining the dried leaf alcoholic extract of Ocimum sanctum.[13]We got5 g of a dark greenish-black and sticky extract (5% dry weight of powdered leaves). The OSE suspension was used in doses of 200 mg/kg BW and 100 mg/kg BW for the respective groups as per previous studies in other models of hepatotoxicity.[9,10]
Standard hepatoprotective
Silymarin (SILY) powder (obtained from Micro Labs Ltd., Bangalore, India) was used to make the suspension in doses of 100 mg/kg BW and 50 mg/kg BW for the respective groups following the method of Mankani et al. and Mansour et al.[14,15]
Hepatotoxin
Paracetamol (PCM) powder (I.P.) (obtained from Bharat Chemicals, Tarapur, Gujarat, India) was used to make the suspension in a dose of 2 g/kg BW for the respective groups.
Methods
The experiment was carried out on 30 healthy albino rats for 10 days. Before starting the experiment, the animals were allowed to acclimatize to the laboratory environment for 1 week.
Grouping and Treatment Schedule
The rats were randomly divided into five groups of six animals each after weighing, recording and numbering. Each group received treatment as follows:
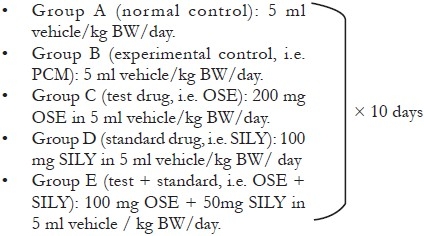
Dosing and Administration of Drugs
The drug suspensions and the vehicle were administered per orally by an intragastric feeding tube at a uniform volume of 5 ml/kg BW.
Induction of Hepatic Injury
A single dose of paracetamol 2 g/kg BW/day was given to groups B, C, D and E on the eighth day of the experiment. It was administered after overnight fasting of the animals, i.e. the diet was restricted 12 h prior to the administration of paracetamol. However, free access to water was permitted.[16]
Laboratory Assessments
On the 10th day, blood was collected from the hearts of the animals under light ether anesthesia. The blood was kept undisturbed for 30 min and the clot was dispersed with a glass rod. The samples were centrifuged for 15–20 min at 2000 rpm to separate the serum and then sent for liver function tests (LFT), namely total serum protein, albumin globulin ratio, alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT).[17–19]
Histopathological Examination
The rats were then sacrificed (on the 10th day) under deep ether anesthesia and the liver samples were excised and washed with normal saline. A record of each liver was made, regarding size and shape, color and presence or absence of any nodule. Then, the livers were fixed immediately in 10% formalin solution. A paraffin embedding technique was carried out and sections were taken at 5-mm thickness, stained with hematoxylin and eosin and examined microscopically for histopathological changes.[20]
Statistical Analysis
The results, obtained from the LFT were presented as mean and standard error of mean (SEM) for each group (mean ± SEM). All groups were subjected to one-way analysis of variance (ANOVA), which was followed by Bonferoni's test to determine the intergroup variability. A comparison was made with the experimental control (paracetamol) group and with the standard (silymarin). We took a P-value of <0.01 (highly significant) as our desired level of significance.
RESULTS
The LFT results are summarized [Table 1] and expressed as mean ± SEM (n = 6). The histopathological examination (HPE) of group A livers showed a normal arrangement of the hepatocytes, with clearly visible nuclei, central vein and portal triad [Figure 1a]. We observed areas of congestion of sinusoids, cloudy swelling, congestion of central vein, centrilobular fatty change and necrosis of hepatocytes in all animals of group B [Figure 1b]. In groups C, D and E, there was marked reduction in sinusoidal congestion, cloudy swelling and fatty change, with areas of regeneration as well [Figure 1c–e].
Table 1.
Effects of the alcoholic leaf extract of Ocimum sanctum on total protein, albumin globulin ratio, serum alkaline phosphatase, aspartate aminotransferase and alanine aminotransferase in paracetamolinduced hepatotoxicity in albino rats (10th day of the experiment)
Figure 1.
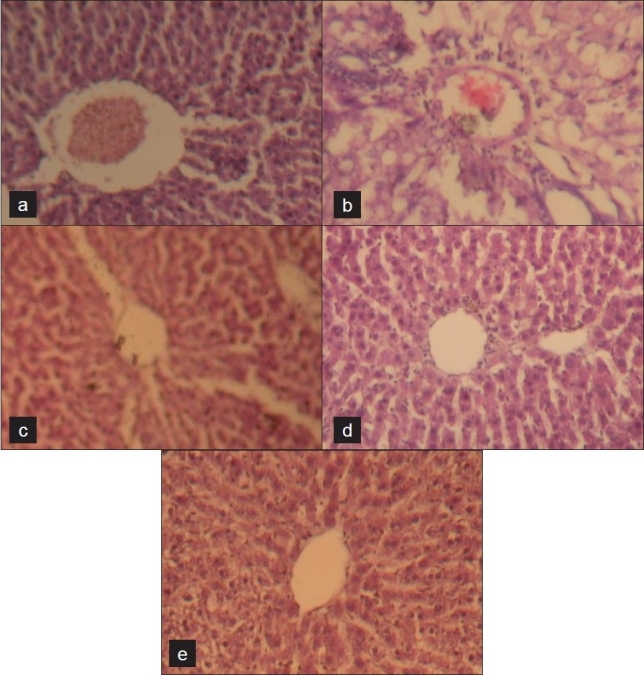
Photomicrographs of rat liver (hematoxylin and eosin) under low power (×100), (A) shows normal hepatic architecture; (B) shows hepatic necrosis; (C, D and E) show varying degrees of hepatic regeneration.
DISCUSSION
The administration of PCM to the animals resulted in a significant fall in the levels of total serum proteins and albumin globulin ratio and a significant rise in serum ALP, AST and ALT. In groups C, D and E, the toxic effect of paracetamol was partly reversed in the animals. Compared with the PCM (experimental control) group, the C and E groups showed a significant increase in the albumin:globulin ratio and a significant decrease in the serum ALP, AST and ALT levels. However, no significant difference was observed in the total protein levels in these groups. Group E in comparison with silymarin (standard) showed a significant decrease in the serum AST and ALT alone. Thus, group C showed greater hepatoprotection than group E, considering the results of the LFT alone. Histology of the control group showed normal hepatic architecture [Figure 1a]. The group B animals exhibited areas of hepatic necrosis induced by paracetamol [Figure 1b]. The animals treated with PCM and OSE (group C), PCM and silymarin (group D) and PCM, OSE and silymarin (group E) [Figure 1c –e, respectively] revealed appreciable protection of hepatic tissue from PCM.
PCM, used as a tool to induce hepatotoxicity in experimental animals, leads to covalent bonding of its toxic metabolite N-acetyl P bezoquinoneimine to sulfydryl groups of proteins. This causes exhaustion of reduced glutathione in the liver, resulting in cell necrosis and lipid peroxidation.[21] An increase in the level of transaminases and ALP is an indication of cellular leakage and loss of functional integrity of the hepatic cell membranes.[22–24]
Administration of the alcoholic extract of Ocimum sanctum leaves showed significant hepatoprotective activity, as shown previously in other studies.[10] Synergistic hepatoprotective activity was seen with the OSE + SILY group. The OSE group showed better hepatoprotection than the OSE + SILY group. But, the OSE and OSE + SILY combination showed lesser efficacy than SILY alone.
Eugenol, flavonoid and ursolic acid components, present in Ocimum sanctum leaves, have free radical scavenging and anti-lipoperoxidative effects. Therefore, the hepatoprotective effect of Ocimum sanctum leaves may be due to the antioxidant properties of its constituents.[25] The membrane stabilizing property of Ocimum sanctum is responsible for its hepatoprotective action.[26]
Moreover, the fixed oil of Ocimum sanctum contains linoleic acid, which is responsible for its anti-inflammatory activity.[27] Hence, linoleic acid may also be responsible for reversing the inflammatory features associated with hepatic injury thus adding to the hepatoprotective effect.
CONCLUSION
Thus, the leaves of Tulsi (Ocimum sanctum) have highly significant (P < 0.01) hepatoprotective activity. When concurrently administered, Ocimum sanctum leaves and silymarin have a highly significant (P < 0.01) synergistic hepatoprotective activity. The Ocimum sanctum group showed better hepatoprotection than the Ocimum sanctum and silymarin combination group. However, in the given doses, the Ocimum sanctum leaf extract alone and in combination with silymarin showed lesser hepatoprotective effect than silymarin alone. Silymarin is a well-known standard hepatoprotective, whereas presence of impurities in the Ocimum sanctum extract may have caused a lower hepatoprotective effect. Moreover, we used lower doses of Ocimum sanctum (100 mg/kg) and standard hepatoprotective silymarin (50 mg/kg) in the combination group (Ocimum sanctum extract and silymarin) than in the silymarin group alone.
Acknowledgments
We express our thanks to the technical and nontechnical support staff of the department of Pharmacology, Pathology and Biochemistry, Assam Medical College and Hospital, Dibrugarh, Assam for help in conducting the study and Dr. Gautam Sahu, department of Pharmacology, Jorhat Medical College, Assam for help in manuscript preparation.
Footnotes
Presentation at a meeting Organization: National Conference of Indian Pharmacological Society
Place: Chennai; Date: December 2005
Source of Support: Dibrugarh University, Assam,
Conflict of Interest: None declared.
REFERENCES
- 1.Dossing M, Sonne J. Drug induced hepatic disorders: Incidence, management and avoidance. Drug Safety. 1993;9:441–9. doi: 10.2165/00002018-199309060-00007. [DOI] [PubMed] [Google Scholar]
- 2.World health organization. WHO media centre. Traditional medicine. WHO Fact sheet N°134. [cited in 2008 Dec]. Available from: http://www.who.int/mediacentre/factsheets/fs134/en/
- 3.Gupta SS. Prospects and perspectives of natural plant products in medicine. Indian J Pharmacol. 1994;2:1–12. [Google Scholar]
- 4.Satyavati GV, Gupta A, Tandon N. Medicinal plants of India (II) New Delhi: Indian Council of Medical Research; 1987. [Google Scholar]
- 5.Handa SS, Chakraborty KK, Sharma A. Antihepatotoxic activity of some Indian herbal formulations as compared to silymarin. Fitoterapia. 1986;57:307. [Google Scholar]
- 6.Ram VJ. Herbal preparations as a source of hepatoprotective agents. Drug News Perspect. 2001;14:353. [PubMed] [Google Scholar]
- 7.Singh S, Majumder DK. Evaluation of anti-inflammatory activity of fatty acids of Ocimum sanctum fixed oil. Indian J Exp Biol. 1997;35:380–3. [PubMed] [Google Scholar]
- 8.Ali M. Indigenous traditional drugs: In Textbook of Pharmacognosy. 1st ed. Delhi: CBS Publishers and Distributors; 1994. pp. 312–3. [Google Scholar]
- 9.Bhargava KP, Singh N. Antistress activity of Ocimum sanctum Linn. Indian J Med Res. 1981;73:443–51. [PubMed] [Google Scholar]
- 10.Chattopadhyay RR, Sarkar SK, Ganguly S, Medda C, Basu TK. Hepatoprotective activity of Ocimum sanctum leaf extract against paracetamol induced hepatic damage in rats. Indian J Pharmacol. 1992;24:163–5. [PubMed] [Google Scholar]
- 11.Ubaid RS, Anantrao KM, Jaju JB, Mateenuddin MD. Effect of Ocimum sanctum leaf extract on hepatotoxicity induced by anti-tubercular drugs in rats. Indian J Physiol Pharmacol. 2003;47:465–70. [PubMed] [Google Scholar]
- 12.CPCSEA guidelines for laboratory animal facility. Committee for the purpose of control and supervision of experiments on animals. Indian J Pharmacol. 2003;35:257–74. [Google Scholar]
- 13.Extraction and extractives. In: Remington’s Pharmaceutical sciences. 14th ed. Easton Pennsylvania: Mack Publishing Company; 1965. pp. 1578–93. [Google Scholar]
- 14.Mankani KL, Krishna V, Manjunatha BK, Vidya SM, Singh SDJ, Manohara YN, Raheman AU, Avinash KR. Evaluation of hepatoprotective activity of stem bark of Pterocarpus marsupium Roxb. Indian J Pharmacol. 2005 Jun;37(3):165–8. [Google Scholar]
- 15.Mansour HH, Hafez HF, Fahmy NM. Silymarin modulates cisplatin-induced oxidative stress and hepatotoxicity in rats. J Biochem Mole Biol. 2006;39:656–61. doi: 10.5483/bmbrep.2006.39.6.656. [DOI] [PubMed] [Google Scholar]
- 16.Dhawan BN, Patnaik GK, Kulshereshtha DK, Sarin VK. Absence of hepatoprotective activity in Laqobo cashminana: An adulterant to Picrorhiza kurrooa. Indian J Pharma. 1991;23(2):121–2. [Google Scholar]
- 17.Reinhold JG. Biuret method. In: Reiner M, editor. Standard Methods of Clinical Chemistry. Vol. 1. Ann Arbor: Academic Press, Inc; 1953. p. 88. [Google Scholar]
- 18.King EJ, Abul-Fadl MAM, Walker PG. King-Armstrong phosphatase estimation by the determination of liberated phosphate. J Clin Pathol. 1951;4:85. doi: 10.1136/jcp.4.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reitman S, Frankel S. Colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 20.Ann Preece. Manual for histologic technicians. 3rd ed. Boston: Little, Brown and Company; 1972. [Google Scholar]
- 21.Burke A, Smyth E, Fitzgerald GA. Analgesic-antipyretic agents: Pharmacotherapy of gout. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological basis of Therapeutics. 11th. USA: McGraw Hill; 2006. p. 694. [Google Scholar]
- 22.Poole A, Leslie GB. A practical approach to toxicological investigations. 1st ed. Cambridge: Cambridge University Press; 1989. pp. 65–6. [Google Scholar]
- 23.Hayes AW, editor. Principles and methods of toxicology. 2nd ed. New York: Raven Press; 1989. pp. 599–628. [Google Scholar]
- 24.Timbrell JA, editor. Principles of biochemical toxicology. 2nd ed. London: Taylor and Francis Ltd; 1982. pp. 184–8. [Google Scholar]
- 25.Prakash P, Gupta N. Therapeutic uses of Ocimum sanctum Linn (tulsi) with a note on eugenol and its pharmacological actions: A short review. Indian J Physiol Pharmacol. 2005;49:125–31. [PubMed] [Google Scholar]
- 26.Sen P, Dewan V, Bhattacharya SK, Gupta VS, Maiti PC, Mediratta PK. In brain and Psychophyioslogy of stress. New Delhi: ICMR Publication; 1988. p. 245. [Google Scholar]
- 27.Singh S, Majumdar DK, Rehan HMS. Evaluation of anti-inflammatory potential of fixed oil of Ocimum sanctum (Holybasil) and its possible mechanism of action. J Ethnopharmacol. 1996;54:19–26. doi: 10.1016/0378-8741(96)83992-4. [DOI] [PubMed] [Google Scholar]


