Abstract
Background:
Ayurvedic formulations are used to treat a wide variety of diseases including diabetes mellitus Standardization of herbal formulation is essential in order to assess the quality of drugs. The present paper reports standardization of eight herbal anti-diabetic drugs–Momordica charantia (seeds), Syzigium cumini (seeds), Trigonella foenum (seeds), Azadirachta indica (leaves), Emblica offi cinalis (fruits), Curcuma longa (rhizomes), Gymnema sylvestre (leaves), Pterocarpus marsupium (heart-wood) individually and in polyherbal marketed samples of Baidyanath Madhumehari Churna
Material and Methods:
Shivayu Madhuhari Churna, Meghdut Madhushoonya Churna and were compared to the in-house preparation for physicochemical properties.
Results and Conclusions:
The limits obtained from the different physicochemical parameters of the individual eight herbal drugs and the marketed formulations could be used as reference standard for standardization of the anti-diabetic drugs in a quality control laboratory.
Keywords: Herbal antidiabetic drugs, physicochemical parameters, polyherbal formulation, standardization
INTRODUCTION
In the present era, market of all commodities has become global. Health has been of utmost importance since ancient times for the mankind. Market of health-related products has been active and these products are manufactured at different parts of the world and sold all over. Standardization is necessary to make sure the availability of a uniform product in all parts of the world. Standardization assures a consistently stronger product with guaranteed constituents.
WHO collaborates and assists health ministries in establishing mechanisms for the introduction of traditional plant medicines into primary healthcare programs, in assessing safety and efficacy, in ensuring adequate supplies, and in the quality control of raw and processed materials.[1] Herbal formulations in general can be standardized schematically as to formulate the medicament using raw materials collected from different localities and a comparative chemical efficacy of different batches of formulation is to be observed. A preparation with better clinical efficacy has to be selected. The routine physical, chemical, and pharmacological parameters are to be checked for all the batches to select the final finished product and to validate the whole manufacturing process.
In India, diabetes is a serious disease due to irrational food habits. Most of the hypoglycemic agents and hypolipidemics used in allopathic practice to treat diabetes mellitus and hyperlipidemia are reported to have side effects in long term use.[2] Hence, there is the need to search for effective and safe drugs for these ailments. Pharmaceutical research across the world shows that natural products are potential sources of novel molecules for drug development.[3]
Based on the above rationale the present study was undertaken with an aim to standardize some herbal antidiabetic drugs based on their physicochemical characteristics and compare them with marketed formulations and in-house developed formulations. The present paper reports the standardisation of herbal antidiabetic drugs based on organoleptic characters, physical characteristics, and physicochemical properties.
MATERIALS AND METHODS
Plant material
Following eight herbal anti-diabetic drugs were chosen: Momordica charantia (seeds), Syzigium cumini (seeds), Trigonella foenum (seeds), Azadirachta indica (leaves), Emblica officinalis (fruits), Curcuma longa (rhizomes), Gymnema sylvestre (leaves) and Pterocarpus marsupium (heart-wood).
All eight herbs were procured from different parts of the state and were authenticated by Dr Anupam Pathak, Head, Department of Pharmacy, Barkatullah Vishwavidyalaya, Bhopal. A herbarium was submitted at the department (Voucher No.: BUPH-4055).
Marketed samples
Three market samples were selected and obtained from the local market-Baidyanath Madhumehari Churna was (Batch no 217, Mfg-2/05, Baidyanath), Shivayu Madhuhari Churna (Batch no CH/4679, Mfg-1/05, Shiv Herbal Research Laboratories), and Meghdut Madhushoonya Churna (Batch no 104, Mfg-10/04, Meghdoot Gramodyog Sewa Sansthan). The market samples were compared to the in-house preparation for physicochemical properties.
Preparation of in-house formulation
In-house formulations were made in triplicate of the same proportions as in marketed products of each brand containing known amount of individual herbs. Formulation 1 was made in the same proportions as the marketed product of Baidyanath Madhumehari Churna containing known amount of individual herbs [Table 1a]. Formulation 2 was made in the same proportions as the marketed product of Shivayu Madhuhari Churna containing known amount of individual herbs [Table 1b]. Formulation 3 was made in the same proportions as the marketed product of Meghdut Madhushoonya Churna containing known amount of individual herbs [Table 1c].
Table 1a.
Ingredients for in-House Formulation 1 as labeled in Baidyanath Madhumehari churna
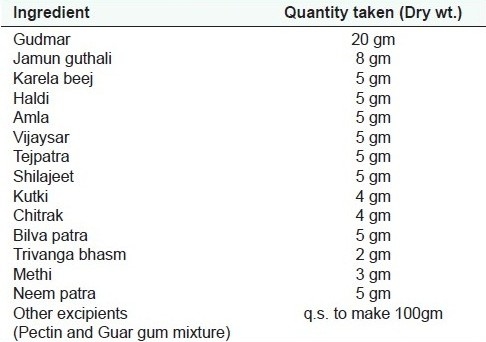
All the procured and authenticated individual drugs were dried in shade and cleaned by hand sorting. The individual drugs were then crushed using willing grinder and passed through mesh no. 40. The individual drugs were then weighed as per the quantity required. The drugs were mixed geometrically using a double cone blender. The mixed formulation was unloaded, weighed, and packed in labeled glass bottles.
Organoleptic evaluation
Organoleptic evaluation refers to evaluation of individual drugs and formulations by color, odor, taste, texture, etc.[4] The organoleptic characters of the samples were carried out based on the method as described by Wallis.[5] For determining the odor of an innocuous material, small portion of the sample was placed in the beaker of suitable size, and examined by slow and repeated inhalation of the air over the material. If no distinct odor was perceptible, the sample was crushed between the thumb and index finger, between the palms of the hands, using gentle pressure or if the material was known to be dangerous, by other suitable means such as pouring a small quantity of boiling water onto the crushed sample placed in a beaker. First, the strength of the odor was determined (none, weak, distinct, strong) and then the odor sensation (aromatic, fruity, musty, mouldy, rancid, etc.) was studied. Taste was distinctively classified as aromatic, pungent, sweet, sour, astringent, mucilaginous, or bitter.
Extractive values[6,7]
Water soluble extractives
Five grams of coarsely powdered air-dried drug was macerated with 100 ml of water in closed conical flask for 24 hours, shaken frequently for the first 6 hours and allowed to stand for 18 hours. This was filtered through Whatman filter paper grade no.100. Twenty-five milliliters of the filtrate was evaporated to dryness in petri dish, dried at 105°C, and weighed. Percentage of water soluble extractive with reference to air-dried material was calculated.
Alcohol soluble extractives
Five grams of air-dried and coarsely powdered drug was macerated with 100 ml of 70% ethanol in a closed conical flask for 24 hours, shaken frequently during the first 6 hours, and allowed to stand for 18 hours. This was filtered rapidly taking precaution against loss of ethanol. Twenty-five milliliters of the filtrate was evaporated to dryness in a petri dish, dried at 105° C, and weighed. Percentage of alcohol soluble extractive was calculated with reference to air-dried drug.
Ether soluble extractives
Five grams of air-dried and coarsely powdered drug was extracted with ethyl ether in a soxhlet extractor for 20 hours. The ether extract was transferred in a petri dish and allowed to evaporate. It was dried at 105° C to constant weight. Percentage of ether soluble extractive was calculated with reference to air-dried drug.
Physicochemical properties
Physical characteristics like moisture content, bulk density, tap density, angle of repose, Hausner ratio, and Carr's index were determined for different formulations.[8]
Moisture Content[9]
The shade-dried drug was grounded in a mixer grinder. The powder passed through #40 and retained on #120. Accurately weighed 10 g of # 40/120 drug powder was kept in a tared evaporating dish. This was dried at 105°C for 5 hours in tray drier and weighed. The drying was continued and weighing was done at one-hour interval until difference between two successive weighings corresponds to not more than 0.25 percent. Drying was continued until a constant weight was reached with two successive weighings after drying for 30 minutes and cooling for 30 minutes in a desiccator was showing not more than 0.01 g difference.
Bulk Density and Tapped Density[10]
In the present study, we had taken the weighed quantity (30 gm) of shade-dried and presieved (#40/120) different drugs, marketed and in-house formulation powders and carefully added them to a cylinder with the aid of a funnel without any losses. The initial volume was noted and the sample was then tapped until no further reduction in volume was noted. The initial volume gave the bulk density value and after tapping the volume reduced, giving the value of tapped density.
Carr's Index
Carr's index has been used as an indirect method of quantifying powder flowability from bulk density; this method was developed by Carr. The percentage compressibility of a powder is a direct measure of the potential powder arch or bridge strength and stability, and is calculated according to following equation.
Carr's index (% compressibility) = 100 × (1 - Db / Dt)
Where Db = Bulk density, Dt = Tapped density
Hausner Ratio
Hausner ratiohas been also used as indirect method of quantifying powder flowability from bulk density. Hausner ratio = Dt / Db. Where Db = Bulk density and Dt = Tapped density.
pH of suspension of the drugs
pH of freshly prepared 1% w/v suspension and 10% w/v suspension in distilled water was determined using simple glass electrode pH meter.
Ash values[7,11]
Total ash
Two grams of grounded air-dried material was accurately weighed in a previously ignited and tared silica crucible. The drug was gradually ignited by raising the temperature to 450°C until it was white. The sample was cooled in a desiccator and weighed. The percentage of total ash was calculated with reference to air-dried drug.
Acid Insoluble ash
The ash was boiled with 25 ml of 2 M hydrochloric acid for 5 minutes, the insoluble matter was collected on an ash less filter paper, washed with hot water, ignited, cooled in a desiccator, and weighed. The percentage of acid insoluble ash was calculated with reference to the air-dried drug.
Water Soluble ash
The ash was boiled with 25 ml of water for 5 minutes, the insoluble matter on ash less filter paper collected, washed with hot water, ignited, cooled in a desiccator, and weighed. The weight of the insoluble matter from the weight of the total ash was subtracted; the difference represents the water soluble ash. The percentage of water insoluble ash was calculated with reference to the air-dried drug.
RESULTS AND DISCUSSION
Organoleptic characteristics
The organoleptic properties are mentioned in Table 2. Madhumehari (Baidyanath) Powder and its developed formulation was buff colored, slightly bitter in taste, and had a characteristic bitter odor. Madhushoonya (Meghdoot) Powder and its developed formulation was brown colored, bitter in taste, and had a characteristic sour odor. Madhuhari (Shivayu) and its developed formulation powder was light brown colored, bitter and acrid in taste, and had a pungent to bitter odor.
Table 1b.
Ingredients for in-House Formulation 2 as labeled in Shivayu Madhuhari churna

Extractive values
Water-soluble extractive value plays an important role in evaluation of crude drugs. Less extractive value indicates addition of exhausted material, adulteration or incorrect processing during drying or storage or formulating. The water-soluble extractive values of marketed formulations and their similar in-house prepared formulations were in the range of 14.05–19.47% [Table 3 and Figure 1]. The in-house Formulation 1 was the developed formulation of Madhumehari (Baidyanath) and its extractable water soluble value (15.43%) was close to its standard drug (16.15%). Formulation 2 was the developed formulation of Madhuhari (Shivayu) and its extractable water soluble value (18.66%) was close to its standard drug (19.47%). Formulation 3 was the developed formulation of Madhushoonya (Meghdoot) and its extractable water soluble value (15.13%) was close to its standard drug (14.05%). The alcohol-soluble extractive value was also indicative for the same purpose as the water-soluble extractive value. Less extractive value indicates addition of exhausted material, adulteration or incorrect processing during drying, or storage or formulating. The ether soluble extractive value signifies the presence of amounts of fats, lipids, and some steroids in the drug. Less extractive value indicates addition of exhausted material, adulteration or incorrect processing during drying, or storage or formulating. The alcohol-soluble extractive values of the different marketed formulations and their similar in-house prepared formulations were in the range of 9.08−11.12% [Table 3 and Figure 1]. Formulation 1 and Madhumehari (Baidyanath) have extractable alcohol-soluble values of 11.12% and 10.14%, respectively. Formulation 2 and Madhuhari (Shivayu) have extractable alcohol-soluble values of 10.98% and 10.36%, respectively. Formulation 3 and Madhushoonya (Meghdoot) have extractable alcohol-soluble values of 9.08% and 9.23%, respectively. The ether-soluble extractive values of the different drug and drug formulations were in the range of 6.86–8.32% [Table 3]. Formulation 1 and Madhumehari (Baidyanath) have extractable ether-soluble values of 8.32% and 8.21%, respectively. Formulation 2 and Madhuhari (Shivayu) have ether-soluble values 7.67% and 8.12%, respectively. Formulation 3 and Madhushoonya (Meghdoot) have extractable ether-soluble values of 6.86% and 7.24%, respectively. It was observed that the % water-soluble extractive values were higher than alcohol- and ether-soluble extractives.
Table 1c.
Ingredients for in-house Formulation 3 as labeled in Meghdoot Madhushoonya churna
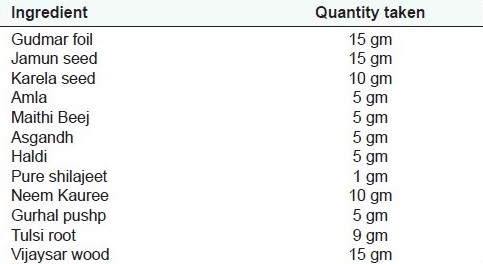
Figure 1.
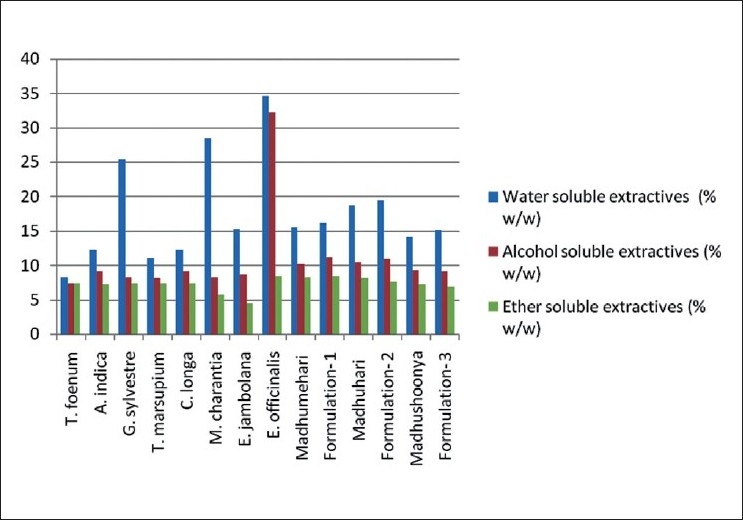
Water-soluble, alcohol-soluble, and ether-soluble extractive values of the individual drug powders and formulations
Comparing the water-soluble, alcohol-soluble, and ether-soluble extractive values of the drugs and formulations, it was concluded that the percent water-soluble extractive values were higher than the two; this indicates presence of more amounts of water-soluble contents in the plants. Also, it was observed that the extractive values of the marketed formulations were matching with the prepared in-house formulations indicating the use of authentic and good quality individual drugs in making those formulations. Further, it was observed that the extractive values of the formulations were matching with the average of individual drugs added.
Moisture content
Moisture is one of the major factors responsible for the deterioration of the drugs and formulations. Low moisture content is always desirable for higher stability of drugs. Moisture contents of the individual drugs, marketed formulations, and in-house prepared formulations were below 10% in the range of 5.21%–7.42% w/w [Table 4 and Figure 2].
Table 2.
Organoleptic characteristics of marketed and prepared in-house formulations
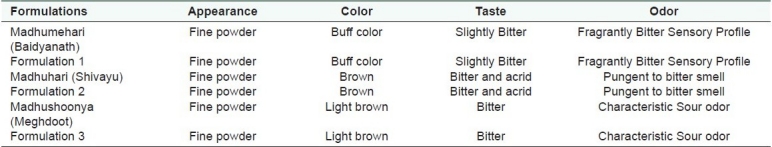
Figure 2.
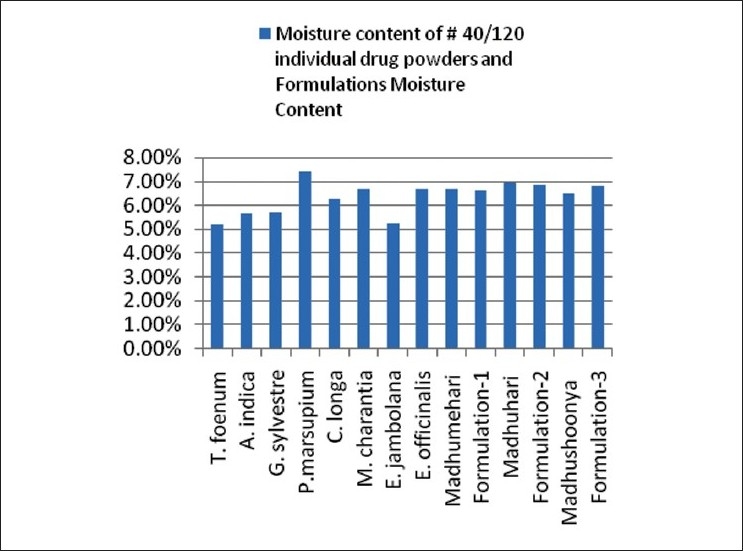
Moisture content of individual drug powders and formulations
Bulk density, Tapped Density, Carr's index, and Hausner Ratio
Study of bulk density and tapped density are important as density of a powder defines its packaging, and are listed in Table 5 and Figure 3, respectively, for individual drugs and formulations. Tapped density gives information on consolidation of a powder. A consolidated powder is likely to have a greater arch strength than a less consolidated one, and may therefore be more resistant to powder flow. Tapped densities of all the eight drug powders were in the range from 0.62 to 0.86 g/ml except Gymnema sylvestre, which was 0.55 g/ml. The three marketed formulations and their prepared in-house formulations had lower densities in the range of 0.50–0.63 g/ml indicating that these formulations were more bulky. There was no significant difference between the densities of the in-house developed formulations and marketed formulations.
Table 3.
Water-soluble, alcohol-soluble, and ether-soluble extractive values of the individual drug powders and formulations
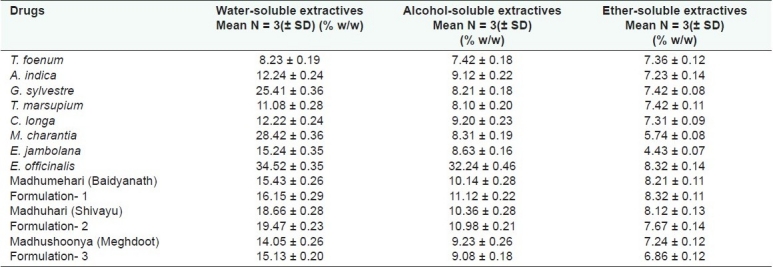
Figure 3.
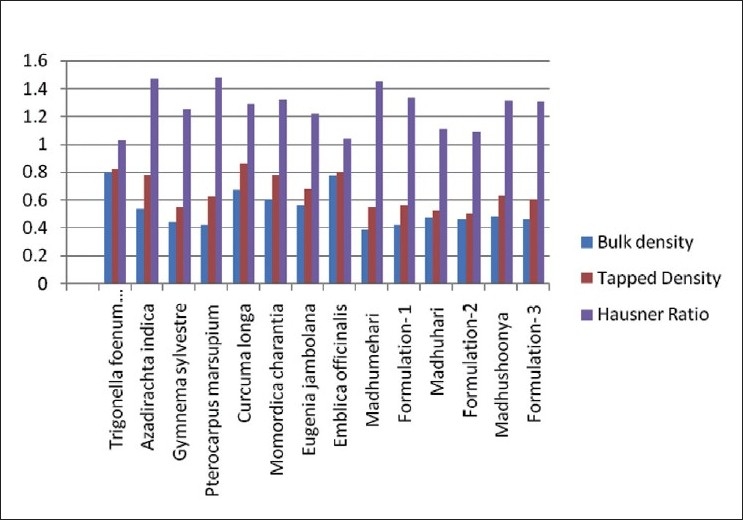
Bulk density, Tapped density, Carr's index, and Hausner Ratio of the individual drugs and formulations
Calculated Hausners ratio and Carr's Index for different drug and formulation are mentioned in the Table 5 for individual drugs and formulations. The Carr's Index and Hausners ratio were 14.63 and 1.025, respectively, for Trigonella foenum graecum indicating good compressibility. Azadirachta indica and Pterocarpus marsupium had Carr's Index of 32.05 and 32.25 and Hausners ratio 1.472 and 1.476 respectively indicating poor compressibility. Eugenia jambolana had Carr's Index and Hausners ratio of 17.6 and 1.21 indicating good compressibility. Gymnema sylvestre and Curcuma longa had values in the range of 20 and 1.25, respectively, for Carr's Index and Hausners ratio indicating fair compressibility. Momordica charantia had values of 27.85 and 1.317, respectively, for Carr's Index and Hausners ratio indicating poor compressibility. The developed formulation had lower values than their respective marketed drug indicating that the developed formulations were better than the marked formulations in terms of compressibility. Madhumehari (Baidyanath) and Madhushoonya (Meghdoot) had a high carr's index indicating poor compressibility. Low Hausner's value of 1.106 and 1.08 were observed for Madhuhari (Shivayu)and its developed formulation indicating good flow.
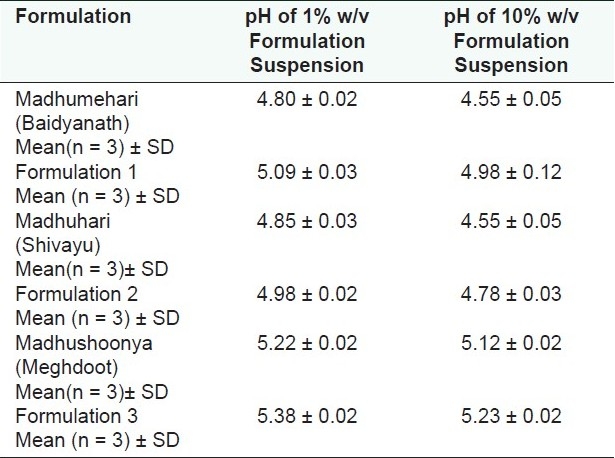
pH of suspension of the drugs
The observed pH values of 1% and 10% suspensions of the drugs were in the range from 4.55 to 5.38 [Table 6 and Figure 4] indicating suitability for human use.
Table 4.
Moisture content of individual drug powders and formulations
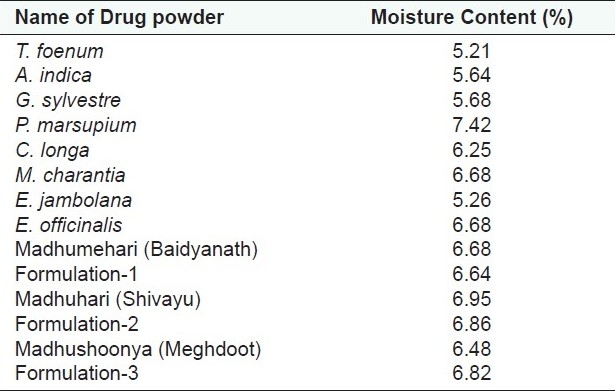
Figure 4.

pH of suspension of the formulations
Table 6.
pH of suspension of the formulations
Ash values
A high ash value is indicative of contamination, substitution, adulteration, or carelessness in preparing the drug or drug combinations for marketing. All the individual drugs were found to have total ash values in the range from 4.18 to 14.47% w/w [Table 7 and Figure 5]. Marketed and prepared in-house formulations were found to have total ash values in the range of 8.25 to 9.28% w/w. These values were found to be reasonably low indicating low contamination. Total ash values of the formulations matches with the average total ash values of the individual drugs. Also, the total ash values of the marketed formulation matches with the prepared in-house formulations.
Table 5.
Bulk density, Tapped Density, Carr's index, and Hausner Ratio of the individual drugs and formulations
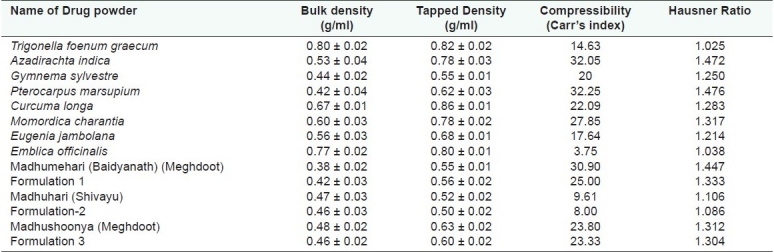
Figure 5.
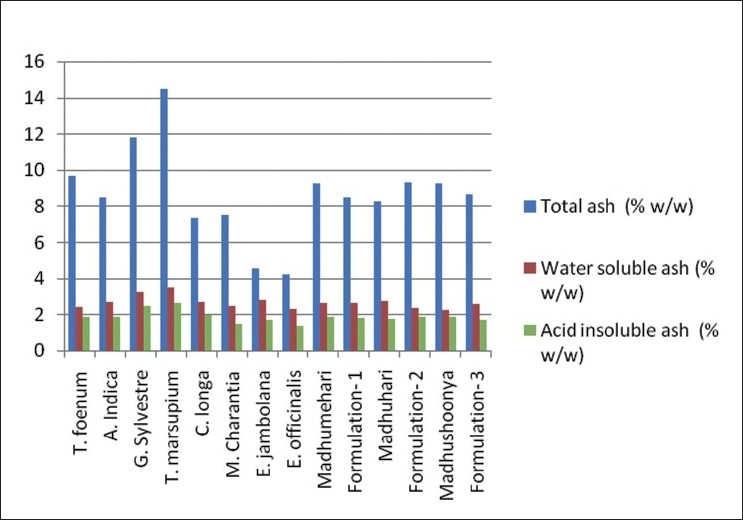
Percentage ash values of individual drugs and formulations (w/w)
Table 7.
Percentage ash values of individual drugs and formulations (w/w)
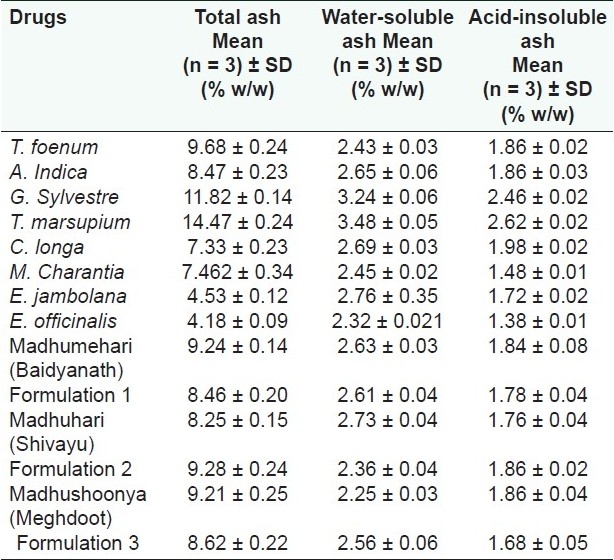
Water-soluble ash is the part of the total ash content, which is soluble in water. It is a good indicator of either previous extraction of water-soluble salts in the drug or incorrect preparation. Thus, it is the difference in weight between the total ash and the residue obtained after treatment of total ash with water. The water-soluble ash values of the individual drugs were in the range of 2.32 to 3.48% w/w. This shows a normal quality of the drugs. Water-soluble ash values of the marketed and prepared in-house formulations were found to be in the range of 2.25 to 2.73% w/w [Table 7 and Figure 5]. These values match with the average water-soluble extractive values of individual drugs. This also signifies that the marketed products also match as far as the water-soluble extractive values are concerned.
The acid-insoluble ash values of the individual drugs ranges from 1.38 to 2.62, and is below 2.0% for all the drugs and formulations except G. Sylvestre and T. marsupium, which were 2.46%and 2.62%. Acid-insoluble extractive values of the marketed and prepared in-house formulations were in the range of 1.68 to 1.86% w/w [Table 6]. These values match with the average acid-insoluble extractive values of individual drugs. This also signifies that the marketed products also match as for as the acid-insoluble extractive values are concerned.
It was observed that the ash values of the marketed formulations were matching with the prepared in-house formulations indicating the use of authentic and good quality individual drugs in making those formulations. Further, it was observed that the ash values of the formulations are matching with the average of individual drugs added. This signifies the ash value determination as an important parameter to standardize the herbal drugs.
CONCLUSION
In the present study it was concluded that the physicochemical parameters such as the water-soluble, alcohol-soluble, and ether-soluble extractive values, moisture content, bulk density, tapped density, Carr's index, Hausner's ratio, pH, water-soluble ash, acid-insoluble ash, and organoleptic characteristics can be efficiently used for standardization of herbal anti-diabetic drugs individually and in a polyherbal formulation. The results obtained from the study could be utilized as a reference for setting limits for the reference standards for the quality control and quality assurance of anti-diabetic drugs.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Shrikumar S, Maheswari MU, Suganthi A, Ravi TK. WHO guidelines for Herbal Drugs standardization. [ast accessed on 2009 Sep 22]. Available from: http://www.pharmainfo.net/exclusive/reviews .
- 2.Khan CR, Shechter Y. Insulin: Oral hypoglycemic agents and the pharmacology of endocrine pancreas, in Goodman and Gillman's The Pharmacological Basis of Therapeutics. 8th ed. New York: Pergaman Press; 1991. p. 1463. [Google Scholar]
- 3.Marles RJ, Farnsworth NR. Plant to patients: An ethnomedical approach. Phytomedicine. 1995;2:137. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 4.Aswatha RH, Kaushik U, Lachake P, Shreedhara CS. Pharmacognosy Research. 2009;4:224–7. doi: 10.4103/0974-8490.72329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallis TE. Text Book of Pharmacognosy. 5th ed. New Delhi: CBS Publishers and Distributors; 2004. p. 578. [Google Scholar]
- 6.Mukharjee PK. Quality Control of Herbal Drugs. New Delhi: Business Horizons Pharmaceutical Publishers; 2008. p. 186. [Google Scholar]
- 7.Indian Herbal Pharmacopoeia, Indian Drug Manufacturers’ Association. Revised ed. 2002. pp. 493–4. [Google Scholar]
- 8.Rajani M, Kanaki NS. Phytochemical Standardization of Herbal Drugs and Polyherbal Formulations, Bioactive Molecules and Medicinal Plants. Berlin, Heidelberg: Springer; 2008. pp. 349–69. [Google Scholar]
- 9.The Ayurvedic Pharmacopoeia of India, Part I. 1st ed. Vol. 2. Govt. of India, Department of Indian System of Medicine and Homoeopathy; 1999. p. 191. [Google Scholar]
- 10.Lachman L, Liberman HA, Kanig JL. The Theory and Practice of Industrial Pharmacy. 3rd ed. Mumbai: Varghese Publishing House; 1991. p. 67. [Google Scholar]
- 11.Mukharjee PK. Quality Control of Herbal Drugs. New Delhi: Business Horizons Pharmaceutical Publishers; 2008. p. 189. [Google Scholar]


