Abstract
Background:
The analgesic activity of petroleum ether, chloroform and methanol extracts of Semecarpus anacardium was investigated by tail flicking and writhing method using acetyl salicylic acid as the standard reference.
Materials and Methods:
The staircase method was adopted for the determination of the acute toxicity. LD50 of the petroleum ether extract and the chloroform extract was 700 mg/kg; however, the LD50 for the methanol extract was 500 mg/kg. After 1 h of oral administration of the extracts, 0.6% acetic acid was administered intraperitoneally and the analgesic activity was evaluated.
Results:
The number of writhing observed in the control group was 73.33 writhes. The methanol extract showed a significant analgesic activity, with 28.33 writhes, than the petroleum ether extract and the chloroform extract. But, all the extracts showed proved to be less potent than the standard drug which showed 2.33 writhes. Animals pretreated with saline did not show a signify cant effect on the latent period of tail-flick response. The analgesic effect of the petroleum ether extract was comparatively less evident. The maximum possible analgesia (MPA) increased up to 9.1% which remained elevated above the basal levels throughout the observation period. The MPA calculated for the chloroform extract increased to 14.03%. However, the analgesic effect of the methanol extract was also observed at 0.5 h following oral administration and the effect remained significant throughout the 3 h observation period, and was increased to 20.43%.
Conclusion:
Consistent analgesic activity of all the three S. anacardium extracts was observed by both the methods. The methanol extract was more potent than the petroleum ether and chloroform extracts but was less effective than the standard drug. This investigation supported the ethnomedicinal claims of S. anacardium.
Keywords: Acute toxicity study, crude extracts, tail-flicking method, writhing method
INTRODUCTION
Pain is the part of a defensive reaction against dysfunction of an organ or imbalance in its functions against potentially dangerous stimulus. The ascending pathway of pain includes the contralateral spinothalamic tract, lateral pons, mid brain to thalamus and ultimately through the somatosensory cortex of the brain that determines the locations, intensity and depth of pain. Many drugs used to relieve the pain and a few drugs like morphine[1] and aspirin[2] have been significantly used for the last three decades. Most of the pain-relieving chemicals produced pronounced side-effects on the physiology of the body. In the indigenous system of medicine, several plants possess an analgesic property and many investigators screened the plant crude extracts for their analgesic property, viz. Glaucium grandiflorum,[3] Basella rubra,[4] Salpichroa rhomboidea,[5] Euphorbia decipiens,[6] Ceriops decandra[7] etc. Reports also indicated that only a few reports were available on screening of the analgesic activity of phytoconstituents such as sesquiterpene dilactone from Mikania cordata,[8] dioclenol and dioflorin from Dioclea grandiflora, [9] divaricatol and hamaudol from Saposhnikovia divaricata[10] etc.
This article reports the analgesic effect of Semecarpus anacardium Linn. crude extract. S. anacardium Linn. belongs to the family Anacardiaceae, also called the “marking nut,” which has found many applications in Indian medicine in the treatment of gout, rheumatic pain and cancer.[11] S. anacardium has been evaluated pharmacologically on isolated tissues and on the whole animal.[12] Anticancer activity, anti-inflammatory, antiarthritic and antioxidant activities have been reported in experimental animals.[13] Very few studies have been reported on the hypolipidemic, hypoglycemic, antiatherogenic, antifungal, antifertility and neuroprotective activities.[14–20] The antiinflammatory and antiarthritic activities of milk extract and chloroform extract have been documented in rats and mice.[21–24] A significant protection against FeSO4 -induced lipid peroxidation with the alcohol extract of S. anacardium has also been demonstrated.[25]
To the best of our knowledge, there are no reports available on the analgesic activity of S. anacardium. In the present investigation, we made an attempt to investigate the analgesic effects of S. anacardium extracts using tail flicking and abdominal writhing methods.
MATERIALS AND METHODS
Plant Resource
S. anacardium plant material was collected from the Bhadra Wild Life Sanctuary of the Western Ghats, Karnataka, India. The stem bark of this plant was chopped finely and was shade dried, powdered mechanically and was subjected to soxhlet extraction using petroleum ether as the solvent system for about 48 h followed by chloroform and then methanol successively. The extracts were filtered and concentrated in vacuum under reduced pressure using a rotary flash evaporator (Buchi, Flawil, Switzerland), allowing the solvent to completely evaporate on a water bath and then finally vacuum dried.
Preparation of the Extracts
The fresh whole plant material was shade dried, powdered mechanically and subjected to soxhlet extraction using petroleum ether as the solvent system for about 48 h followed by chloroform and then methanol successively. The extracts were filtered and concentrated in vacuum under reduced pressure using a rotary flash evaporator (Buchi), allowing for complete evaporation of the solvent on a water bath and then finally vacuum dried. The yield of petroleum ether (black pasty in nature), chloroform (dark brown dry solid in nature) and methanol crude (maroon pasty in nature) extracts for 1 kg of the powdered whole plant material was 28.5 g, 20 g and 36.5 g, respectively.
Animals
Male Swiss albino mice weighing 25-30 g were procured from Central Animal House, National College of Pharmacy, Shivamogga and were maintained at standard housing conditions. The animals were fed with a commercial diet (Durga Feeds and Foods, Bangalore, India) and water ad libitum during the experiment. The Institutional Animal Ethical Committee (Reg. No. NCP/IAEC/CLEAR/06/2007-08) permitted the study.
The staircase method[26] was adopted for the determination of the acute toxicity. Healthy albino mice of either sex weighing 20-25 g were used to determine the safer dose. Gum tragacanth (1% w/v) was used as a vehicle to suspend the extracts and was administered orally.
Analgesic Activity by the Abdominal Writhing Method
The analgesic activity of petroleum ether, chloroform and methanol extracts of S. anacardium was carried out using adult Swiss albino mice by the abdominal writhing method.[27] Five groups of six mice each (25-30 g) were selected and 0.6% acetic acid (dose 10 ml/kg) was injected intraperitoneally. The number of writhes was counted for 20 min after 5 min of injection of acetic acid into each mouse. This reading was taken as control. The next day the same groups of mice were used for evaluating the analgesic activity. Group I was maintained as control, group II was administered orally with the suspension of the crude petroleum ether extract at the dose of 70 mg/kg, group III with chloroform extract (70 mg/kg) and group IV was administered with methanol extract (50 mg/kg) in gum tragacanth (1% w/v). Group V was administered with acetyl salicylic acid (asprin) used as standard for the comparison of analgesic activity. The crude extract and the standard drug were treated 1 h before administration of acetic acid. After 5 min, each group of mice was observed for the number of writhes for a duration of 20 min. The mean value for each group was calculated and compared with the control.
Analgesic Activity by the Tail-Flick Method
To support the analgesic activity by the writhing method, petroleum ether, chloroform and methanol extracts of S. anacardium were evaluated for analgesic activity by the tail-flick method using adult Swiss albino mice. Male or female albino mice weighing between 20 and 25 g were fasted for 24 h with water given ad libitum, maintained at room temperature and were divided into four groups of six mice. Group I mice were treated with gum tragacanth (1% w/v), Group II with petroleum ether extract (70 mg/kg), Group III with chloroform extract (70 mg/kg) and group IV were administered with methanol extract (50 mg/kg). The analgesic effect of the test samples was determined by the hot tail-flick method described by Sewell and Spencer (1976). One to 2 cm of the tail of mice was immersed in warm water kept constant at 50C. The reaction time was the time taken by the mice to deflect their tails. The first reading is discarded and the reaction time was taken as a mean of the next two readings. The latent period of the tail-flick response was taken as the index of analgesia and was determined before and at 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 h after the administration of drugs. The maximum reaction time was fixed at 0.5 h (30 min). The maximum possible analgesia (MPA) was calculated.[28]
Statistical Analysis
The data of the analgesic activity by number of writhes and tail flicks were expressed as mean ± SEM of six animals in each group. The statistical analysis was carried out using one-way ANOVA followed by Tukey's t-test. The difference in values at P ? 0.01 was considered as statistically significant.
RESULTS
Acute Toxicity Studies
After 72 h of observation, a plot of mortality values vs. log dose showed that the LD50 of the petroleum ether extract and the chloroform extract was 700 mg/kg. However, the methanol extract had an LD50 of 500 mg/kg body weight for oral administration. One-tenth of these doses were considered as a safer dose for administration.
Analgesic Activity by the Writhing Method
The number of writhing observed during a 20-min period in the control group was 73.33±1.16. The methanol extract (28.33 ± 2.25) showed a significant analgesic activity than the petroleum ether extract (51 ± 2.62) and the chloroform extract (45 ± 1.81). But, all the extracts proved to be less potent than the standard drug acetyl salicylic acid (2.33 ± 0.3) [Table 1]. We also observed that animals treated with petroleum ether, chloroform and methanol extracts showed a delayed onset of writhes (after 5-16 min) as compared with the control. And, the animals treated with the standard drug showed a delayed onset of writhes after 19-20 min. The results of the analgesic activity are depicted in Figure 1.
Table 1.
Analgesic activity of extracts of S. anacardium by the writhing method.
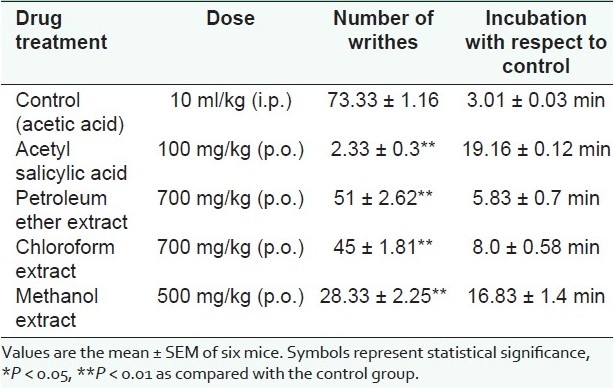
Figure 1.
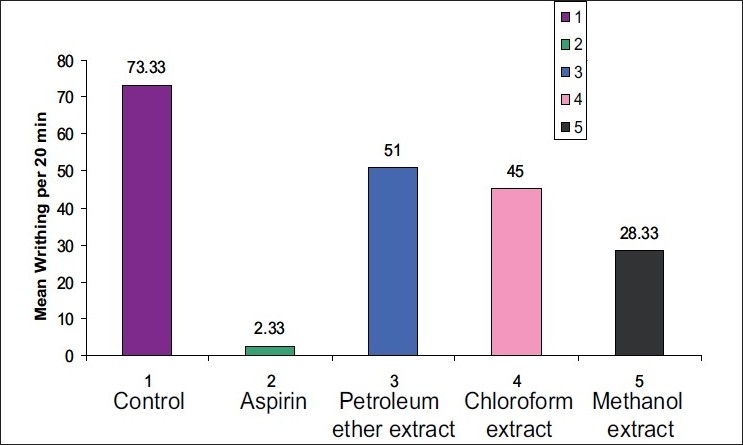
Graph showing the analgesic activity of the extracts of S. anacardium based on the mean of the writhes.
Analgesic Activity by the Tail-Flick Method
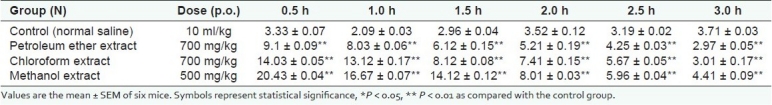
Throughout the 3-h observation, animals pretreated with saline did not show a significant effect on the latent period of the tail-flick response. The analgesic effect of the petroleum ether extract was comparatively less evident within 0.5 h following oral administration. The MPA increased up to 9.1 ± 0.09%, which remained elevated above the basal levels throughout the observation period [Figure 2 and Table 2]. Likewise, the chloroform extract also exhibited significant analgesia, which began within 0.5 h following oral administration and the effect remained significant throughout the 3-h observation period (p <0.01). The MPA calculated for the chloroform extract increased to 14.03 ± 0.05%. Similarly, the analgesic effect of the methanol extract was also observed at 0.5 h following oral administration and the effect remained significant throughout the 3-h observation period. The MPA calculated for the methanol extract increased to 20.43 ± 0.04%.
Figure 2.
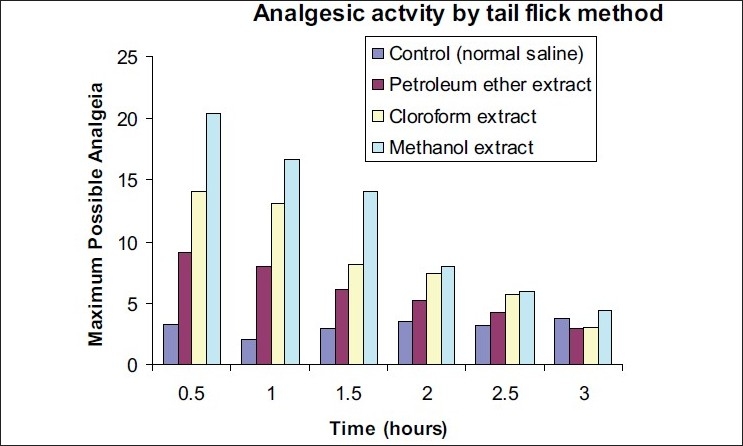
Graph showing the maximum possible analgesia (%) of S. anacardium extracts
Table 2.
Analgesic effects of S. anacardium extracts on mice by the tail flick method.
DISCUSSION
In the present investigation, petroleum ether, chloroform and methanol crude extracts of S. anacardium were evaluated for their analgesic activity by the tail-flick and abdominal writhing methods to determine the therapeutic efficacy of S. anacardium. Tail-flick and writhing methods are the most common tests for evaluating the analgesic efficacy of drugs/compound/crude extracts in rodents. The abdominal constriction response induced by glacial acetic acid is a sensitive procedure to establish peripherally acting analgesics. This response is thought to involve local peritoneal receptors.
We used the tail-flick and the writhing method to assess of the analgesic activity. The methanol extract of S. anacardium possessed a significant analgesic activity by increase of delayed onset of writhing and decrease of the number of writhes as compared with the control group. However, the petroleum ether and chloroform extracts showed a moderate activity.
On the other hand, the hot tail-flick method is shown here as incapable of differentiating between opiate and non-opiate analgesics, or between peripherally acting and centrally acting substances. In the report of Jansen and Prast (1988), the authors stated that nalorphine did not antagonize the effect caused by alkaloid from Mitragyna speciosa. Consistent analgesic activity was elicited by all the three S. anacardium extracts by both the methods used in the present study. The methanol extract showed consistent results than the petroleum ether and chloroform extracts. But, this was less effective than the standard reference acetyl salicylic acid.
CONCLUSION
This investigation revealed that all the extracts of S. anacardium showed an antinociceptive activity. Results obtained by the abdominal writhing and tail-flick methods indicated that among all the three extracts, the methanol extract was more efficient than the petroleum ether and the chloroform extracts in its antinociceptive action. This investigation supports the use of S. anacardium as an analgesic medicinal tree by the traditional practitioners of south India.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Brune K. New Pharmacological and Epidemiological Data in Analgesics Research. Basel, Switzerland: Birkhauser Verlag; 1990. [Google Scholar]
- 2.Willete RE, Delgado JN, Remers WA. Wilson and Gisvold's Textbook of Organic Medicinal and Pharmaceutical Chemistry. 1987;17:657. [Google Scholar]
- 3.Morteza-Semnani K, Saeedi M, Hamidian M. Anti-inflammatory and analgesic activity of the topical preparation of Glaucium grandiflorum. Fitotherpia. 2004;75:123–9. doi: 10.1016/j.fitote.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Hukkeri VI, Patil BS, Savadi RV, Nagathan CV. Analgesic, Antipyretic and Diuretic Activities of Basella rubra Linn. Indian Drugs. 2004;41:536–9. [Google Scholar]
- 5.Luigi M, Paola M, Giancarlo B, Bruno T. Preliminary studies on anti-inflammatory and analgesic activities of Salpichroa rhomboidea Miers extract. J Nat Remedies. 2004;4:32–5. [Google Scholar]
- 6.Viqar UA, Hidayat H, Ishfaq A, Bukhari JH, Amir RJ, Ahsana D. Antinociceptive diterpene from Euphorbia decipiens. Fitotherpia. 2005;76:230–2. doi: 10.1016/j.fitote.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Uddin SJ, Shilpi JA, Barua J, Rouf R. Antinociceptive activity of Ceriops decandra leaf and pneumatophore. Fitotherpia. 2005;76:261–3. doi: 10.1016/j.fitote.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed M, Rahman MT, Alimuzzaman M, Shilpi JA. Analgesic sesquiterpene dilactone from Mikania cordata. Fitotherpia. 2001;72:919–21. doi: 10.1016/s0367-326x(01)00318-5. [DOI] [PubMed] [Google Scholar]
- 9.Almeida ER, Almeida RN, Navarro DS, Bhattacharryya J, Silva BA, Birnbaum JS. Central antinociceptive effect of a hydroalcoholic extract of Dioclea grandifloraseeds in rodents. J Ethnopharmacol. 2003;88:1–4. doi: 10.1016/s0378-8741(03)00180-6. [DOI] [PubMed] [Google Scholar]
- 10.Okuyama E. Analgesic Components of Saposhnikovia Root (Saposhnikovia divaricata) Chem Pharm Bull. 2001;49:154–60. doi: 10.1248/cpb.49.154. [DOI] [PubMed] [Google Scholar]
- 11.Nadkarni AK. Indian Materia Medica. 3rd ed. Mumbai: Popular Book, Depot, Ltd; 1954. p. 119. [Google Scholar]
- 12.Bose BC, Mathur VS, Vijayavargiya R. Study of chemical and pharmacological properties of Semecarpus anacardium (Linn.) Indian J Med Res. 1967;55:155. [PubMed] [Google Scholar]
- 13.Premlatha B. Semecarpus anacardium Linn. Nuts – A boon in alternative medicine, Indian J Exp Biol. 2000;38:1177. [PubMed] [Google Scholar]
- 14.Tripathi YB, Pandey RS. Semecarpus anacardium Linn, nuts inhibit lipopolysaccharide induced NO production in rat macrophages along with its hypolipidemic property. Indian J Exp Biol. 2004;42:432. [PubMed] [Google Scholar]
- 15.Sharma A, Mathur R, Dixit VP. Hypocholesterolemic activity of nut shell extract of semecarpus anacardium (Bhilawa) in cholesterol fed rabbits. Indian J Exp Biol. 1995;33:444. [PubMed] [Google Scholar]
- 16.Arun B, Kothai R, Chruistina AJ. Hypoglycemic and antihyperglycemic effect of Semecarpus anacardium Linn. in normal and strepozotcin-induced diabetic rats. Exp Clin Pharmacol. 2004;26:759. doi: 10.1358/mf.2004.26.10.872556. [DOI] [PubMed] [Google Scholar]
- 17.Mary NK, Babu BH, Padikkala J. Antiatherogenic effect of Caps HT2, a herbal Ayurvedic medicine formulation. Phytomed. 2003;10:474. doi: 10.1078/094471103322331412. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A, Verma PK, Dixit VP. Effect of Semecarpus anacardium fruits on reproductive function of male albino rats. Asian J Androl. 2003;5:121–4. [PubMed] [Google Scholar]
- 19.Sharma K, Shukla SD, Mehta P, Bhatnagar M. Fungistatic activity of Semecarpus anacardium Linn nut extract. Indian J Exp Biol. 2002;40:314. [PubMed] [Google Scholar]
- 20.Shukla SD, Jain S, Sharma K, Bhatnagar M. Stress induced neuron degeneration and protective effects of Semecarpus anacardium Linn. and Withania somnifera Dunn. In hippocampus of albino rats: An ultrastructural study. Indian J Exp Biol. 2000;38:1007. [PubMed] [Google Scholar]
- 21.Satyavati GV, Prasad DN, Das PK, Singh HD. Antinflammatory activity of Semecarpus anacardium Linn. A preliminary study. Indian J Physiol Pharmacol. 1969;13:37. [PubMed] [Google Scholar]
- 22.Saraf MN, Ghooi RB, Patwardhan BK. Studies on the mechanism of action of Semecarpus anacardium in rheumatoid arthritis. J Ethnopharmacology. 1989;25:159. doi: 10.1016/0378-8741(89)90017-2. [DOI] [PubMed] [Google Scholar]
- 23.Vijayalaxmi T, Muthalaxmi V, Sachdanadam P. Salubrious effect of Semecarpus anacardium against lipid peroxidase changes in adjuvant arthritis studied in rats. Mol Cell Biochem. 1997a;175:65. doi: 10.1023/a:1006837312145. [DOI] [PubMed] [Google Scholar]
- 24.Vijayalaxmi T, Muthulaxmi V, Sachdanandam P. Effect of the milk extract of Semecarpus anacardium nuts on glycohydrolases and lysosomal stability in adjuvant arthritis in rats. J Ethnopharmacol. 1997b;58:1. doi: 10.1016/s0378-8741(97)00074-3. [DOI] [PubMed] [Google Scholar]
- 25.Tripathi YB, Singh AV. Effect of Semecarpus anacardium nuts on lipid peroxidation. Indian J Exp Biol. 2000;39:798. [PubMed] [Google Scholar]
- 26.Ghosh MN. Fundamentals of Experimental Pharmacology. Kolkata: Scientific Book Agency; 1984. [Google Scholar]
- 27.Collier HD, Dinnin LC, Johnson CA, Schneider C. The abdominal response & its suppression by analgesic drugs in the mouse. Br J Pharmacol. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Idid SZ, Norehan K, Roslan A. The involvement of the noradrenergic system in analgesia induced by the alkaloidal extract of Mitragyna speciosa in the rat. UKM: Proc 3rd Medical Colloquim; 1992. pp. 337–40. [Google Scholar]
- 29.Hendershot LC, Forsaith J. Antagonism of the frequency of phenylquinone-induced writhing in mouse by peak analgesics and non analgesics. J Pharmacol Exp Ther. 1959;125:237–41. [PubMed] [Google Scholar]
- 30.Sewell RD, Spencer PS. Antinociceptive activity of narcotic agonist and partial agonist analgesics and other agents in the tail-immersion test in mice and rats. Neuropharmacology. 1976;15:683. doi: 10.1016/0028-3908(76)90037-x. [DOI] [PubMed] [Google Scholar]
- 31.Jansen KL, Prast CJ. Ethnopharmacology of Kratom and the Mitragyna alkaloids. J. Ethnopharmacol. 1988;23:115–9. doi: 10.1016/0378-8741(88)90121-3. [DOI] [PubMed] [Google Scholar]


