Abstract
Background:
Streptozotocin (STZ) selectively destroys the pancreatic insulin secreting cells, leaving less active cells and resulting in a diabetic state. The present study was designed to investigate the antihyperglycemic effect of the ethanolic seed extract of Swietenia macrophylla (SME) in normal and STZ-diabetic rats.
Materials and Methods:
The experimental groups were rendered diabetic by intraperitoneal injection of a single dose of STZ (40 mg/kg body weight [BW]). Rats with glucose levels > 200 mg/dL were considered diabetic and were divided into 5 groups. Three groups of diabetic animals were orally administered, daily with seed extract at a dosage of 50, 100, and 200 mg/kg BW. One group of STZ rats was treated as diabetic control and the other group was orally administered 600 μg/kg BW glibenclamide daily.
Results:
Graded doses of seed extract and glibenclamide showed a significant reduction in blood glucose levels and improvement in serum insulin levels. The extract also improved body weight and promoted liver glycogen content. After treatment, hemoglobin (Hb) level increased and glycosylated Hb level significantly decreased in diabetic rats. The activities of the carbohydrate metabolic enzymes showed significant changes in the rats. Of the 3 doses, 100 mg dose showed maximum activity. Histological investigations of pancreas also supported the biochemical findings.
Conclusions:
Thus, our findings indicate the folklore use of the seed for diabetes and the mechanism seems to be insulin secretion.
Keywords: Glucose, insulin, Swietenia macrophylla, strepotozotocin
INTRODUCTION
The chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction, and failure of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels.[1] The therapeutic measures for the treatment of hyperglycemia include the use of insulin and other agents, such as amylin analogs, and alpha-glucosidase inhibitors such as acarbose and miglitol, voglibiose, sulfonylureas, and biguanides. These drugs have certain adverse effects, such as causing hypoglycemia at higher doses, liver problems, lactic acidosis, and diarrhea.[2,3] Therefore, there is a necessity to look for newer agents that meet the requirement of an ideal antidiabetic compound. Nature has been a source of medicinal substances for thousands of years, and plant-based systems continue to play an essential role in the primary health care of 80% of the world's underdeveloped and developing countries.[4] There is an increasing demand for natural products with antidiabetic activity for use by diabetic patients. Ethnopharmacological surveys indicate that more than 1200 plants are used worldwide in traditional medicine for their alleged hypoglycemic activity.[5,6] The investigation of antidiabetic agents of plant origin, which are used in traditional medicine, is thus of great significance.
Swietenia macrophylla is a beautiful, lofty, evergreen large tree, native to tropical America, Mexico, and South America, usually 30 – 40 m in height, and 3 m in girth.[7] The seeds of S. macrophylla have been reported for their anti-inflammatory, anti-mutagenic, and anti-tumor activities.[8] The seeds of S. macrophylla are traditionally used by the local healers of Azhagar hills, Madurai, Tamil Nadu, India, for curing diabetes. Hence, the present study was undertaken to evaluate the antidiabetic potential of the alcoholic seed extract of S. macrophylla experimentally in normal and streptozotocin (STZ)-induced diabetic rats, to prove its use by the tribes in folk medicine.
MATERIALS AND METHODS
Animals
Male albino (nine-week-old) rats of the Wistar strain, with a body weight ranging from 180 to 200 g, were procured from the Central Animal House, Department of Experimental Medicine, Rajah Muthiah Medical College and Hospital, Annamalai University, Annamalainagar. The animals were maintained at the Central Animal House and the animals were fed on a standard diet (Hindustan Lever Ltd., Bangalore) and water ad libitum. The animals were housed in polypropylene cages and maintained in a controlled environment, under standard conditions of temperature and humidity, with alternating 12 hour light / dark cycles. The studies were carried out in accordance with the Indian National Law on Animal Care and Use and ethical clearance was provided by the Committee for the Purpose of Control and Supervision of Experiments on Animals, of the Rajah Muthiah Medical College and Hospital (Reg. No.160 / 1999 / CPCSEA P. No. 493), Annamalai University, Annamalainagar.
Experimental induction of diabetes
The animals were rendered diabetic by a single intraperitoneal injection of STZ (40 mg / kg BW) in freshly prepared citrate buffer (0.1 M, pH 4.5) after an overnight fast. The STZ-injected animals were given 20% glucose solution for 24 hours, to prevent initial drug-induced hypoglycemic mortality. The STZ-injected animals exhibited massive glycosuria (determined by the Benedict's qualitative test) and hyperglycemia (by the glucose oxidase method). The diabetes in STZ rats was confirmed by measuring the fasting blood glucose concentration 96 hours after injection with STZ. The animals with blood glucose of more than 200 mg / dL were considered diabetic and used for the experiment.
Plant material
Seeds of S. macrophylla were collected from the months of October to December from Azhagar hills, Madurai, Tamil Nadu, India. The plant was botanically identified and authenticated in the Department of Botany, Annamalai University, Annamalainagar, Tamil Nadu, India.
Preparation of the seed extract
The plant seeds were dried and pulverized into a fine powder, and 100 g of dry powder was suspended in 400 mL of 95% ethanol for 72 hours. The extract was filtered using a muslin cloth and concentrated at 40°C ± 5°C. Then the extract was refluxed with petroleum ether to remove the lipid content of the seed. The fine powder was stored in a desiccator until use.
Experimental design
The animals were randomly divided into seven groups of six animals each, as described a little later in the text. Glibenclamide and S. macrophylla were administered orally using vehicle solution (2% Tween 80).
Chemicals
Streptozotocin was purchased from Sigma Chemical Co., St. Louis, MO, USA. All other chemicals and solvents were of analytical grade and purchased from E. Merck and Himedia Laboratories Pvt. Ltd., Mumbai, India.
Biochemical estimations
Blood glucose was estimated by the method of Trinder[9] using the reagent kit. The insulin in the rat plasma was measured using the method of Burgi et al.10 Hemoglobin (Hb) and glycosylated Hb (HbA1C ) were estimated by following the methods of Drabkin and Austin,[11] and Sudhakar and Pattabiraman,[12] respectively. The activities of glucokinase, glucose-6-phosphatase, and fructose-1,6-bisphosphatase were assayed by the methods of Brandstrup et al.,[13] Koide and Oda,[14] and Gancedo and Gancedo,[15] respectively. All quantitative measurements were expressed as means ° SD for control and experimental animals. The data were analyzed using one-way analysis of variance on the SPSS / * (statistical package for social sciences, personal computer) and the group means were compared by Duncan's multiple range test. The results were considered statistically significant if the P value was less than 0.05.
RESULTS
Table 1 shows the effect of the alcoholic seed extract of S. macrophylla (SME) on the body weight and blood glucose levels of control and STZ-diabetic rats. Diabetic rats showed decreased body weight and elevated blood glucose level. Oral administration of SME and glibenclamide in diabetic rats showed an improvement in body weight and decreased blood glucose level and the effect of SME was more pronounced at the 100 mg dose.
Table 1.
Effect of Swietenia macrophylla on the body weight of Streptozotocin-diabetic rats
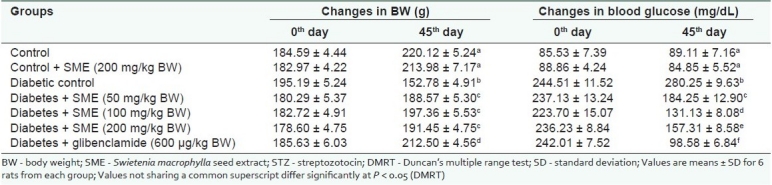
Table 2 shows the levels of insulin, Hb, HbA1C, and liver glycogen. Hb, plasma insulin levels decreased, whereas, the HbA1C level significantly increased in diabetic rats and oral administration of SME and glibenclamide significantly increased the Hb, insulin and decreased the HbA1C levels. The liver glycogen of diabetic rats treated with S. macrophylla and glibenclamide was brought to a normal level.
Table 2.
Effect of Swietenia macrophylla on insulin, blood Hb, glycosylated Hb, and liver glycogen of Streptozotocin-diabetic rats
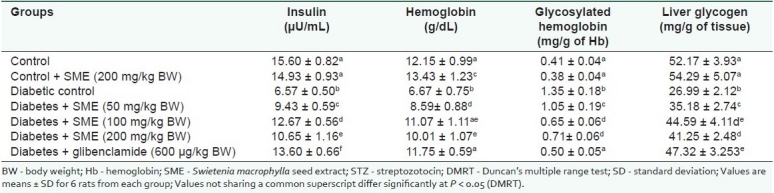
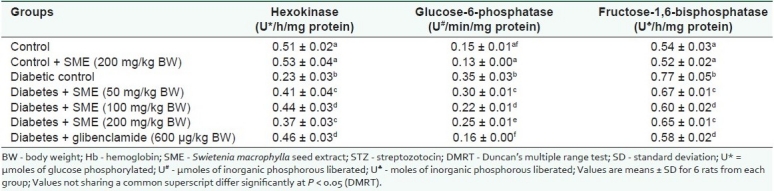
Table 3 shows the effect of SME on the activities of carbohydrate metabolic enzymes in the liver of control and STZ-diabetic rats. The activity of glucokinase reduced, whereas, the activities of gluconeogenic enzymes glucose-6-phosphatase, and fructose-1,6-bisphosphatase increased in diabetic rats. Oral administration of SME and glibenclamide significantly increased the hexokinase activity and decreased glucose-6-phosphatase and fructose-1,6-bisphosphatase activities.
Table 3.
Effect of SME on the activities of carbohydrate metabolic enzymes in the liver of Streptozotocindiabetic rats
DISCUSSION
Streptozotocin-induced diabetes is characterized by severe loss in body weight,[16] due to the degradation of structural proteins, which are responsible for the changes in body weight. In the present study, significant weight loss has been observed in diabetic rats. Oral administration of SME and glibenclamide significantly reduced the body weight. This may be due to the ability of SME to reduce hyperglycemia.
The mechanism underlying hyperglycemia in diabetes mellitus involves overproduction (excessive hepatic glycogenolysis and gluconeogenesis) and decreased utilization of glucose by the tissues.[17] As STZ destroys the β cells, insulin production is decreased, and therefore, there is an increase in the blood glucose level. Administration of S. macrophylla and glibenclamide lowers the blood glucose concentration in diabetic rats and no hypoglycemic activity is observed in control rats treated with S. macrophylla. The increase in insulin level may be due to increased pancreatic secretion from the existing β cells.
During diabetes, the excess glucose present in the blood reacts with Hb to form HbA1C. The rate of glycation is proportional to the concentration of blood glucose.[17] The lower levels of total Hb observed in diabetic rats may be due to the increased formation of HbA1C. Diabetic rats treated with SME show a significant decrease in the HbA1C levels and a significant increase in the Hb levels, when compared with diabetic rats. This may be due to the glucose lowering effect of SME.
Glycogen is the primary intracellular storable form of glucose. Glycogen deposition from glucose is impaired in diabetic animals,[18] in proportion to the severity of insulin deficiency.[19] S. macrophylla has brought the liver glycogen to normalcy in diabetic rats, which may be due to the increased secretion of insulin.
Glucokinase is the prime enzyme catalyzing glucose phosphorylation. Impairment of hexokinase activity suggests that the impaired oxidation of glucose via glycolysis, leads to its accumulation, resulting in hyperglycemia. A partial or total deficiency of insulin causes a derangement in carbohydrate metabolism that decreases the activity of several key enzymes, including glucokinase, phosphofructokinase, and pyruvate kinase,[20] resulting in impaired peripheral glucose utilization and augmented hepatic glucose production. In our study, the glucokinase activity has been found to be decreased in the liver of diabetic rats, which may be due to a deficiency of insulin, and treatment with S. macrophylla and glibenclamide elevates the activity of glucokinase. S. macrophylla administration increases the insulin level, which in turn activates glucokinase, thereby increasing the utilization of glucose, leading to decreased blood sugar level.
Glucose-6-phosphatase and fructose-1,6-bisphosphatase are the regulatory enzymes of gluconeogenesis. The increased activities of these enzymes in the liver may be due to insulin insufficiency. Insulin decreases gluconeogenesis by decreasing the activities of key enzymes, such as, glucose-6-phosphatase, fructose-1,6-bisphosphatase, phosphoenolpyruvate carboxykinase, and pyruvate carboxylase. In S. macrophylla- and glibenclamide-treated rats, the activities of these two enzymes were significantly reduced. Thus, our results show a sequential metabolic correlation between increased glycolysis and decreased gluconeogenesis, stimulated by S. macrophylla, suggesting the possible biochemical mechanism via insulin secretion, through which glucose homeostasis is regulated.
In conclusion, the results of this investigation reveal that the alcoholic extract has antidiabetic properties, which provides the rationale for the use of SME as an antidiabetic drug by traditional healers. It has been found that the alcoholic seed extract is devoid of the hypoglycemic effect on normal rats and the maximum effect of antihyperglycemic activity is found at the 100 mg dose. The histological studies support the biochemical findings. Further research has to be carried out, to fractionate and purify the extract, to find out the active compound(s) responsible for the antidiabetic activity.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Diagnosis and classification of diabetes mellitus. Diabetes care. 2008;31:S55–60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson MA, Maclaren NK. The pathogenesis of insulin dependent diabetes mellitus (review) N Engl J Med. 1994;31:1428–36. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 3.Yki-Jarvinen H. Action of insulin action on glucose metabolism in vivo. Bailliere's Clin Endocrinol Metab. 1993;7:903–27. doi: 10.1016/s0950-351x(05)80239-3. [DOI] [PubMed] [Google Scholar]
- 4.Grover JK, Vats V. Shifting paradigm from conventional to alternate medicine.An introduction to traditional Indian medicine. Asia Pacific Biotech News. 2001;5:28–32. [Google Scholar]
- 5.Gupta RK, Kesari AN, Murthy PS, Chandra R, Tandon V, Watal G. Hypoglycemic and antidiabetic effect of ethanolic extract of leaves of Annona squamosa L. Experimental animals. J Ethnopharmacol. 2005;99:75–81. doi: 10.1016/j.jep.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 6.Kesari AN, Gupta RK, Singh SK, Diwakar S, Watal G. Hypoglycemic and antihyperglycemic activity of Aegle marmelos seed extract in normal and diabetic rats. J Ethnopharmacol. 2006;107:374–9. doi: 10.1016/j.jep.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Rastogi RP, Mehrotra BN. Compendium of Indian Medicinal plants. New Delhi: India, PID; 1990. p. 1937. [Google Scholar]
- 8.Guevera AP, Apilado A, Sakarai H, Kozuka M, Tokunda H. Anti-inflammatory, antimutagenicity and antitumor activity of mahogany seeds Swietenia macrophylla (Meliaceae) Phill J Sci. 1996;125:271–8. [Google Scholar]
- 9.Trinder P. Determination of blood glucose using an oxidase peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969;22:158–61. doi: 10.1136/jcp.22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgi W, Briner M, Franken N, Kessler AC. One-step sandwich enzyme immunoassay for insulin using monoclonal antibodies. Clin Biochem. 1988;21:311–4. doi: 10.1016/s0009-9120(88)80087-0. [DOI] [PubMed] [Google Scholar]
- 11.Drabkin DL, Austin JM. Spectrophotometric constants for common haemoglobin derivatives in human, dog and rabbit blood. J Biol Chem. 1932;98:719–33. [Google Scholar]
- 12.Sudhakar NS, Pattabiraman TN. A new colorimetric method for the estimation of glycosylated haemoglobin. Clin Chim Acta. 1981;109:267–74. doi: 10.1016/0009-8981(81)90312-0. [DOI] [PubMed] [Google Scholar]
- 13.Brandstrup N, Kirk JE, Bruni C. The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J Gerontol. 1957;12:166–71. doi: 10.1093/geronj/12.2.166. [DOI] [PubMed] [Google Scholar]
- 14.Koide H, Oda T. Pathological occurrence of glucose-6-phosphate in serum liver diseases. Clin Chim Acta. 1992;4:554–61. doi: 10.1016/0009-8981(59)90165-2. [DOI] [PubMed] [Google Scholar]
- 15.Gancedo JM, Gancedo C. Fructose 1,6-bisphosphatase, phosphofructokinase and glucose 6-phosphate dehydrogenase from fermenting and non-fermenting yeasts. Arch Mikrobiol. 1971;76:132–8. doi: 10.1007/BF00411787. [DOI] [PubMed] [Google Scholar]
- 16.Chen V, Ianuzzo CD. Dosage effect of streptozotocin on rat tissue enzyme activities and glycogen concentration. Can J Physiol Pharmacol. 1982;60:1251–6. doi: 10.1139/y82-183. [DOI] [PubMed] [Google Scholar]
- 17.Paulsen EP. Haemoglobin ALC in childhood of diabetes. Metabolism. 1973;22:269–71. doi: 10.1016/0026-0495(73)90170-4. [DOI] [PubMed] [Google Scholar]
- 18.Bollen M, Keppens S, Stalmans W. Specific features of glycogen metabolism in the liver. Biochem J. 1998;336:19–31. doi: 10.1042/bj3360019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gannon MC, Nuttall FQ. Effect of feeding, fasting and diabetes on liver glycogen synthase activity protein and mRNA in rats. Diabetologia. 1997;40:758–63. doi: 10.1007/s001250050746. [DOI] [PubMed] [Google Scholar]
- 20.Hikino H, Kobayashi M, Suzuki Y, Konno C. Mechanisms of hypoglycemic activity of aconitan A, a glycan from Aconitum carmichaeli roots. J Ethnopharmacol. 1989;25:295–304. doi: 10.1016/0378-8741(89)90035-4. [DOI] [PubMed] [Google Scholar]


