Abstract
Background:
The phytochemical and pharmacological activities of Annona reticulata components suggest a wide range of clinical application in lieu of cancer chemotherapy.
Materials and Methods:
Ethanol and aqueous extracts of roots of Annona reticulata Linn were studied for their in vitro antiproliferative activity on A-549 (human lung carcinoma), K-562 (human chronic myelogenous leukemia bone marrow), HeLa (human cervix) and MDA-MB (human adenocarcinoma mammary gland) cancer cell lines by MTT [3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide] colorimetric assay.
Results:
The ethanol extract exhibited a prominent inhibitory effect against A-549, K-562, HeLa and MDA-MB human cancer cell lines at a concentration range between 10 and 40 μg/ml, whereas the aqueous extract showed a lower activity at the same concentration. Simultaneously, the effect of the ethanol extract toward the inhibition of Vero cell line proliferation was lower in comparison with the cancer cell lines.
Conclusion:
The significant antiproliferative activity of the ethanol extract of Annona reticulata roots against A-549, K-562, HeLa and MDA-MB human cancer cell lines may be attributed toward the collective presence of acetogenins, alkaloids and lower inhibitory effect on Vero cell line, which suggests Annona reticulata be used as a chemopreventive agent in cancer therapy.
Keywords: A-549, Annona reticulata, antiproliferative activity, HeLa and MDA-MB human cancer cell lines, K-562, MTT assay
INTRODUCTION
Cancer is a disease in which there is infinite multiplication and spread within the body of abnormal forms of the body's own cells. In spite of much progress in the treatment of cancer by modern system of medicines and therapy using synthetic drugs, search for newer natural drugs continues because of several complications like cell injury, bone marrow depression, impaired growth, sterility and hair loss that are associated with the prevalent modern cancer chemotherapy.[1] Annona reticulata Linn, commonly known as bullock's heart or raamphal plant, is widely distributed all over India and are tall, with many branches, bearing nutritious fruits. The leaves are used as insecticides, anthelmintic, styptic and are also used externally as suppurant. The bark as a powerful astringent is used as antidysentric and vermifuge. Root bark, leaves and stem possess isoquinoline alkaloids.[2] Acetogenins from the leaves were found to be selectively cytotoxic to certain human tumors.[3] The phytochemical and pharmacological activities of Annona reticulata components suggest a wide range of clinical application in lieu of cancer chemotherapy. Recent reports revealed that the plant exerted selective cytotoxicity and that the acetogenins present in the leaves is responsible for this specific cytotoxic effect.[4] These studies suggest that Annona reticulata possesses a selective antiproliferative effect, although its inhibitory effect on different cancer cell lines by the roots remains to be evaluated. In this study, we have made an attempt to confirm the need to evaluate the antiproliferative activity of different extracts of roots on A-549 (human lung carcinoma), K-562 (human chronic myelogenous leukemia bone marrow), HeLa (human cervix) and MDA-MB (human adenocarcinoma mammary gland), which are the possible different human cancer cell lines. In addition, we further aim to define the phytoconstituents responsible for the claimed in vitro antiproliferative activity.
MATERIALS AND METHODS
Plant Material
The roots of Annona reticulata were collected from local areas of North Karnataka and a voucher specimen has been deposited at the departmental herbarium. The roots (500 g) were dried and pulverized to particle size (#) 40 and then extracted with ethanol in the soxhlet apparatus for 48 h, and 200 g of fresh drug was subjected to cold maceration with chloroform water (I.P.) to obtain the ethanol and aqueous extracts, respectively. The filtrate of both the extracts was concentrated to dryness at 40°C under reduced pressure in a rota evaporator. The yield of the ethanol and aqueous extracts was found to be 150 g (30% w/w) and 16 g (8% w/w), respectively.
Preliminary Phytochemical Studies
The phytochemical screening of the ethanol extract indicated the presence of alkaloids, acetogenins, carbohydrates, proteins and flavonoids. Similarly, the aqueous extract showed the presence of tannins, carbohydrates, flavonoids and proteins.[5]
Proliferation Kit, Cell Lines and Reagents
The MTT assay kit was purchased from Roche Applied Sciences, Germany. A-549 (human lung carcinoma), K-562 (human chronic myelogenous leukemia bone marrow), HeLa (human cervix) and MDA-MB (human adenocarcinoma mammary gland) and Vero (African green monkey kidney normal cell) cell lines, free from any bacterial and fungal contamination, were procured from NCCS, Pune, India. All the chemicals and reagents, viz. Propanol (Qualigens), Fetal Bovine Serum (Bioclot) and MTT dye are used for the study.
Microculture Tetrazolium (MTT) Assay
Both the ethanol extract and the aqueous extract were evaluated for the in vitro cytotoxicity study on A-549 (human lung carcinoma), K-562 (human chronic myelogenous leukemia bone marrow), HeLa (human cervix) and MDA-MB (human adenocarcinoma mammary gland) and Vero (African green monkey kidney normal cell) cell lines at the concentrations of 10, 20, 30 and 40 μg by performing the MTT assay.[6] This colorimetric assay is based on the capacity of mitochondria succinate dehydrogenase enzymes in living cells to reduce the yellow water-soluble substrate 3-(4, 5-dimethyl thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) into an insoluble, colored formazan product, which was measured spectrophotometrically. The monolayer cell culture was trypsinized and the cell count was adjusted to 3-lakh cells/ml using a medium containing 10% newborn calf serum. To each well of the 96-well microtitre plates, 0.1 ml of the diluted cell suspension of the different cell lines were added separately. After 24 h, when the monolayer formed, the supernatant was flicked off and 100 μl of the ethanol and aqueous extracts, each at the concentration of 10, 20, 30 and 40 μg in buffered DMSO, were added to the cells in the microtitre plates separately and kept for incubation at 37ΊC in a 5% CO2 incubator for 72 h and the cells were periodically checked for granularity, shrinkage and swelling. After 72 h, the sample dilution in the wells was flicked off and 50 μl of the MTT dye was added to each well. The plates were gently shaken and incubated for 4 h at 37°C in a 5% CO 2 incubator. The supernatant was removed, 50 μl of Propanol was added and the plates were gently shaken to solubilize the formed formazan. The absorbance was measured using a microplate reader at a wavelength of 490 nm.[7]
A graph of absorbance against the concentration of the drug was plotted and the inhibitory effect (IC50) was calculated as the drug concentration that is required to reduce absorbance to half that of the control, based on the dose–response curve for the different extracts. The reduction of MTT can only occur in metabolically active cells, the level of activity being a measure of viability of the cells. Absorbance values that are lower than the control cell lines reveal the decline in the rate of cell proliferation. Conversely, a higher absorbance indicates increase in the cell proliferation. Untreated microtitre plates of cell lines with only vehicle (0.3% v/v DMSO in water) is considered as proliferative control.
The percent inhibition of cell proliferation by the extracts is calculated based on the difference in the inhibitory effect between the treated cell lines and their respective controls, where 100% cell proliferation is taken from the corresponding controls.
Statistical analysis
All the results are expressed as mean ± SD of triplicate. The difference in the inhibitory effect at different doses between the treated and the corresponding controls was analyzed for statistical significance by performing a Student's t-test.[8] P <0.05 implies significance.
RESULTS
In the present study, the inhibitory effect of the ethanol and aqueous extracts were assessed for their in vitro antiproliferative activity on different human cancer cell lines (A-549, K-562, HeLa and MDA-MB). The IC50 and percent inhibition of cell proliferation of the cancer cell lines of the above extracts were calculated and are represented in Table 1.
Table 1.
Antiproliferative effect of the extracts of Annona reticulata roots on human cancer cell lines
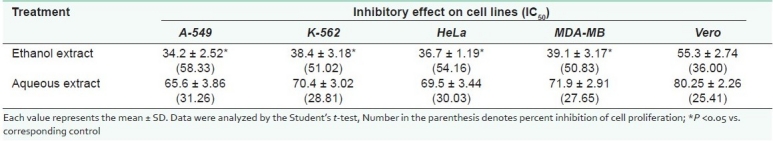
The ethanol extract exhibited a significant dose-dependent antiproliferative activity against the different cancer cell lines at dilutions ranging from 10 to 40 μg/ml, whereas the aqueous extract is less prominent in suppression of proliferation of the above cancer cell lines at similar concentrations. No inhibitory effect on the cancer cell lines is observed by both the extracts at dilutions less than 10 μg/ml. The antiproliferative activity of the ethanol extract on the Vero cell line is constantly less at the experimented dilutions as compared with the cancer cell lines. The prominent antiproliferative effect of the ethanol extract of the roots of Annona reticulata on A-549, K-562, HeLa and MDA-MB cancer cell lines, as revealed by its IC50 based on the MTT assay, was found to be 34.2 ± 2.52, 38.4 ± 3.18, 36.7 ± 1.19 and 39.1 ± 3.17, respectively. The IC50 of the ethanol extract was specifically less significant on the Vero cell line, i.e. 55.3 ± 2.74. The phytochemical investigation of the ethanol extract was revealed to contain acetogenins, alkaloids, carbohydrates, flavonoids and proteins.
DISCUSSION
The IC50 values of the extracts using the MTT assay against A-549, K-562, HeLa, MDA-MB and Vero cell lines revealed that the ethanol extract of Annona reticulata roots exerted a significant antiproliferative effect on the A-549, K-562, HeLa and MDA-MB cancer cell lines. Simultaneously, it is evident that the ethanol extract exhibited a less-prominent antiproliferative activity on the Vero cell line. However, the activity of the aqueous extract is less significant on both the cancer and the normal cell lines. Apoptosis is a physiologically programmed process of active cellular self-destruction responsive to gene expression.[9] The ethanol extract-mediated antiproliferative activity is more confined to the cancer cell lines rather than to the normal cell lines. This indicates that the specific inhibitory effect may be due to the apoptosis-inducing ability of the ethanol extract in response to the defective gene expression in cancer cell lines rather than the normal cell line. This suggests the drug to be used as an antiproliferative against cancer cell lines rather than to possess cytotoxicity. With the significant antiproliferative activity of the ethanol extract of the roots of Annona reticulata against A-549, K-562, HeLa and MDA-MB cancer cell lines, the mechanisms of action could, possibly, be due to the dose-dependent apoptosis-inducing ability, by necrosis of cancer cell lines, by enhanced neoplastic transformation followed by apoptosis or by any other mechanisms related to epigenetic and signal transduction pathways.
From the phytochemical investigation, it was found that the major chemical constituents of the ethanol extract were acetogenins, alkaloids, flavonoids, proteins, carbohydrates. etc. Acetogenins are reported to possess a specific cytotoxic effect.[10,11] Alkaloids are also known to possess cytotoxic properties.[12,13] The ethanol extract exhibited a significant antiproliferative activity against all experimented cancer cell lines. Hence, the collective presence of acetogenins and alkaloids in the ethanol extract could be attributed to the observed significant antiproliferative activity against human cancer cell lines. The less-prominent antiproliferative activity of the ethanol extract on the Vero cell line elicits the use of the ethanol extract of the roots of Annona reticulata as a chemopreventive agent in cancer therapy. However, this is a preliminary research work and the precise mechanism(s) of action influenced by potent bioactive constituents of the ethanol extract for antiproliferative activity in the human cancer cell lines is yet to be investigated.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. New Delhi: Elsevier; 2003. pp. 698–9. [Google Scholar]
- 2.Nadkarni KM. Indian Materia Medica. Vol. 1. Mumbai: Popular Prakashan; 2002. pp. 115–6. [Google Scholar]
- 3.Xu L, Sun N, Kong J. Alkaloids of Annona reticulata L. Zhonqquo Zhong Yao Za Zhi. 1992;17:295–6. [PubMed] [Google Scholar]
- 4.Cassady JM, Baird WM, Chang CJ. Natural products as a source of potential cancer chemotherapeutic and chemopreventive agents. Mol Cancer Ther. 2008;7:2662–71. doi: 10.1021/np50067a003. [DOI] [PubMed] [Google Scholar]
- 5.Clarke EG. Isolation and identification of drugs. Vol. 2. London: Pharmaceuticals Press; 1975. p. 905. [Google Scholar]
- 6.Patel SS, Gheewala N, Suthar A, Shah A. In vitro Cytotoxicity activity of Solanum nigrum extracts against Hela and Vero cell lines. Int J Pharma Pharmaceut Sci. 2009;1:38–46. [Google Scholar]
- 7.Masters RW. Animal cell culture. Cytotox Viability Assays. 2000;3:207. [Google Scholar]
- 8.Kulkarni SK. Hand book of Experimental Pharmacology. Vol. 5. New Delhi: Vallabh Prakashan; 1993. pp. 78–81. [Google Scholar]
- 9.Deodhare SG. General pathology and pathology of systems. Vol. 2. Mumbai: Popular Prakashan; 2002. pp. 1572–4. [Google Scholar]
- 10.Coothankandaswamy V, Liu Y, Mao SC, Morgan JB, Mahdi F, Jekabsons MB, et al. The alternate medicine PawPaw and its acetogenins constituents supress tumour angiogenesis via the HIF-I/ VEGF pathway: J Nat Prod. 2010;73:956–61. doi: 10.1021/np100228d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardhasaradhi BV, Reddy M, Ali AM, Kumari AL, Khar A. Differential cytotoxic effects of Annona squamosa seed extracts on human tumour cell lines: Role of reactive oxygen species and glutathione. J Biosci. 2005;30:237–44. doi: 10.1007/BF02703704. [DOI] [PubMed] [Google Scholar]
- 12.Griffin C, Sharda N, Sood D, Nair J, McNulty J, Pandey S. Selective cytotoxicity of Pancratistatin-related natural Amaryllidaceae alkaloids: Evaluation of the activity of two new compounds. Cancer Cell Int. 2007;7:10. doi: 10.1186/1475-2867-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chougule M, Patel AR, Sachdeva P, Jackson T, Singh M. Anticancer activity of Noscapine an Opoid alkaloid in combination with Cisplatin in human non-small cell lung cancer. Lung Cancer. 2010;6:2. doi: 10.1016/j.lungcan.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


