Abstract
There is substantial scientific evidence to support the notion that bovine spongiform encephalopathy (BSE) has contaminated human beings, causing variant Creutzfeldt–Jakob disease (vCJD). This disease has raised concerns about the possibility of an iatrogenic secondary transmission to humans, because the biological properties of the primate-adapted BSE agent are unknown. We show that (i) BSE can be transmitted from primate to primate by intravenous route in 25 months, and (ii) an iatrogenic transmission of vCJD to humans could be readily recognized pathologically, whether it occurs by the central or peripheral route. Strain typing in mice demonstrates that the BSE agent adapts to macaques in the same way as it does to humans and confirms that the BSE agent is responsible for vCJD not only in the United Kingdom but also in France. The agent responsible for French iatrogenic growth hormone-linked CJD taken as a control is very different from vCJD but is similar to that found in one case of sporadic CJD and one sheep scrapie isolate. These data will be key in identifying the origin of human cases of prion disease, including accidental vCJD transmission, and could provide bases for vCJD risk assessment.
The recognition of a variant of the human transmissible spongiform encephalopathy (TSE) Creutzfeldt–Jakob Disease (vCJD) in the U.K. in 1996 raised the major concern that it would correspond to human infection with the agent responsible for bovine spongiform encephalopathy (BSE; ref. 1). Transmission of BSE to macaques provided the first experimental evidence as it produced a disease close to vCJD in humans (2). Strain typing in inbred mice (consisting of measuring the incubation period and establishing lesion profiles corresponding to the strain-specific distribution of brain vacuolation) allows reliable identification of TSE strains (3). This method, together with biochemical methods, has revealed a single phenotype for the agents of BSE and the British cases of vCJD (4–6). Mice expressing only the bovine prion protein (PrP) were highly susceptible to vCJD and BSE, which induced the same disease (7). Thus, it is now well established that BSE has caused vCJD, probably by alimentary contamination. In this respect, the finding of abnormal PrP labeling in the gastrointestinal tract and lymphatic tissues of orally BSE-contaminated lemurs shows that the BSE agent can infect primates by the oral route (8). About 1 million contaminated cattle may have entered the human food chain, and the future number of vCJD cases could range from 63 to 136,000 depending on the incubation period of BSE in humans (9). Unlike sporadic CJD (sCJD) and iatrogenic CJD (iCJD) linked to the administration of contaminated growth hormone extracted from human hypophyses, in vCJD, the infectious agent seems to be widely distributed in lymphoid organs, as pathological PrP (PrPres) can be detected in tonsils, lymph nodes, spleen, and appendix even in the preclinical phase of the disease (10, 11). This raises a public health issue with regard to the risk of iatrogenic transmission of vCJD through surgical instruments, grafts, blood transfusion, or parenteral administration of biological products of human origin. However, this risk is difficult to assess, because it largely depends on factors such as the virulence of the BSE agent adapted to primates and the efficiency of secondary transmission to humans by a peripheral route such as the i.v. one. A further issue is whether vCJD accidentally acquired from humans would be recognized. The latter poses the question of a phenotypic variation of the BSE agent after successive transmissions in humans: does it retain its strain characteristics, and does it induce a pathology similar to that observed in the previous host? A 9-year history of transmission of BSE to primates and mice enables us today to clarify a number of these important points.
Although BSE has mainly affected the U.K., two definite cases and one probable case of vCJD have now been reported in France in people who have never resided in the U.K. (12, 13). We strain-typed the first of these cases to establish its origin. Strain typing in C57BL/6 mice of BSE, French, and British vCJD was compared with that of BSE passaged in nonhuman primates, thus allowing us to study the effect of serial passages in primates. Comparisons were also made with French cases of sCJD and iCJD and two strains of scrapie (one of French and one of U.S. origin). Our findings provide experimental demonstration that the same agent, namely that responsible for the cattle disease BSE, has caused vCJD both in France and in the U.K., in line with biochemical data and with the fact that, until 1996, about 10% of the beef consumed in France was imported from the U.K. We found that the BSE agent in nonhuman primates is similar to that causing vCJD in humans and tends to evolve rapidly toward a primate-adapted variant. Furthermore, we showed that the strain responsible for iCJD is closely related to that of one patient with sCJD, and, more unexpectedly, that these agents were similar to the French scrapie strain studied (but different from the U.S. scrapie strain). This finding requires a cautious interpretation for several reasons, not least because of the inevitably limited number of TSE strains that can be studied by such a cumbersome method as strain typing. Nonetheless, it also prompts reconsideration of the possibility that, in some instances, sheep and human TSEs can share a common origin.
Materials and Methods
Inocula.
Transmissions to C57BL/6 mice were set up from the first French patient with vCJD, two British patients with vCJD, BSE cattle, and macaques experimentally infected with BSE (first and second passage). This protocol was designed to detect any change in the phenotypic expression of the BSE agent because of its replication in primates. The French patient was a 26-year-old man who died of CJD in 1996. This case has been classified as vCJD on the basis of the observation of florid plaques and PrPres typing (12, 13). One British vCJD patient was a 42-year-old woman who had sensory symptoms at onset of the disease; the other was a 31-year-old man whose clinical history began with memory impairment. Cynomolgus macaques (Macaca fascicularis) were inoculated intracerebrally (i.c.) with brain of BSE-infected cattle from the U.K. They were killed 3 years later at the terminal stage of the disease, and the brain of the youngest (2) was used for inoculation of a second set of macaques.
As controls, primary transmissions were also performed from two French patients affected by sCJD and iCJD (linked to the subcutaneous injection of pituitary-extracted growth hormone), respectively, and from two Romanov sheep of the same flock at the clinical stage of scrapie, whose brains were kindly provided by P. Sarradin, Institut National de la Recherche Agronomique, Nouzilly, France. Both human patients were methionine homozygotes at codon 129 of the PrP gene, like the patients with vCJD. They had been classified as type 1 (corresponding to high molecular weight PrPres; refs. 5 and 14). The sheep were VRQ/ARQ heterozygotes at codons 136, 154, and 171. Scrapie appeared suddenly in this experimental Romanov flock after oral challenge with nematode parasites in 1994 (15). The mouse-adapted C506 M3 scrapie strain was used as a further control. It is derived from a U.S. case of scrapie in a Cheviot sheep (16) and was kindly provided by P. Brown, National Institutes of Health, Bethesda.
Inocula from nonhuman primates and humans consisted of gray matter from the cerebellum or the forebrain. British vCJD, iCJD, and sCJD transmissions were achieved with the brain region that had maximum levels of PrPres deposition (refs. 1 and 17 and Table 1). Cattle inoculum consisted of brainstem and was kindly provided by R. Bradley and M. Dawson (Central Veterinary Laboratory, Weybridge, U.K.).
Table 1.
Incubation periods of C57BL/6 mice inoculated with TSEs
| Strain | Donor* | Incubation
period,† days
|
|||||
|---|---|---|---|---|---|---|---|
| i.c.
|
i.c. +
i.p.
|
i.c.
|
i.c. + i.p.
|
||||
| Cerebellum | Frontal cortex | Cerebellum | Frontal cortex | Brainstem | Brainstem | ||
| Scrapie | Sheep FR | — | — | — | — | 773 ± 97§ | 705 ± 89§ |
| iCJD | Human FR | 729 ± 119¶ | — | 688 ± 114¶ | — | — | — |
| sCJD | Human FR | — | 701 ± 98¶ | — | 668 ± 85¶ | — | — |
| vCJD | Human FR | 461 ± 34¶ | 514 ± 61¶ | — | — | — | — |
| Human UK1 | 469 ± 42¶ | — | 474 ± 44¶ | — | — | — | |
| Human UK2 | 449 ± 25¶ | — | 465 ± 69¶ | — | — | — | |
| BSE | Cattle UK | — | — | — | — | 514 ± 40§ | 525 ± 47¶ |
| M. fascicularis | 286 ± 15¶ | 297 ± 15¶ | — | 277 ± 14§ | — | — | |
| 2P M. fascicularis | 277 ± 45‡¶ | — | — | — | — | — | |
Fr, French case; UK, British case; 2P, second passage in the same host.
Incubation periods are given as mean ± SD for the indicated route and source of inoculum.
The i.c. inoculation was performed with 10% brain homogenate; 1% homogenate gave an incubation period of 330 ± 28 days by the i.c. route, 372 ± 35 days by the i.p. route.
§n = 15.
¶n = 10.
Transmissions to cynomolgus macaques were set up from one British and the first French case of vCJD, experimental macaque BSE, and experimental macaque kuru. The patient with kuru (Enage) was M/M at codon 129 of the PrP gene; the disease was transmitted first to rhesus macaques (8 years of incubation), then to cynomolgus macaques (ref. 16; the inoculum was a gift from P. Brown and C. Gibbs, National Institutes of Health, Bethesda).
Transmission Experiments.
Adult female C57BL/6 mice (R. Janvier, Le Genest-Saint-Isle, France) were inoculated either with 20 μl of 20% (vol/vol) brain homogenate in glucose solution i.c. or with 20 μl of 10% (vol/vol) i.c. and 100 μl of 2% (vol/vol) brain homogenate i.p. (n = 10–15 per group). The experiments were performed under containment level 3. Mice were housed in an independent environmental cabinet for animal storage, and their cages were handled under a flow hood. Ten control mice were each inoculated i.c. with brain homogenates from a healthy macaque and mouse, respectively, and kept under the same conditions. Mice were examined weekly for neurological signs until the prodromic phase; from there on, they were observed every other day. Mice were killed at the terminal stage of disease by cervical fracture. For each animal, one brain hemisphere was fixed in 10% (vol/vol) phosphate-buffered formalin for histology, whereas the other hemisphere was frozen in liquid nitrogen and stored at −80°C for Western blotting.
Captive-bred macaques were purchased from Centre de Recherche en Primatologie (Mauritius) and were checked for the absence of common primate pathogens, in particular tuberculosis, filoviruses, herpes B, simian immunodeficiency virus, simian retrovirus, simian T lymphotropic virus 1, hepatitis B, and measles. Macaques were inoculated i.c. with 400 μl of brain homogenate at the appropriate concentration. For i.v. administration, 400 μl of 10% (vol/vol) brain homogenate were adjusted to a volume of 1 ml with an isotonic glucose solution. Inoculations were performed under ketamine anesthesia, and the animals recovered within a few hours after the inoculation procedure. Each incubation period indicated in Fig. 1 corresponds to that of one individual animal. Details of the i.c. tritration are given in the figure legend. Kuru was inoculated to one animal. To avoid any interindividual contamination, animals were housed in separate cages within containment level 3 modules in our animal care facility. Each module consists of four individual cages between which the animals can communicate without direct contact with each other to form a social group. The animals are grouped several weeks before inoculation to verify their social compatibility.
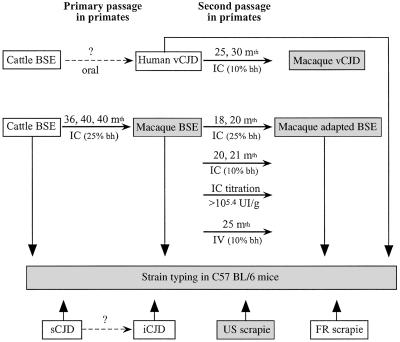
Figure 1.
Transmission scheme of TSEs in cynomolgus macaques and C57BL/6 mice. Open frames, natural or accidental disease; hatched frames, experimental disease in primates; stippled frames, experimental disease in mice. IC, intracerebral inoculation; IV, intravenous inoculation; bh, brain homogenate; mth, month. The incubation periods indicated on the arrows each correspond to one inoculated macaque. One macaque was inoculated with British vCJD (25 months), and the other was inoculated with the first French case of vCJD (30 months). The incubation periods of the i.c. titration are as follows: 10−1 dilution: 20 and 21 months; 10−2 dilution: 28 months; 10−3 dilution: 33 months; 10−5 dilution: 38 months (the second macaque inoculated presents slight behavioral changes at 48 months after inoculation).
Pathology and Immunohistochemistry.
Formalin-fixed whole brain hemispheres were inactivated with 98% (vol/vol) formic acid for 30 min, paraffin-embedded, and cut into 5-μm sagittal sections. The lesion profile system was adapted from the original Edinburgh system (3) with the differences being that the scored brain areas were examined on sagittal sections, with two additional areas (pons and striatum), and that only one score was used for the cerebral cortex instead of two. As a result, a total of 10 gray-matter areas were examined for vacuolation after hematoxylin–eosin staining in 6–10 mice per inoculum; readings were done blinded and repeated at least three times. Astrogliosis was detected after glial fibrillary acidic protein (GFAP) immunohistochemistry with a mix of three monoclonal anti-GFAP antibodies (PharMingen) diluted to 1/10,000, and a biotin-labeled goat anti-mouse secondary antibody followed by horseradish peroxidase detection with streptavidin amplification (Dako). PrP immunochemistry of macaque brains was done on sections treated according to the consensus U.K. recommendations for PrP immunocytochemical procedures with slight modifications (18) with 3F4 (Clinisciences, Montrouge, France) and 8G8 (kindly provided by G. Hunsmann, Göttingen, Germany) PrP monoclonal antibodies. The reaction product was visualized by using the diaminobenzidine staining kit containing a goat biotin-labeled secondary antibody as described above (Dako).
PrPres Analysis.
PrPres was purified according to a scrapie-associated fibrils protocol and detected by Western blotting as described (19). Briefly, PrPres samples were separated by electrophoresis on an 12%/SDS polyacrylamide gel, blotted onto a nitrocellulose membrane, and revealed with an anti-PrP rabbit polyclonal serum (JB007) produced in the laboratory (20).
Results
Transmissions to Mice.
All mice (but none of the control animals) developed a neurological disease after variably long incubation periods (Table 1). With the vCJD and BSE inocula, disease was mainly characterized by nervousness, a hunched posture, hindlimb paresis and subsidence, rigidity of the tail, and urinary retention. The clinical signs were more homogeneous after transmission of French vCJD, British vCJD, and primate BSE than of cattle BSE (where, for example, obesity occurred in some mice). The incubation periods after transmission from either of the vCJD cases were remarkably similar to each other and to BSE (transmissions from the frontal cortex of French vCJD and the brainstem of BSE cattle yielded the same incubation period of 514 ± 40 days, Table 1).
Transmission of macaque BSE yielded incubation periods shorter by about 200 days, with no significant difference between passages 1 and 2 (Table 1). Macaque BSE also transmitted to other mouse strains with the classical BSE pattern, except that the incubation periods were shorter than with cow BSE (data not shown). A likely explanation is that titers were higher in the brains of i.c.-inoculated monkeys than in cattle and humans presumably contaminated by the oral route. Interim results from end-point titration experiments in mice aimed at verifying this hypothesis indicate that the infectivity is at least 100-fold lower in the brain of the French vCJD patient than of BSE macaques (data not shown).
Primary transmissions from French cases of sheep scrapie and sporadic or iatrogenic CJD could be readily distinguished from BSE and vCJD transmissions, as they yielded much longer incubation periods and different clinical features. In the case of sCJD and iCJD, the main clinical features encompassed spread hindlimbs with a lowering of the back and dorsal kyphosis. Scrapie mice showed arched backs, rigidity of the tail and limbs, and withdrawal of the posterior legs. In sCJD, iCJD, and scrapie, a frequent symptom was the early appearance of an exophthalmos associated later with keratitis rapidly leading to blindness. The scrapie strain C506 M3 derived from a U.S. scrapie case gave an incubation period of 170 ± 5 days.
Lesion Profiles in C57BL/6 Mice of BSE and vCJD.
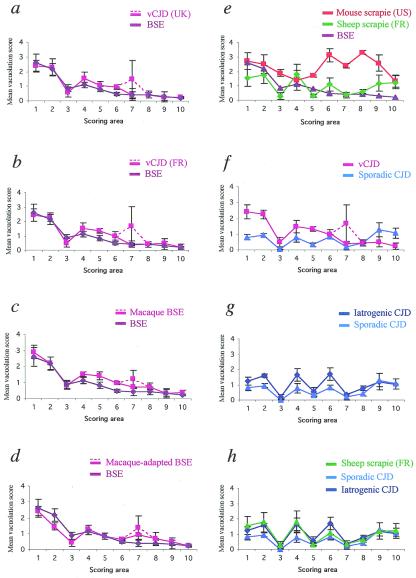
In BSE, we observed the typical caudorostral decrease in severity of the lesions described in natural and experimental BSE (21, 22), confirmed by the general shape of the lesion profile (Fig. 2). Spongiosis was most prominent in the brainstem and the pons, whereas the cortex was almost spared with few vacuoles. The lesion profiles of French vCJD, British vCJD, and macaque BSE were identical to that of BSE except that a polymorphism was observed in the hippocampus, with some mouse brains disclosing severe vacuolation localized to the superior region of Ammon's horn, whereas in others the severity of vacuolation was strictly superimposable with that of BSE (Fig. 2 a, b, and c). In second-passage macaque BSE, the “hippocampal signature” of the BSE agent passaged in primates was stronger, with all mice showing higher vacuolation scores in this region, whereas the lesions in the posterior regions of the brain were slightly milder (Fig. 2d). Thus, the infectious agent passaged twice in primates was still the BSE strain, but the latter had undergone small variations revealed by the lesion profile, which may have an impact in terms of biological virulence.
Figure 2.
Lesion profiles in C57BL/6 mice after transmission of vCJD, BSE, sCJD, iCJD, and scrapie. Lesion profiles are (a) cattle BSE and British vCJD (two pooled cases); (b) cattle BSE and the first of the three French vCJD cases; (c) cattle BSE and BSE after primary transmission to macaques; (d) cattle BSE and BSE after secondary transmission to macaques; (e) cattle BSE, the mouse-adapted C506 M3 scrapie strain (derived from U.S. sheep scrapie), natural sheep scrapie (two pooled cases from a single French flock); (f) French vCJD and a French case of sCJD; (g) the former sCJD and one French case of iCJD linked to the administration of growth hormone; and (h) same as in g plus the natural sheep scrapie. The dotted lines belong to the lesion profiles of vCJD (in a and b) and of macaque-BSE (in c and d). Vacuolation was scored on a scale of 0–5 in the following brain areas: 1, medulla; 2, pons/mesencephalon; 3, cerebellar cortex; 4, colliculi; 5, hypothalamus; 6, thalamus; 7, hippocampus; 8, septum; 9, cerebral cortex; and 10, striatum.
Lesion Profiles in C57BL/6 Mice of sCJD, iCJD, and Natural Scrapie.
Both French natural scrapie and the U.S. scrapie strain C506 M3 produced lesion profiles that were clearly distinct from that of BSE (Fig. 2e). Also, sCJD was readily distinguishable from vCJD (Fig. 2f). However, the lesion profiles of iCJD and sCJD had a similar shape despite a slight difference in intensity (Fig. 2g), and the latter shape was also identical to that of the French natural scrapie strain (Fig. 2h).
PrPres Analyses.
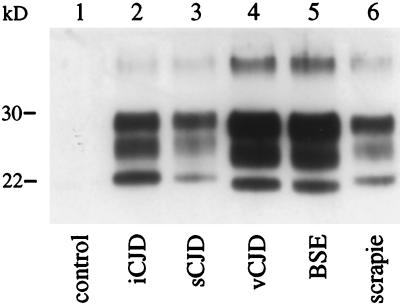
We were ideally placed to perform biochemical strain typing because we could analyze the PrPres induced by the different agents in syngeneic C57BL/6 mice carrying a single PrP sequence. We clearly distinguished two types of PrPres: that of cattle/macaque BSE and vCJD (corresponding to a type 2 or 4 depending on the classification used; refs. 5 and 14) and that of scrapie, sCJD, and iCJD, which had a higher molecular weight most clearly seen in the un- and monoglycosylated bands (type 1 PrPres, Fig. 3, lanes 4 and 5 versus lanes 2, 3, and 6).
Figure 3.
Electrophoretic analysis of PrPres of vCJD, BSE, sCJD, iCJD, and scrapie transmitted to C57BL/6 mice. Western blot detection of PrPres purified from the brains of C57BL/6 mice at the terminal stage of the disease (except for the control) after inoculation of normal mouse brain homogenate (lane 1); French case of iCJD linked to the administration of growth hormone (lane 2); French case of sCJD (lane 3); first of the three French vCJD cases (lane 4); cattle-BSE (lane 5); and natural sheep scrapie (French Romanov flock) (lane 6). The mouse brain equivalent loads were 0.6 mg (lanes 4 and 6), 1.2 mg (lanes 3 and 5), and 12 mg (lanes 1 and 2).
Transmissions of vCJD and BSE to Nonhuman Primates.
Secondary transmission of BSE to macaques by the i.c. route led to a shortening of the incubation period to half that of the primary passage (18 versus 36 months, Fig. 1). The vCJD-inoculated primates died 25 and 30 months after inoculation, as compared to the 20 and 21 months observed with macaque BSE at the same inoculation concentration. Macaque-adapted kuru transmitted after 24 months.
Titration by homologous i.c. inoculation of a limited number of macaques revealed the presence of >105.4 infectious units/g of BSE-infected macaque brain. BSE-infected macaque brain (40 mg) was also injected i.v. into another macaque, which died of the disease after 25 months.
The duration of the clinical phase was as follows: 1–3.5 months for BSE (i.c.), 4 months (i.v.); 5.5 months for vCJD (i.c.); and 3.5 months for kuru (i.c.). For vCJD and BSE, clinical signs were similar to the first passage of BSE (2); the disease began with behavioral signs (with variable relative intensity depending on the animals: depression, nervousness, teeth grinding, yawning, alterations of the grooming activity, voracious appetite) and evolved rapidly to a truncal ataxia with tremors, incoordination of movements, and imbalance. Later on, the animals became aggressive; the ataxia was extremely severe; and myoclonic jerks were observed. In the monkey inoculated with kuru, cerebellar signs were prominent, and behavioral changes (which were early signs of illness for BSE) appeared later in the course of the disease, apparently as a consequence of wasting.
Phenotype in Nonhuman Primates of vCJD and BSE After Secondary Transmission.
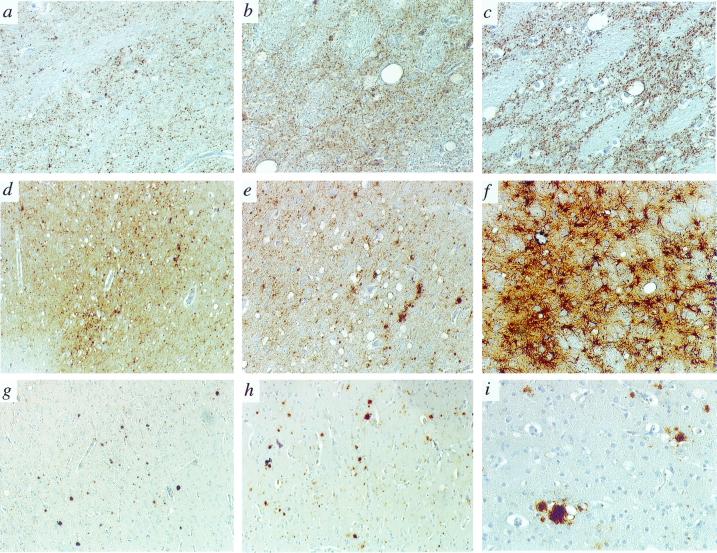
The pathology of the second passage of BSE in nonhuman primates was similar to that of vCJD transmitted to macaques and resembled that described for the first passage in macaques (2). Thus, the second passage of BSE in primates is characterized pathologically by severe vacuolation and astrocytosis in the thalamus (Fig. 4 b, c, and f); vacuolation and PrP deposition in the granular and molecular layers of the cerebellum; a patchy distribution of vacuolation in the cortex (mainly cingulate and occipital) accompanied by the presence of dense plaques, some of them harboring a florid morphology (Fig. 4 g, h, and i); and cellular and granular PrP deposition in deep cortical layers, in the basal ganglia and thalamus (Fig. 4 b and c). No difference in pathology was observed between macaques inoculated i.c. and i.v. (Fig. 4 g and h).
Figure 4.
Immunopathology of BSE, vCJD, and kuru in cynomolgus macaques. All panels show the pattern of PrP deposition with the 3F4 antibody, except f, which depicts glial fibrillary acidic protein immunohistochemistry. (a, b, and c) Thalamus in kuru, BSE, and vCJD, ×20. (d and e) Cerebral cortex in kuru, ×2.5 and ×10. (f) Thalamus in BSE, ×10. (g) Cerebral cortex in BSE (i.v.), ×2.5. (h) Cerebral cortex in BSE (i.c.), ×2.5. (i) Immature florid plaque with a dense core of PrP surrounded by few vacuoles in the cerebral cortex (BSE, i.c.), ×20.
The pattern of PrP deposition and plaque morphology were identical to those observed at first passage of BSE in the animals inoculated as adults. There were many dense plaques with no or few vacuoles, quite obviously representing immature forms of florid plaques. Typical florid plaques were more numerous in the young animal inoculated as a neonate (2). This may be related to the longer duration of the clinical phase in the young animal, which allowed greater pathology development.
In contrast, in kuru there were few vacuoles in the thalamus and cerebellum (notwithstanding the presence of PrP deposition, Fig. 4a); vacuolation was more severe than in BSE and was uniform throughout the cortex (Fig. 4d); and PrP immunohistochemistry revealed mainly punctate deposits in all cortical layers with few plaques (Fig. 4 d and e).
Discussion
One aim of this study was to determine the risk of secondary transmission to humans of vCJD, which is caused not by a primarily human strain of TSE agent but by the BSE strain having passed the species barrier to humans. This risk is tightly linked to the capacity of the BSE agent to adapt to primates and harbor enhanced virulence (i.e., induce disease after a short incubation period and provoke disease even if highly diluted) and to its pathogenicity after inoculation by the peripheral route. With respect to the latter, there are huge variations between different TSE agent strains and hosts. For example, the BSE agent is pathogenic to pigs after i.c. inoculation but not after oral administration (23). Thus, we wanted to know to what extent the BSE/vCJD agent is pathogenic to humans by the i.c. and i.v. routes. To achieve this, we used the macaque model. To monitor the evolution of the BSE agent in primates, but also to verify the identity of French vCJD, we conducted parallel transmission to C57BL/6 mice, allowing strain-typing. The experimental scheme is depicted in Fig. 1.
Characterization of the BSE Agent in Primates.
The identity of the lesion profiles obtained from the brains of the French patient with vCJD, two British patients with vCJD, and nonhuman primates infected with BSE provides experimental demonstration of the fact that the BSE agent strain has been transmitted to humans both in the U.K. and in France. Further, it lends support to the validity of the macaque model as a powerful tool for the study of vCJD. As far as the evolution of the BSE agent in primates is concerned, we observed an interesting phenomenon: at first passage of BSE in macaques and with vCJD, there was a polymorphism of the lesion profile in mice in the hippocampal region, with about half of them harboring much more severe vacuolation than the mice inoculated with cattle BSE. At second passage, the polymorphism tended to disappear, with all mice showing higher vacuolation scores in the hippocampus than cattle BSE mice. This observation suggests the appearance of a variant of the BSE agent at first passage in primates and its clonal selection during second passage in primates. The lesion profiles showed that it was still the BSE agent, but the progressive appearance of a “hippocampal signature” hallmarked the evolution toward a variant by essence more virulent to primates.
Characterization of the CJD and Scrapie Strains.
Controls were set up by transmitting one French and one U.S. scrapie isolate from ruminants as well as French sCJD and iCJD cases from humans. None of these revealed a lesion profile or transmission characteristics similar or close to those of BSE or vCJD, respectively, thus extending to the present French scrapie isolate the previous observation that the BSE agent was different from all known natural scrapie strains (4, 24).
The lesion profiles of sCJD and iCJD differed only slightly in severity of the lesions, but not in shape of the profile, revealing the identity of the causative agents. One of us reported the absence of similarity between sCJD (six cases) and U.K. scrapie (eight cases) in transmission characteristics in mice (4). Herein, we made the striking observation that the French natural scrapie strain (but not the U.S. scrapie strain) has the same lesion profile and transmission times in C57BL/6 mice as do the two human TSE strains studied. This strain “affiliation” was confirmed biochemically. There is no epidemiological evidence for a link between sheep scrapie and the occurrence of CJD in humans (25). However, such a link, if it is not a general rule, would be extremely difficult to establish because of the very low incidence of CJD as well as the existence of different isolates in humans and multiple strains in scrapie. Moreover, scrapie is transmissible to nonhuman primates (26). Thus, there is still a possibility that in some instances TSE strains infecting humans do share a common origin with scrapie, as pointed out by our findings.
Transmission of vCJD and BSE to Nonhuman Primates.
vCJD transmitted readily to the cynomolgus macaque after 2 years of incubation, which was comparable to the transmission obtained from first-passaged macaque BSE and much shorter than the interspecies transmission of BSE. Starting with 100 mg of BSE–macaque brain material, dilutions up to 4 μg still provoked disease. These data suggest that the BSE agent rapidly adapts to primates accompanied by enhanced virulence.
Examination of macaque brain inoculated with vCJD revealed a similar pathology to that with second-passage BSE. The distribution of vacuolation and gliosis, as well as the pattern of PrP deposition, including the dense, sometimes florid plaques, were similar to the human vCJD and the BSE hallmarks of the first passage (1, 2). These data show that the phenotype of BSE in primates is conserved over two passages. Moreover, they confirm that the BSE agent behaves similarly in humans and macaques, a precious finding that will prove useful in the near future for the design of pathogenesis or therapeutic studies.
Because of the number of macaques examined in this study, we can now reliably state that the pathology, in particular the PrP deposition pattern provoked by BSE, is similar in older and very young animals. However, plaque deposition is greater, and mature florid plaques were more numerous, in the young, which may be correlated with a longer duration of the clinical phase observed in this animal (2). This is important with regard to the fact that vCJD has been diagnosed mainly in teenagers and young adults, which raises the concern that older patients may have been misdiagnosed because of an alternative phenotype of the disease.
One should bear in mind, however, that cynomolgus macaques are all homozygotes for methionine at codon 129 of the PrP gene. Thus, our observations may not be relevant to humans carrying one or both valine alleles; however, all patients with vCJD reported to date have been M/M at this position (27).
Intravenous Transmissions to Nonhuman Primates.
Brain pathology was identical in macaques inoculated i.c. and i.v. The i.v. route proved to be very efficient for the transmission of BSE, as shown by the 2-year survival of the animals, which is only 5 months longer than that obtained after inoculating the same amount of agent i.c. As the i.v. injection of the infectious agent implies per se a delayed neuroinvasion compared with a direct inoculation in the brain, this slight lengthening of the incubation period cannot, at this stage, be interpreted as a lower efficiency of infection as regards the i.c. route.
These data should be taken into account in the risk assessment of iatrogenic vCJD transmission by i.v. administration of biological products of human origin. They also constitute an incentive for a complete i.v. titration.
Conclusions
From BSE and vCJD transmissions in nonhuman primates, a number of conclusions can be drawn that are of major importance for human health: (i) human-adapted BSE appears to be a variant of the BSE agent that is more virulent for humans than cattle BSE and is efficiently transmitted by the peripheral route; (ii) the detection of vCJD in unusually young patients is probably not because of a lack of diagnosis of cases in older patients, thus raising the question of the source of human contamination with BSE early in life; and (iii) iatrogenic transmissions from patients with vCJD would be readily recognized by using the same diagnostic criteria as those applied to vCJD [clinical and pathological criteria (27) comprising neuronal loss and gliosis in the thalamus correlated with high MRI signal (28, 29)], whether such contaminations had occurred by the central or i.v. route. Primary and iatrogenic cases of vCJD could be distinguished on the basis of the patient's clinical history.
The risk assessment of biological products of human origin, notably those derived from blood, has been deeply modified by the appearance of vCJD. We confirm that the BSE agent has contaminated humans not only in the U.K. and the Republic of Ireland but also in France, and we show that its pathogenic properties for primates are being enhanced by a primary passage in humans. Considering the flow of potentially contaminated bovine-derived products between 1980 and 1996, it is obvious that further vCJD cases may occur outside the U.K. Thus, and in the light of the present study, it is necessary to sustain worldwide CJD surveillance regardless of national BSE incidence and to take all precautionary measures to avoid iatrogenic transmissions from vCJD.
Acknowledgments
We are very grateful to N. Salès for helpful scientific discussions, as well as to R. Rioux, D. Farrant, S. Jacquin, and J.-C. Mascaro for the quality of the animal care. We are very much indebted to R. Bradley and M. Dawson (Central Veterinary Laboratory, U.K.) for the BSE cattle brain, to P. Sarradin (Institut National de la Recherche Agronomique, France) for providing us with scrapie sheep brain, and to P. Brown (National Institutes of Health) for supply of kuru material and the U.S. strain of scrapie. We also thank R. G. Will for access to clinical data on the British patients with vCJD. The European Concerted Action on TSEs (BMH4-CT98-6015) has to be acknowledged for providing support to scientific collaborations and exchange. This work was supported by a grant from the European Union (BMH4-CT98-6029) and from the Programme Interministériel de Recherche sur les Prions (Paris, France).
Abbreviations
- BSE
bovine spongiform encephalopathy
- CJD
Creutzfeldt–Jakob disease
- vCJD
variant CJD
- sCJD
sporadic CJD
- iCJD
iatrogenic CJD
- TSE
transmissible spongiform encephalopathy
- PrP
prion protein
- PrPres
pathological PrP
- i.c.
intracerebral(ly)
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Will R G, Ironside J W, Zeidler M, Cousens S N, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith P G. Lancet. 1996;347:921–925. doi: 10.1016/s0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- 2.Lasmézas C I, Deslys J-P, Robain O, Demaimay R, Adjou K T, Lamoury F, Ironside J, Hauw J-J, Dormont D. Nature (London) 1996;381:743–744. doi: 10.1038/381743a0. [DOI] [PubMed] [Google Scholar]
- 3.Fraser H, Dickinson A G. J Comp Pathol. 1968;78:301–311. doi: 10.1016/0021-9975(68)90006-6. [DOI] [PubMed] [Google Scholar]
- 4.Bruce M E, Will R G, Ironside J W, McConnell I, Drummond D, Suttle A, McCardle L, Chree A, Hope J, Birkett C, et al. Nature (London) 1997;389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 5.Collinge J, Sidle K C L, Meads J, Ironside J, Hill A F. Nature (London) 1996;383:685–690. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- 6.Hill A F, Desbruslais M, Joiner S, Sidle K C L, Gowland O, Collinge J. Nature (London) 1997;389:448–450. doi: 10.1038/38925. [DOI] [PubMed] [Google Scholar]
- 7.Scott M R, Will R, Ironside J, Nguyen H-O B, Tremblay P, DeArmond S J, Prusiner S B. Proc Natl Acad Sci USA. 1999;96:15137–15142. doi: 10.1073/pnas.96.26.15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bons N, Mestre-Frances N, Belli P, Cathala F, Gajdusek D C, Brown P. Proc Natl Acad Sci USA. 1999;96:4046–4051. doi: 10.1073/pnas.96.7.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghani A C, Ferguson N M, Donnelly C A, Anderson R M. Nature (London) 2000;406:583–584. doi: 10.1038/35020688. [DOI] [PubMed] [Google Scholar]
- 10.Hill A F, Butterworth R J, Joiner S, Jackson G, Rossor M N, Thomas D J, Frosh A, Tolley N, Bell J E, Spencer M, et al. Lancet. 1999;353:183–189. doi: 10.1016/s0140-6736(98)12075-5. [DOI] [PubMed] [Google Scholar]
- 11.Ghani A C, Donnelly C A, Ferguson N M, Anderson R M. Proc R Soc London Ser B. 2000;267:23–29. doi: 10.1098/rspb.2000.0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chazot G, Broussolle E, Lapras C, Blätter T, Aguzzi A, Kopp N. Lancet. 1996;347:1181. doi: 10.1016/s0140-6736(96)90638-8. [DOI] [PubMed] [Google Scholar]
- 13.Deslys J-P, Lasmézas C I, Streichenberger N, Hill A, Collinge J, Dormont D, Kopp N. Lancet. 1997;349:30–31. doi: 10.1016/s0140-6736(05)62163-0. [DOI] [PubMed] [Google Scholar]
- 14.Parchi P, Capellari S, Chen S G, Petersen R B, Gambetti P, Kopp N, Brown P, Kitamoto T, Tateishi J, Giese A, Kretzschmar H. Nature (London) 1997;386:232–233. doi: 10.1038/386232a0. [DOI] [PubMed] [Google Scholar]
- 15.Clouscard C, Beaudry P, Elsen J-M, Milan D, Dussaucy M, Bounneau C, Schelcher F, Chatelain J, Launay J-M, Laplanche J-L. J Gen Virol. 1995;76:2097–2101. doi: 10.1099/0022-1317-76-8-2097. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs C J, Gajdusek D C, Amyx H. In: Slow Transmissible Diseases of the Nervous System. Prusiner S B, Hadlow W J, editors. Vol. 2. New York: Academic; 1979. pp. 87–110. [Google Scholar]
- 17.Deslys J P, Lasmézas C, Dormont D. Lancet. 1994;343:848–849. doi: 10.1016/s0140-6736(94)92046-x. [DOI] [PubMed] [Google Scholar]
- 18.Bell J E, Gentleman S M, Ironside J W, McCardle L, Lantos P L, Doey L, Lowe J, Fergusson J, Luthert P, McQuaid S, Allen I V. Neuropathol Appl Neurobiol. 1997;23:26–35. [PubMed] [Google Scholar]
- 19.Lasmézas C I, Deslys J-P, Robain O, Jaegly A, Beringue V, Peyrin J-M, Fournier J-G, Hauw J-J, Rossier J, Dormont D. Science. 1997;275:402–405. doi: 10.1126/science.275.5298.402. [DOI] [PubMed] [Google Scholar]
- 20.Demaimay R, Adjou K T, Beringue V, Demart S, Lasmézas C I, Deslys J-P, Seman M, Dormont D. J Virol. 1997;71:9685–9689. doi: 10.1128/jvi.71.12.9685-9689.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells G A H, Wilesmith J W. Brain Pathol. 1995;5:91–103. doi: 10.1111/j.1750-3639.1995.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 22.Lasmézas C I, Deslys J-P, Demaimay R, Adjou K T, Hauw J-J, Dormont D. J Gen Virol. 1996;77:1601–1609. doi: 10.1099/0022-1317-77-7-1601. [DOI] [PubMed] [Google Scholar]
- 23.Ministry of Agriculture, Fisheries, and Food. Bovine Spongiform Encephalopathy in Great Britain. Fisheries, Food, London: Minist. Agric.; 1998. [Google Scholar]
- 24.Bruce M, Chree A, McConnell I, Foster J, Pearson G, Fraser H. Philos Trans R Soc London. 1994;343:405–411. doi: 10.1098/rstb.1994.0036. [DOI] [PubMed] [Google Scholar]
- 25.van Duijn C M, Delasnerie-Laupretre N, Masullo C, Zerr I, de Silva R, Wientjens D P, Brandel J P, Weber T, Bonavita V, Zeidler M, et al. Lancet. 1998;351:1081–1085. doi: 10.1016/s0140-6736(97)09468-3. [DOI] [PubMed] [Google Scholar]
- 26.Gibbs C J, Amyx H L, Bacote A, Masters C, Gajdusek D C. J Infect Dis. 1980;142:205–208. doi: 10.1093/infdis/142.2.205. [DOI] [PubMed] [Google Scholar]
- 27.Will R G, Zeidler M, Stewart G E, Macleod M A, Ironside J W, Cousens S N, Mackenzie J, Estibeiro K, Green A J, Knight R S. Ann Neurol. 2000;47:575–582. [PubMed] [Google Scholar]
- 28.Zeidler M, Sellar R J, Collie D A, Knight R, Stewart G, Macleod M A, Ironside J W, Cousens S, Colchester A F, Hadley D M, Will R G. Lancet. 2000;355:1412–1418. doi: 10.1016/s0140-6736(00)02140-1. [DOI] [PubMed] [Google Scholar]
- 29.Oppenheim C, Brandel J P, Hauw J J, Deslys J P, Fontaine B. Lancet. 2000;356:253–254. doi: 10.1016/S0140-6736(05)74505-0. [DOI] [PubMed] [Google Scholar]