Abstract
(See the editorial commentary by Altfeld and Goulder, on pages 753–5.)
Background. The Step study was a randomized trial to determine whether an adenovirus type 5 (Ad5) vector vaccine, which elicits T cell immunity, can lead to control of human immunodeficiency virus (HIV) replication in participants who became HIV-infected after vaccination.
Methods. We evaluated the effect of the vaccine on trends in HIV viral load, CD4+ T cell counts, time to initiation of antiretroviral therapy (ART), and AIDS-free survival in 87 male participants who became infected with HIV during the Step study and who had a median of 24 months of post-infection follow-up.
Results. There was no overall effect of vaccine on mean log10 viral load (estimated difference between groups, -0.11; P = .47). In a subset of subjects with protective HLA types (B27, B57, B58), mean HIV-1 RNA level over time was lower among vaccine recipients. There was no significant difference in CD4+ T cell counts, time to ART initiation, or in AIDS-free survival between HIV-1–infected subjects who received vaccine versus those who received placebo.
Conclusions. HIV RNA levels, CD4+ T cell counts, time to initiation of ART, and AIDS-free survival were similar in vaccine and placebo recipients. There may have been a favorable effect of vaccine on HIV-1 RNA levels in participants with HLA types associated with better control of HIV-1.
Cell-mediated immunity in human immunodeficiency virus (HIV)–infected patients may control viral replication and slow HIV disease progression [1–8]. In preclinical primate models, most vaccines that elicit cell-mediated immunity do not prevent infection, but they do lower post-infection viral loads and slow retroviral disease progression [9–11]. Therefore, a number of HIV-1 vaccine candidates have been developed that aim to elicit cell-mediated immunity. The goal of these vaccines is to improve immune control of viral replication after infection, to decrease HIV-1 RNA levels, and to thereby slow HIV-1 disease progression and reduce sexual transmission to others.
The Step study was a randomized trial of the Merck adenovirus type 5 (Ad5) trivalent HIV-1 vaccine that was designed to elicit cell-mediated immunity. Three thousand adults in North America, the Caribbean, South America, and Australia were randomized to receive placebo or the vaccine. Vaccinations were halted and the study was unblinded in 2007, when the first interim analysis indicated that the vaccine would not achieve efficacy for the two primary study end points, HIV-1 acquisition and plasma HIV-1 RNA level 3 months after the diagnosis of HIV infection [12].
In the current analysis, we evaluate the effect of the vaccine on HIV/AIDS disease progression in subjects who became HIV-1–infected during the Step trial. Measures of disease progression include HIV-1 RNA levels, CD4+ T cell counts, time to antiretroviral therapy (ART), and AIDS-free survival during post-infection follow-up [13–15].
METHODS
Step Study Design and Vaccine
The Step study was a multicenter, double-blind, randomized, placebo-controlled study of the MRKAd5 HIV-1 gag/pol/nef vaccine. The study was performed at 34 sites in North America, the Caribbean, South America, and Australia, in regions where clade B HIV-1 is predominant. Complete details on the study design are provided elsewhere [12]. Three thousand HIV-1–negative participants 18-45 years of age who were at high risk of HIV-1 acquisition were enrolled and randomized to receive vaccine or placebo in a 1:1 ratio, stratified by Ad5 antibody titers at enrollment (≤18 [lower limit of detection of assay], 19–200, 201–1000, and >1000), sex, and study site. The vaccine consisted of a 1:1:1 mixture of 3 replication-defective Ad5 vectors, one each containing the gag, pol, and nef gene inserts from HIV-1 clade B strains [16]. The placebo consisted of the diluent only. The trial opened in December of 2004. Subjects received vaccinations at day 1 (enrollment), week 4, and week 26 and were observed through week 208.
The institutional review board at each clinical site approved the protocol, and participants completed a thorough written informed consent process before enrolment.
Testing for HIV-1 Infection
Subjects were tested for HIV-1 infection at day 1; weeks 12 30, and 52; and every 26 weeks thereafter through week 208. All HIV-1 tests were done at a central laboratory. Screening HIV-1 testing was performed with an immunoassay that contained only HIV envelope antigens that were not included in the vaccine (Uni-Gold Recombigen HIV test [Trinity BioTech] or the Multispot HIV-1/HIV-2 Rapid Test [Bio-Rad]). Subjects who tested positive by the screening test had confirmatory testing by HIV-1 Western blot and HIV-1 plasma viral RNA assays (Amplicor Monitor, version 1 [Roche]). Stored plasma samples from earlier time points were tested to determine the onset of HIV-1 infection. A blinded adjudication committee consisting of 3 independent experts in HIV diagnostics made the final decision regarding HIV-1 infection status. We define the date of HIV-1 diagnosis as the date of the first positive immunoassay result that was confirmed by Western blot or viral RNA assay.
Follow-up of HIV-1–Infected Subjects
HIV-1–infected participants received no additional study vaccinations, were counseled, and were linked to local HIV medical care. Participants infected with HIV-1 underwent clinical and laboratory assessment at 1, 2, 8, 12, and 26 weeks after their initial HIV-1 diagnosis and every 26 weeks thereafter. (The original protocol called for 78 weeks of follow-up; this was extended to 4 years in a protocol amendment in May 2007. Because this amendment had not been implemented at all sites, follow-up for some HIV-infected participants was terminated at 78 weeks.) HIV-1 RNA levels and CD4+ T cell counts (Becton Dickenson) were measured at each post-infection study visit. All new medical conditions were documented in case report forms. HIV clinical events were classified according to the 1993 Centers for Disease Control and Prevention (CDC) system [17].
All HIV-1–infected participants were assured access to ART [18]. HIV-1–infected participants were treated according to the clinical judgment of their primary care clinician and guidelines in their country. Study-specified antiretroviral regimens or guidelines were not used. Some clinicians opted to treat acute HIV infection. Others initiated therapy using the guidelines of the United States Department of Health and Human Services [19], the World Health Organization [20], or the International AIDS Society-USA [21].
Subjects Included in Analysis of HIV/AIDS Progression
We include in the analysis all male subjects in the Step trial who were HIV-1–uninfected at randomization, who received at least 1 dose of study vaccine or placebo, and who became HIV-1–infected between December 2004, when the study opened, and 17 October 2007, shortly before the study was unblinded. We exclude women from this analysis because of the very small number of HIV-1–infected women in the Step study prior to unblinding, and because of some suggestion that HIV disease progression may be different in women than in men [22]. We include follow-up data on subjects through 22 September 2009.
Statistical Methods
We describe the baseline characteristics of the study subjects, including age, sex, race, country of residence, Ad5 seropositivity, herpes simplex virus type 2 (HSV-2) serostatus, self-reported circumcision status, and HLA group. The distribution of these baseline characteristics is compared between the vaccine and placebo groups using Wilcoxon rank-sum tests for continuous variables, χ2 tests for categorical variables, and tests of proportions for binary variables. Individuals missing a baseline characteristic are excluded from the analysis, and P values are adjusted for multiple comparisons using the Holm method [23].
Baseline characteristics are also included in the multivariate models described below. Ad5 serostatus is included in all analyses, because it was a pre-specified stratification variable. Circumcision status is included in multivariate analyses, because it modified the effect of vaccination status on HIV acquisition in the Step trial [12] and is therefore included to control for selection bias of HIV-infected patients [24–26]. Four-digit resolution HLA types are included, because they have been strongly associated with HIV-1 RNA set point and HIV disease progression. Participants are categorized on the basis of previous publications as possessing protective (B27, B57, B5801), unfavorable (B*3502, *3503, *3504, B53, or homozygous in at least 1 locus for nonprotective alleles), and neutral haplotypes (all others) [27–31]. We also adjust for other characteristics that are plausibly associated with post-infection outcomes. For consistency with previous analyses of the Step study data, we use dichotomous versions of the following variables: age (>30 years v ≤ 30 years), race (white vs non-white), region of residence (North America vs Other), circumcision status, HSV-2 serostatus, and Ad5 seropositivity (titers >18 vs ≤18). Two North American subjects with missing circumcision status data are regarded as circumcised in all multivariate analyses. One subject with missing HLA type and 1 subject with missing HSV-2 serostatus data are excluded from analyses that include HSV-2 serostatus and HLA type.
We report the status of study participants at the time of analysis (22 September 2009). This includes the number of subjects who were lost to follow-up and the number of missed visits during the follow-up period.
During the study period, ART-naive CD4+ T cell and HIV-1 RNA data are unavailable at some time points, because subjects had already initiated ART, missed a visit, or were lost to follow-up prior to week 78. In total, 43% of the potential pre-ART biomarker values are not available for analysis (15% due to initiation of ART; 12% due to loss to follow-up while ART naive, and 16% due to missed visits while ART naive and still under follow-up).
For descriptive analyses, we plotted log10 pre-ART HIV-1 RNA levels. Missing HIV-1 RNA values were imputed through week 78. Missing values were due to subjects who missed a visit, initiated ART, or dropped out of the study prior to week 78. Imputation was based on other (non-missing) measurements of viral load and CD4+ T cell counts, as well as treatment assignment, age, race, region, circumcision status, HSV-2 seropositivity, Ad5 serostatus, and HLA group [32, 33]. Imputed values represent averages across 10 imputations.
For inferential analyses, we estimated the difference in mean pre-ART log viral load between vaccine and placebo groups using weighted generalized estimating equation (GEE) models [34–36]. Here, also, viral load values are censored at the time of ART initiation. We accounted for the missing viral load data by weighting each observation with respect to the inverse probability of being observed (see Supplementary Materials). For comparison, we also considered a standard unweighted analysis that ignored the missing data, as well as a full likelihood-based analysis (see Supplementary Materials). We estimated the difference in means after adjusting for visit week and Ad5 serostatus and after additional adjustment for age, race, region, circumcision status, HSV-2 serostatus, and HLA group. We tested for changes in the vaccine and HLA effects over time and for modification of the vaccine effect by HLA group, circumcision status, and Ad5 serostatus using generalized Wald tests of interaction.
We plotted the observed pre-ART CD4+ T cell counts, showing the mean of all observed values at each study time point. Missing values were filled in through week 78 using the multiple imputation approach described above for descriptive analyses. CD4+ T cell counts were square-root transformed for analysis to create a more symmetrical distribution. We estimated the difference in mean square-root CD4+ T cell count between vaccine and placebo groups using the weighted GEE, unweighted GEE, and likelihood-based approaches described above in relation to the analysis of viral load.
We detailed the time of antiretroviral initiation using Kaplan-Meier survival plots, log-rank tests, and Cox proportional hazards models. We described the number of patients who progressed to AIDS according to the 1993 CDC classification system. We compared AIDS-free survival between vaccine and placebo groups using the log rank test.
RESULTS
Study Subjects and Characteristics
Of the 3000 study participants enrolled in the Step trial, 90 were HIV-uninfected at randomization, received at least 1 dose of vaccine or placebo, and became HIV-infected from December 2004 through November 2007. Only 2 of these participants were female, and they are excluded from analysis. One male subject used post-exposure antiretroviral prophylaxis and was receiving antiretroviral drugs at the time of HIV-1 diagnosis. He is excluded from all analyses of post-infection outcomes.
Characteristics of the 87 subjects who were included in the analysis of HIV/AIDS disease progression are described in Table 1. The majority of patients came from the United States. The majority of patients were white; the average age was 31 years. Subjects were randomized from March 2005 through November 2006 (median date of randomization, January 2006) and received a diagnosis of HIV-1 infection between 28 and 751 days after the first vaccination (median time to diagnosis, 287 days). There are no significant differences in baseline characteristics between treatment arms.
Table 1.
Baseline Characteristics of the 87 Human Immunodeficiency Virus (HIV)–Infected Participants
| Variable | Overall (n = 87) | Vaccine (n = 52) | Placebo (n = 35) | Pa |
| Age, mean years (range) | 31 (19–45) | 31 (19–45) | 30 (19–44) | >.99 |
| Race | >.99 | |||
| White | 45 (52) | 26 (50) | 19 (54) | |
| Mestizo | 17 (20) | 11 (21) | 6 (17) | |
| Black | 10 (11) | 5 (10) | 5 (14) | |
| Hispanic | 9 (10) | 8 (15) | 1 (3) | |
| Multi-race | 2 (2) | 1 (2) | 1 (3) | |
| Other | 4 (5) | 1 (2) | 3 (9) | |
| Country | >.99 | |||
| United States | 65 (75) | 38 (73) | 27 (77) | |
| Peru | 17 (20) | 11 (21) | 6 (17) | |
| Canada | 4 (5) | 2 (6) | 2 (6) | |
| Haiti | 1 (1) | 1 (2) | 0 (0) | |
| Circumcised | 0.26 | |||
| No | 30 (34) | 23 (44) | 7 (20) | |
| Yes | 55 (63) | 28 (54) | 27 (77) | |
| Missing data | 2 (2) | 1 (2) | 1 (3) | |
| Baseline Ad5 titer | >.99 | |||
| ≤18 | 42 (48) | 22 (42) | 20 (57) | |
| >18 | 45 (52) | 30 (58) | 15 (43) | |
| HLA groupb | >.99 | |||
| Unfavorable | 31 (36) | 20 (38) | 11(31) | |
| Neutral | 41 (47) | 23 (44) | 18 (51) | |
| Protective | 14 (16) | 9 (17) | 5 (15) | |
| Missing data | 1 (1) | 0 (0) | 1 (3) | |
| Baseline HSV-2 | >.99 | |||
| Seronegative | 51 (59) | 33 (63) | 18 (51) | |
| Seropositive | 35 (40) | 19 (37) | 16 (46) | |
| Missing data | 1 (1) | 0 (0) | 1 (3) |
NOTE. Data are no. (%) of patients, unless otherwise indicated. Ad5, adenovirus serotype 5.
P values for tests comparing the distribution of baseline characteristics between the vaccine group and the placebo group.
HLA types are categorized on the basis of their association with HIV disease progression as protective (B27, B57, and B58), unfavorable (B35 and B53), or neutral (all others).
Subject Status and Data Available at the Time of Analysis
The median length of post-infection follow-up was 732 days. (The median time from diagnosis to date of last visit was 749 days for vaccine recipients and 660 days for placebo recipients.) Twenty (23%) of the subjects were lost to follow-up prior to planned study completion or prior to September 2009. The rate of dropout did not differ between treatment arms (P = .37, by log-rank test).
HIV-1 RNA Levels
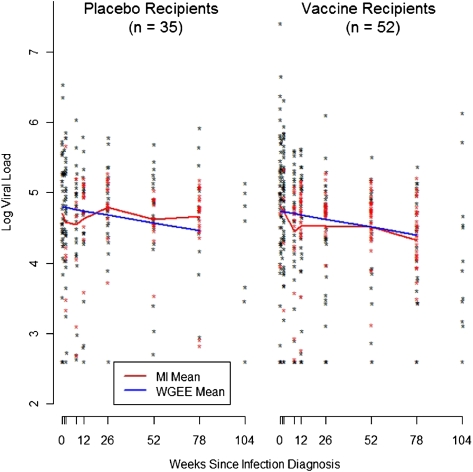
There was an average of 5 pre-ART HIV-1 RNA levels determined for each volunteer (range, 1–11 measures). At weeks 0, 1, 2, 8, 12, 26, 52, and 78 average pre-ART log10 viral load was 4.79, 4.85, 4.72, 4.53, 4.49, 4.55, 4.48, and 4.43 copies/mL, respectively, pooling across the vaccine and placebo groups. There was no significant effect of vaccine on log HIV-1 RNA levels among study participants overall (Figure 1). The estimated difference in mean log10 viral load between vaccine recipients and placebos recipients is -0.15 (95% confidence interval [CI], -0.47 to 0.17; P = .36). After adjusting for age, race, region, circumcision status, HSV-2 serostatus, Ad5 serostatus, and HLA group, the estimated difference is -0.11, (95% CI, -0.40 to 0.18, P = .47). We found no evidence that the effect of HLA or vaccine on viral load changed over time (P = .20 and P = .95, respectively). Neither do we find evidence that the vaccine effect is modified by circumcision status (P = .32) or Ad5 serostatus (P = .82). Estimates based on the unweighted analysis that ignored the missing data were very similar (see Supplementary Materials).
Figure 1.
Pre-antiretroviral therapy log10 viral load measurements at each post-infection visit. Black points are observed values, and red points are averages of 10 imputed values. The red curve shows the mean value at each time point (MI mean), which was estimated using both the observed and imputed data. The blue curve shows the mean value as estimated using the weighted generalized estimating equation model (WGEE mean).
HLA group modified the vaccine effect on longitudinal HIV-1 RNA levels (P = .03), and there is some evidence that the vaccine had an effect in a subset of subjects. The evidence of a vaccine effect was concentrated in the group of participants with protective HLA alleles. In subjects with protective HLA types (B27, B57, B58), the mean HIV-1 RNA level over time was 0.86 log10 lower in the vaccine group than in the placebo group (95% CI, -1.52 to -0.20). In the unfavorable HLA group, there was a 0.19 higher log10 HIV-1 RNA level in vaccine recipients than in placebo recipients (95% CI, -0.46 to 0.84), and in the neutral HLA group there was a 0.15 lower log viral load in vaccine recipients than in placebo recipients (95% CI, -0.50 to 0.20). Estimates based on the unweighted analysis that ignored the missing viral load data were very similar (see Supplementary Materials).
CD4+ T cell Counts
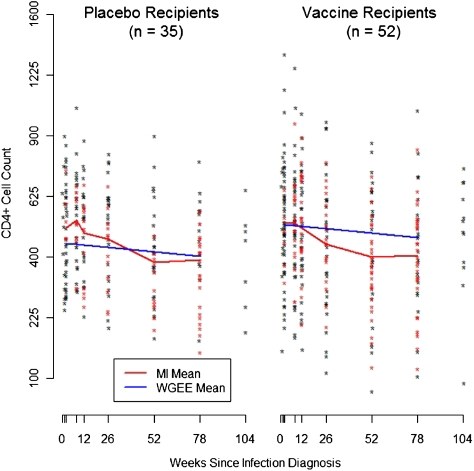
There was an average of 4 pre-ART CD4+ T cell counts for each volunteer, (range, 0–10 counts). At weeks 1, 2, 8, 12, 26, 52, and 78, the average pre-ART observed CD4+ T cell count was 489, 535, 539, 515, 477, 460, and 474 cells/mL, respectively, pooling across the 2 treatment groups. There was no significant effect of vaccine on CD4+ T cell count (Figure 2). The estimated difference in mean square-root CD4+ T cell count between vaccine and placebo groups is 1.11, which is 0.2 standard deviations (95% CI, -0.95 to 3.17; P = .29). After adjusting for age, race, region, circumcision status, HSV-2 serostatus, Ad5 serostatus, and HLA group, the estimated difference was 1.33, which is still less than 0.3 standard deviations (95% CI, -0.52 to 3.17; P = .16). We found no evidence that the vaccine effect on CD4+ T cell count was modified by HLA group (P = .88), circumcision status (P = .24), or Ad5 serostatus (P = .34). Neither did we find evidence that the HLA or vaccine effect on CD4+ T cell counts changed over time (P = .58 and P = .89, respectively). Estimates from the unweighted analysis that ignored the missing data were very similar (see Supplementary Materials).
Figure 2.
Pre-antiretroviral therapy CD4+ cell count measurements at each post-infection visit. Black points are observed values, and red points are averages of 10 imputed values. The red curve shows the mean value at each time point, which was estimated using both the observed and imputed data. The blue curve shows the mean as estimated using the weighted generalized estimating equation model.
Time to Initiation of Antiretroviral Therapy
Thirty-four (39%) of the 87 participants initiated ART between 27 and 800 days after HIV diagnosis (median time to ART, 275 days) and the frequency of ART use was similar in the vaccine and placebo groups. In the vaccine group, 19 (37%) of 52 subjects started ART, including 3 who received antiretroviral drugs during acute HIV infection. In the placebo group, 15 (43%) of 35 started ART, including 1 who received antiretroviral drugs during acute HIV infection.
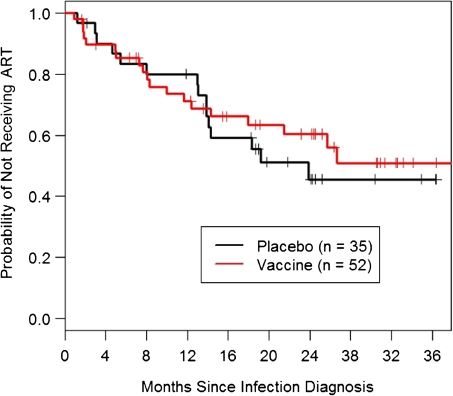
Figure 3 displays Kaplan-Meier curves for time to ART initiation by treatment assignment. A log-rank test comparing time to initiation of therapy between groups, stratified on Ad5 serostatus, was not significant (P = .77). Using a Cox proportional hazards regression model adjusting for age, race, region, circumcision status, HSV-2 serostatus, Ad5 serostatus, and HLA group, we estimated that the ratio of hazards in the vaccine and placebo groups was 1.14 (95% CI, 0.55–2.37; P = .72). There is no evidence of significant modification of the vaccine effect by circumcision status (P = .53) or Ad5 serostatus (P = .62). A formal test of effect modification by HLA is not possible because of sparse strata; 1 (20%) of the 5 placebo recipients versus 0 of 9 vaccine recipients in the protective HLA group initiated ART. There is no evidence that the vaccine effect differed after treatment unblinding in November 2007 (P= .46). Among participants who initiated ART, 9 of 15 placebo recipients and 7 of 19 vaccine recipients began ART after unblinding. Finally, when the 4 subjects who started ART for acute HIV infection were censored, there was still no significant difference between vaccine and placebo groups.
Figure 3.
Kaplan-Meier curves for the time between human immunodeficiency virus infection diagnosis and initiation of antiretroviral therapy (ART).
AIDS-free Survival
Fourteen (16%) of the 87 patients satisfied the CDC case definition of AIDS during the study period, 9 in the vaccine group and 5 in the placebo group. Four subjects developed a CDC stage C AIDS clinical event; 10 other subjects developed a CD4+ T cell count of < 200 cells/mm3. None of the 87 patients died. There was no difference in AIDS-free survival between the vaccine and placebo groups (P = .69).
DISCUSSION
Among 87 male study participants who acquired HIV infection during the Step study, there was no difference in HIV disease progression between vaccine and placebo recipients during 2 years of follow-up. HIV RNA levels, CD4+ T cell counts, time to initiation of ART, and AIDS-free survival were the same for vaccine and placebo recipients. There may have been a favorable effect of the vaccine on HIV-1 RNA levels in a subset of participants with HLA types associated with better immune control of HIV-1.
These results are consistent with the primary analysis of the Step study that was conducted when the study was halted in October 2007 [12]. The earlier analysis found no vaccine effect on set point viral load, defined as the average log10 viral load at weeks 8 and 12 after infection. Our analysis includes the full set of additional follow-up, through October 2009, and incorporates the longitudinal viral load and CD4+ T cell count measurements, as well as time to initiation of ART and AIDS-free survival. The breadth, magnitude, or functionality of the immune response elicited by the MRKAd5 HIV-1 gag/pol/nef vaccine may not have been sufficient to affect HIV-1 viral load set point and disease progression [37–41]. Furthermore, rapid HIV-1 escape from immunologic control and exhaustion of cell-mediated immunity may also explain the inability of the MRKAd5 HIV-1 gag/pol/nef vaccine to affect HIV-1 disease progression [8, 42–44].
The failure of MRKAd5 HIV-1 gag/pol/nef to control viremia is perplexing in light of the robust CD8+ T cell responses elicited by the vaccine [37]. Studies of cellular immunity in elite controllers [6] and in vaccinated and non-vaccinated non-human primates suggest that HIV-specific CD8+ T cells play a central role in maintaining effective control of viral replication. We are conducting additional studies to characterize the nature of the cellular response in Step study vaccine recipients to elucidate why it failed to control viral replication; this information may guide development of more-efficacious T cell vaccines. Results from a large trial in Thailand, RV144, which used a vaccine regimen comprising a series of 4 priming injections of recombinant canarypox vaccine accompanied by a booster injection of a recombinant bivalent (B/E) glycoprotein 120 subunit protein on the final 2 vaccination occasions, showed modest protection against HIV acquisition [45]. The immune profile elicited by this regimen differed substantially from that seen in response to the Merck vaccine; it too was ineffective in controlling early viremia or maintaining CD4+ T cell count among infected study participants.
We found no effect of Ad5 serostatus or circumcision status on HIV disease progression. There may have been a modest effect of the MRKAd5 HIV-1 gag/pol/nef vaccine on HIV-1 RNA levels among a subset of subjects with HLA types known to be associated with lower viral load set points and a slower course of disease progression (HLA B27, B57, and B58). In a previous study of recombinant canarypox ALVAC-HIV vCP205, vaccinated healthy volunteers with HLA-B27 or –B57 developed earlier and greater magnitude CTL responses than did vaccine recipients with other class I alleles, suggesting that HLA alleles can favorably alter HIV-specific immune responses following vaccination [46]. The number of patients was small, and the trend was modest; therefore, these data need to be interpreted with caution.
Several participants started ART during acute HIV infection, and ∼30% of the participants started ART by 1 year. Of note, international HIV treatment guidelines are moving towards earlier initiation of therapy. Future HIV vaccine trials that plan to observe HIV disease progression in infected volunteers should design their studies to anticipate that a large proportion of participants will start ART within 1 year of diagnosis of HIV infection.
In HIV-infected participants in the Step study, there was no difference in HIV RNA levels, CD4+ T cell counts, time to ART initiation, and AIDS-free survival between the vaccine and placebo recipients. There may have been a favorable effect of the vaccine on HIV-1 RNA levels in a subset of participants. Additional clinical testing of vaccine candidates that aims to elicit robust cellular immunity, alone or in combination with antigens eliciting a humoral response, is warranted to build on these early findings in selecting regimens that will reduce HIV acquisition and effectively control viral replication.
Supplementary Data
Supplementary data are available at http://www.oxfordjournals.org/our_journals/jid/online.
Funding
Merck Research Laboratories; the Division of AIDS, National Institute of Allergy and Infectious Diseases, in the US National Institutes of Health (NIH); and the NIH-sponsored HIV Vaccine Trials Network (HVTN).
Supplementary Material
Acknowledgments
This study is registered with ClinicalTrials.gov, number NCT00095576.
References
- 1.Altfeld M, Kalife ET, Qi Y, et al. HLA alleles associated with delayed progression to AIDS contribute strongly to the initial CD8(+) T cell response against HIV-1. PLoS Med. 2006;3:e403. doi: 10.1371/journal.pmed.0030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emu B, Sinclair E, Hatano H, et al. HLA class I-restricted T cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J Virol. 2008;82:5398–5407. doi: 10.1128/JVI.02176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein MR, van Baalen CA, Holwerda AM, et al. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koup RA, Safrit JT, Cao Y, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197:563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg ES, Billingsley JM, Caliendo AM, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 8.Streeck H, Jolin JS, Qi Y, et al. Human immunodeficiency virus type 1-specific CD8+ T cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells. J Virol. 2009;83:7641–7648. doi: 10.1128/JVI.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amara RR, Villinger F, Altman JD, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 10.Barouch DH, Santra S, Schmitz JE, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 11.Shiver JW, Fu TM, Chen L, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 12.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurunathan S, Habib RE, Baglyos L, et al. Use of predictive markers of HIV disease progression in vaccine trials. Vaccine. 2009;27:1997–2015. doi: 10.1016/j.vaccine.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 14.Margolick JB, Farzadegan H, Hoover DR, Saah AJ. Relationship between infectious cell-associated human immunodeficiency virus type 1 load, T lymphocyte subsets, and stage of infection in homosexual men. J Infect Dis. 1996;173:468–471. doi: 10.1093/infdis/173.2.468. [DOI] [PubMed] [Google Scholar]
- 15.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 16.Priddy FH, Brown D, Kublin J, et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46:1769–1781. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA. 1993;269:729–730. [PubMed] [Google Scholar]
- 18.Fitzgerald DW, Marotte C, Verdier RI, Johnson WD, Jr., Pape JW. Comprehension during informed consent in a less-developed country. Lancet. 2002;360:1301–1302. doi: 10.1016/S0140-6736(02)11338-9. [DOI] [PubMed] [Google Scholar]
- 19.Panel on Antiretroviral Guidelines for Adults, Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2008. pp. 1–139. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 1 June 2009. [Google Scholar]
- 20.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a Public Health approach, 2006 Revision. Geneva, Switzerland: World Health Organization; 2006. [PubMed] [Google Scholar]
- 21.Hammer SM, Eron JJ, Jr., Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 22.Jarrin I, Geskus R, Bhaskaran K, et al. Gender differences in HIV progression to AIDS and death in industrialized countries: slower disease progression following HIV seroconversion in women. Am J Epidemiol. 2008;168:532–540. doi: 10.1093/aje/kwn179. [DOI] [PubMed] [Google Scholar]
- 23.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- 24.Halloran ME, Struchiner CJ. Causal inference in infectious diseases. Epidemiology. 1995;6:142–151. doi: 10.1097/00001648-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum PR. The consequences of adjustment for a concomitant variable that has been affected by the treatment. Journal of the Royal Statistical Society, Ser A. 1984;147:656–666. [Google Scholar]
- 26.Shepherd BE, Gilbert PB, Jemiai Y, Rotnitzky A. Sensitivity analyses comparing outcomes only existing in a subset selected post-randomization, conditional on covariates, with application to HIV vaccine trials. Biometrics. 2006;62:332–342. doi: 10.1111/j.1541-0420.2005.00495.x. [DOI] [PubMed] [Google Scholar]
- 27.Fellay J, Shianna KV, Ge D, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaslow RA, Carrington M, Apple R, et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 29.Migueles SA, Sabbaghian MS, Shupert WL, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A. 2000;97:2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miura T, Brockman MA, Schneidewind A, et al. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte [corrected] recognition. J Virol. 2009;83:2743–2755. doi: 10.1128/JVI.02265-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneidewind A, Brockman MA, Yang R, et al. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J Virol. 2007;81:12382–12393. doi: 10.1128/JVI.01543-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, New Jersey: John Wiley and Sons; 1987. [Google Scholar]
- 33.Van Buuren S, Brand JP, Groothuis-Oudshoorn CGM, Rubin DB. Fully conditional specification in multivariate imputation. J Stat Comput Simulation. 2006;76:1049–1064. [Google Scholar]
- 34.Hughes JP. Mixed effects models with censored data with application to HIV RNA levels. Biometrics. 1999;55:625–629. doi: 10.1111/j.0006-341x.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 35.Liang KY, Zeger SL. Longitudinal data analysis using the generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 36.Robins JM, Rotnitzky A, Zhao LP. Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. Am Stat Assoc. 1995;90:106–121. [Google Scholar]
- 37.McElrath MJ, De Rosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Almeida JR, Price DA, Papagno L, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Streeck H, Brumme ZL, Anastario M, et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 2008;5:e100. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harari A, Cellerai C, Enders FB, et al. Skewed association of polyfunctional antigen-specific CD8 T cell populations with HLA-B genotype. Proc Natl Acad Sci U S A. 2007;104:16233–16238. doi: 10.1073/pnas.0707570104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brumme ZL, Brumme CJ, Carlson J, et al. Marked epitope- and allele-specific differences in rates of mutation in human immunodeficiency type 1 (HIV-1) Gag, Pol, and Nef cytotoxic T-lymphocyte epitopes in acute/early HIV-1 infection. J Virol. 2008;82:9216–9217. doi: 10.1128/JVI.01041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao J, McNevin J, Malhotra U, McElrath MJ. Evolution of CD8+ T cell immunity and viral escape following acute HIV-1 infection. J Immunol. 2003;171:3837–3846. doi: 10.4049/jimmunol.171.7.3837. [DOI] [PubMed] [Google Scholar]
- 44.Li B, Gladden AD, Altfeld M, et al. Rapid reversion of sequence polymorphisms dominates early human immunodeficiency virus type 1 evolution. J Virol. 2007;81:193–201. doi: 10.1128/JVI.01231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2210. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 46.Kaslow RA, Rivers C, Tang J, et al. Polymorphisms in HLA class I genes associated with both favorable prognosis of human immunodeficiency virus (HIV) type 1 infection and positive cytotoxic T-lymphocyte responses to ALVAC-HIV recombinant canarypox vaccines. J Virol. 2001;75:8681–8689. doi: 10.1128/JVI.75.18.8681-8689.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.