Abstract
The ectonucleotide pyrophosphatase phosphodiesterase 1 (ENPP1) has documented roles in mineralization, nucleotide recycling, and insulin resistance. While ENPP1 was first identified as an alloantigen on mouse plasma cells (PCs), later studies revealed expression in many tissues. Previously described monoclonal antibodies against ENPP1 expressed at the cell surface recognized cells only from mice bearing the a allotype, ENPP1a, precluding studies of mice bearing the alternative allele, ENPP1b. Here, we characterize a novel anti-ENPP1 monoclonal antibody that recognizes both alleles and can be used for flow cytometry.
Introduction
The ectonucleotide pyrophosphatase phosphodiesterase 1 (ENPP1) was discovered in 1970 by Takahashi, Old, and Boyse as a membrane alloantigen expressed by neoplastic plasma cells (PCs), termed plasmacytomas, and normal PCs, termed PC-1. PC-1 was shown to be 115 kDa under reducing conditions and 230 kDa under non-reducing conditions.(1) They identified two alleles of PC-1. PC-1a, recognized by alloantisera, was expressed by strains including BALB/c, NZB, and AKR. Strains that did not express the alloantigen (C57BL/6, C58, DBA/2, and others) were later designated as expressing the PC-1b allele following molecular identification of the alternative allele. PC-1a and PC-1b differ by two amino acids (aa) (H650 and R679) in the extracellular domain.(2) Later studies by Goding and colleagues showed that PC-1, encoded by the gene Enpp1, is also expressed in many non-lymphoid tissues including chondrocytes, liver, distal convoluted tubules of the kidney, salivary gland, and brain capillary endothelium.(3) They succeeded in generating a monoclonal antibody (MAb) (IR518) that recognized the a allele(4) at the H650 position.(2)
The function of ENPP1 is multifaceted. First, ENPP1 catalyzes 5′-phosphodiesterase bonds in nucleotide triphosphates to generate pyrophosphate (PPi),(5,6) an important inhibitor of calcification and bone formation. Consistent with this, mice with inactivating mutant alleles of Enpp1(7,8) or a genetically engineered null allele(9) exhibited “stiff joints” and “tiptoe walking” due to pathologic calcification of the joints and paraspinal ligaments. In addition, mutations of Enpp1 also cause blood vessel calcification in both mice(7,10) and humans.(11–14) Second, ENPP1 mediates nucleotide recycling by breaking down ATP to AMP, which is then converted to adenosine by 5′-nucleotidase.(5) Adenosine is then transported freely into cells for metabolism. Third, ENPP1 is involved in regulation of cell adhesion(15) and adipocyte differentiation.(16) Finally, ENPP1 has been shown to modulate insulin receptor signal transduction(17) and purinoceptor signaling(18) such that overexpression of ENPP1 is associated with obesity and insulin resistance (reviewed by Bacci et al.(19)).
Although expression of ENPP1 on PCs has been recognized for four decades, little is known about the function of this molecule in B lineage cells. The lack of MAb with specificity for ENPP1b has impeded study of this molecule in mice bearing the b allele. Remarkably, Takei generated a rat MAb, YE1/19.1, against the C57BL/6 EL4 T cell lymphoma that recognized a homodimer of 115 kDa under reducing conditions and 230 kDa under non-reducing conditions.(20) The antigen was expressed on a subset of normal T cells, and at high levels on the aberrantly expanded T cell population of Fas and Fasl mutant mice and a non-secretory BALB/c PCT.(21) In this report, we further characterize YE1/19.1, showing that the MAb recognizes both alleles of ENPP1 and can be used for flow cytometry.
Materials and Methods
The anti-ENPP1 monoclonal antibodies
The rat [IgG2b, κ] MAb YE1/19.1 was described previously.(20) The mouse [IgG2a, κ] anti-ENPP1a MAb (clone IR518) was generated by Goding and colleagues.(22) Both antibodies were purified from culture supernatants and labeled with allophycocyanin (APC) using standard procedures from the Custom Antibody Facility, Research Technological Branch (NIAID). A mouse IgG2a isotype control antibody labeled with APC was purchased from Southern Biotech (Birmingham, AL). Purified normal rat IgG (Southern Biotech) was also labeled with APC.
Mice and cells
C57BL/6J (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Enpp1–/– mice, described previously,(9) were generously provided by Dr. Robert Terkeltaub (University of California–San Diego). Mouse plasmacytoma (PCT) cell lines MPC11 (originated from BALB/c)(23) and BPC4 (originated from B6; generated by Dr. Michael Potter in the National Cancer Institute) were used in this study. All animal studies were performed under protocols of LIP-4 approved by the NIAID IACUC.
Immunoprecipitation and protein identification
The MPC11 and BPC4 PCT cells were cultured in RPMI 1640 supplemented with 10% FBS, 50 μM 2-mercaptoethanol, 2 mM L-glutamine, and 100 U/mL penicillin. Cells were lysed with lysis buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, proteinase inhibitors (Roche Molecular Systems, Branchburg, NJ), and 1% Triton X-100. The cell lysate was pre-cleared with protein G beads (Invitrogen, Carlsbad, CA) for 2 h at 4°C and incubated with 20 μg of YE1/19.1 MAb. Immune complexes were precipitated by incubation with protein G beads (Thermo Scientific, Rockford, IL) and washed seven times before being resolved on a NuPAGE Novex 4–12% Bis-Tris Gel (Invitrogen). After staining with Coomassie Blue, the protein bands between 110 and 260 kDa were dissected and processed for in-gel digestion with trypsin. The peptides extracted from the gel digestion were analyzed by LC-MS (Q-Star, Applied Biosystems, Carlsbad, CA). The LC-MS data were analyzed using NCBI database.
Flow cytometry
Single-cell suspensions prepared from bone marrows (BM) and spleens of B6 mice (8–24 weeks old) were prepared and stained with fluorochrome-labeled MAbs using standard procedures. All antibodies, except as indicated, were purchased from BD Biosciences (San Diego, CA). Cells were analyzed using a FACSCalibur (BD Biosciences, Mountain View, CA), or a LSR II (BD Biosciences).
Transfection and expression of Enpp1
HEK 293 cells were transfected with an Enpp1b-pcDNA3.1 vector or the pcDNA3.1 vector only (provided by Dr. Robert Terkeltaub, University of California–San Diego) using Lipofectamine LTX reagents (Invitrogen, Carlsbad, CA). Stable transfectants were selected with neomycin and tested by FACS.
Results
Characterization of the anti-ENPP1 MAb
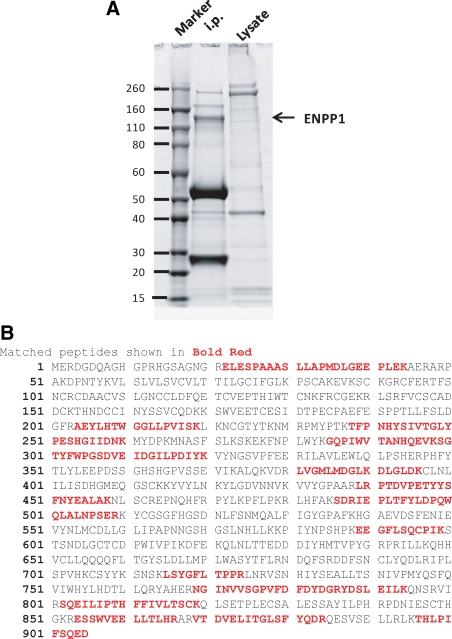
ENPP1 has two allotypic alleles, a and b, distinguished by two aa.(2) Mouse strains including BALB/c, C3H, A/J, and NZB express allele a, while strains including C57BL/6, DBA/2, and 129 express allele b. The YE1/19.1 MAb was raised by immunization of rats with the EL4 T cell lymphoma-derived cell line (B6 origin) and precipitated a molecule of 230 kDa under native and 115 kDa under reducing conditions.(20) This MAb also precipitated a protein of 115 kDa from the MPC11 PCT cell line expressing allele a (Fig. 1A). We then used mass spectroscopy to determine the aa composition of peptides generated by digestion of the excised band and confirmed the identity of the protein as ENPP1 (Fig. 1B).
FIG. 1.
Identification of YE1/19.1 target protein as ENPP1. (A) Murine MPC11 plasmacytoma cells were lysed and precipitated with YE1/19.1. The precipitates were resolved by SDS-PAGE followed by staining with Coomassie Blue. The bands between 260 and 110 kDa were dissected and analyzed by mass spectrometry. The molecular weight standard is shown on left. (B) Peptide sequences of mass spectrometry matched the amino acid sequence of ENPP1.
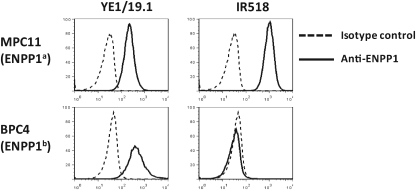
To compare the binding of YE1/19.1 with the well-characterized anti-ENPP1a MAb IR518, we stained PCT cell lines of BALB/c origin (MPC11) and of B6 origin (BPC4) with the two anti-ENPP1 MAbs. As shown in Figure 2, YE1/19.1 bound to both cell lines, whereas IR518 bound only to MPC11, indicating that YE1/19.1 recognizes both a and b alleles. Preincubation of MPC11 cells with YE1/19.1 partially blocked subsequent binding to APC-labeled IR518 or YE1/19.1 (data not shown). Whether both antibodies could share an overlapping epitope (not the H650) requires further examination. In subsequent analyses using YE1/19.1, we found positive binding of the antibody to peritoneal B1a cells of all strains tested (H. Wang and H.C. Morse III, unpublished data), confirming that YE1/19.1 and IR518 react with at least partially different epitopes of the ENPP1 protein.
FIG. 2.
Binding of anti-ENPP1 MAbs to PCT cell lines MPC11 and BPC4. Cells were stained with indicated antibodies directly labeled with APC and analyzed by FACS. Dead cells were excluded by propidium iodide.
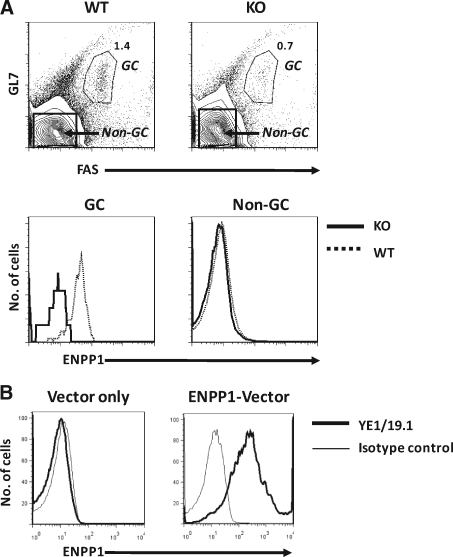
Analyses of splenic B cells from normal B6 mice showed that germinal center (GC) B cells, defined as GL7+FAS+, expressed ENPP1 at considerably higher levels than non-GC B cells (Fig. 3A). To further confirm the binding specificity of YE1/19.1, we proceeded to analyze the reactivity of YE1/19.1 with spleen cells of mice homozygous for a null allele of ENPP1. These mice were generated by insertion of a Neo gene into exon 9, resulting in disruption of transcription of the Enpp1 gene.(9) The results showed that reactivity of the YE1/19.1 MAb with both GC and non-GC B cells of the knockout mice was at background levels (Fig. 3A).
FIG. 3.
YE1/19.1 binding to germinal centers (GCs) and ENPP1-overexpressing cells (A). Splenic cells from PC-1–/– (KO) and WT mice were stained with GL7-FITC, anti-FAS-PE, and YE1/19.1-APC. The cells were analyzed by flow cytometry. The top shows gating of GCs and non-GCs. The numbers are percentages of cells falling in each gate. The bottom shows overlays of ENPP1 expression in GCs (left) and non-GCs (right). (B) HEK 293 cells were transfected with an Enpp1b expression vector and analyzed by flow cytometry.
Finally, studies of human HEK 293 cells transfected with an ENPP1b expression vector revealed high-level cell surface expression of ENPP1 detected by YE1/19.1 (Fig. 3B). Together, these analyses led to the conclusion that YE1/19.1 recognizes ENPP1a and ENPP1b and can be used for flow cytometric studies of ENPP1 expression in all strains of mice carrying either allele.
ENPP1 is differentially expressed in immune cells
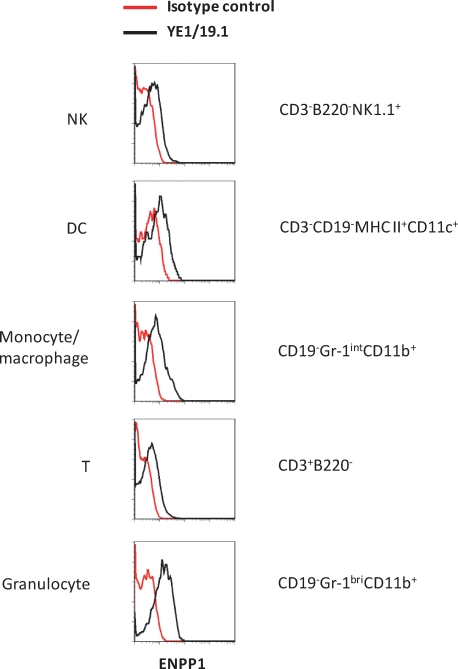
To our knowledge, the expression patterns of ENPP1 in different subsets of hematopoietic cells have not been reported. This prompted us to determine the levels of ENPP1 expression on spleen cell subpopulations defined by use of a series of lineage-specific markers and sophisticated flow cytometric analyses. As shown in Figure 4, ENPP1 was expressed at similarly low levels on natural killer (NK) cells, dendritic cells (DC), monocytes/macrophages, and naïve T cells but at higher levels on neutrophils.
FIG. 4.
ENPP1 expression in immune cells. Splenic cells of B6 mice were stained with indicated antibodies and analyzed by flow cytometry. Subsets of immune cells were identified by lineage-specific markers as indicated.
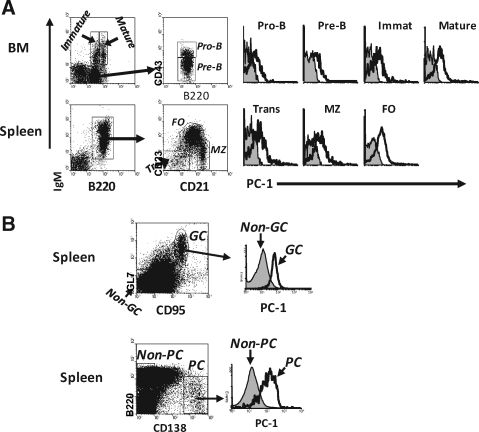
As ENPP1 was first described as a marker for plasma cells, we next focused our analysis on B lineage cells. Studies of BM pro-B, pre-B, and immature B cells, as defined by the Hardy nomenclature,(24) showed that ENPP1 was expressed at levels slightly higher than background (Fig. 5A). Levels of ENPP1 expression were slightly higher on more mature splenic B cell subsets including transitional, marginal zone (MZ), and follicular (FO) B cells. As noted above, GC B cells (GL7+CD95+) expressed significantly higher levels of ENPP1 than non-GC cells (Fig. 5B). As expected from previous studies,(20) PCs (B220lo/-CD138bri) expressed high levels of ENPP1 (Fig. 5B).
FIG. 5.
ENPP1 expression in B lineage cells. (A) Bone marrow and splenic cells from B6 mice were stained with antibodies indicated and analyzed by flow cytometry. Each subpopulation of B cells is indicated. The closed line is isotype control background. (B) Splenic cells from immunized (KLH/alum) mice were stained with indicated antibodies and analyzed by flow cytometry.
Discussion
This study shows that the MAb YE1/19.1 recognizes both the a and b alleles of ENPP1 and can be used for flow cytometric analyses of ENPP1 expression in all strains of mice. While ENPP1 has been well studied in mineralization during bone formation and insulin resistance and diabetes, the correlates of its expression are poorly understood in the immune system, especially in the B cell lineage. This antibody could help to reveal functions of ENPP1 in different types of immune cells under physiological and pathological conditions.
In comparisons with a well-characterized anti-ENPP1a MAb, IR518, we found that YE1/19.1 recognizes a different epitope of the ENPP1 protein. IR518 binds to a region that includes the aa H650, one of the two aa responsible for the difference between the a and b alleles. The capability of YE1/19.1 to react with both a and b allotypes indicates that YE1/19.1 binds to an epitope other than that defined by H650 and R679. Clearly, further work is required to map the binding site of YE1/19.1.
ENPP1 has several documented functions. While expression of ENPP1 in chondrocytes has been attributed to its function in generating PPi, a factor suppressing calcium precipitation,(5,6) expression of ENPP1 in liver is thought to modulate insulin-mediated sugar metabolism.(25) Although ENPP1 is expressed at relatively low levels on a range of hematopoietic cells including NK cells, DCs, monocytes/macrophages, and naïve T and B cells, the function of ENPP1 on these cells remains to be determined. Previous work suggested that the expression of ENPP1 was upregulated in activated T cells and activated B cells(26) (H. Wang et al., unpublished data). The fact that ENPP1 is highly expressed by GC B cells and PCs suggests that it may play important roles in the development and function of late-stage B cells. Characterization of YE1/19.1 in this study allows us to further investigate the function of ENPP1 in B cells.
Acknowledgments
This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We are indebted to Dr. Fumio Takei at the Terry Fox Laboratory (Vancouver, Canada) for providing the YE1/19.1 antibody. We thank Larry Lantz at NIAID for technical assistance. We are grateful to Drs. Alexander Kovalchuk and Yong-Soo Kim for reagents. We thank NIAID editor Brenda Rae Marshall for manuscript assistance.
Because we are government employees and this is a government work, the work is in the public domain in the United States. The authors have no conflicting financial interests to report.
Author Disclosure Statement
The authors have no financial conflicts to declare.
References
- 1.Takahashi T. Old LJ. Boyse EA. Surface alloantigens of plasma cells. J Exp Med. 1970;131:1325–1341. doi: 10.1084/jem.131.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banakh I. Sali A. Dubljevic V. Grobben B. Slegers H. Goding JW. Structural basis of allotypes of ecto-nucleotide pyrophosphatase/phosphodiesterase (plasma cell membrane glycoprotein PC-1) in the mouse and rat, and analysis of allele-specific xenogeneic antibodies. Eur J Immunogenet. 2002;29:307–313. doi: 10.1046/j.1365-2370.2002.00330.x. [DOI] [PubMed] [Google Scholar]
- 3.Harahap AR. Goding JW. Distribution of the murine plasma cell antigen PC-1 in non-lymphoid tissues. J Immunol. 1988;141:2317–2320. [PubMed] [Google Scholar]
- 4.Stearne PA. van Driel IR. Grego B. Simpson RJ. Goding JW. The murine plasma cell antigen PC-1: purification and partial amino acid sequence. J Immunol. 1985;134:443–448. [PubMed] [Google Scholar]
- 5.Goding JW. Grobben B. Slegers H. Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim Biophys Acta. 2003;1638:1–19. doi: 10.1016/s0925-4439(03)00058-9. [DOI] [PubMed] [Google Scholar]
- 6.Terkeltaub RA. Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol Cell Physiol. 2001;281:C1–C11. doi: 10.1152/ajpcell.2001.281.1.C1. [DOI] [PubMed] [Google Scholar]
- 7.Babij P. Roudier M. Graves T. Han CY. Chhoa M. Li CM. Juan T. Morony S. Grisanti M. Li X. Yu L. Dwyer D. Lloyd DJ. Bass MB. Richards WG. Ebeling C. Amato J. Carlson G. New variants in ENPP1 and Ptpn6 genes cause low bone density, crystal-related arthropathy and vascular calcification. J Bone Miner Res. 2009;24:1552–1564. doi: 10.1359/jbmr.090417. [DOI] [PubMed] [Google Scholar]
- 8.Okawa A. Nakamura I. Goto S. Moriya H. Nakamura Y. Ikegawa S. Mutation in Npps in a mouse model of ossification of the posterior longitudinal ligament of the spine. Nat Genet. 1998;19:271–273. doi: 10.1038/956. [DOI] [PubMed] [Google Scholar]
- 9.Sali A. Favaloro JM. Terkeltaub R. Goding JW. Ecto-ATPases and Related Ectoenzymes. Shaker Publishing; Maastricht, The Netherlands: 1999. Germline deletion of the nucleoside triphosphate pyrophosphohydrolase (NTPPPH) plasma cell membrane glycoprotein-1 (PC-1) produces abnormal calcification of periarticular tissues; pp. 267–282. [Google Scholar]
- 10.Johnson K. Polewski M. van Etten D. Terkeltaub R. Chondrogenesis mediated by PPi depletion promotes spontaneous aortic calcification in NPP1–/– mice. Arterioscler Thromb Vasc Biol. 2005;25:686–691. doi: 10.1161/01.ATV.0000154774.71187.f0. [DOI] [PubMed] [Google Scholar]
- 11.Crade M. Lewis DF. Nageotte MP. In utero appearance of idiopathic infantile arterial calcification: ultrasound study of a 28-week fetus. Ultrasound Obstet Gynecol. 1991;1:284–285. doi: 10.1046/j.1469-0705.1991.01040284.x. [DOI] [PubMed] [Google Scholar]
- 12.Reitter A. Fischer D. Buxmann H. Nitschke Y. Rutsch F. Mottok A. Hansmann ML. Harms E. Louwen F. Schlosser R. Fetal hydrops, hyperechogenic arteries and pathological Doppler findings at 29 weeks: prenatal presentation of generalized arterial calcification of infancy—a novel mutation in ENPP1. Fetal Diagn Ther. 2009;25:264–268. doi: 10.1159/000223683. [DOI] [PubMed] [Google Scholar]
- 13.Rutsch F. Ruf N. Vaingankar S. Toliat MR. Suk A. Hohne W. Schauer G. Lehmann M. Roscioli T. Schnabel D. Epplen JT. Knisely A. Superti-Furga A. McGill J. Filippone M. Sinaiko AR. Vallance H. Hinrichs B. Smith W. Ferre M. Terkeltaub R. Nurnberg P. Mutations in ENPP1 are associated with 'idiopathic' infantile arterial calcification. Nat Genet. 2003;34:379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- 14.Samon LM. Ash KM. Murdison KA. Aorto-pulmonary calcification: an unusual manifestation of idiopathic calcification of infancy evident antenatally. Obstet Gynecol. 1995;85:863–865. doi: 10.1016/0029-7844(94)00362-h. [DOI] [PubMed] [Google Scholar]
- 15.Hosoda N. Hoshino SI. Kanda Y. Katada T. Inhibition of phosphodiesterase/pyrophosphatase activity of PC-1 by its association with glycosaminoglycans. Eur J Biochem. 1999;265:763–770. doi: 10.1046/j.1432-1327.1999.00779.x. [DOI] [PubMed] [Google Scholar]
- 16.Liang J. Fu M. Ciociola E. Chandalia M. Abate N. Role of ENPP1 on adipocyte maturation. PLoS One. 2007;2:e882. doi: 10.1371/journal.pone.0000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maddux BA. Goldfine ID. Membrane glycoprotein PC-1 inhibition of insulin receptor function occurs via direct interaction with the receptor alpha-subunit. Diabetes. 2000;49:13–19. doi: 10.2337/diabetes.49.1.13. [DOI] [PubMed] [Google Scholar]
- 18.Grobben B. Claes P. Roymans D. Esmans EL. Van Onckelen H. Slegers H. Ecto-nucleotide pyrophosphatase modulates the purinoceptor-mediated signal transduction and is inhibited by purinoceptor antagonists. Br J Pharmacol. 2000;130:139–145. doi: 10.1038/sj.bjp.0703289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacci S. De Cosmo S. Prudente S. Trischitta V. ENPP1 gene, insulin resistance and related clinical outcomes. Curr Opin Clin Nutr Metab Care. 2007;10:403–409. doi: 10.1097/MCO.0b013e3281e386c9. [DOI] [PubMed] [Google Scholar]
- 20.Takei F. Unique surface phenotype of T cells in lymphoproliferative autoimmune MRL/Mp-lpr/lpr mice. J Immunol. 1984;133:1951–1954. [PubMed] [Google Scholar]
- 21.Zhang JQ. Okumura C. McCarty T. Shin MS. Mukhopadhyay P. Hori M. Torrey TA. Naghashfar Z. Zhou JX. Lee CH. Roopenian DC. Morse HC., III Davidson WF. Evidence for selective transformation of autoreactive immature plasma cells in mice deficient in Fasl. J Exp Med. 2004;200:1467–1478. doi: 10.1084/jem.20041575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goding JW. Terkeltaub R. Maurice M. Deterre P. Sali A. Belli SI. Ecto-phosphodiesterase/pyrophosphatase of lymphocytes and non-lymphoid cells: structure and function of the PC-1 family. Immunol Rev. 1998;161:11–26. doi: 10.1111/j.1600-065x.1998.tb01568.x. [DOI] [PubMed] [Google Scholar]
- 23.Laskov R. Lanzerotti R. Scharff MD. Synthesis, assembly and secretion of gamma globulin by mouse myeloma cells. II. Assembly of IgG2b immunoglobulin by MPC 11 tumor and culture cells. J Mol Biol. 1971;56:327–339. doi: 10.1016/0022-2836(71)90468-2. [DOI] [PubMed] [Google Scholar]
- 24.Hardy RR. Carmack CE. Shinton SA. Kemp JD. Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong H. Maddux BA. Altomonte J. Meseck M. Accili D. Terkeltaub R. Johnson K. Youngren JF. Goldfine ID. Increased hepatic levels of the insulin receptor inhibitor, PC-1/NPP1, induce insulin resistance and glucose intolerance. Diabetes. 2005;54:367–372. doi: 10.2337/diabetes.54.2.367. [DOI] [PubMed] [Google Scholar]
- 26.Dumont FJ. Habbersett RC. Coker LZ. Nichols EA. Treffinger JA. High level expression of the plasma cell antigen PC.1 on the T-cell subset expanding in MRL/MpJ-lpr/lpr mice: detection with a xenogeneic monoclonal antibody and alloantisera. Cell Immunol. 1985;96:327–337. doi: 10.1016/0008-8749(85)90364-8. [DOI] [PubMed] [Google Scholar]