Abstract
The lipopolysaccharide (LPS) of Francisella tularensis (Ft), the Gram negative bacterium that causes tularemia, has been shown to be a main protective antigen in mice and humans; we have previously demonstrated that murine anti-Ft LPS IgG2a monoclonal antibodies (MAbs) can protect mice against otherwise lethal intranasal infection with the Ft live vaccine strain (LVS). Here we show that four IgG2a anti-LPS MAbs are specific for the O-polysaccharide (O-antigen [OAg]) of Ft LPS. But whereas three of the MAbs bind to immunodominant repeating internal epitopes, one binds to a unique terminal epitope of Ft OAg. This was deduced from its even binding to both long and short chains of the LPS ladder in Western blots, its rapid decrease in ELISA binding to decreasing solid-phase LPS concentrations, its inability to compete for LPS binding with a representative of the other three MAbs, and its inability to immunoprecipitate OAg despite its superior agglutination titer. Biacore analysis showed the end-binding MAb to have higher bivalent avidity for Ft OAg than the internal-binding MAbs and provided an immunogenicity explanation for the predominance of internal-binding anti-Ft OAg MAbs. These findings demonstrate that non-overlapping epitopes can be targeted by antibodies to Ft OAg, which may inform the design of vaccines and immunotherapies against tularemia.
Introduction
Francisella tularensis (Ft), the Gram negative intracellular bacterium that causes tularemia, has been classified by the Centers for Disease Control and Prevention as a Category A select agent, a likely bioweapon, due to its low infectivity dose (<10 CFU) and the high mortality rate associated with respiratory tularemia (30–60% in untreated patients). Two of the F. tularensis subspecies, tularensis (type A) and holarctica (type B), cause most cases of human disease; type A, found predominantly in North America, is the more virulent of the two.(1–3) Ft types A and B have high genomic sequence homology (BioHealthBase BioDefense Public Health Database, www.biohealthbase.org) and the same LPS structure, with an OAg consisting of four sugar repeats, connected at its reducing end to a core oligosaccharide, which in turn is connected at its reducing end to lipid A.(4–8) An attenuated Ft type B strain, designated live vaccine strain (LVS), partially protects against pathogenic Ft in humans,(9) but is virulent in mice.(10)
Tularemia is usually treated with intravenous and later oral antibiotics, but infection is still associated with considerable morbidity and up to 2% mortality in treated patients.(2,3,11) LVS, the partially protective vaccine, is not currently licensed due to safety concerns.(2,9) These considerations, combined with the threat of engineered multiple antibiotic-resistant strains for bioterrorism, suggest the need for additional strategies to combat tularemia, including vaccines and immunotherapeutics, and hence an understanding of the immune response to Ft.
Based on literature reports, immune protection against Ft involves a dominant role for CD8 and TH1-type CD4 Ft-specific T cells,(12–14) and the cytokines IL-12, IFN-γ, and TNF-α.(12,13,15,16) Despite the critical role of T cells, B cells are required for generation of memory to Ft,(17) and polyclonal IgG antibodies to Ft or to Ft LPS have been reported to transfer resistance against Ft to naïve hosts, including humans.(10,18–26) Furthermore, the protective immune response to Ft in mice correlates with generation of antibodies of the IgG2a isotype,(27) the mouse analog of human IgG1,(28) which binds better than other isotypes to the activating Fc receptor FcγRI.(28–30) This was confirmed by our studies, which have shown that anti-Ft LPS MAbs of the mouse IgG2a isotype, but not of the IgM, IgG3, or IgG1 isotypes, can protect mice against intranasal (i.n.) lethal LVS challenge.(31)
To gain insight into the specificities and avidities of protective anti-Ft LPS antibodies, we now compared the binding characteristics of four anti-Ft LPS IgG2a MAbs. We show that all four MAbs are specific for the O-polysaccharide (OAg) of Ft LPS; but whereas three of the MAbs bind to repeating internal OAg epitopes, one MAb binds with higher avidity to a unique terminal epitope.
Materials and Methods
Bacterial strains
F. tularensis holarctica strain LVS was obtained from Jeannine Petersen (Centers for Disease Control and Prevention, Fort Collins, CO). F. tularensis tularensis strain SchuS4 was obtained from BEI Resources (Manassas, VA). Escherichia coli strain TG1 was purchased from Stratagene (La Jolla, CA). For experiments, LVS and SchuS4 bacteria were grown on chocolate agar plates (Remel, Lenexa, KS); TG1 bacteria were grown on LB plates at 37°C in a humidified environment of 100% air for 2.5 days (LVS and SchuS4) or overnight (TG1) and pools of single colonies were scraped and resuspended in PBS. Heat-killed bacterial samples were prepared by 2 h incubation at 80°C. Heat-killed (80°C, 2 h) WbtIG191V (WbtI), an OAg-deficient LVS mutant,(32) was obtained from Dr. Thomas Inzana of Virginia Polytechnic Institute (Blacksburg, VA).
Hybridoma and recombinant antibodies
Four IgG2a hybridoma antibodies (Ab) specific for Ft LPS were used in this study (FB11, Ab3, Ab52, and Ab54). Protein G-purified FB11(33) was purchased from GeneTex (Irvine, CA) and dialyzed against PBS on a Centricon YM-30 centrifugal filter (Millipore, Billerica, MA) to remove the sodium azide used as preservative by the manufacturer. An IgG2a hybridoma antibody (GTX40330) to E. coli J5 LPS was purchased from GeneTex as a positive control for E. coli TG1. The Ab3 hybridoma, generated in our laboratory from LVS-infected mice, was previously described.(31) The Ab52 and Ab54 hybridomas were generated in our laboratory from BALB/c mice repeatedly immunized with LVS and Ft LPS admixed with CpG ODN 1826 (TCC ATG ACG TTC CTG ACG TT) (an oligodeoxynucleotide containing unmethylated CpG dinucleotides with fully phosphorothioate backbone [CPG-ODN, Coley Pharmaceutical Group, Wellesley, MA]) by fusion of splenocytes with the Sp2/0-Ag14 mouse myeloma cell line as described previously.(31) The anti-Ft GroEL IgG2a hybridoma Ab53 was similarly generated in our laboratory from mice immunized and boosted with LVS (Lu et al., unpublished results). Mouse hybridoma cell line CO17-1A,(34) producing an IgG2a antibody specific for the human tumor associated Ag EpCam,(35) used as isotype control, was obtained from Dr. Dorothee Herlyn of the Wistar Institute (Philadelphia, PA). Mouse IgG2a (mIgG2a, a MAb specific for non-human PBLs), used as isotype control in ELISA, was purchased from Sigma (St. Louis, MO).
A recombinant IgA version of hybridoma antibody Ab3 (Ab3IgAR) was generated by RT-PCR amplification of the expressed VH and VL region genes from Ab3 hybridoma RNA, as previously described, for generation of recombinant polyclonal antibodies from immunized mice(36) and subcloned into an intermediate bidirectional vector containing a head-to-head mammalian expression cassette.(36) The DNA segment, including the VH and VL region genes and the expression cassette, was then inserted into the bidirectional mouse pMDVIgA mammalian expression vector containing the Cκ and Cα genes (derived from pMDVIgG2b(36) by substitution of the Cγ2b gene with the Cα gene obtained from the J558.1 mouse myeloma cell line, which produces an anti-α(1 → 3) dextran IgA antibody) and the resulting construct transfected into Sp2/0-Ag14 myeloma cells by spheroplast fusion(36) to obtain an IgA-producing transfectoma.
Hybridoma and transfectoma cells were cultured in IMDM (Gibco, Grand Island, NY) supplemented with 10% FBS or SFM (Hyclone, Logan, UT) supplemented with 2% FBS at 37°C in a humidified environment of 5% CO2/95% air. Ab3, Ab52, Ab53, Ab54, and CO17-1A were separately purified from culture supernatants on Protein G-Sepharose (Pierce, Rockford, IL) according to the manufacturer's instructions and their purity verified by SDS-PAGE.
ELISA and Western blot analysis
ELISA was performed as previously described.(31) Briefly, EIA/RIA microtiter plates were coated with Ags diluted in 50 mM sodium carbonate buffer (pH 9.6). Coating with heat-killed (80°C for 2 h) LVS or WbtI, at 0.04 OD600/mL, was by overnight air-drying in a laminar flow hood, and with various concentrations of Ft LPS (previously described(31)) by overnight incubation at 4°C. Coating with 10 μg/mL of Ft OAg (Sussex Research, Ottawa, Canada) was by overnight air-drying in a laminar flow hood. After washing and blocking, the plates were incubated sequentially with 3-fold serial dilutions of mouse IgG2a MAbs and HRP-conjugated anti-mouse IgG2a (SouthernBiotech, Birmingham, AL). Assays were developed by the addition of 60 μL/well TMB substrate (Kirkegaard & Perry Labs, Gaithersburg, MD) and 15 min incubation at room temperature in the dark. The reaction was stopped with 60 μL/well of 0.2 M H2SO4, and absorbance at 450 nm measured in a microplate reader.
For isotype-specific competition ELISA, plates were coated with 5 μg/mL of Ft LPS, and the binding of a fixed concentration (1 μg/mL) of Ab3IgAR, in the presence of graded concentrations of IgG2a competitor MAb (starting at 1 mg/mL), was determined using HRP-conjugated anti-mouse IgA secondary antibody (Sigma, St. Louis, MO). The presence/binding of all IgG2a anti-LPS competitor MAbs to LPS was verified in a duplicate plate by developing the ELISA with HRP-conjugated anti-mouse IgG2a secondary Ab.
Western blot analysis was performed as previously described,(31) using precast preparative 4 − 15% polyacrylamide gradient gels (2-D/Prep) and broad range pre-stained SDS-PAGE molecular weight standards (Bio-Rad Laboratories, Hercules, CA). After electrotransfer to nitrocellulose membranes and blocking, the membranes were cut into strips and each strip incubated sequentially with a MAb and alkaline phosphatase-conjugated anti-mouse IgG2a secondary Ab (SouthernBiotech), then developed with Western Blue stabilized substrate for alkaline phosphatase (Promega, Madison, WI).
Immunoprecipitation and microagglutination
Immunoprecipitation by double immunodiffusion in agar was performed using 8-hexagons Ouchterlony plates (MP Biomedicals, Solon, OH). Purified MAb, starting at 6 mg/mL followed by 2-fold serial dilutions, was added clockwise to the outer wells of each Ouchterlony hexagon beginning in the top well. After 8 h, 1 mg/mL of Ft OAg (14 kDa average molecular mass, Sussex Research) was added to the center wells, and the plate incubated at room temperature in a humidified chamber overnight. Visible precipitin lines were recorded and photographed.
Microagglutination assays were performed using 4 × 109/mL fixed/crystal violet-stained LVS prepared as previously described.(37) Thirty μL per well of fixed/stained LVS were added to a 96-well round-bottom plate (Corning, Corning, NY) containing 30 μL/well of 2-fold serial dilutions of MAbs in SFM, and incubated overnight at room temperature. Wells showing a carpet-like pattern were recorded as positive and those showing a small, tight button were recorded as negative. Microagglutination titers were calculated as the reciprocal of the last MAb dilution that showed agglutination.
Biacore analysis
Surface plasmon resonance (Biacore) analysis to determine equilibrium dissociation constants (KD) of MAbs was performed by Precision Antibody (Columbia, MD) using two assay formats. In the first format, MAbs were immobilized on anti-mouse IgG-coated chips and probed with Ft OAg (avg. molecular mass 14 kDa, Sussex Research). In the second format, purified MAb Ab52 was immobilized on anti-mouse IgG-coated chips and used to capture Ft LPS; the chip was then probed with anti-Ft OAg MAbs.
Results
Ft OAg targeted by anti-LPS IgG2a MAbs
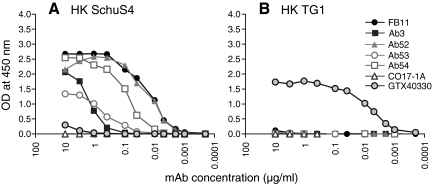
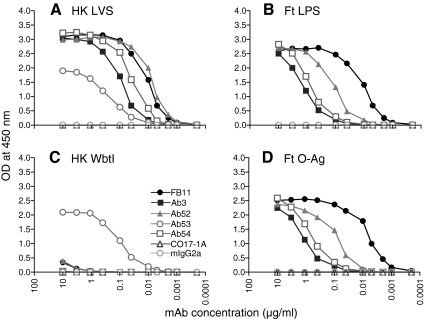
Four anti-Ft LPS IgG2a(κ) MAbs, one commercial (FB11), and three in-house-generated (Ab3,(31) Ab52 and Ab54) were characterized for their Ag-binding specificity by ELISA. All four MAbs bound to both heat-killed LVS and SchuS4 Ft strains, which have the same LPS structure,(4–7) but did not bind to the TG1 strain of E. coli, demonstrating their specificity for Ft (Fig. 1). As expected, all four MAbs bound to Ft LPS. In addition, all four MAbs failed to bind to a heat-killed preparation of the WbtI OAg deficient LVS mutant and bound to purified Ft OAg (Fig. 2), demonstrating their OAg specificity. (Although Ft OAg is uncharged and has been reported not to adhere to ELISA plates,(4) we found that it can be immobilized on EIA/RIA plates by air-drying, which facilitated the identification of OAg as the target of all four MAb.) Reassuringly, the anti-Ft GroEL IgG2a MAb Ab53, used as Ag specificity control, bound only to LVS and WbtI, and the CO17-1A and mIgG2a MAbs, used as isotype controls, did not bind to any of the ELISA plates. The order of binding potency of the four anti-LPS IgG2a MAbs to immobilized OAg was FB11 > Ab52 > Ab54 > Ab3 (Fig. 2D), suggesting that FB11 has the highest and Ab3 has the lowest avidity for OAg.
FIG. 1.
All four anti-LPS IgG2a MAbs are specific for virulent type A strain by ELISA. ELISA plates were coated with heat-killed (HK) SchuS4 (A) or TG1 (B), as described in Materials and Methods. IgG2a MAbs Ab53 (anti-GroEL), CO17-1A (anti-human EpCam), and GTX40330 (mouse monoclonal IgG2a to Gram negative endotoxin) were used as specificity and isotype controls.
FIG. 2.
All four anti-LPS IgG2a MAbs are specific for OAg by ELISA. ELISA plates were coated with heat-killed (HK) LVS (A), Ft LPS (B), HK WbtI (C), or Ft OAg (D), as described in Materials and Methods. IgG2a MAbs Ab53 (anti-GroEL), CO17-1A (anti-human EpCam), and mIgG2a (anti-non-human PBLs) were used as specificity and isotype controls. Data shown are from one of two (A, C) or three (B, D) experiments with similar results.
Anti-OAg MAbs distinguished by Western blot reactivity to LPS ladder
The differences in binding potency observed in ELISA on Ft OAg (and Ft LPS) were also evident in Western blots on LVS lysate. There, Ab3, Ab52, and Ab54 required 100-fold higher concentrations to show similar intensity binding to the longer chains of the LPS ladder, and, even so, only FB11 showed binding to the shortest LPS chains (Fig. 3A). In contrast to the (fairly) equal binding intensity of FB11 to all rungs of the LPS ladder, the binding intensity of the other three MAbs decreased with decreasing chain size (Fig. 3A). Because the ladder pattern formed by LPS on Western blots is due to the varying numbers of four-sugar repeats in the LPS OAg chains,(38) we reasoned that the longer OAg chains will accommodate a higher number of antibody molecules than the shorter chains if the antibody molecules bind to repeating internal epitopes. On the other hand, head-on binding to the OAg non-reducing end, or binding to the unique reducing end of OAg, which, by currently known isolation procedures, still contains the (reduced) core oligosaccharide,(39) will be independent of chain size because all OAg chains contain a single non-reducing end and a single reducing end. This suggests that FB11 binds to a terminal epitope of LPS OAg on both long and short OAg chains whereas Ab3, Ab52, and Ab54 bind to internal LPS OAg repeating epitopes on the longer OAg chains only (as illustrated in Fig. 3B for the hypothetical binding of Ab52 to internal epitopes and of FB11 to the non-reducing end of OAg).
FIG. 3.
FB11 binds with equal intensity to all rungs of the LPS ladder, whereas the binding intensity of the other three anti-OAg IgG2a MAbs decreases with decreasing chain size. (A) Western blot analysis of the indicated MAbs, at the indicated concentrations, on LVS lysate. The positions of pre-stained molecular weight standards (in kDa) are indicated. (B) Model of antibody binding (only one antibody arm shown) to terminal or internal OAg epitopes for the lower rungs of the LPS ladder in the Western blot. Schematic of Ft LPS and the four sugar repeating unit of OAg is shown at top. QNF, QuiN4Fm, 4,6-dideoxy-4-formamido-D-glucose; GNA, GalNAcAN, 2-acetamido-2-deoxy-D-galacturonamide; QN, QuiNAc, 2-acetamido-2,6-dideoxy-D-glucose.(5,6) The higher sensitivity and different laddering pattern of FB11 compared with the other three IgG2a anti-Ft OAg MAbs were observed on numerous Western blots.
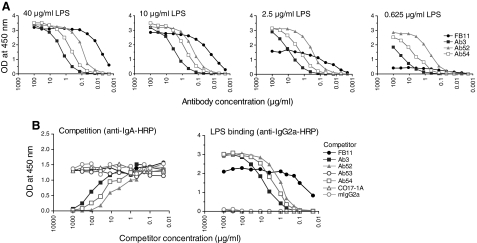
Different OAg target epitopes supported by differential MAb reactivity in decreasing Ag and competition ELISAs
Consistent with recognition of a single terminal epitope of OAg by FB11, the LPS ELISA binding of FB11 decreased much quicker than that of Ab3, Ab52, and Ab54 with decreasing LPS coating concentrations, as the terminal but not the internal OAg epitopes become limiting (Fig. 4A). Furthermore, Ab3, Ab52, and Ab54, but not FB11 (or the control MAbs Ab53, CO17-1A, and mIgG2a) inhibited the LPS binding of a recombinant mouse IgA version of Ab3 used as reporter in an isotype-specific competition ELISA (Fig. 4B, left). This indicates that Ab3, Ab52, and Ab54 bind to the same or overlapping epitope(s) of OAg. The LPS binding of all four anti-LPS IgG2a MAbs in the competition ELISA was verified in a duplicate plate by developing the reaction with anti-mouse IgG2a secondary antibody (Fig. 4B, right). Nucleotide sequence analysis of the H and L chain V region genes of the three in-house generated anti-Ft OAg IgG2a MAbs revealed that Ab52 and Ab54 are partially encoded by the same VL germline gene and, except for one amino acid difference, share the same VL region complementarity determining regions (data not shown). This suggests that Ab52 and Ab54 may in fact be binding to the same Ft OAg epitope.
FIG. 4.
FB11 binds to a different OAg epitope than Ab3, Ab52 and Ab54. (A) The binding of FB11 to decreasing concentrations of Ft LPS decreases at a much higher rate than the binding of the other IgG2a anti-OAg MAbs. Reactivity of IgG2a anti-OAg MAbs was tested by ELISA on plates coated with the indicated concentrations of LPS. Data from one of three experiments with similar results are shown. (B) Ab3, Ab52, and Ab54, but not FB11, bind to overlapping OAg epitopes. Isotype-specific competition ELISA was performed with graded concentrations of the indicated IgG2a competitors and a fixed concentration of Ab3IgAR, and the ELISA was developed with anti-IgA secondary Ab. The left panel shows the competition assay. The presence/binding of all IgG2a anti-LPS competitor MAbs to LPS was verified in a duplicate plate by developing the reaction with HRP-conjugated anti-mouse IgG2a secondary Ab (right panel). Data from one of three experiments with similar results are shown.
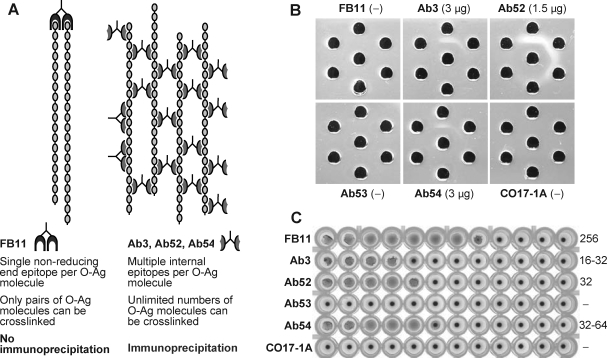
End-binding and internal-binding MAbs confirmed by linear carbohydrate immunoprecipitation assay
To confirm that FB11 binds to a unique terminal epitope whereas the other three MAbs bind to repeating internal epitopes of OAg, we used the linear carbohydrate immunoprecipitation assay,(40) the principle of which is depicted in Figure 5A. Because OAg is a linear carbohydrate containing a single non-reducing end and a single reducing end per molecule, the binding of an OAg molecule to one molecule of a MAb specific for one of the ends will leave no exposed epitopes for binding to other molecules of this MAb and therefore will result in no immunoprecipitation (Fig. 5A, left). However, because OAg contains many repeating internal epitopes, the binding of an OAg molecule to one molecule of a MAb specific for internal epitopes will still leave many exposed internal epitopes for binding to other molecules of this MAb, resulting in immunoprecipitation (Fig. 5A, right). Indeed, Ouchterlony double immunodiffusion in agar, using OAg in the center well and serial dilutions of each MAb in the outer wells (Fig. 5B), showed formation of precipitin lines with Ab3, Ab52, and Ab54 but not with FB11 or the control MAbs Ab53 and CO17-1A. Of the immunoprecipitating MAbs, Ab52 showed thicker precipitin lines and a 2-fold higher titer than Ab3 and Ab54 (Fig. 5B), consistent with its higher binding potency than Ab3 and Ab54 in ELISA (Figs. 1, 2, 4) and Western blot assays (Fig. 3).
FIG. 5.
FB11 cannot immunoprecipitate OAg despite its higher bacterial agglutination titer compared to the other three MAbs. (A) Schematic of end-binding by FB11 versus internal-binding by Ab52 (or Ab3 or Ab54) to OAg chains. (B) Immunoprecipitation by double immunodiffusion in agar. The contrast and brightness of the photograph were globally increased to allow better visualization of the thin precipitin lines formed with Ab3 and Ab54, which were clearly visible with the naked eye. The lowest antibody concentration that showed a precipitin line (the immunoprecipitation titer) is indicated in parentheses. −, no precipitin line. The Ouchterlony set shown is one of two performed with similar results. (C) Microagglutination of fixed/crystal violet-stained LVS by 2-fold serial dilutions of MAbs, starting with a final concentration of 0.5 mg/mL in well 1. Wells showing a carpet-like pattern were recorded as positive and those showing a small, tight button were recorded as negative. Microagglutination titers (shown on the right side of the plate) were calculated as the reciprocal of the last antibody dilution that showed agglutination. The microagglutination assay with fixed/stained cells was repeated once with the same results. Unfixed LVS bacteria also yielded the same results but were much less visible on photographs.
In contrast to its inability to immunoprecipitate OAg, FB11 was better than Ab3, Ab52, and Ab54 at agglutinating LVS bacteria, which have many OAg ends per bacterial particle, with a 4- to 8-fold higher agglutination titer (Fig. 5C). The anti-Ft GroEL MAb Ab53 did not show agglutination, nor did the isotype control MAb CO17-1A (Fig. 5C). It is noteworthy that FB11 has an 8-fold higher LVS agglutination titer than Ab52 (Fig. 5C) yet shows the same or even slightly lower potency at binding to LVS in ELISA (Figs. 1A, 2A). An explanation for this apparent inconsistency is that bacterial agglutination reflects the ability to hold on to LPS chains on two bacterial particles in suspension and is therefore a measure of antibody affinity, which favors FB11. However, binding to immobilized LVS bacteria in ELISA favors the internal-binding Ab52, which can easily cross-link neighboring densely packed LPS chains on the same bacterial particle.
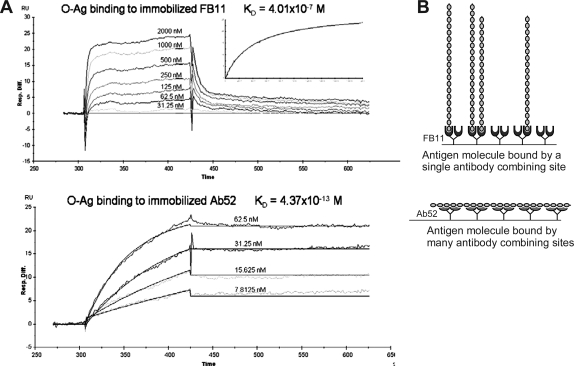
Biacore analysis confirms higher avidity of end-binding MAb
Biacore analysis, using chip-immobilized MAb and soluble Ft OAg, was performed in an attempt to determine the affinities of the end-binding MAb FB11 and of the strongest internal-binding MAb Ab52 for their OAg epitopes. The affinity (KD) of FB11 for Ft OAg was found to be 4.0 × 10−7 M, with a fast on-rate and a fast off-rate (Fig. 6A, top). The affinity (KD for the interaction of a single antibody combining site with a single epitope) of Ab52 could not be determined using chip-immobilized Ab52 due to the multivalent interaction of many molecules of Ab52 with the multiple internal epitopes on each OAg molecule. However, the KD for this multivalent interaction was 4.4 × 10−13 M (Fig. 6A, bottom), indicating an essentially irreversible reaction.
FIG. 6.
The affinity of FB11, but not of Ab52, can be determined using immobilized MAb and soluble Ft OAg. (A) Biacore analysis of the binding of immobilized FB11 (top) and Ab52 (bottom) to Ft OAg. MAbs were captured on anti-mouse IgG-coated chips and probed with Ft OAg. (B) Schematic of the monovalent binding of immobilized FB11 to Ft OAg (top) and of the multivalent binding of immobilized Ab52 to Ft OAg (bottom).
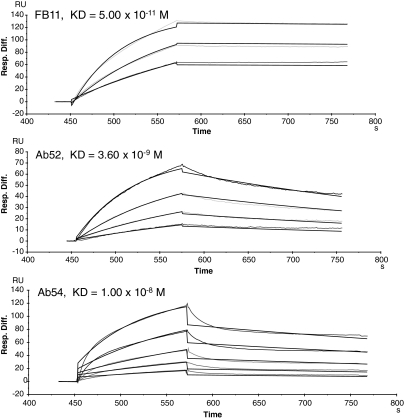
To compare the bivalent avidities of the end-binding and internal-binding Ft OAg MAbs, we used a Biacore assay format in which the antigen is immobilized and probed with soluble MAbs. The assay takes advantage of the strong interaction of immobilized Ab52 with Ft OAg to capture and immobilize the antigen (except that Ft LPS rather than OAg is used because LPS molecules tend to aggregate via their lipid A portions) allowing binding of OAg chains to both immobilized Ab52 and soluble MAbs. As shown in Figure 7, FB11 has the highest avidity, with a KD 72-fold lower than that of Ab52 and 200-fold lower than that of Ab54. Although the avidity of Ab3 could not be determined with this assay, the order of avidities of the other three Ft OAg MAbs correlates directly with their potency of binding in ELISA (Fig. 2) and Western blot (Fig. 3), suggesting that Ab3 has the lowest avidity.
FIG. 7.
The avidity of FB11 is higher than those of Ab52 and Ab54. Biacore analysis of the binding of soluble FB11, Ab52, and Ab54 to immobilized Ft LPS. Ab52, captured on anti-mouse IgG-coated chips, was used to capture Ft LPS, which was then probed with FB11, Ab52, or Ab54. MAb concentration ranges used in the assays were 0 − 10 nM for FB11, 0 − 20 nM for Ab52, and 0 − 100 nM for Ab54.
Discussion
The characterization of four anti-Ft LPS IgG2a MAbs in the current study showed that all bind to OAg, with FB11 binding to a terminal epitope, and Ab3, Ab52, and Ab54 binding to internal epitopes. Binding of FB11 to a terminal epitope of OAg in contrast to the binding of the other three MAbs to internal OAg epitopes is supported by three independent experiments: (1) Western blot analysis on Ft LVS, showing that FB11 binds with equal intensity to both long and short LPS chains whereas binding of the other three MAbs decreases with decreasing LPS chain length; (2) ELISA on plates coated with decreasing concentrations of LPS, showing that the binding of FB11 decreases much quicker than the binding of the other three MAbs with decreasing LPS concentrations; and (3) the linear carbohydrate immunoprecipitation assay showing the inability of FB11, but not of the other three MAbs, to immunoprecipitate Ft OAg despite the superiority of FB11 in agglutinating LVS bacteria.
The linear carbohydrate immunoprecipitation assay was first used by Cisar and colleagues(40) to distinguish between end-binding and internal-binding anti-carbohydrate MAbs. In the original assay, an end-binding anti-dextran myeloma antibody was distinguished from an internal-binding anti-dextran myeloma antibody by the inability of the end-binding antibody to immunoprecipitate a linear α(1 → 6)-linked dextran (which contains a single non-reducing end per molecule) despite the ability of both antibodies to immunoprecipitate a branched dextran (95% α(1 → 6)/5% α(1 → 3), which contains multiple non-reducing ends per molecule). Based on this assay, Cisar and colleagues(40) suggested that the antibody binding to the terminal epitope has a “cavity-like” binding site whereas the antibody binding to a non-terminal (internal) epitope has a “groove-like” binding site. Cavity-like and groove-type sites for anti-carbohydrate antibodies, including antibodies to LPS OAg from Shigella flexneri, Vibrio cholerae, and Brucella abortus, were supported by immunochemical, computer modeling, and x-ray crystallographic studies.(41–50) These showed a maximum of five sugar residues accommodated by cavity-type sites and six to eight sugar residues accommodated by groove-type sites. In the current study, FB11 may have a cavity-type site and the other three anti-Ft OAg MAbs likely have groove-type sites. Consistent with a cavity-type (or pocket-like) site, which buries more of the epitope and is therefore expected to have higher complementarity, and hence affinity and avidity, for Ag than groove-type sites,(40) the avidity of FB11 for OAg is higher than those of Ab3, Ab52, and Ab54, as suggested by ELISA and Western blot analysis and demonstrated by Biacore analysis. The affinity of FB11 (4.01 × 10−7 M), determined by Biacore analysis, is in the upper range of affinities reported for MAbs specific for the OAg of Shigella flexneri (2.7 × 10−5 – 2.9 × 10−6 M) and Vibrio cholerae (4.0 × 10−4 – 4.5 × 10−7 M) or for α(1 → 6) dextran (1.5 × 10−4 – 5.9 × 10−6 M).(40,42,43,50–52)
The affinity of Ab52 could not be determined by Biacore analysis of immobilized MAb binding to soluble Ft OAg because of the multivalent binding of immobilized Ab52 to each soluble OAg molecule. Instead, the equilibrium dissociation constant for this multivalent interaction was calculated to be 4.37 × 10−13 M, which describes an essentially irreversible reaction. This very tight “Velcro-like” interaction of soluble OAg with immobilized Ab52 illustrates the in vivo advantage of B cells expressing (immobilized) internal-binding surface B cell receptors at holding onto LPS and becoming activated. The prevalence of internal-binding anti-Ft LPS antibodies is therefore not surprising: although FB11 was reported to have been obtained from BALB/c mice immunized with LVS or with Ft LPS in complete Freund's adjuvant, using “different immunization schemes,”(33) no other anti-Ft LPS MAb, obtained by us or described by others, shows binding to the short chains of the LPS ladder in Western blot analyses.(31,53–56) This suggests that the repeating internal epitopes of Ft-LPS OAg are much more immunogenic than the OAg ends, even though, evidently, BALB/c mice have the ability to produce FB11-like antibodies.
Thus, the current findings demonstrate that antibodies to non-overlapping epitopes of Ft OAg can be generated and suggest that antibodies targeting terminal epitopes of Ft OAg are more difficult to induce but may have higher avidities than those targeting internal OAg epitopes. End-binding antibodies may therefore be beneficial in defense against Ft. Because end-binding anti-Ft LPS antibodies are distinguished from internal-binding anti-LPS antibodies by their ability to bind to short LPS chains, our results support the use of short-chain LPS or short-chain OAg as a tularemia vaccine component.
Acknowledgments
This work was supported by grant U19 AI 56543 and contract HHSN272200900054C from the National Institutes of Health.
Author Disclosure Statement
The authors have no financial conflicts to declare.
References
- 1.McLendon MK. Apicella MA. Allen LA. Francisella tularensis: taxonomy, genetics, and immunopathogenesis of a potential agent of biowarfare. Annu Rev Microbiol. 2006;60:167–185. doi: 10.1146/annurev.micro.60.080805.142126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sjostedt A. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann NY Acad Sci. 2007;1105:1–29. doi: 10.1196/annals.1409.009. [DOI] [PubMed] [Google Scholar]
- 3.Tarnvik A. Chu MC. New approaches to diagnosis and therapy of tularemia. Ann N Y Acad Sci. 2007;1105:378–404. doi: 10.1196/annals.1409.017. [DOI] [PubMed] [Google Scholar]
- 4.Conlan JW. Shen H. Webb A. Perry MB. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine. 2002;20:3465–3471. doi: 10.1016/s0264-410x(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 5.Gunn JS. Ernst RK. The structure and function of Francisella lipopolysaccharide. Ann NY Acad Sci. 2007;1105:202–218. doi: 10.1196/annals.1409.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prior JL. Prior RG. Hitchen PG. Diaper H. Griffin KF. Morris HR. Dell A. Titball RW. Characterization of the O antigen gene cluster and structural analysis of the O antigen of Francisella tularensis subsp. tularensis. J Med Microbiol. 2003;52:845–851. doi: 10.1099/jmm.0.05184-0. [DOI] [PubMed] [Google Scholar]
- 7.Thirumalapura NR. Goad DW. Mort A. Morton RJ. Clarke J. Malayer J. Structural analysis of the O-antigen of Francisella tularensis subspecies tularensis strain OSU 10. J Med Microbiol. 2005;54:693–695. doi: 10.1099/jmm.0.45931-0. [DOI] [PubMed] [Google Scholar]
- 8.Vinogradov EV. Shashkov AS. Knirel YA. Kochetkov NK. Tochtamysheva NV. Averin SF. Goncharova OV. Khlebnikov VS. Structure of the O-antigen of Francisella tularensis strain 15. Carbohydr Res. 1991;214:289–297. doi: 10.1016/0008-6215(91)80036-m. [DOI] [PubMed] [Google Scholar]
- 9.Griffin KF. Oyston PC. Titball RW. Francisella tularensis vaccines. FEMS Immunol Med Microbiol. 2007;49:315–323. doi: 10.1111/j.1574-695X.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- 10.Fortier AH. Slayter MV. Ziemba R. Meltzer MS. Nacy CA. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991;59:2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennis DT. Inglesby TV. Henderson DA. Bartlett JG. Ascher MS. Eitzen E. Fine AD. Friedlander AM. Hauer J. Layton M. Lillibridge SR. McDade JE. Osterholm MT. O'Toole T. Parker G. Perl TM. Russell PK. Tonat K. Tularemia as a biological weapon: medical and public health management. Jama. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 12.Elkins KL. Cowley SC. Bosio CM. Innate and adaptive immunity to Francisella. Ann NY Acad Sci. 2007;1105:284–324. doi: 10.1196/annals.1409.014. [DOI] [PubMed] [Google Scholar]
- 13.Elkins KL. Rhinehart-Jones TR. Culkin SJ. Yee D. Winegar RK. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect Immun. 1996;64:3288–3293. doi: 10.1128/iai.64.8.3288-3293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu TH. Hutt JA. Garrison KA. Berliba LS. Zhou Y. Lyons CR. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun. 2005;73:2644–2654. doi: 10.1128/IAI.73.5.2644-2654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leiby DA. Fortier AH. Crawford RM. Schreiber RD. Nacy CA. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992;60:84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenmark S. Sunnemark D. Bucht A. Sjostedt A. Rapid local expression of interleukin-12, tumor necrosis factor alpha, and gamma interferon after cutaneous Francisella tularensis infection in tularemia-immune mice. Infect Immun. 1999;67:1789–1797. doi: 10.1128/iai.67.4.1789-1797.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkins KL. Bosio CM. Rhinehart-Jones TR. Importance of B cells, but not specific antibodies, in primary and secondary protective immunity to the intracellular bacterium Francisella tularensis live vaccine strain. Infect Immun. 1999;67:6002–6007. doi: 10.1128/iai.67.11.6002-6007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drabick JJ. Narayanan RB. Williams JC. Leduc JW. Nacy CA. Passive protection of mice against lethal Francisella tularensis (live tularemia vaccine strain) infection by the sera of human recipients of the live tularemia vaccine. Am J Med Sci. 1994;308:83–87. doi: 10.1097/00000441-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Foshay L. Tularemia: a summary of certain aspects of disease including methods for early diagnosis and the results of serum treatment in 600 patients. Medicine. 1940;19:1–83. [Google Scholar]
- 20.Fulop M. Mastroeni P. Green M. Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001;19:4465–4472. doi: 10.1016/s0264-410x(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 21.Kirimanjeswara GS. Golden JM. Bakshi CS. Metzger DW. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol. 2007;179:532–539. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]
- 22.Rhinehart-Jones TR. Fortier AH. Elkins KL. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect Immun. 1994;62:3129–3137. doi: 10.1128/iai.62.8.3129-3137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenmark S. Lindgren H. Tarnvik A. Sjostedt A. Specific antibodies contribute to the host protection against strains of Francisella tularensis subspecies holarctica. Microb Pathog. 2003;35:73–80. doi: 10.1016/s0882-4010(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 24.Sebastian S. Dillon ST. Lynch JG. Blalock LT. Balon E. Lee KT. Comstock LE. Conlan JW. Rubin EJ. Tzianabos AO. Kasper DL. A defined O-antigen polysaccharide mutant of Francisella tularensis live vaccine strain has attenuated virulence while retaining its protective capacity. Infect Immun. 2007;75:2591–2602. doi: 10.1128/IAI.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirimanjeswara GS. Olmos S. Bakshi CS. Metzger DW. Humoral and cell-mediated immunity to the intracellular pathogen Francisella tularensis. Immunol Rev. 2008;225:244–255. doi: 10.1111/j.1600-065X.2008.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klimpel GR. Eaves-Pyles T. Moen ST. Taormina J. Peterson JW. Chopra AK. Niesel DW. Carness P. Haithcoat JL. Kirtley M. Nasr AB. Levofloxacin rescues mice from lethal intra-nasal infections with virulent Francisella tularensis and induces immunity and production of protective antibody. Vaccine. 2008;26:6474–6482. doi: 10.1016/j.vaccine.2008.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eyles JE. Unal B. Hartley MG. Newstead SL. Flick-Smith H. Prior JL. Oyston PC. Randall A. Mu Y. Hirst S. Molina DM. Davies DH. Milne T. Griffin KF. Baldi P. Titball RW. Felgner PL. Immunodominant Francisella tularensis antigens identified using proteome microarray. Proteomics. 2007;7:2172–2183. doi: 10.1002/pmic.200600985. [DOI] [PubMed] [Google Scholar]
- 28.Murphy K. Travers P. Walport M. Janeway C. Janeway's Immunobiology. 7th. Garland Science; New York: 2008. [Google Scholar]
- 29.Nimmerjahn F. Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 30.Ravetch JV. Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 31.Lu Z. Roche MI. Hui JH. Unal B. Felgner PL. Gulati S. Madico G. Sharon J. Generation and characterization of hybridoma antibodies for immunotherapy of tularemia. Immunol Lett. 2007;112:92–103. doi: 10.1016/j.imlet.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J. Ryder C. Mandal M. Ahmed F. Azadi P. Snyder DS. Pechous RD. Zahrt T. Inzana TJ. Attenuation and protective efficacy of an O-antigen-deficient mutant of Francisella tularensis LVS. Microbiology. 2007;153:3141–3153. doi: 10.1099/mic.0.2007/006460-0. [DOI] [PubMed] [Google Scholar]
- 33.Khlebnikov VS. Golovlev IR. Tokhtamysheva NV. Averin SF. Kulevatskii DP. Grechko GK. Averina AA. Vetchinin SS. The determination of the antigenic determinant of protective monoclonal antibodies specific to the Francisella tularensis lipopolysaccharide. Zh Mikrobiol Epidemiol Immunobiol. 1993:83–88. [PubMed] [Google Scholar]
- 34.Herlyn DM. Steplewski Z. Herlyn MF. Koprowski H. Inhibition of growth of colorectal carcinoma in nude mice by monoclonal antibody. Cancer Res. 1980;40:717–721. [PubMed] [Google Scholar]
- 35.Schlimok G. Gottlinger H. Funke I. Swierkot S. Hauser H. Riethmuller G. In vivo and in vitro labelling of epithelial tumor cells with anti 17–1A monoclonal antibodies in bone marrow of cancer patients. Hybridoma. 1986;5(Suppl 1):S163–170. [PubMed] [Google Scholar]
- 36.Sharon J. Sompuram SR. Yang CY. Williams BR. Sarantopoulos S. Construction of polyclonal antibody libraries using phage display. Methods Mol Biol. 2002;178:101–112. doi: 10.1385/1-59259-240-6:101. [DOI] [PubMed] [Google Scholar]
- 37.Porsch-Ozcurumez M. Kischel N. Priebe H. Splettstosser W. Finke EJ. Grunow R. Comparison of enzyme-linked immunosorbent assay, Western blotting, microagglutination, indirect immunofluorescence assay, and flow cytometry for serological diagnosis of tularemia. Clin Diagn Lab Immunol. 2004;11:1008–1015. doi: 10.1128/CDLI.11.6.1008-1015.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palva ET. Makela PH. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107:137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- 39.Chalabaev S. Kim TH. Ross R. Derian A. Kasper DL. 3-deoxy-D-manno-octulosonic acid (Kdo) hydrolase identified in Francisella tularensis, Helicobacter pylori, and Legionella pneumophila. J Biol Chem. 2010;285:34330–34336. doi: 10.1074/jbc.M110.166314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cisar J. Kabat EA. Dorner MM. Liao J. Binding properties of immunoglobulin combining sites specific for terminal or nonterminal antigenic determinants in dextran. J Exp Med. 1975;142:435–459. doi: 10.1084/jem.142.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziegler T. Kovac P. Glaudemans CP. A synthetic heptasaccharide reveals the capability of a monoclonal antibody to read internal epitopes of a polysaccharide antigen. Carbohydr Res. 1990;203:253–263. doi: 10.1016/0008-6215(90)80022-u. [DOI] [PubMed] [Google Scholar]
- 42.Vyas NK. Vyas MN. Chervenak MC. Johnson MA. Pinto BM. Bundle DR. Quiocho FA. Molecular recognition of oligosaccharide epitopes by a monoclonal Fab specific for Shigella flexneri Y lipopolysaccharide: X-ray structures and thermodynamics. Biochemistry. 2002;41:13575–13586. doi: 10.1021/bi0261387. [DOI] [PubMed] [Google Scholar]
- 43.Villeneuve S. Souchon H. Riottot MM. Mazie JC. Lei P. Glaudemans CP. Kovac P. Fournier JM. Alzari PM. Crystal structure of an anti-carbohydrate antibody directed against Vibrio cholerae O1 in complex with antigen: molecular basis for serotype specificity. Proc Natl Acad Sci USA. 2000;97:433–8438. doi: 10.1073/pnas.060022997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharon J. Kabat EA. Morrison SL. Immunochemical characterization of binding sites of hybridoma antibodies specific for alpha (1 leads to 6) linked dextran. Mol Immunol. 1982;19:375–388. doi: 10.1016/0161-5890(82)90203-6. [DOI] [PubMed] [Google Scholar]
- 45.Rose DR. Przybylska M. To RJ. Kayden CS. Oomen RP. Vorberg E. Young NM. Bundle DR. Crystal structure to 2.45 A resolution of a monoclonal Fab specific for the Brucella A cell wall polysaccharide antigen. Protein Sci. 1993;2:1106–1113. doi: 10.1002/pro.5560020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Padlan EA. Kabat EA. Model-building study of the combining sites of two antibodies to alpha (1–6)dextran. Proc Natl Acad Sci USA. 1988;85:6885–6889. doi: 10.1073/pnas.85.18.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oomen RP. Young NM. Bundle DR. Molecular modeling of antibody-antigen complexes between the Brucella abortus O-chain polysaccharide and a specific monoclonal antibody. Protein Eng. 1991;4:427–433. doi: 10.1093/protein/4.4.427. [DOI] [PubMed] [Google Scholar]
- 48.Nashed EM. Perdomo GR. Padlan EA. Kovac P. Matsuda T. Kabat EA. Glaudemans CP. Binding characteristics of IgA 16.4.12E, a monoclonal antibody with specificity for the nonreducing terminal epitope of alpha-(1–6)-dextrans. Comparisons between IgA hybridoma 16.4.12E and myeloma W3129. J Biol Chem. 1990;265:20699–20707. [PubMed] [Google Scholar]
- 49.Glaudemans CP. Kovac P. Rao AS. The subsites of monoclonal anti-dextran IgA W3129. Carbohydr Res. 1989;190:267–277. doi: 10.1016/0008-6215(89)84130-8. [DOI] [PubMed] [Google Scholar]
- 50.Carlin NI. Bundle DR. Lindberg AA. Characterization of five Shigella flexneri variant Y-specific monoclonal antibodies using defined saccharides and glycoconjugate antigens. J Immunol. 1987;138:4419–4427. [PubMed] [Google Scholar]
- 51.Sharon J. Kabat EA. Morrison SL. Association constants of hybridoma antibodies specific for alpha (1 leads to 6) linked dextran determined by affinity electrophoresis. Mol Immunol. 1982;19:389–397. doi: 10.1016/0161-5890(82)90204-8. [DOI] [PubMed] [Google Scholar]
- 52.Liao X. Poirot E. Chang AH. Zhang X. Zhang J. Nato F. Fournier JM. Kovac P. Glaudemans CP. The binding of synthetic analogs of the upstream, terminal residue of the O-polysaccharides (O-PS) of Vibrio cholerae O:1 serotypes Ogawa and Inaba to two murine monoclonal antibodies (MAbs) specific for the Ogawa lipopolysaccharide (LPS) Carbohydr Res. 2002;337:2437–2442. doi: 10.1016/s0008-6215(02)00278-1. [DOI] [PubMed] [Google Scholar]
- 53.Fulop MJ. Webber T. Manchee RJ. Kelly DC. Production and characterization of monoclonal antibodies directed against the lipopolysaccharide of Francisella tularensis. J Clin Microbiol. 1991;29:1407–1412. doi: 10.1128/jcm.29.7.1407-1412.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gubbins MJ. Berry JD. Schmidt L. Cabral T. Kabani A. Tsang RS. Production and characterization of a monoclonal antibody to Francisella tularensis lipopolysaccharide. Hybridoma. 2007;26:98–103. doi: 10.1089/hyb.2006.049. [DOI] [PubMed] [Google Scholar]
- 55.Hotta A. Uda A. Fujita O. Tanabayashi K. Yamada A. Preparation of monoclonal antibodies for detection and identification of Francisella tularensis. Clin Vaccine Immunol. 2007;14:81–84. doi: 10.1128/CVI.00057-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narayanan RB. Drabick JJ. Williams JC. Fortier AH. Meltzer MS. Sadoff JC. Bolt CR. Nacy CA. Immunotherapy of tularemia: characterization of a monoclonal antibody reactive with Francisella tularensis. J Leukoc Biol. 1993;53:112–116. doi: 10.1002/jlb.53.1.112. [DOI] [PubMed] [Google Scholar]