Summary
The PR domain containing 1a, with ZNF domain factor, gene (prdm1a) plays an integral role in the development of a number of different cell types during vertebrate embryogenesis, including neural crest cells, Rohon-Beard (RB) sensory neurons and the cranial neural crest-derived craniofacial skeletal elements. To better understand how Prdm1a regulates the development of various cell types in zebrafish, we performed a microarray analysis comparing wild type and prdm1a mutant embryos and identified a number of genes with altered expression in the absence of prdm1a. Rescue analysis determined that two of these, sox10 and islet1, lie downstream of Prdm1a in the development of neural crest cells and Rohon-Beard neurons, respectively. In addition, we identified a number of other novel downstream targets of Prdm1a that may be important for the development of diverse tissues during zebrafish embryogenesis.
Keywords: zebrafish, cell fate, gene regulatory network, neural plate border, transcription factor
Introduction
In vertebrates, neural crest cells are a transient embryonic population that derive from the border between the neural plate and the non-neural ectoderm. They subsequently migrate throughout the embryo to form multiple derivates, including neurons and glia of the peripheral nervous system, melanocytes, and cartilage and bone of the face (For review, see (Knecht and Bronner-Fraser, 2002; Le Douarin, 1982). Neural crest cells form at the region of the neural plate border, and it has been demonstrated that interactions between the neural and non-neural ectoderm are required for cell fate acquisition (Mancilla and Mayor, 1996; Mayor et al., 1995; Selleck and Bronner-Fraser, 1995). Once specified, neural crest cells express foxd3, snail2, sox10, and ap-2alpha, and then express sox9, sox10 and crestin as they migrate to their final destination (Meulemans and Bronner-Fraser, 2004). In zebrafish and Xenopus, Rohon-Beard (RB) sensory neurons are also born within the border domain and require a similar inductive mechanism as the neural crest, but do not migrate; RB neurons remain in the dorsal spinal cord and function as proprioceptive sensory neurons which mediate the mechanosensory touch response (Lamborghini, 1980) (Rossi et al., 2008). RB neurons express several genes required for development of all primary neurons including huC, neurog1, dlx3b/4b and neuroD and islet1, and these genes are also required for RB neurons (Rossi et al., 2009).
The molecular mechanisms by which neural crest cells and RB sensory neurons are specified and differentiate remain unclear, but several genes that are required for these processes have been identified. Both cell fates require BMP signaling for their induction, where an intermediate level of BMP signaling is required at the neural plate border (Neave B, 1997; Nguyen et al., 1998; Nguyen et al., 2000). Notch signaling is also important for the segregation of neural crest cells and RB sensory neurons. These cell fates form within the same equivalence domain at the neural plate border and RB neuron cell fate is promoted at the expense of neural crest cell fates when Notch signaling is defective. This was demonstrated in several zebrafish mutations, including mind bomb and deltaA (Cornell and Eisen, 2000; Itoh et al., 2003; Jiang et al., 1996) where Notch signaling is reduced. The gene regulatory network downstream of these primary inducers is currently being assembled, and includes many transcription factors required in specific lineages. For neural crest cells, sox10, a member of the Sry-related transcription factor family, plays an important role in neural crest development. Mutations in both mouse and zebrafish have defects in pigmentation and peripheral nervous system derivatives (Britsch et al., 2001; Carney et al., 2006; Dutton et al., 2001; Herbarth et al., 1998). islet1 is a LIM homeodomain transcription factor that plays a role in the specification and determination of a specific motorneuron subtype (Hutchinson and Eisen, 2006) (Pfaff et al., 1996). Although islet1 is expressed in RB sensory neurons, not much is known about the role of this protein in RB development.
Previous studies have identified the transcription factor, prdm1a, in the development of both neural crest and RB neurons. Zebrafish embryos with a mutation in prdm1a, known as narrowmindedm805 mutants, fail to develop RBs or display an escape response when touched on the trunk (Artinger et al., 1999; Hernandez-Lagunas et al., 2005). In addition, neural crest cells and their derivatives are reduced. The predicted Prdm1a protein in narrowminded mutants contains no DNA binding domain, thus preventing the ability of the protein to act as a transcription factor. prdm1a has been implicated in the development of several other cell types as well, including cells in craniofacial cartilage, slow twitch muscle cells, germ cells and immune cells (For review see (John and Garrett-Sinha, 2009) (Bikoff et al., 2009). In these systems, Prdm1a acts as a canonical transcriptional repressor to regulate cell fate (Ohinata et al., 2005). A conditional knockout of Blimp1 in the mouse, which is a homolog of prdm1a in zebrafish, has defects in posterior forelimb, secondary heart field, sensory vibrissae and, importantly, in caudal pharyngeal arches in which neural crest cells contribute to the head mesenchyme (Robertson, et al 2007). In zebrafish, Prdm1a functions at several points in embryogenesis, including: during gastrulation, formation of head structures, and fin development (Mercader et al., 2006; Wilm and Solnica-Krezel, 2005), and as a Hedgehog regulated switch between slow twitch and fast twitch muscle development (Baxendale et al., 2004; Elworthy et al., 2008; von Hofsten et al., 2008). The expression of prdm1a begins at midgastrulation at the neural plate border and continues to be expressed until around the 6 somite stage. By 24 hours post fertilization (hpf), prdm1a is expressed in a large domain covering the posterior pharyngeal arches, which give rise to the posterior viscerocranium (Hernandez-Lagunas et al., 2005) (Wilm and Solnica-Krezel, 2005) (Birkholz et al., 2009). While it is clear that prdm1a is required for the development of RB neurons and neural crest cells, the genes that act downstream of prdm1a are still unknown.
In order to begin to understand the genetic hierarchy of Prdm1a in neural crest, RB neuron and pharyngeal arch development, we determined the differential gene expression profiles between wild type and prdm1a mutant embryos focusing on 25 hpf because it is a key time point in the development of both neural crest cells and RB neurons. Using microarray analysis, we have identified potential downstream effectors of Prdm1a in the development of neural crest and RB neuron development. Further, in rescue experiments, we find that sox10 is a primary effector of prdm1a in the neural crest, while islet1 lies downstream of Prdm1a in the development of RB neurons.
Results
Placing prdm1a in the neural crest and RB sensory neuron gene regulatory network
To determine the genetic hierarchy of Prdm1a in the neural crest (NC), RB and pharyngeal arch domain, we performed microarray analysis comparing gene expression in whole wildtype and prdm1a mutant zebrafish embryos at 25 hpf. Analysis revealed a large number of differentially expressed genes, including several genes specifically down- or up-regulated in the NC, RB and pharyngeal arch domains. Based on its role as a transcriptional repressor, we expected that loss of Prdm1a would result in upregulated genes. Because we observe downregulated genes in the microarray, it suggests that Prdm1 may activate genes or that prdm1a represses genes that encode repressors. We confirmed the results of the microarray analysis by whole mount in situ hybridization. Here, we report our findings for a small number of genes expressed within the pharyngeal arches, RB neurons or in the NC (Table 1). We found in total 796 genes that are significantly (p<0.05) upregulated and 1197 genes that are significantly downregulated in prdm1a mutants at 25 hpf.
Table 1.
Quantification of in situ hybridization results
| Gene ID | Upregulated or downregulated |
Fold change |
Number of embryos affected |
Total number of embryos |
% affected* |
|---|---|---|---|---|---|
| agr2 | upregulated | 5.16 | 17 | 64 | 26.6% |
| col9a2 | upregulated | 1.53 | 13 | 55 | 23.6% |
| crestin | downregulated | 2.85 | 15 | 68 | 22.1% |
| cxcr4a | downregulated | 1.73 | 18 | 81 | 22.2% |
| dlx2a | downregulated | 1.5 | 20 | 86 | 23.3% |
| hey1 | downregulated | 2.72 | 16 | 66 | 24.2% |
| inta5 | downregulated | 1.73 | 15 | 66 | 22.7% |
| isl1 | downregulated | 1.23 | 17 | 70 | 24.3% |
| isl2a | downregulated | 1.46 | 18 | 77 | 23.4% |
| isl2b | downregulated | 1.23 | 16 | 64 | 25.0% |
| rab32 | downregulated | 1.5 | 17 | 71 | 23.9% |
| runx3 | downregulated | 1.6 | 20 | 78 | 25.6% |
| sdf1a | downregulated | 1.3 | 16 | 64 | 25.0% |
| sox10 | downregulated | 1.6 | 16 | 68 | 23.5% |
Mating between two heterozygote narrowminded fish will result in approximately 25% embryos displaying a mutant phenotype. The percent affected describes the observed number of mutant embryo in which we observe either up or down regulated expression which is close to the expected number and between 22–27%.
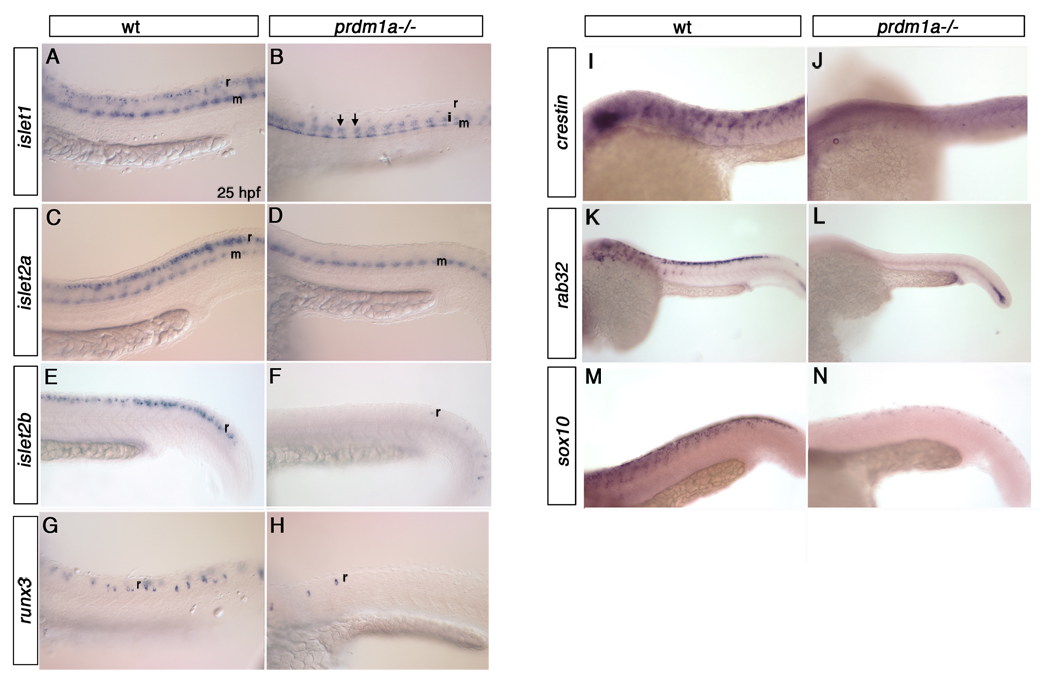
Among the genes we identified as downregulated in prdm1a mutants are several known regulators of trunk neural crest cells and RB neurons. islet1 is normally expressed in RB neurons, motor neurons and a small subset of P2 interneurons (Inoue et al., 1994). In prdm1a mutant embryos, islet1 expression was lost specifically in the RB neuron domain, but its expression increased in the interneuron domain (Figure 1 A, B; arrows indicate interneurons). Islet2a and islet2b, both expressed in RB neurons of wild type embryos, exhibited decreased expression within the RB domain of prdm1a mutant embryos (Figure 1 C–F) (Appel et al., 1995) (Hutchinson and Eisen, 2006). Similarly, expression of runx3, normally expressed in a subset of RB neurons (Horsfield et al., 2007; Park and Saint-Jeannet, 2010) is severely reduced in prdm1a mutant embryos (Figure 1 G , H). The expression of several neural crest markers, including crestin (Luo et al., 2001) and rab32, a member of the ras oncogene family, was also reduced in prdm1a mutant embryos (Figure 1 I- L). Similarly, expression of the SRY-box containing gene 10 (sox10), normally strong in trunk and nonectomesenchymal cranial neural crest cells (Dutton et al., 2001) (Blentic et al., 2008), was reduced dramatically in trunk neural crest cells of prdm1a mutant embryos (Figure 1 M, N).
Figure 1. prdm1a mutant embryos exhibit reduced expression of neural crest and RB neuron markers.
Lateral views of wildtype and prdm1a mutant embryos at 25hpf. islet1 is expressed in RB neurons (r) and motor neurons (m) of wild type embryos at 25hpf (A). prdm1a mutants lose expression of islet1 within the RB neuron domain but have ectopic islet1 expression in the interneuron (i) domain (arrows, B). islet2a and islet2b are both expressed in the RB neurons of wild type embryos (C, E respectively), but are reduced in the RB neuron domain of prdm1a mutant embryos (D,F). runx3 is expressed in a subset of RB neurons in wild type embryos at 25hpf (G) but is reduced in prdm1a mutant embryos (H). crestin is lost in the trunk neural crest cells of prdm1a mutant embryos (J) as compared to wild type controls (I). rab32 and sox10 are expressed within the trunk neural crest cells at 25hpf (K, M respectively) but are lost in the trunk neural crest cells of prdm1a mutant embryos (L, N respectively). i, interneurons; m, motor neurons; r, Rohon-Beard sensory neurons.
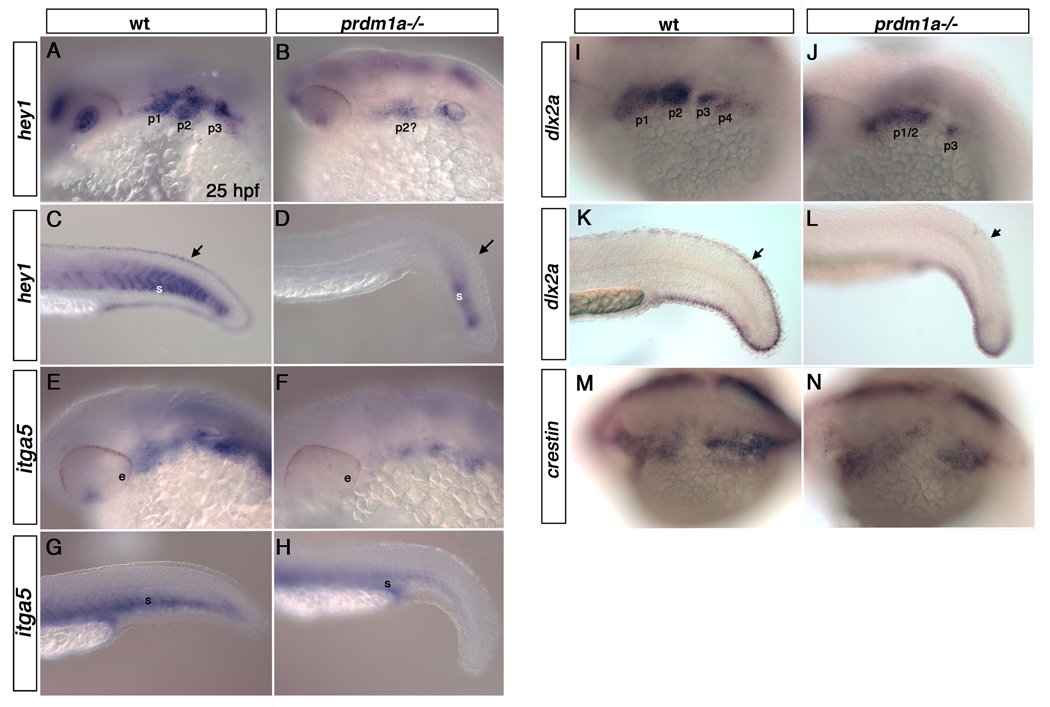
Since prdm1a also functions in posterior pharyngeal arch development, we analyzed expression of genes in the craniofacial region that were downregulated in our microarray. At 25 hpf, hairy/enhancer-of-split related with YRPW motif 1(hey1) is expressed in the posterior somites (Winkler et al., 2003), pharyngeal arches, retina and fin mesenchyme. In prdm1a mutants, expression of hey1 was markedly reduced in each of these tissues (Figure 2 A–D). Integrin alpha 5 (itga5) is expressed throughout the pharyngeal arches (Crump et al., 2004) and caudal somites in wild type 25hpf embryos, but was reduced in both the arches and caudal somites of prdm1a mutant embryos (Figure 2 E–H). distal-less homeobox gene 2a (dlx2a) is expressed throughout the pharyngeal arches (Kimmel and Eberhart, 2008) (Sperber and Dawid, 2008) and fin mesenchyme. In prdm1a mutant embryos at 25 hpf, dlx2a was reduced in the anterior arches and absent in the posterior pharyngeal arches and dorsal fin mesenchyme (Figure 2 I–L) (Birkholz et al., 2009). Although reduced in trunk neural crest cells, crestin remained expressed throughout cranial neural crest cells in prdm1a mutant embryos (Figure 2 M–N). Expression of the chemokine Sdf1 and its receptor Cxcr4 were unaffected in the anterior arch in prdm1a mutant embryos (not shown (Olesnicky Killian et al., 2009); however, their expression in the lateral line, fin mesenchyme and caudal somites was reduced (Figure 3 A–D).
Figure 2. prdm1a mutant embryos exhibit reduced expression of multiple genes within the pharyngeal arches.
Lateral views of wildtype and prdm1a mutant embryos at 25hpf embryos. hey1 is expressed throughout the pharyngeal arches (A) and within the fin mesenchyme and caudal somites (C) of WT embryos at 25hpf, but is reduced in the pharyngeal arches (B), somites and fin mesenchyme (arrows) of prdm1a mutant embryos (D). inta5 is expressed throughout the pharyngeal arches (E) and ventral region of the somites (G) of wild type embryos but is dramatically reduced within the pharyngeal arches (F) and modestly reduced within the somites (H) of prdm1a mutant embryos. dlx2a marks the cranial neural crest cells of the pharyngeal arches (I) and the fin mesenchyme (K) in 25 hpf wild type embryos. prdm1a mutants have reduced expression of dlx2a in the anterior arches and loss of dlx2a in the posterior pharyngeal arches (J). dlx2a is also reduced in the dorsal fin mesenchyme (arrows) of prdm1a mutant embryos (L). crestin expression remains unchanged in the cranial neural crest cells: wild type (M) and prdm1a mutant embryos (N). e, eye; p1, p2, p3,p4, pharyngeal arches 1–4; s, somite.
Figure 3. Chemokine expression is downregulated in prdm1a mutant embryos.
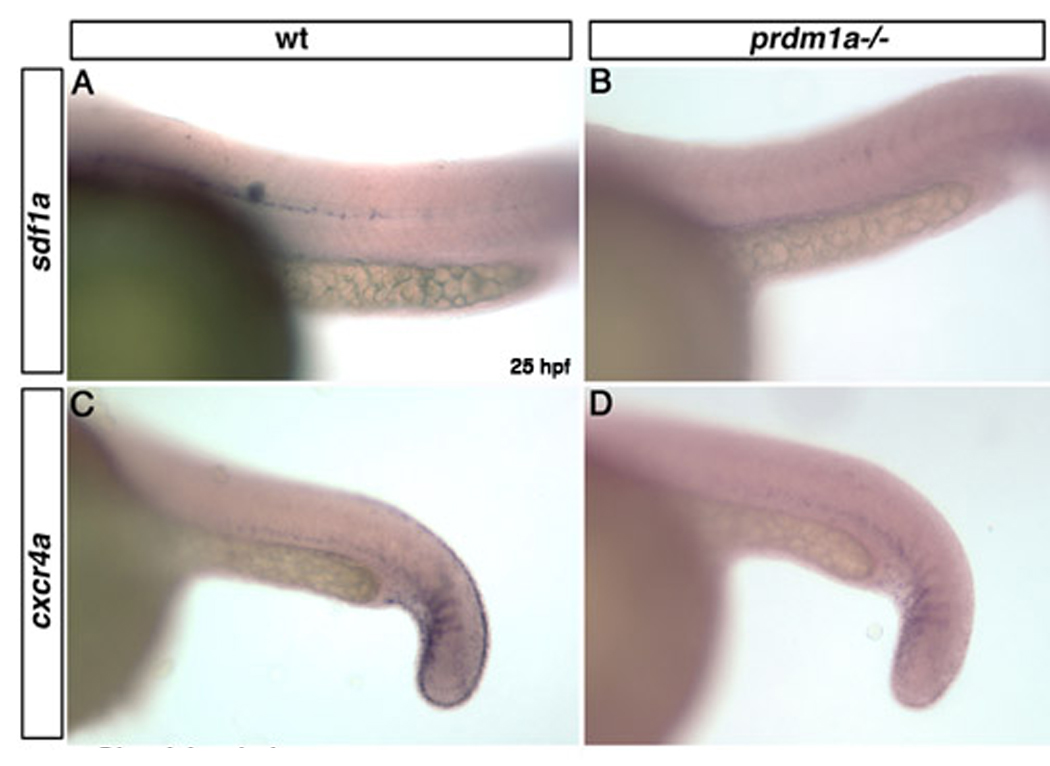
Wild type expression of sdf1a within the pathway of the migrating lateral line (A). sdf1a expression is absent in the lateral line migration pathway in prdm1a mutant embryos (B). cxcr4a is expressed in the caudal somites and fin mesenchyme in wild type embryos at 25 hpf (C) but is lost in the fin mesenchyme and reduced within the caudal somites of prdm1a mutant embryos (D).
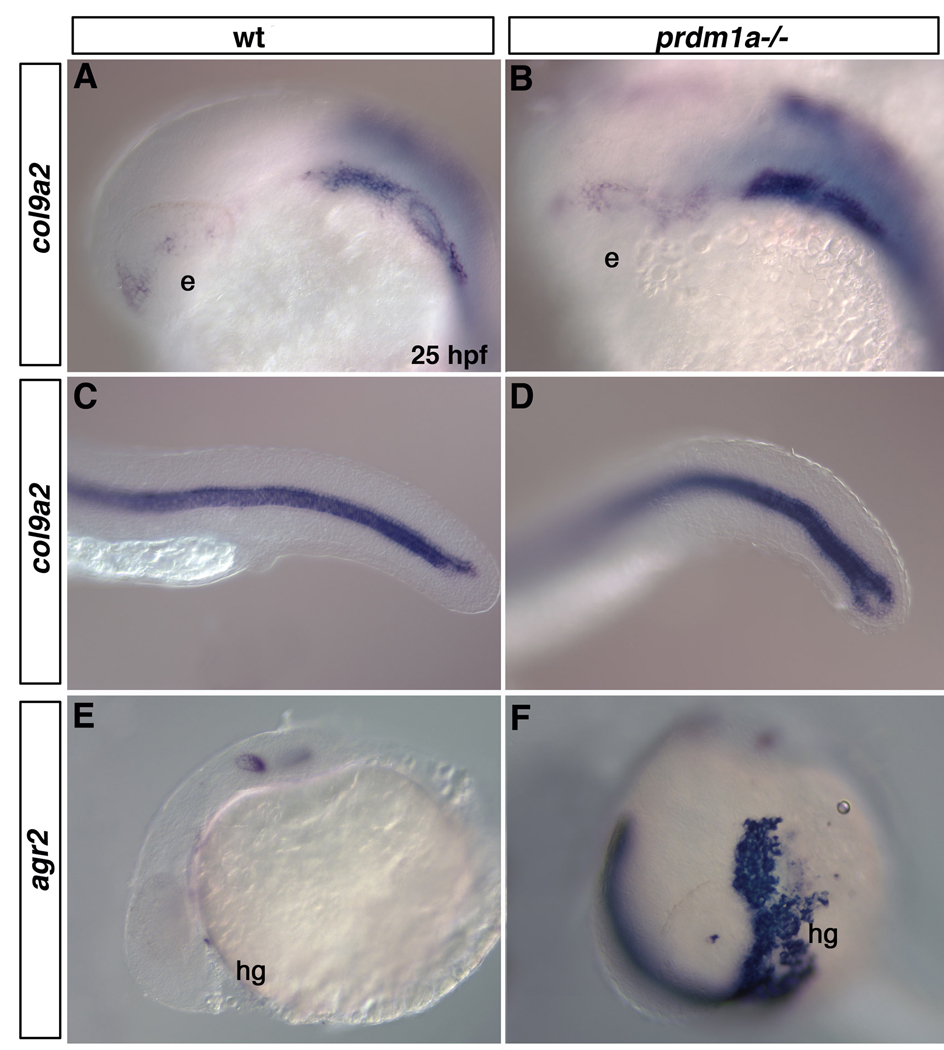
As expected based on Prdm1a’s role as a transcriptional repressor, we observed increased expression of a number of genes in prdm1a mutants. procollagen type IX, alpha 2 (col9a2), a chondrocyte specific marker (de Crombrugghe et al., 2000), is expressed in the pharyngeal arches and notochord of wild type embryos at 25 hpf. In prdm1a mutant embryos at 25hpf, expression of col9a2 is increased within the pharyngeal arches and otic vesicle, with only a modest increase in expression in the caudal notochord (Figure 4 A–D). anterior gradient homolog 2 (agr2) is expressed within the otic vesicle and at low levels within the hatching gland of zebrafish embryos at 25 hpf (Shih et al., 2007). We found dramatic increases in agr2 expression specifically within the hatching gland of prdm1a mutant embryos, while expression within the otic vesicle was unaffected (Figure 4 E,F). In addition, genes involved in muscle specification were upregulated in prdm1a mutants (Table 1). These results confirm genes previously known to be regulated by Prdm1a and identify new candidate Prdm1a target genes.
Figure 4. col9a2 and agr2 are upregulated in prdm1a mutant embryos.
Lateral views of wildtype and prdm1a mutant embryos at 25hpf embryos. col9a2 is expressed in the posterior pharyngeal arches, posterior to the otic vesicle (A) and within the notochord (C) in wild type embryos. col9a2 is upregulated in the posterior pharyngeal arches, otic vesicle (B) and slightly within the notochord (D) of prdm1a mutant embryos. agr2 is modestly expressed within the hatching gland and otic vesicle of wild type embryos (E), but is dramatically upregulated in the hatching gland of prdm1a mutant embryos (F). e, eye; hg, hatching gland.
Sox10 is a key downstream effector of prdm1a in the neural crest development
sox10 has been shown to be integral to neural crest cell fate specification and subsequent differentiation in the zebrafish embryo. Expression of sox10 commences in premigratory NC and is required for differentiation of nonectomesenchymal cranial and trunk neural crest cells(Blentic et al., 2008; Carney et al., 2006; Dutton et al., 2001). Because sox10 expression is reduced in prdm1a mutant embryos, we tested whether sox10 is a downstream effector of prdm1a by asking whether sox10 can rescue the trunk neural crest cell defects of prdm1a mutant embryos, using crestin expression as a marker for neural crest cells. Injection of sox10 mRNA at the one-cell stage in wild type embryos results in an increase in neural crest cells throughout the trunk of the embryo at 25hpf (Figure 5A–B). Injection of sox10 mRNA into prdm1a mutant embryos rescues crestin-expressing neural crest cells throughout the trunk of the embryo (Figure 5C–D). 26% (or 19 of 72) of prdm1a mutant embryos injected with control RNA exhibited mutant neural crest cell phenotype, comparable to uninjected mutants. However, following injection of sox10, only 9% (16 of 169) displayed mutant neural crest cell phenotype, indicating that sox10 rescued neural crest cells in the absence of prdm1a (Figure 5N). To determine whether NC derivatives are also rescued, we examined pigment cells, which are decreased in prdm1a mutants. Injection of sox10 mRNA rescues neural crest-derived pigment cells in prdm1a mutant embryos, similar to what we observe for neural crest cells (Figure 5 E–J; 7% with 3 of 44 displaying the mutant phenotype). These results strongly suggest that sox10 is a key downstream effector of prdm1a in trunk neural crest cell specification. However, conserved domains within the sox10 enhancer region contained no canonical prdm1a binding sites, previously described in prdm1a targets in mouse and zebrafish muscle (Lord et al., 2009; von Hofsten et al., 2008). This suggests that sox10 may not be a direct transcriptional target of prdm1a. rab32 was also decreased in prdm1a mutants; however, injection of rab32 mRNA did not rescue the neural crest cell phenotype (not shown).
Figure 5. sox10 and islet1 lie downstream of prdm1a in neural crest cell and RB neuron fate specification.
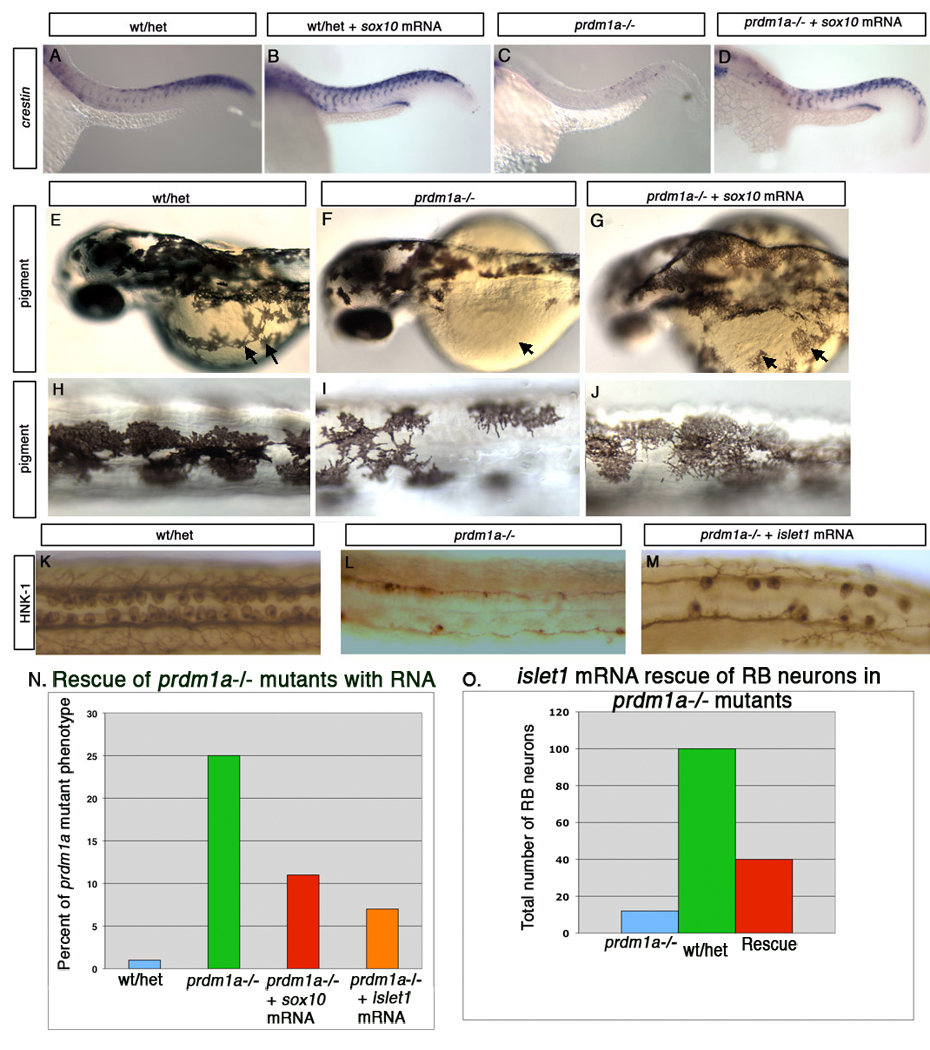
Lateral views of wildtype and prdm1a mutant embryos at 25hpf. Crestin expression in a wild type embryo at 25 hpf (A). sox10 overexpression in a wild type embryo results in increased neural crest cell number as assessed by crestin expression (B). prdm1a mutant embryos have reduced crestin expression at 25hpf (C). sox10 overexpression in prdm1a mutant embryos rescues the neural crest cell defects but not the kinked tail or U-shaped somite phenotypes (D). Pigment is rescued following injection of sox10 mRNA. Low (E) and high (H) magnification of the same embryo showing wildtype pigment pattern (arrows point to pigment on yolk). Low (F) and high (I) magnification of the same prdm1a mutant embryo shows reduced pigment on the dorsal side and especially on the yolk (arrows). (G, J) Injection of sox10 mRNA partially rescues the prdm1a pigment phenotype, restoring pigment cells both on the dorsal side and yolk (arrows). (K) RB neurons stained with HNK-1 antibody within the dorsal trunk of a wild type embryo at 24 hpf. (L) prdm1a mutants lack RB neurons. (M.) islet1 overexpression within prdm1a mutant embryos results in partial rescue of RB neurons. (N,O) Quantification of sox10 and islet1 rescue of RB phenotype of prdm1a mutants. (N) Percentage of prdm1a mutants rescued after injection of either sox10 or islet1 RNA. Uninjected and GFP mRNA control injected prdm1a mutant embryos do not exhibit rescue, while on average, 58% of sox10 RNA injected (red) and 64% of islet1 RNA injected (orange) embryos display significant rescue. (O) Wild type embryos have an average of 100 RB sensory neurons (green) while prdm1a mutant embryos have an average of 12 RB neurons (blue) per embryo. islet1 mRNA injection partially rescues RB neurons, resulting in an average of 40.6 RB sensory neurons per embryo (red).
Islet1 functions downstream of prdm1a in RB sensory neuron development
islet1 is expressed in RB neurons immediately after they appear (Rossi et al., 2009) (Inoue et al., 1994) and depletion of islet1 via Morpholino injection results in a loss of primary motor neurons (Hutchinson and Eisen, 2006). In these morphants, some RB sensory neurons fail to differentiate, suggesting that islet1 plays an important role in RB neuron development (J Eisen and S Hutchinson, personal communication). We therefore asked whether injection of islet1 mRNA can rescue RB neurons in prdm1a mutant embryos. In wildtype embryos, overexpression of islet1 mRNA does not induce more RB neurons (not shown). However, injection of islet1 mRNA into prdm1a mutant embryos at the one-cell stage results in a partial rescue of RB neurons at 25hpf, assessed by antibody staining for the RB neuron marker, HNK-1 (Figure 5 K–M; quantification in Figure 5 O). Conserved domains within the islet1 enhancer contain two canonical prdm1a binding sites (GAAAG), suggesting that islet1 is a direct target of prdm1a. These results provide evidence that islet1 lies downstream and is likely a key effector of prdm1a in RB neuron development. By contrast, injection of islet2 or runx3 mRNAs did not rescue the RB phenotype of prdm1a mutants (not shown).
prdm1a overexpression expands sox10 and islet1 expression
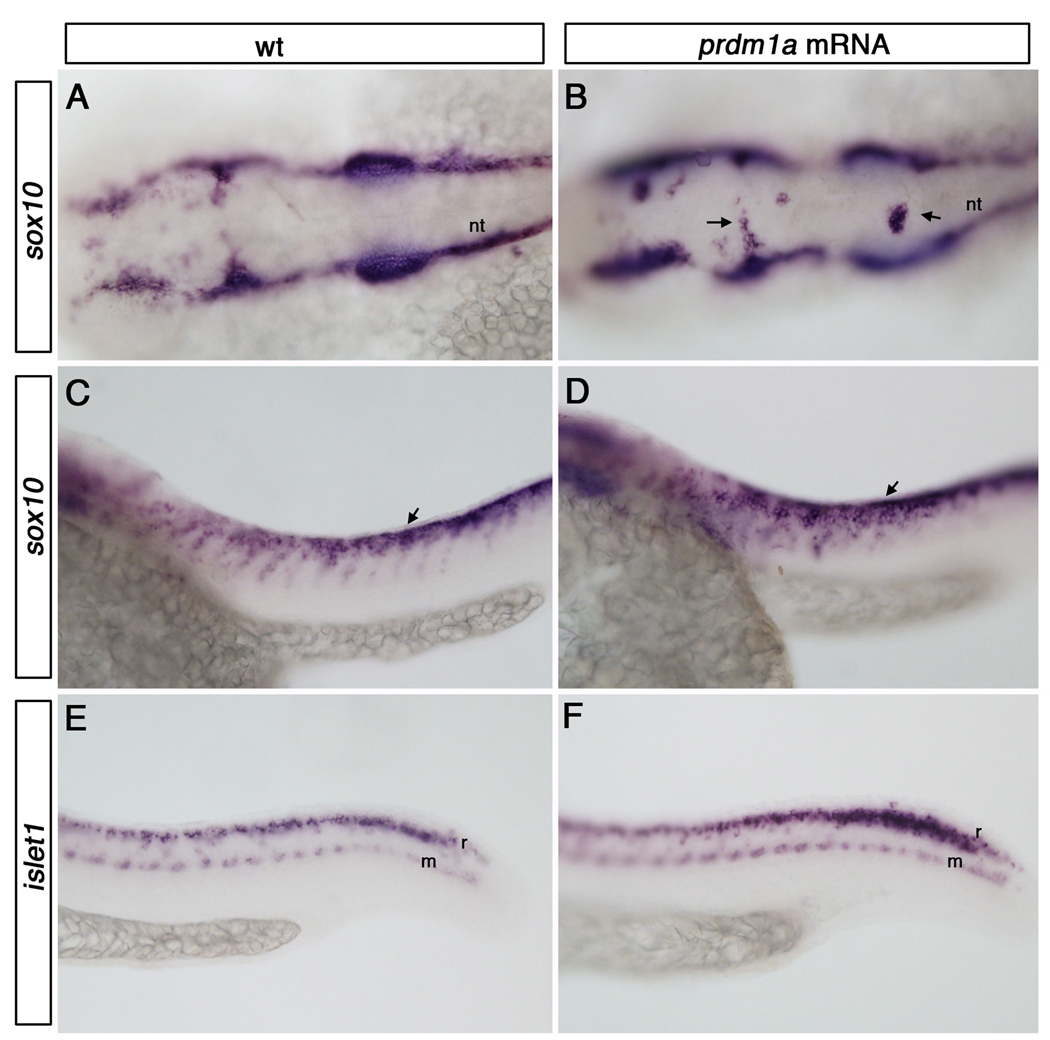
Our rescue experiments provide evidence that sox10 and islet1 lie downstream of Prdm1a in the formation of neural crest cells and RB neurons, respectively. To determine the epistatic relationships, we asked whether overexpressing prdm1a in wild type zebrafish embryos increases expression of islet1 and sox10. Injection of prdm1a mRNA at the one-cell stage results in upregulation of both sox10 and islet1 expression at 25hpf. Prdm1a overexpression results in ectopic cranial neural crest cells along the dorsal midline of the embryo (Figure 6 A,B; arrows). In the trunk, striking upregulation of sox10 expression appears in ectopic neural crest cells, which migrate as a sheet rather than in streams corresponding to each somite (Figure 6 C,D). Prdm1a overexpression also increased islet1 expression specifically within the RB neuron domain, not within motorneurons or interneurons (Figure 6 E,F). These results are consistent with previous reports using crestin and HNK-1 staining to show increases in NC and RB neurons, respectively (Hernandez-Lagunas et al., 2005).
Figure 6. prdm1a overexpression causes upregulation of sox10 and islet1 expression.
Dorsal and lateral views of wildtype and prdm1a mutant embryos at 25hpf embryos. A dorsal view of sox10 expression within the cranial region of a wild type embryo at 25 hpf (A). prdm1a overexpression causes ectopic clusters of cranial neural crest cells within the dorsal midline (B, arrows). Lateral view of wild type sox10 expression in the trunk showing neural crest cells migrating in streams corresponding to each somite (C). prdm1a overexpression causes upregulation of sox10 expression and neural crest cells migrate as a sheet along the trunk instead of in streams (arrow, D). islet1 expression in a wild type embryo at 25 hpf (E). prdm1a overexpression results in an upregulation of islet1 expression specifically within the RB sensory neuron domain (F). nt, neural tube; m, motor neurons; r, Rohon-Beard sensory neurons.
Discussion
Our results demonstrate that Prdm1a functions upstream of islet1 in RB neuron development, while sox10 is the primary effector of prdm1a in neural crest development. Analysis of the prdm1a mutant phenotype in zebrafish reveals a variety of neural crest defects, including reduced peripheral nervous system derivatives and pigment cell number in addition to a complete loss of RB neurons (Artinger et al., 1999; Hernandez-Lagunas et al., 2005). Closer examination of prdm1a mutant embryos shows that cranial neural crest cells are initially reduced in number but at later time points recover to a number comparable to wild type. Nonetheless, the posterior pharyngeal arches fail to execute their normal developmental program, resulting in loss of the ceratobranchial skeletal elements (Birkholz et al., 2009). The results presented here confirm that cranial neural crest cells do reach the pharyngeal arches, as crestin expression in the head is unchanged between wild type and prdm1a mutants at 25hpf. Instead, expression of genes that are important for condensing neural crest and craniofacial skeleton development, such as dlx2a and itga5, and the chondrocyte specific marker col9a2, is misregulated in prdm1a mutant embryos.
In contrast to cranial neural crest cells, the trunk crest cells in prdm1a mutant embryos remain reduced in prdm1a mutant embryos. This supports the idea that neural crest cell populations are differentially specified and maintained along the rostro-caudal axis. Other zebrafish mutants also show differential defects in neural crest specification along this axis. For example, mind bomb mutants have a more severe defect in neural crest development in the trunk then in the head (Itoh et al., 2003; Jiang et al., 1996). Neural crest cells form normally in the cranial region of mind bomb mutant embryos whereas trunk neural crest are completely absent, replaced by an increase in RB sensory and other primary neurons (Cornell and Eisen, 2000, 2002). Other mutations such as foxd3, sox10, also show different affects along the rostro-caudal axis (Dutton et al., 2001; Li and Cornell, 2007; Stewart et al., 2006). sox10 mutant embryos exhibit similar rostro-caudal defects in the non-ectomesenchymal derivatives such as neurons, pigment and glia. However, these mutant embryos show normal development of the craniofacial skeleton, a cranial NC derivative (Kelsh, 2006). There are also examples of mutants that have similar affects along the entire rostrocaudal axis, such as ap-2 alpha, and embryos with a knockdown of ap-2 alpha+gamma via Morpholino injection, where both cranial and trunk neural crest are absent (Knight et al., 2003; Li and Cornell, 2007; O'Brien et al., 2004). As Sox10 is reduced in prdm1a mutants, overexpression of prdm1a causes an increase in sox10 expression, suggesting that the prdm1a regulates sox10 during the development of neural crest cells. Using rescue experiments, we find that sox10 can rescue the prdm1a neural crest phenotype and thus lies downstream and is likely a key effector of Prdm1a in neural crest cell specification.
Consistent with prdm1a playing a role as a NPB specifier gene, RB sensory neurons, which are also derived from the NPB, are lost in prdm1a mutant embryos (Artinger et al., 1999; Hernandez-Lagunas et al., 2005; Roy and Ng, 2004). We find that expression of runx3 and expression of members of the islet gene family including islet1, islet2a and islet2b are lost within the RB domain of prdm1a mutant embryos. Interestingly, prdm1a mutant embryos also show an increase in islet1 expression within the ventral interneuron domain, suggesting that prdm1a might repress the interneuron cell fate and instead promote formation of RB sensory neurons. Rescue experiments suggest that islet1 plays a role downstream of Prdm1a in RB neuron specification, since islet1 expression in prdm1a mutants partially rescues RB neurons.
In conclusion, we have identified genes that play a role downstream of prdm1a in the specification of neural crest cells and RB neurons, within the developing zebrafish embryo. prdm1a is a key element in the gene regulatory networks responsible for both neural crest cells and RB sensory neurons.
Methods
Animals
Zebrafish were maintained according to Westerfield (1993) and staged by hours post fertilization (hpf) and morphology according to Kimmel (1995). The zebrafish prdm1a mutant has been previously described (Artinger et al., 1999) (Hernandez-Lagunas et al., 2005) (Rossi et al., 2009) (Birkholz et al., 2009).
Single embryo genotyping and prdm1a mutant identification
Single embryo genotyping in prdm1a (narrowmindedm805) clutches was performed in the rescue experiments as previously described (Rossi et al., 2009). For microarray analysis and rescue experiments, we also determined mutants based on phenotype. At 25 hpf prdm1a mutant embryos can easily be identified by an obvious kinked tail and U shaped somite phenotype. Normally, a mating between two heterozygote narrowminded fish will result in 25% mutant embryos. We scored embryos as rescued if we observed neural crest, pigment, or RB neurons in more then 7 somite lengths. Table 1 describes the percentage of mutant embryos from such a cross and the number as expected of mutant embryo in which we observe either up or down regulated expression.
Embryo manipulation and analysis
Whole-mount in situ hybridization was adapted from Thisse and Thisse (1998) and Brent and colleagues (2003) (Brent et al., 2003). Immunohistochemistry was performed as described (Ungos et al., 2003) and the following antibodies were used: HNK-1 antibody (Sigma) was used at a 1:1000 dilution. For overexpression, the prdm1a ORF was cloned into the pCS2 vector. RNA was prepared using the mMessage mMachine capped RNA transcription kit (Ambion). 60–100pg of Capped RNA total was injected into 1-cell-stage embryos together with rhodamine dextran for observation of efficiency of injection. (Molecular Probes). For rescue experiments, 25 hpf prdm1a mutant embryos were identified by an obvious kinked tail and U shaped somite phenotypes and/or by genotyping. 100- 200pg of sox10 mRNA and 100–200 pg of islet1 mRNA were injected into 1 cell stage embryos. At least three experiments in separate clutches were done for each experimental condition.
Microarray Analysis
RNA was isolated from whole zebrafish embryos, 3 replicates each for wildtype and prdm1a mutant embryos, using the RNAqueous-Micro Micro Scale RNA Isolation kit (Ambion). Purity of each sample was determined based on the ratio of A260 to A280. The integrity of total RNA samples was examined by Agilent 2100 Bioanalyzer. Total RNA was converted to double-stranded cDNA (ds-cDNA) using the cDNA synthesis kit (Affymetrix). ds-cDNA was purified and recovered using GeneChip sample cleanup module (Affymetrix). Three biological replicates were constructed for wildtype and prdm1a −/− embryos. In vitro transcription was performed to generate biotin-labeled cRNA using an RNA Transcript Labeling Kit (Affymetrix or Enzo, Farmingdale, New York, USA). Biotin-labeled cRNA was purified using GeneChip sample cleanup module (Affymetrix). To ensure optimal hybridization to the oligonucleotide array, the cRNA was fragmented. Hybridization was performed by incubating 200 µL of the sample with Affymetrix Zebrafish GeneChip® arrays (Affymetrix Inc., Santa Clara, California, USA). Arrays were read at a resolution of 2.5 to 3 microns using the GeneChip Scanner 3000 (Affymetrix).
Data Analysis
All available raw gene expression data (probe-level) was taken from Affymetrix CEL files. The perfect-match (PM) data was background corrected, normalized, and summarized using the RMA (robust-multichip average) algorithm as previously described (Bolstad et al., 2003; Irizarry et al., 2003a; Irizarry et al., 2003b).
A Principal Component Analysis (PCA) was performed on the normalized data to identify any outlier samples. No distinct outlier was determined. Prior to performing statistical analysis between the two groups, the normalized dataset was filtered for low variance genes across all samples. Genes with zero statistical variance to a variance p-value of 0.01 across all samples are considered to be “flat”, with no change in gene expression, and were filtered out from the dataset.
An ANOVA was performed on the filtered dataset to determine statistically significant gene regulation between the two experimental groups. The data was log base 2 (log2) transformed prior to running the ANOVA. After the ANOVA, the log2 ratios were converted into a linear scale fold change. p-values were calculated determining the most statistically significant gene changes. The dataset was then sorted by p-values and then fold changes to identify the genes with the most robust up- and down-regulation between the two experimental groups.
Supplementary Material
Acknowledgements
We would like to thank ZIRC (P40 RR012546-NIH-NCRR) for reagents; Bruce Appel, Linda Barlow, and Lee Niswander for helpful comments on the manuscript; Morgan Singleton for extraordinary fish care; We gratefully acknowledge the support of NIH P30 NS04815 zebrafish and imaging core grant, NIH F32 HD056779 to E.C.O and NIH HD050698 and DE017699 to K.B.A.
References
- Appel B, Korzh V, Glasgow E, Thor S, Edlund T, Dawid IB, Eisen JS. Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development. 1995;121:4117–4125. doi: 10.1242/dev.121.12.4117. [DOI] [PubMed] [Google Scholar]
- Artinger KB, Chitnis AB, Mercola M, Driever W. Zebrafish narrowminded suggests a genetic link between formation of neural crest and primary sensory neurons. Development. 1999;126:3969–3979. doi: 10.1242/dev.126.18.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxendale S, Davison C, Muxworthy C, Wolff C, Ingham PW, Roy S. The B-cell maturation factor Blimp-1 specifies vertebrate slow-twitch muscle fiber identity in response to Hedgehog signaling. Nat Genet. 2004;36:88–93. doi: 10.1038/ng1280. [DOI] [PubMed] [Google Scholar]
- Bikoff EK, Morgan MA, Robertson EJ. An expanding job description for Blimp-1/PRDM1. Curr Opin Genet Dev. 2009;19:379–385. doi: 10.1016/j.gde.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Birkholz DA, Killian EC, George KM, Artinger KB. Prdm1a is necessary for posterior pharyngeal arch development in zebrafish. Dev Dyn. 2009;238:2575–2587. doi: 10.1002/dvdy.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blentic A, Tandon P, Payton S, Walshe J, Carney T, Kelsh RN, Mason I, Graham A. The emergence of ectomesenchyme. Dev Dyn. 2008;237:592–601. doi: 10.1002/dvdy.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney TJ, Dutton KA, Greenhill E, Delfino-Machin M, Dufourcq P, Blader P, Kelsh RN. A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development. 2006;133:4619–4630. doi: 10.1242/dev.02668. [DOI] [PubMed] [Google Scholar]
- Cornell RA, Eisen JS. Delta signaling mediates segregation of neural crest and spinal sensory neurons from zebrafish lateral neural plate. Development. 2000;127:2873–2882. doi: 10.1242/dev.127.13.2873. [DOI] [PubMed] [Google Scholar]
- Cornell RA, Eisen JS. Delta/Notch signaling promotes formation of zebrafish neural crest by repressing Neurogenin 1 function. Development. 2002;129:2639–2648. doi: 10.1242/dev.129.11.2639. [DOI] [PubMed] [Google Scholar]
- Crump JG, Maves L, Lawson ND, Weinstein BM, Kimmel CB. An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development. 2004;131:5703–5716. doi: 10.1242/dev.01444. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000;19:389–394. doi: 10.1016/s0945-053x(00)00094-9. [DOI] [PubMed] [Google Scholar]
- Dutton KA, Pauliny A, Lopes SS, Elworthy S, Carney TJ, Rauch J, Geisler R, Haffter P, Kelsh RN. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development. 2001;128:4113–4125. doi: 10.1242/dev.128.21.4113. [DOI] [PubMed] [Google Scholar]
- Elworthy S, Hargrave M, Knight R, Mebus K, Ingham PW. Expression of multiple slow myosin heavy chain genes reveals a diversity of zebrafish slow twitch muscle fibres with differing requirements for Hedgehog and Prdm1 activity. Development. 2008;135:2115–2126. doi: 10.1242/dev.015719. [DOI] [PubMed] [Google Scholar]
- Herbarth B, Pingault V, Bondurand N, Kuhlbrodt K, Hermans-Borgmeyer I, Puliti A, Lemort N, Goossens M, Wegner M. Mutation of the Sry-related Sox10 gene in Dominant megacolon, a mouse model for human Hirschsprung disease. Proc Natl Acad Sci U S A. 1998;95:5161–5165. doi: 10.1073/pnas.95.9.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lagunas L, Choi IF, Kaji T, Simpson P, Hershey C, Zhou Y, Zon L, Mercola M, Artinger KB. Zebrafish narrowminded disrupts the transcription factor prdm1 and is required for neural crest and sensory neuron specification. Dev Biol. 2005;278:347–357. doi: 10.1016/j.ydbio.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsfield JA, Anagnostou SH, Hu JK, Cho KH, Geisler R, Lieschke G, Crosier KE, Crosier PS. Cohesin-dependent regulation of Runx genes. Development. 2007;134:2639–2649. doi: 10.1242/dev.002485. [DOI] [PubMed] [Google Scholar]
- Hutchinson SA, Eisen JS. Islet1 and Islet2 have equivalent abilities to promote motoneuron formation and to specify motoneuron subtype identity. Development. 2006;133:2137–2147. doi: 10.1242/dev.02355. [DOI] [PubMed] [Google Scholar]
- Inoue A, Takahashi M, Hatta K, Hotta Y, Okamoto H. Developmental regulation of islet-1 mRNA expression during neuronal differentiation in embryonic zebrafish. Dev Dyn. 1994;199:1–11. doi: 10.1002/aja.1001990102. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003a;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003b;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, Weissman AM, Lewis J, Chandrasekharappa SC, Chitnis AB. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Jiang YJ, Brand M, Heisenberg CP, Beuchle D, Furutani-Seiki M, Kelsh RN, Warga RM, Granato M, Haffter P, Hammerschmidt M, Kane DA, Mullins MC, Odenthal J, van Eeden FJ, Nusslein-Volhard C. Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio. Development. 1996;123:205–216. doi: 10.1242/dev.123.1.205. [DOI] [PubMed] [Google Scholar]
- John SA, Garrett-Sinha LA. Blimp1: a conserved transcriptional repressor critical for differentiation of many tissues. Exp Cell Res. 2009;315:1077–1084. doi: 10.1016/j.yexcr.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Kelsh RN. Sorting out Sox10 functions in neural crest development. Bioessays. 2006;28:788–798. doi: 10.1002/bies.20445. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Eberhart JK. The midline, oral ectoderm, and the arch-0 problem. Integrative and Comparative Biology. 2008;48:668–680. doi: 10.1093/icb/icn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht AK, Bronner-Fraser M. Induction of the neural crest: a multigene process. Nat Rev Genet. 2002;3:453–461. doi: 10.1038/nrg819. [DOI] [PubMed] [Google Scholar]
- Knight RD, Nair S, Nelson SS, Afshar A, Javidan Y, Geisler R, Rauch GJ, Schilling TF. lockjaw encodes a zebrafish tfap2a required for early neural crest development. Development. 2003;130:5755–5768. doi: 10.1242/dev.00575. [DOI] [PubMed] [Google Scholar]
- Lamborghini JE. Rohon-beard cells and other large neurons in Xenopus embryos originate during gastrulation. J Comp Neurol. 1980;189:323–333. doi: 10.1002/cne.901890208. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM. The neural crest. New York: Cambridge Univ Press; 1982. [Google Scholar]
- Li W, Cornell RA. Redundant activities of Tfap2a and Tfap2c are required for neural crest induction and development of other non-neural ectoderm derivatives in zebrafish embryos. Dev Biol. 2007;304:338–354. doi: 10.1016/j.ydbio.2006.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CA, Savitsky D, Sitcheran R, Calame K, Wright JR, Ting JP, Williams KL. Blimp-1/PRDM1 mediates transcriptional suppression of the NLR gene NLRP12/Monarch-1. J Immunol. 2009;182:2948–2958. doi: 10.4049/jimmunol.0801692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, An M, Arduini BL, Henion PD. Specific pan-neural crest expression of zebrafish Crestin throughout embryonic development. Dev Dyn. 2001;220:169–174. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1097>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Mancilla A, Mayor R. Neural crest formation in Xenopus laevis: mechanisms of Xslug induction. Dev Biol. 1996;177:580–589. doi: 10.1006/dbio.1996.0187. [DOI] [PubMed] [Google Scholar]
- Mayor R, Morgan R, Sargent MG. Induction of the prospective neural crest of Xenopus. Development. 1995;121:767–777. doi: 10.1242/dev.121.3.767. [DOI] [PubMed] [Google Scholar]
- Mercader N, Fischer S, Neumann CJ. Prdm1 acts downstream of a sequential RA, Wnt and Fgf signaling cascade during zebrafish forelimb induction. Development. 2006;133:2805–2815. doi: 10.1242/dev.02455. [DOI] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Dev Cell. 2004;7:291–299. doi: 10.1016/j.devcel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Neave BHN, Patient R. A graded response to BMP-4 spatially coordinates patterning of the mesoderm and ectoderm in the zebrafish. Mech Dev. 1997;62:183–195. doi: 10.1016/s0925-4773(97)00659-x. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, Mullins MC. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Trout J, Connors SA, Andermann P, Weinberg E, Mullins MC. Dorsal and intermediate neuronal cell types of the spinal cord are established by a BMP signaling pathway. Development. 2000;127:1209–1220. doi: 10.1242/dev.127.6.1209. [DOI] [PubMed] [Google Scholar]
- O'Brien EK, d'Alencon C, Bonde G, Li W, Schoenebeck J, Allende ML, Gelb BD, Yelon D, Eisen JS, Cornell RA. Transcription factor Ap-2alpha is necessary for development of embryonic melanophores, autonomic neurons and pharyngeal skeleton in zebrafish. Dev Biol. 2004;265:246–261. doi: 10.1016/j.ydbio.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Payer B, O'Carroll D, Ancelin K, Ono Y, Sano M, Barton SC, Obukhanych T, Nussenzweig M, Tarakhovsky A, Saitou M, Surani MA. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436:207–213. doi: 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- Olesnicky Killian EC, Birkholz DA, Artinger KB. A role for chemokine signaling in neural crest cell migration and craniofacial development. Dev Biol. 2009;333:161–172. doi: 10.1016/j.ydbio.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BY, Saint-Jeannet JP. Expression analysis of Runx3 and other Runx family members during Xenopus development. Gene Expr Patterns. 10:159–166. doi: 10.1016/j.gep.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- Robertson EJ, *, Charatsi Iphigenie, 1, Joyner Clive J, 1, Koonce Chad H, 1, Morgan Marc, 1, Islam Ayesha, 1, Carol Paterson EL, 1, Arnold Sebastian J, 1, Kallies Axel, 2, Nutt Stephen L, 2, Bikoff Elizabeth K., 1,* Blimp1 regulates development of the posterior forelimb, caudal pharyngeal arches, heart and sensory vibrissae in mice. Development. 2007;134:4335–4345. doi: 10.1242/dev.012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi CC, Hernandez-Lagunas L, Zhang C, Choi IF, Kwok L, Klymkowsky M, Artinger KB. Rohon-Beard sensory neurons are induced by BMP4 expressing non-neural ectoderm in Xenopus laevis. Dev Biol. 2008;314:351–361. doi: 10.1016/j.ydbio.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi CC, Kaji T, Artinger KB. Transcriptional control of Rohon-Beard sensory neuron development at the neural plate border. Dev Dyn. 2009;238:931–943. doi: 10.1002/dvdy.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Ng T. Blimp-1 specifies neural crest and sensory neuron progenitors in the zebrafish embryo. Curr Biol. 2004;14:1772–1777. doi: 10.1016/j.cub.2004.09.046. [DOI] [PubMed] [Google Scholar]
- Selleck MA, Bronner-Fraser M. Origins of the avian neural crest: the role of neural plate-epidermal interactions. Development. 1995;121:525–538. doi: 10.1242/dev.121.2.525. [DOI] [PubMed] [Google Scholar]
- Shih LJ, Lu YF, Chen YH, Lin CC, Chen JA, Hwang SP. Characterization of the agr2 gene, a homologue of X. laevis anterior gradient 2, from the zebrafish, Danio rerio. Gene Expr Patterns. 2007;7:452–460. doi: 10.1016/j.modgep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Sperber SM, Dawid IB. barx1 is necessary for ectomesenchyme proliferation and osteochondroprogenitor condensation in the zebrafish pharyngeal arches. Dev Biol. 2008;321:101–110. doi: 10.1016/j.ydbio.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RA, Arduini BL, Berghmans S, George RE, Kanki JP, Henion PD, Look AT. Zebrafish foxd3 is selectively required for neural crest specification, migration and survival. Dev Biol. 2006;292:174–188. doi: 10.1016/j.ydbio.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Ungos JM, Karlstrom RO, Raible DW. Hedgehog signaling is directly required for the development of zebrafish dorsal root ganglia neurons. Development. 2003;130:5351–5362. doi: 10.1242/dev.00722. [DOI] [PubMed] [Google Scholar]
- von Hofsten J, Elworthy S, Gilchrist MJ, Smith JC, Wardle FC, Ingham PW. Prdm1- and Sox6-mediated transcriptional repression specifies muscle fibre type in the zebrafish embryo. EMBO Rep. 2008;9:683–689. doi: 10.1038/embor.2008.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilm TP, Solnica-Krezel L. Essential roles of a zebrafish prdm1/blimp1 homolog in embryo patterning and organogenesis. Development. 2005;132:393–404. doi: 10.1242/dev.01572. [DOI] [PubMed] [Google Scholar]
- Winkler C, Elmasri H, Klamt B, Volff JN, Gessler M. Characterization of hey bHLH genes in teleost fish. Dev Genes Evol. 2003;213:541–553. doi: 10.1007/s00427-003-0360-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.