Abstract
This study examined reflex mechanisms that mediate urinary bladder and external urethral sphincter (EUS) coordination in female Sprague-Dawley urethane-anesthetized rats under empty and distended bladder conditions. The bladder was distended either by a small balloon or a saline filled catheter inserted through the body of the bladder. Stimulation of the entire pudendal nerve elicited short latency (8–12 ms) responses in the EUS and short (3–8 ms) and long latency responses (16–20 ms) in contralateral pudendal nerve. The long latency pudendal–pudendal reflex was reduced by 36.7% in area during bladder distension with the balloon catheter. However, there was no significant change in the area of pudendal–EUS reflex during bladder distension. Peak amplitudes of both reflexes were reduced 32% by bladder distension. The effects of glutamatergic receptor antagonists on the reflexes were also examined. MK801 (0.3–5 mg/kg, i.v.), an N-methyl-D-aspartate glutamatergic receptor antagonist, markedly depressed the pudendal–pudendal reflex, but LY215490 (3 mg/kg, i.v.), an alpha-amino-5-methyl isoxazole-4-propionate antagonist, had a minimal inhibitory effect. Both glutamatergic receptor antagonists significantly suppressed the pudendal–EUS reflex. These results indicate that the EUS is innervated by multiple pathways and that glutamatergic excitatory transmission is important in the neural mechanisms underlying bladder–sphincter coordination in the rat.
Keywords: Pudendal nerve, Electrical stimulation, External urethral sphincter, Reflex
1. Introduction
The storage and periodic elimination of urine are regulated by a complex neural control system located in the brain and spinal cord that coordinates the activities of the reservoir (urinary bladder) and the urethral outlet including bladder neck, urethra and external urethral sphincter (EUS). Normally, these two parts of the lower urinary tract (LUT) exhibit reciprocal activity. During urine storage the reservoir is quiescent and intravesical pressure remains low, whereas activity in the outlet gradually increases during bladder filling to maintain continence. During voiding the pattern of activity is reversed.
The functions of the LUT are regulated by three sets of peripheral nerves: sacral parasympathetic (pelvic nerves) and thoracolumbar sympathetic nerves that innervate the bladder and urethra, and sacral somatic nerves (pudendal nerves) that innervate the urethra and external urethral sphincter (de Groat, 1993; de Groat et al., 1993). Neurological disorders such as spinal cord injury disrupt the coordination between the bladder and the urethral outlet leading to voiding dysfunction (detrusor-sphincter-dyssynergia). Studies of the relationship between bladder dynamics (e.g., bladder pressure) and reflex activity in the pudendal nerves innervating the urethral sphincter are essential for developing pharmacological and electrical stimulation-based treatments to control neurogenic voiding dysfunction in spinal cord injured (SCI) patients (Jezernik et al., 2002).
Sacral neuromodulation which involves electrical stimulation of the sacral spinal nerves carrying pudendal nerve afferent axons to the spinal cord is an effective method for suppressing neurogenic bladder problems (Jezernik et al., 2002). In addition modulation of pudendal nerve activity by electrical stimulation or blocking techniques as well as local application of pharmacological agents has been useful for treating voiding dysfunction. This is because the pudendal nerves contain motor pathways to the EUS and also afferent pathways mediating urethra–bladder and somato–bladder reflexes (Thor et al., 1989a, b). Low frequency electrical stimulation of afferent axons in the pudendal nerve in humans or the deep perineal nerve, a caudal branch of the pudendal nerve, in cats can initiate reflex bladder contractions and voiding (Boggs et al., 2005; Shefchyk and Buss, 1998). On the other hand, high frequency stimulation of pudendal nerve efferent axons or local application of 5% phenol solution can effectively block axonal conduction, suppress EUS contractions and in turn decrease in intraurethral pressure (Tai et al., 2004; Tsai et al., 2002). Treatment with phenol solution is useful in reducing detrusor-sphincter-dyssynergia and promoting voiding in SCI subjects. Complex methods for controlling pudendal nerve stimulation have also been proposed. One scheme involves the use of bladder sensory nerve activity recorded on sacral nerve roots to trigger pudendal nerve stimulation and thereby create an artificial circuit to modulate voiding function (Jezermik et al., 2000, 2001).
To support the further development of neuromodulatory techniques for controlling the LUT it is essential to conduct electrophysiological studies to examine the role of pudendal nerve reflexes in coordinating urethra and bladder function. However, very few studies have focused on EUS related pudendal nerve reflexes under empty and distended bladder conditions. Most studies have been conducted on the reflex control of the anal sphincter by pudendal nerve efferent pathways, because the anal canal is more accessible than the urethra for stimulation and recording (McMahon et al., 1982). Another potential problem in studies of LUT function is that bladder distension is usually induced by injection of fluid through a urethral catheter. A catheter can continuously stimulate urethral afferents and induce urethral–bladder reflexes. Leakage of fluid from the bladder around the catheter and into the urethra could trigger similar reflexes (Floyd et al., 1982; Mackel, 1979; McMahon and Morrison, 1982; McMahon and Spillane, 1982).
The aim of this study was to directly examine pudendal nerve reflexes that mediate bladder and EUS coordination under empty and distended bladder conditions. To distend the bladder without urethral catheterization which could trigger undesired urethral–bladder reflexes, a catheter with a small balloon on the end was inserted through the apex of the bladder dome. Electrophysiological methods were used to record reflexes to the EUS and to pudendal nerve efferent axons elicited by stimulation of pudendal nerve afferent axons. The role of glutamatergic excitatory transmission in EUS and pudendal nerve reflexes was examined using glutamatergic receptor antagonists.
2. Materials and methods
2.1. Animal preparation
Female Sprague-Dawley rats (N = 17, 250–330 g) were anesthetized by subcutaneous injection of urethane (1.2 mg/kg) and supplemental doses of urethane were given throughout the experiments. The experimental protocols were approved by University of Pittsburgh Institutional Animal Care and Use Committee.
A polyethylene tube (PE-50) filled with physiological saline was inserted into the jugular vein for intravenous administration of drugs. The urinary bladder was exposed via a midline abdominal incision and two catheters were inserted into the bladder. One catheter, a PE-50 polyethylene tube filled with saline, was inserted through an incision in the lateral side of the bladder and secured with cotton thread. A double-lumen catheter with a small balloon attached to the tip was inserted through the apex of the bladder. Injection of saline (0.2 ml) or injection of air (0.2 or 0.5 ml) into the balloon was used to distend the bladder. Half of the pubic symphysis was removed to expose the middle urethra and the EUS. To record the EUS electromyogram (EMG), two fine, insulated silver wire electrodes (0.05 mm diameter) with exposed tips were inserted into the muscle on both sides of the middle urethra, 5–8 mm from the bladder neck. Then the wound was closed, and the animal was turned over on its abdomen.
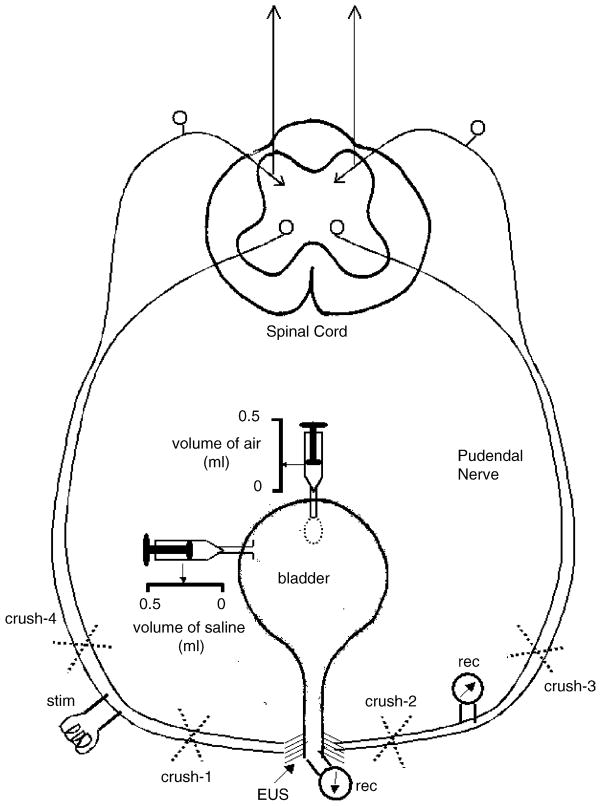
Two incisions were made on both sides of the spine to access the pudendal nerves from the dorsal side. The pudendal nerves were dissected bilaterally (McKenna and Nadelhaft, 1986). By retracting skin flaps a pool was formed around the pudendal nerves, and filled with mineral oil (37 °C) to prevent drying. For electrical stimulation and recording of reflexes, bipolar silver-wire electrodes were positioned bilaterally on the pudendal nerves, at a location 30–35 mm central to the insertion of the nerve into the urethra and at a point central to the division of the nerve into skin and urethral branches. At this site the nerve contains efferent and afferent axons. The experimental setup for modulating the bladder pressure as well as for measuring pudendal reflexes and EUS–EMG is depicted in Fig. 1.
Fig. 1.
Schematic diagram of the experimental setup. Stimulation electrodes (stim) were positioned on the pudendal nerve on one side and recording electrodes (rec) were positioned on the nerve on the contralateral side approximately 35 mm from the junction of the nerves with the urethra. During the experiments the pudendal nerves were crushed peripheral (#1 and #2) or central (#3 and #4) to the electrodes to determine the pathways for evoking the reflexes. The EUS–EMG was recorded by inserting single electrodes into the striated muscle on either side of the urethra. Catheters were inserted into the bladder lumen to distend the bladder. One catheter was connected to a small balloon that could be filled with air; the other catheter was filled with saline.
2.2. Electrophysiological examination and analysis of glutamatergic receptor antagonists
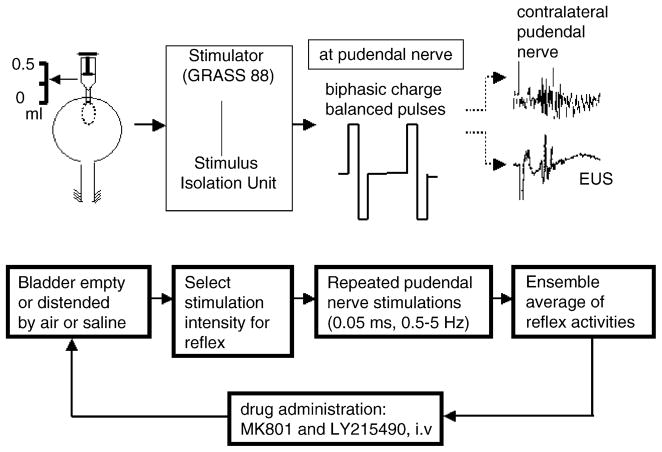
The flowchart of the entire experimental procedure for electrophysiological and pharmacological protocols is depicted in Fig. 2. Two pudendal nerve-related reflexes, pudendal–pudendal and pudendal–EUS reflexes, were compared when the bladder was distended or empty. Bladder pressure was manipulated by either inflating the balloon inside the bladder or by injecting saline into the bladder.
Fig. 2.
Flowchart of the experimental protocol for the electrophysiological and pharmacological studies. Reflex activity in pudendal efferent nerves and EUS–EMG were elicited by electrical stimulation of pudendal afferent axons using biphasic charge balanced pulses. The bladder was distended with air or saline. After completing the physiological studies reflexes were recorded before and after injection of glutamatergic receptor antagonists administered as single doses to individual animals or administered sequentially, MK801 followed by LY215490. Recordings were obtained 25–45 min after drug injection.
The pudendal–pudendal reflex was elicited by single shocks (Grass S88 stimulator) to the pudendal nerve and recorded from the contralateral pudendal nerve (McKenna and Nadelhaft, 1989) as well as from the EUS on the both sides. The electrical stimulation consisted of biphasic charge balanced pulses at frequencies between 0.5 and 5 Hz with pulse width of 0.05 ms. Submaximal stimulus intensities which ranged between 0.5 and 20 V in different experiments were set at two to three times the threshold for eliciting a detectable reflex. The evoked pudendal–pudendal reflex activity was amplified 50,000-fold with a preamplifier (P511AC, Grass Instruments) and filtered (high-frequency cutoff at 3000 Hz and low-frequency cutoff at 3 Hz with a 60 Hz notch filter). The pudendal nerve reflex signal was sampled at 5000 Hz and then averaged on a personal computer (PC). Similarly, EUS–EMG signals were amplified 20,000-fold and filtered (high-frequency cutoff at 3000 Hz and low-frequency cutoff at 100 Hz) for further ensemble averaging of at least 10 evoked responses. At least 10–20 averaged responses were recorded with the bladder empty or distended. In some experiments the pudendal nerves were crushed either on the side ipsilateral or contralateral to the site of stimulation to determine if the evoked responses were mediated by peripheral or central reflex pathways (Fig. 1).
Following the electrophysiological studies pharmacological experiments were conducted according to two protocols to observe the effects of glutamatergic receptor antagonists on the EUS and pudendal nerve reflexes evoked by electrical stimulation. MK801 (dizocilpine, Merck, Sharp & Dohme Res. Labs., West Point, PA), a noncompetitive NMDA glutamate antagonist and LY215490 ((3SR, 4aRS, 6RS, 8aRS)-6-[2-(1H-tetrazol-5-yl-)ethyl]-decahydroisoquinoline-3-carboxylic acid, Lilly Res. Labs.), a potent competitive AMPA glutamate receptor antagonist were dissolved in normal saline for i.v. administration. Drug doses were calculated for the base of each compound. In some experiments the reflexes were recorded for 30 min before and after single doses of either MK801 (0.3–5 mg/kg, i.v.) or LY215490 (1–3 mg/kg, i.v.). In other experiments the drugs were administered sequentially: MK801, 1 h before LY215490.
2.3. Data analysis
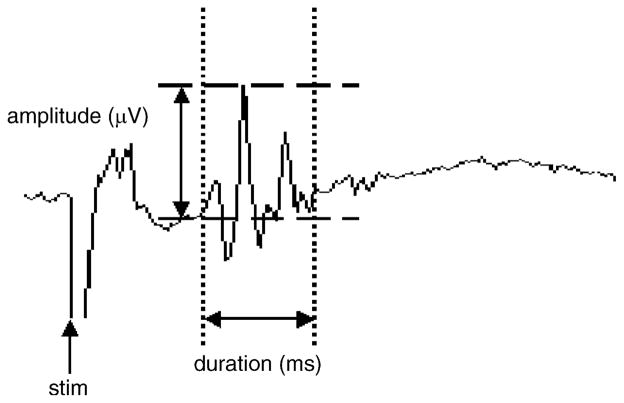
The areas of averaged pudendal–pudendal reflexes and EUS–EMG activity were measured by integrating the area under the curve over the duration of the reflex responses with a PC in μV ms (Fig. 3, Stalberg et al., 1986). Compared to reflex amplitude, reflex area was more consistent over long periods of time and therefore more suitable for representing the intensity of reflex activity. The results are given as mean values ± S.E.M. The reflex areas obtained before and after bladder distension as well as drug administration were statistically compared using the Student’s t-test. P < 0.05 was considered statistically significant.
Fig. 3.
Measurement of the magnitude of the pudendal nerve reflex. Reflex area represents area (μV ms) under the upward deflection of the evoked potential between two cursors set on the baseline at the beginning and the end of the potential. Usually the potential consisted of several peaks of varying amplitude. Amplitude measurements reflect the amplitude of the largest peak (μV).
3. Results
3.1. Pudendal–pudendal and pudendal–EUS reflexes
Fig. 4 depicts typical examples of the pudendal–pudendal reflex when evoked by single-shock electrical stimulation of one intact pudendal nerve and recording the reflex on the contralateral intact pudendal nerve under empty and distended bladder conditions. The pudendal–pudendal reflex consists of two components at different latencies: an early reflex with smaller amplitude and a late component with larger amplitude. When the bladder was empty, the latency of the early response ranged from 3 to 8 ms (mean 3.4 ± 0.03 ms, N = 6). This early component only occurred in 40–50% of stimulus trials in each animal and was not detected in some recordings. The late component ranged from 16 to 20 ms (mean 17.8 ± 0.3 ms, N = 6), and the duration was 14.8 ± 0.13 ms when the bladder was empty. The duration was 15.5 ± 0.39 ms when the bladder was distended.
Fig. 4.
Effect of bladder distension on the pudendal nerve reflex elicited by a single shock to the contralateral pudendal nerve (arrow) (A) when the bladder was empty, (B) when the bladder was distended with 0.2 ml of saline, and (C) after recovery following bladder distension. Upper trace in each pair of recordings is the control activity in the absence of stimulation. Lower trace shows the response to stimulation.
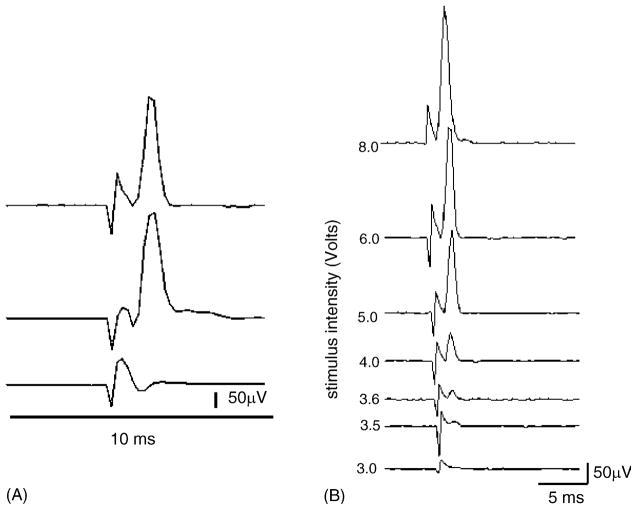
The early component was abolished after crushing the pudendal nerve distal to the point of stimulation (crush-1, Fig. 1) or crushing the contralateral nerve distal to the point of recording (crush-2, Fig. 1). Therefore the early component is likely to be mediated by a peripheral pathway that makes a connection between the right and left pudendal nerves rather than a central reflex mechanism. Similar short latency responses could be elicited on the pudendal nerve by direct stimulation (0.7 Hz with pulse width 0.05 ms) of the EMG electrodes inserted in the EUS (Fig. 5A). The latencies were 0.8 to 2.2 ms. The responses were elicited at threshold voltages between 3 and 3.5 V, and increased in magnitude at voltages up to three times threshold (Fig. 5B). As shown in Fig. 6C the late component of pudendal–pudendal reflex was completely eliminated after the contralateral pudendal nerves were crushed central to the site of stimulation (crush-4, Fig. 1) or the ipsilateral pudendal nerve was crushed at a point central to the recording site (crush-3, Fig. 1). The reflex latency and amplitude were similar at different frequencies of stimulation between 0.5–5 Hz.
Fig. 5.
EUS–pudendal response. (A) Upper trace: the pudendal nerve response (average of 10 individual responses) was elicited by electrical stimulation of the EUS (6 V, 0.7 Hz, pulse width 0.05 ms). Middle trace: the EUS–pudendal response was not changed after the contralateral pudendal nerve was crushed at the site marked crush-1 in Fig. 1. Lower trace: the pudendal response was totally abolished after the pudendal nerve was crushed at the site marked crush-2 in Fig. 1. (B) Changes in the EUS–pudendal response with stimulus intensity. Series of recordings showing the EUS–pudendal response at increasing stimulus intensities. The threshold was identified as 3.5 V in this animal. Only the stimulation artifact occurs below the threshold (3.0 V, the lowest trace). The first trace shows the maximum amplitude of this response elicited by 8 V. Each trace represents the average of 10 individual responses. The stimulus duration was constant at 0.05 ms.
Fig. 6.
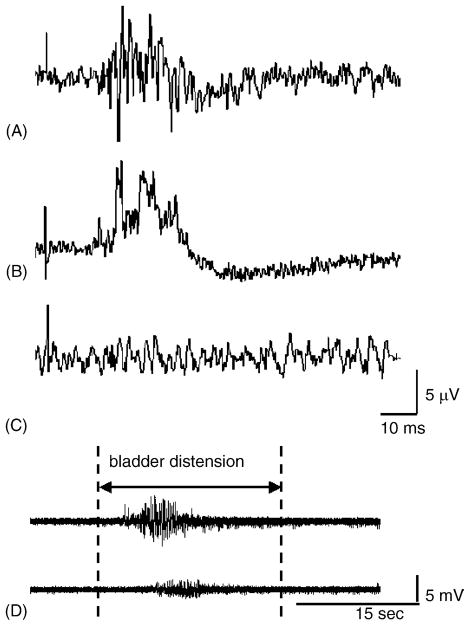
Effect of crushing the pudendal nerves at various sites on the pudendal–pudendal (A) and bladder–EUS reflexes. (A) The pudendal–pudendal reflex was elicited by single-shock stimulation (9 V, 0.5 Hz, 0.05 ms pulse duration) when the bladder was distended. (B) The pudendal–pudendal reflex remained after the pudendal nerve was crushed at the site marked crush-2 in Fig. 1. (C) The pudendal–pudendal reflex was abolished after pudendal nerves were crushed bilaterally at sites marked crush-3 and crush-4 in Fig. 1. (D) After bilateral crushing of pudendal nerves, the EUS–EMG activity induced by bladder distension was reduced but not completely eliminated. Upper panel: EUS–EMG firing induced by distending the bladder with saline when the pudendal nerves were intact. Lower panel: after crushing pudendal nerves bilaterally the amplitude of EUS–EMG activity during bladder distension was markedly reduced.
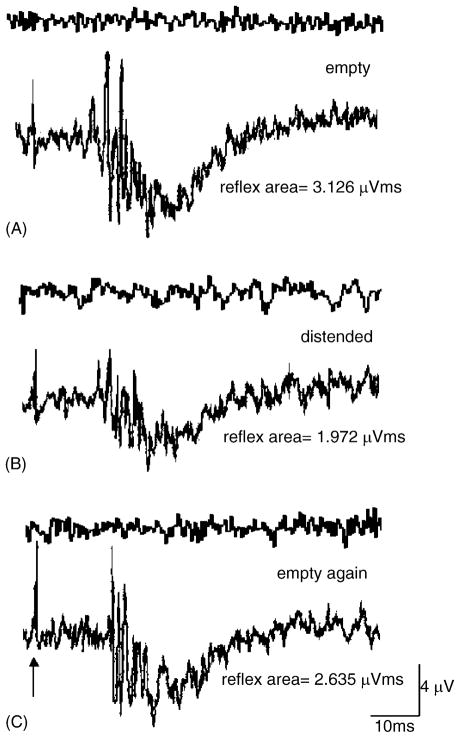
The pudendal–EUS reflex elicited by stimulation of pudendal nerve afferents occurred at latencies ranging between 8 and 12 ms (mean 9.1 ± 0.04 ms, N = 6) (Fig. 7). The reflex duration was 8.5 ± 0.33 ms when the bladder was empty and 13.5 ± 0.84 ms when the bladder was distended. The reflex was abolished when the pudendal nerves were crushed bilaterally peripheral to the stimulation and recording electrodes (crush-1 and crush-2, Fig. 1) or crushed bilaterally central to the electrodes at the sites marked as crush-3 and crush-4 in Fig. 1. After bilateral pudendal nerves were crushed, the EUS activity induced by bladder distension was reduced but not completely abolished (Fig. 6D).
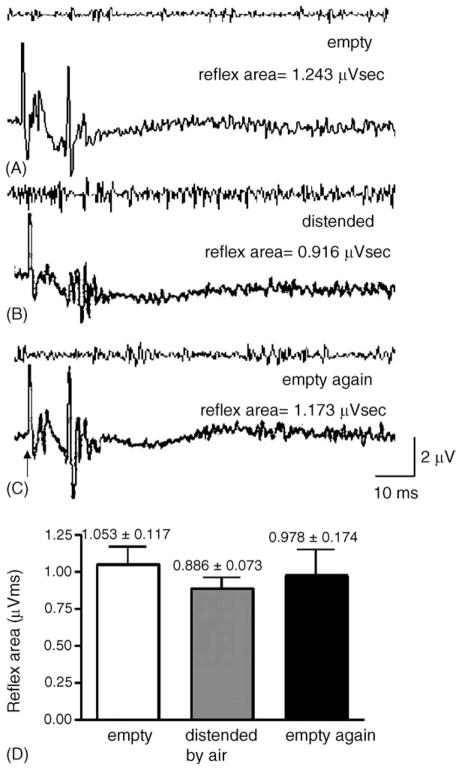
Fig. 7.
Examples of the pudendal–EUS reflex when the bladder was (A) empty, (B) distended by air, and (C) empty after distension. (D) Statistical analysis indicated no significant change in the reflex area of the pudendal–EUS reflex when the bladder was distended by air or empty (N = 6). However the amplitude of the reflex was reduced during bladder distension (B).
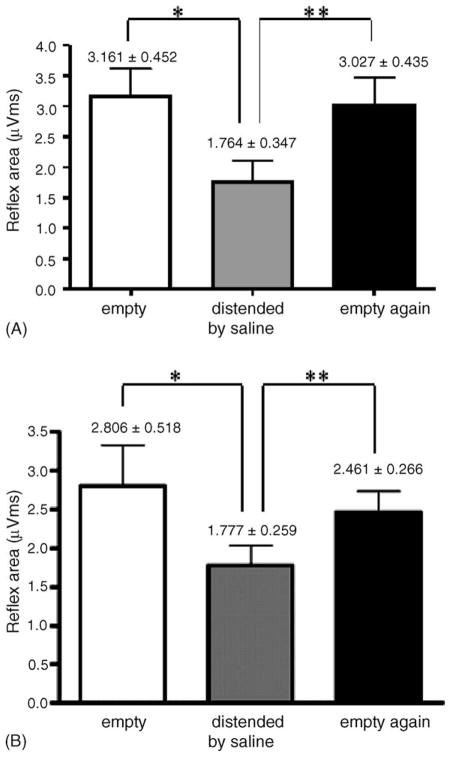
As illustrated in Fig. 4A and B, the reflex area of the late component of pudendal–pudendal reflex was significantly reduced (Fig. 8A) when the bladder was distended with 0.2 ml of saline injected into the bladder via the catheter and allowed to flow out through the urethra. Similar changes in the pudendal–pudendal reflex occurred after distending the bladder by injecting air (Fig. 8B) into the balloon catheter. The reflex area of the late pudendal–pudendal reflex was reduced from 3.161 ± 0.452 to 1.764 ± 0.347 μV ms or an average of 44.2% reduction when the bladder was distended by injecting 0.2 ml saline (Fig. 8A); whereas when the bladder was distended with 0.2 ml (N = 3) or 0.5 ml (N = 6) of air injected into the intravesical balloon (Fig. 8B) the reflex area was decreased by 50% and 36.7%, respectively. The reflex area returned to control levels within 5 min after the bladder was emptied.
Fig. 8.
Effect of bladder distension with different volumes of saline and air on the pudendal–pudendal reflex (A). The reflex area of the pudendal–pudendal reflex was compared when the bladder was empty, distended by saline (0.2 ml) and emptied again. Statistical analysis indicated that the pudendal–pudendal reflex was significantly decreased by distension of the urinary bladder (N = 6, P < 0.05, Student’s t-test). (B) Similarly, the reflex area was significantly changed when the bladder was distended by 0.5 ml of air (*). The reflex area was significantly increased after the bladder was emptied (**). There was no significant difference between control and empty conditions.
The amplitude of the reflex was also significantly reduced from 2.85 ± 0.06 μV when the bladder was empty to 1.95 ± 0.09 μV when the bladder was distended. In most animals bladder distension evoked a large amplitude bladder contraction that we interpret as a micturition reflex. After the bladder was emptied and bladder pressure returned to control levels, the amplitude and area of the pudendal–pudendal reflex recovered, as shown in Fig. 4C.
Fig. 7B shows that the reflex area of pudendal–EUS reflex was not significantly decreased when the bladder was distended by air (0.5 ml). However the amplitude was reduced from 2.87 ± 0.57 μV when the bladder was empty to 1.96 ± 0.16 μV when the bladder was distended. Although there was a reduction of 32% in the amplitude of EUS–EMG reflex during distension of the bladder, the change in reflex area was not statistically significant (Fig. 7D) due to the longer reflex duration when the bladder was distended. Table 1 shows the electrophysiological properties of pudendal–pudendal and pudendal–EUS reflex under the empty and distended bladder conditions.
Table 1.
Electrophysiological properties of pudendal–pudendal and pudendal–EUS reflexes
| Reflexes | Pudendal–pudendal | Pudendal–EUS |
|---|---|---|
| Short latency (ms) | 3.4 ± 0.03 | N/A |
| Late latency | 17.8 ± 0.3 | 9.1 ± 0.04 |
| Amplitude/bladder empty (μV) | 2.85 ± 0.06* | 2.87 ± 0.57* |
| Amplitude/bladder distended | 1.95 ± 0.09* | 1.96 ± 0.16* |
| Amplitude/emptied again | 3.07 ± 0.2 | 2.75 ± 0.14 |
| Duration/bladder empty (ms) | 14.85 ± 0.13 | 8.50 ± 0.33* |
| Duration/bladder distended | 15.50 ± 0.39 | 13.5 ± 0.84 |
The numbers in each category represent the mean ± S.E.M. and an average of six experiments. The amplitudes of both pudendal–pudendal and pudendal–EUS reflexes were significantly reduced by bladder distension. The duration of pudendal–EUS reflex was significantly increased when the bladder was distended.
P < 0.05.
3.2. Role of glutamatergic mechanisms in reflex pathways
Glutamatergic receptor antagonists were tested alone in individual animals or administered sequentially to the same animals at approximately a 1 h interval. Drugs were tested in a range of doses that were shown in previous studies to suppress voiding (Yokoyama et al., 2001; Yoshiyama et al., 1994, 1997). MK801, an NMDA receptor antagonist (0.3 mg/kg, i.v.) suppressed the area of the pudendal–pudendal reflex by an average of 23%, whereas the administration of a larger dose of MK801 (5.0 mg/kg, i.v.) suppressed the reflex area by an average of 45%. The effect of MK801 was evident within 10 min after administration and persisted for at least 1 h. When LY215490 (3.0 mg/kg, i.v.) was given alone, it suppressed the reflex area by only 9%. When the low dose of MK801 (0.3 mg/kg, i.v.) was given prior to LY215490 (3.0 mg/kg, i.v.), the reflex area was suppressed by 27% and the subsequent injection of LY215490 elicited only a small additional decrease of 4% in the reflex. Fig. 9 summarizes the effects of MK801 and LY215490 alone and in combination on the pudendal–pudendal reflex.
Fig. 9.
The effects MK801 and LY2155490 on the reflex area of the pudendal–pudendal reflex (N = 12). The animals were separated into three groups: group 1 (N = 3) receiving LY215490 alone (3.0 mg/kg, i.v.), group 2 (N = 6) first receiving MK801 (0.3 mg/kg, i.v.) and followed by LY215490 (3.0 mg/kg, i.v.), and group 3 (N = 3) receiving MK801 alone (5.0 mg/kg, i.v.). The reflex area was decreased by an average of 23% with MK801 (0.3 mg/kg, i.v.), and 45% with MK801 (5.0 mg/kg, i.v.). When LY215490 (3.0 mg/kg, i.v.), was administered after MK801 (0.3 mg/kg, i.v.) the reflex area exhibited only a small further reduction (4%). LY215490 (3.0 mg/kg, i.v.) alone had a small suppressant effect (9%).
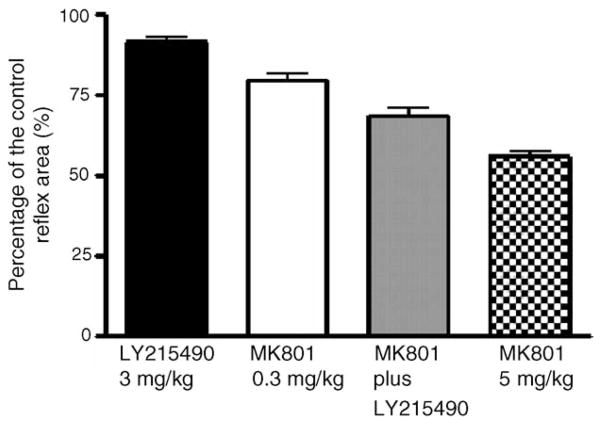
The pudendal–EUS reflex was more sensitive to the effects of the drugs. This reflex was abolished 98% decrease either by MK801 (0.3 mg/kg, i.v.) or by LY215490 (3 mg/kg, i.v.). In this study, we observed that the effects of the drugs could last up to an hour after i.v. administration.
4. Discussion
In the present study, we examined various properties of the pudendal–pudendal and pudendal–EUS reflexes including reflex latencies, the sensitivity of the reflexes to bladder distension and responses to glutamatergic receptor antagonists. The results indicate that reflexes evoked by electrical stimulation of afferent fibers in the pudendal nerve are modulated by afferent input from the bladder and are dependent in part on glutamatergic excitatory transmission in the central nervous system. Because the EUS receives an innervation from motor axons in the pudendal nerves it was expected that pudendal–pudendal and pudendal–EUS reflexes would exhibit similar properties. However our experiments revealed important differences between these two reflexes indicating: (1) that the pudendal nerves carry only part of the EUS innervation and that other nerves are also important or (2) that EUS–EMG recordings detect electrical signals from additional muscles that are innervated by other motor nerves.
Previous studies in female rats (McKenna and Nadelhaft, 1986, 1989) showed that stimulation of the sensory branch of the pudendal nerve elicited several reflexes on the motor branch of the ipsilateral pudendal nerve including a short latency (11.8 ms latency) large amplitude discharge and two longer latency (25–30 and 100–140 ms) small amplitude discharges. Reflexes on the contralateral pudendal nerve occurred at 1–1.5 ms longer latencies. The latency of pudendal–pudendal nerve reflex in cats was 7.8 ± 1.2 ms (Thor et al., 1989a, b). In our experiments the pudendal–pudendal contralateral reflex occurred at a slightly longer latency (16–20 ms) but was presumably mediated by a spinal polysynaptic pathway similar to the one underlying the short latency reflex identified by McKenna and Nadelhaft. In approximately 40% of our recordings, the 16 ms latency reflex was preceded (at 3–8 ms latency) by a small discharge that is most likely due to activation of a peripheral axonal pathway crossing between the pudendal nerves or due to initiation of an afferent discharge in the contralateral nerve following contraction of striated sphincter muscles that would occur at short latency following pudendal nerve stimulation. The early response was eliminated by crushing the pudendal nerve peripheral to the stimulation or recording sites whereas the 16 ms latency reflex was unaffected. On the other hand the latter reflex was eliminated by crushing the pudendal nerves central to the site of stimulation or recording (Fig. 6C), indicating that this response originated in the central nervous system and that the 3–8 ms latency response was mediated by a peripheral pathway. Direct electrical stimulation of the EUS induced a volley in the pudendal nerve at a short latency (0.8–2.2 ms) (Fig. 5A) supporting the idea that fast conducting axons from the EUS could contribute to the 3–8 ms early discharge.
In our experiments the pudendal–EUS reflex was of shorter latency (8–12 ms) than the contralateral pudendal–pudendal reflex (16–20 ms). The latency of pudendal–EUS reflex in cats was 8.6 ± 1.9 ms (Thor et al., 1989a, b). Because efferent nerve firing should precede muscle contractions, this finding raises questions about the role of pudendal nerve efferent axons in the pudendal–EUS reflex. However it should be noted that the EUS reflex could be mediated by efferent pathways ipsilateral as well as contralateral to the site of stimulation; and since McKenna and Nadelhaft (1989) reported contralateral reflexes had longer latencies this may account for the discrepancy between latency of the pudendal–EUS and pudendal–pudendal reflexes in our experiments. On the other hand EUS reflex activity was not completely eliminated by crushing the pudendal nerves bilaterally (Fig. 6D, also see Kamo et al., 2004) indicating that the EUS or adjacent periurethral striated muscles that contribute to the EMG activity are innervated by other motor nerves, e.g., the muscular branch of the pelvic nerve to the illiococcygeus/pubococcygeus muscle (Kamo et al., 2004; Pacheco et al., 1989, 1997). These nerves could be involved in the pudendal–EUS reflex.
Differences between the pudendal–pudendal and pudendal–EUS reflexes were also noted in the sensitivity to bladder distension (Figs. 4 and 7). The area of pudendal–pudendal reflex was reduced by 44.2% and 36.7% when the bladder was distended by saline infusion or balloon inflation, respectively. On the other hand the area of the pudendal–EUS was not significantly reduced by bladder distension. However the amplitude of both reflexes was reduced (32% decrease) by bladder distension (Table 1). The reason for the difference between amplitude and area measurements in regard to the effect of bladder distension on the pudendal–EUS is unknown. However it is possible that the increase in baseline EUS–EMG activity during bladder distension (Fig. 7B) complicated the measurement of the area of the pudendal nerve evoked EUS reflex area. The other possibility is that the duration of pudendal–EUS reflex was increased from 8–10 to 13–15 ms when the bladder was distended. Hence, the area of pudendal–EUS reflex did not significantly change due to increased duration and decreased amplitude when the bladder was distended. Despite the differences in responses of EUS and pudendal nerve reflexes to bladder distension it is clear that some reflexes evoked by pudendal afferent nerve stimulation are modulated by afferent input from the bladder and therefore these reflexes are likely to be altered during voiding.
To examine the effect of bladder distension on the pudendal–pudendal reflex two methods were used: injection of saline into the bladder and distension of a balloon in the bladder. When the bladder is distended with saline, the saline can also pass into the urethra which can activate urethral afferents and in turn modulate bladder and urethral reflexes as reported in previous studies (Floyd et al., 1982). Our other method of using a small balloon for bladder distension would not cause the additional urethral stimulation and therefore the effects induced by this method must be due to activation of bladder afferents. Because both methods produced a similar suppression of the pudendal–pudendal reflex we conclude that this suppression must be due primarily to bladder distension and not to stimulation of urethral afferents. Our findings are consistent with previous reports in cats that a reflex in the anal or urethral branch of the pudendal nerve evoked by electrical stimulation of the contralateral pudendal nerve was suppressed by increasing intravesical and intracolonic pressure (McMahon and Morrison, 1982; McMahon et al., 1982; Thor et al., 1989a, b). These data indicate that pudendal–pudendal reflexes to the anal and urethral sphincters are both suppressed by afferent stimuli that evoke micturition and defecation. Thus pudendal–pudendal reflexes may be linked with urinary and distal bowel continence mechanisms.
The pudendal–pudendal and pudendal–EUS reflexes exhibited quantitatively different responses to glutamatergic receptor antagonists. The pudendal–pudendal reflex was depressed by 45% after the largest dose of MK801, an NMDA glutamatergic receptor antagonist, but was relatively insensitive to LY215490, an AMPA glutamatergic receptor antagonist administered alone or in combination with MK801. On the other hand the pudendal–EUS reflex was suppressed by 98% by either MK801 or LY215490. These data indicate that glutamatergic excitatory transmission involving both NMDA and AMPA receptors is essential in the pudendal–EUS reflex pathway and that NMDA but not AMPA mechanisms contribute to the pudendal–pudendal reflex. These findings support the conclusions based on the responses to bladder distension that pudendal–pudendal and pudendal–EUS reflexes represent distinct reflex mechanisms. The considerable sensitivity of the pudendal–EUS reflex to both types of glutamatergic receptor antagonists is consistent with previous reports that EUS reflex activity during micturition is also dependent upon glutamatergic excitatory transmission mediated by NMDA and AMPA receptors (Yokoyama et al., 2001; Yoshiyama et al., 1993, 1997, 1999).
In summary, physiological and pharmacological experiments indicate that pudendal–pudendal and pudendal–EUS reflexes are mediated in part by separate central pathways that vary in their sensitivity to bladder distension and glutamatergic receptor antagonists. It seems likely that these reflexes play a role in the central control of voiding and that a more detailed understanding of the properties of these reflexes could promote the development of electrical stimulation and pharmacological-based treatments for lower urinary tract dysfunction.
Acknowledgments
This work is supported by NIH grants (NIDDK-49430) to William C. de Groat, a grant from the Li Foundation, USA, to H.-Y. Chang and by National Science Council (NSC-91-2213-E-075A-002) and Industrial Technology Research Institute grants in Taiwan to C.-L. Cheng and J.-J.J. Chen, respectively.
References
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol. 2005;93:2688–97. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Anatomy and physiology of the lower urinary tract. Urol Clin North Am. 1993;20:383–401. [PubMed] [Google Scholar]
- de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of humane disease. In: Maggi CA, editor. The autonomic nervous system: nervous control of the urogenital system. Vol. 3. London, UK: Harwood Academic Publishers; 1993. pp. 227–89. [Google Scholar]
- Floyd K, McMahon SB, Morrison JFB. Inhibitory interactions between colonic and vesical afferents in the micturition reflex of the cat. J Physiol. 1982;322:45–52. doi: 10.1113/jphysiol.1982.sp014021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezernik S, Craggs M, Grill WM, Creasey G, Rijkhoff NJ. Electrical stimulation for the treatment of bladder dysfunction: current status and future possibilities. Neurol Res. 2002;24:413–30. doi: 10.1179/016164102101200294. [DOI] [PubMed] [Google Scholar]
- Jezermik S, Grill WM, Sinkjaer T. Detection and inhibition of hyperrelexia-like bladder contraction in the cat by sacral nerve root recordings and electrical stimulation. Neurourol Urodyn. 2001;20:215–30. doi: 10.1002/1520-6777(2001)20:2<215::aid-nau23>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Jezermik S, Wen JG, Rijkhoff NJM, Djurhuus JC, Sinkjaer T. Analysis of bladder related nerve cuff electrode recordings from preganglionic pelvic nerve and sacral roots in pigs. J Urol. 2000;163:1309–14. [PubMed] [Google Scholar]
- Kamo I, Cannon TW, Conway DA, Torimoto K, Chancellor MB, de Groat WC. The role of bladder-to-urethral reflexes in urinary continence mechanisms in rats. Am J Physiol Renal Physiol. 2004;287:F434–41. doi: 10.1152/ajprenal.00038.2004. [DOI] [PubMed] [Google Scholar]
- Mackel R. Segmental and descending control of the external urethral and anal sphincters in the cat. J Physiol. 1979;294:105–22. doi: 10.1113/jphysiol.1979.sp012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–49. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The pudendo–pudendal reflex in male and female rats. J Auton Nerv Syst. 1989;27:67–77. doi: 10.1016/0165-1838(89)90130-6. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Morrison JF. Factors that determine the excitability of parasympathetic reflexes to the cat bladder. J Physiol. 1982;322:35–43. doi: 10.1113/jphysiol.1982.sp014020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Morrison JF, Spillane K. An electrophysiological study of somatic and visceral convergence in the reflex control of the external sphincters. J Physiol. 1982;328:379–87. doi: 10.1113/jphysiol.1982.sp014271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Spillane K. Brain stem influences on the parasympathetic supply to the urinary bladder of the cat. Brain Res. 1982;234:237–49. doi: 10.1016/0006-8993(82)90865-4. [DOI] [PubMed] [Google Scholar]
- Pacheco P, Camacho MA, Garcia LI, Hernandez ME, Carrillo P, Manzo J. Electrophysiological evidence for the nomenclature of the pudendal nerve and sacral plexus in the male rat. Brain Res. 1997;763:202–8. doi: 10.1016/s0006-8993(97)00408-3. [DOI] [PubMed] [Google Scholar]
- Pacheco P, Martinez-Gomez M, Whipple B, Beyer C, Komisaruk BR. Somato-motor components of the pelvic and pudendal nerves of the female rat. Brain Res. 1989;490:85–94. doi: 10.1016/0006-8993(89)90433-2. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neurosci Lett. 1998;244:137–40. doi: 10.1016/s0304-3940(98)00155-4. [DOI] [PubMed] [Google Scholar]
- Stalberg E, Andreassen S, Falck B, Lang H, Rosenfalck A, Trojaborg W. Quantitative analysis of individual motor unit potentials: a proposition for standardized terminology and criteria for measurement. J Clin Neurophysiol. 1986;3:313–48. doi: 10.1097/00004691-198610000-00003. [DOI] [PubMed] [Google Scholar]
- Tai C, Roppolo JR, de Groat WC. Block of external urethral sphincter contraction by high frequency electrical stimulation of pudendal nerve. J Urol. 2004;172:2069–72. doi: 10.1097/01.ju.0000140709.71932.f0. [DOI] [PubMed] [Google Scholar]
- Thor KB, Hisamitsu T, Roppolo JR, Tuttle P, Nagel J, deGroat WC. Selective inhibitory effects of ethylketocyclazocine on reflex pathways to the external urethral sphincter of the cat. J Pharmacol Exp Ther. 1989a;248:1018–25. [PubMed] [Google Scholar]
- Thor KB, Morgan C, Nadelhaft I, Houston M, de Groat WC. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989b;288:263–79. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Lew HL, Date E, Bih LI. Treatment of detrusor-sphincter dyssynergia by pudendal nerve block in patients with spinal cord injury. Arch Phys Med Rehabil. 2002;83:714–7. doi: 10.1053/apmr.2002.31609. [DOI] [PubMed] [Google Scholar]
- Yokoyama O, Yoshiyama M, Namiki M, de Groat WC. Interaction between D2 dopaminergic and glutamatergic excitatory influences on lower urinary tract function in normal and cerebral-infarcted rats. Exp Neurol. 2001;169:148–55. doi: 10.1006/exnr.2001.7639. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Erickson KA, Erdman SL, de Groat WC. Effects of N-methyl-D-aspartate (dizocilpine) and alpha-amino-3-hydroxy -4-isoxazolepropionate (LY215490) receptor antagonists on the voiding reflex induced by perineal stimulation in the neonatal rat. Neuroscience. 1999;90:1415–20. doi: 10.1016/s0306-4522(98)00545-4. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Roppolo JR, de Groat WC. Effects of MK-801 on the micturition reflex in the rat—possible sites of action. J Pharmacol Exp Ther. 1993;265:844–50. [PubMed] [Google Scholar]
- Yoshiyama M, Roppolo JR, de Groat WC. Alteration by urethane of glutamatergic control of micturition. Eur J Pharmacol. 1994;264:417–25. doi: 10.1016/0014-2999(94)00505-2. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Roppolo JR, de Groat WC. Effects of LY215490, a competitive alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor antagonist, on the micturition reflex in the rat. J Pharmacol Exp Ther. 1997;280:894–904. [PubMed] [Google Scholar]