Abstract
P-glycoprotein (P-gp) antagonists inhibit ceramide metabolism at the juncture of glycosylation. The purpose of this study was to test whether targeting P-gp would be a viable alternative to targeting glucosylceramide synthase (GCS) for enhancing ceramide cytotoxicity. A2780 wild-type, and multidrug resistant 2780AD and NCI/ADR-RES human ovarian cancer cell lines and the cell-permeable ceramide analog, C6-ceramide (C6-cer), were employed. Compared to P-gp-poor A2780 cells, P-gp-rich 2780AD cells converted 3.7-fold more C6-cer to nontoxic C6-glucosylceramide (C6-GC), whereas cell-free GCS activities were equal. 2780AD cells displayed resistance to C6-cer (10 μM) that was reversed by inclusion of the P-gp antagonist tamoxifen (5 μM) but not by inclusion of a GCS inhibitor. Co-administration of C6-cer and P-gp antagonists was also effective in NCI/ADR-RES cells. For example, C6-cer, VX-710 (Biricodar), and cyclosporin A (cyc A) exposure resulted in viabilities of ~90% of control; however, C6-cer/VX-710 and C6-cer/cyc A additions were synergistic and resulted in viabilities of 22 and 17%, respectively. Further, whereas C6-ceramide and cyc A imparted 1.5- and zero-fold increases in caspase 3/7 activity, the combination produced a 3.5-fold increase. Although the upstream elements of cell death have not been elucidated, the novel C6-ceramide/P-gp antagonist combination merits further study and assessment of clinical translational potential.
Keywords: Ceramide, C6-ceramide, P-glycoprotein, P-glycoprotein antagonists, drug resistance, ovarian cancer
Introduction
This investigation was conducted to evaluate the effect of P-glycoprotein (P-gp) antagonists on ceramide cytotoxicity in cancer cells. We administered ceramide exogenously by introducing a short-chain, cell-permeable analog, C6-ceramide [1] to cells in culture instead of stimulating ceramide generation in situ. Generally, ceramide cytotoxicity can be increased by limiting ceramide clearance; this is usually accomplished by introduction of inhibitors of ceramide-metabolizing enzymes [2]. However, because multidrug transporters like P-gp, when located intracellularly, also regulate short-chain ceramide metabolism [3–6], they could serve as targets for regulating ceramide cytotoxicity.
Overexpression of P-gp is one of the most consistent biological alterations in drug resistance [7–9]. P-gp is a member of the ATP-binding cassette (ABC) superfamily of membrane-resident transporter proteins [7]. It is a 170 kDa protein encoded in humans by the MDR1 gene (gene symbol ABCB1). Other prominent members of the ABC super family of transporter proteins include multidrug resistance protein 1 (MRP1) (gene symbol ABCC1), and breast cancer resistance protein (BCRP, gene symbol ABCG2) [10–12]. These proteins reduce the intracellular concentration of anticancer agents via ATP-dependent effluxing and in this way greatly limit therapeutic efficacy.
Drug transporters continue to be a major focus of laboratory and clinical studies aimed at improving cancer therapy. With few expectations, research in this area has been directed to overcome the chemotherapy efflux capacity of the multidrug transporters with the goal of attaining lethal drug concentrations at the intended site [13–15]. In ovarian cancer, clinical studies have employed P-gp antagonists in combination with chemotherapy as a means to overcome drug resistance [16–18]. Whereas this remains a promising line of investigation, clinical use of antagonists has provided little progress in treating drug resistance [19]. In the present study we have employed drug-resistant ovarian cancer cell lines as our model.
The expression of P-gp and its relationship to prognosis and treatment outcome is striking in ovarian cancer. Overexpression of P-gp has been shown to be correlated with disease progression during first-line chemotherapy [20]. In a retrospective survival analysis study, it was shown that expression of the MDR1 phenotype was a powerful influence on paclitaxel response [21]. In a similar study using immunohistochemistry, P-gp expression was detected in 47% of untreated cases and correlated with unfavorable prognostic factors such as presence of ascites and larger residual disease after surgery [22]. From these and other studies [23–25], it becomes clear that P-gp expression occurs with high frequency in ovarian cancer and plays a major role in prognosis and treatment response. In spite of this, the employ of P-gp antagonists to increase anticancer drug retention in ovarian cancer patients has not met with clinical success [16, 26, 27].
Apoptosis is a major pathway by which cytotoxic agents induce cell death, and a growing body of evidence shows that alterations in apoptotic pathways are important effectors of response to chemotherapy [28]. Ceramide has been shown to play a central role in both apoptotic and mitogenic pathways [2, 29–32]. Ceramide is generated in mass in response to various stressors including cytokines, radiation, and chemotherapy [33–36]. Control of ceramide metabolism is an effective means for increasing sensitivity to various therapeutic agents [2, 33, 36]. For example, up- and downregulation of glucosylceramide synthase (GCS) has been demonstrated to confer drug resistance and sensitize cancer cells to chemotherapy, respectively [37, 38]. In addition to enzymatic regulation of ceramide glycosylation, we have demonstrated that antagonists of multidrug transporters inhibit GC synthesis [39]. This finding interfaces well with discoveries by Eckford and Sharom [3], Borst et al [4], van Helvoort et al [5], and De Rosa et al [6] who showed that P-gp and other drug transport proteins function in glycolipid trafficking. We hypothesize that drug transporters can be effective targets for augmenting ceramide-governed cell death. Therefore, P-gp, which is a constituent of ovarian tumors regardless of disease stage and drug resistance status, presents us with an alternative to GCS for enhancing ceramide-related therapies, specifically in the present work, short-chain ceramide.
Materials and methods
Materials
[3H]UDP-glucose (40 Ci/m mol), [9, 10-3H(N)]palmitic acid (60 Ci/mmol), and N-hexanoyl[1-14C]-D-erythro-sphingosine (C6-ceramide) (55 mCi/mmol) were purchased from American Radiolabeled Chemicals (St. Louis, MO). The GCS inhibitor ethylenedioxy-P4 [40], a phenyl ring substituted analog of parent P4, D-threo-1-phenyl-2-hexadecanoylamino-3-pyrrodilino-1-propanol (molecular weight 509), was a gift from Dr. James Shayman, University of Michigan, Ann Arbor. C6-ceramide, glucosyl-C8-ceramide (D-glucosyl-β-1-1′-N-octanoyl-D-erythro-sphingosine), egg yolk L-α-phosphatidylcholine, Lα-phosphatidylcholine (dioleoyl), and brain sulfatide, ammonium salt, were purchased from Avanti Polar Lipids, Inc (Alabaster, AL). Short-chain sphingomyelin, N-hexanoyl-sphingosylphosphorylcholine (C6) was from Matreya (Pleasant Gap, PA). Tamoxifen, verapamil, and cyclosporin A were purchased from Sigma-Aldrich (St. Louis). VX-710 (Biricodar) was provided by Vertex Pharmaceuticals (Cambridge, MA). SP600125, a specific inhibitor of c-Jun N-terminal kinase (JNK), SB20358, an inhibitor of p38 MAPK, and PD98059, a selective MAPK inhibitor (MEK), were purchased from Enzo Life Sciences (Plymouth Meeting, PA), and Fumonisin B1 (FB1) was purchased from EMD Chemicals (Gibbstown, NJ). Monoclonal antibody C219 against human P-glycoprotein was a product of EMD Chemicals (Gibbstown, NJ). Silica Gel G prescored thin-layer chromatography (TLC) plates were purchased from Analtech (Newark, DE), and solvents certified A.C.S. or HPLC grade, were purchased from Fischer Scientific. EcoLume for liquid scintillation counting (LSC) was purchased from ICN (Costa Mesa, CA).
Cell Lines
The human ovarian cancer cell line A2780 was obtained from the American Type Culture Collection (Bethesda, MD) and a doxorubicin-resistant counterpart, 2780AD, was provided by Dr. Thomas C. Hamilton (Fox Chase Cancer Center, Philadelphia, PA). These cell lines were maintained in RPMI 1640 medium containing 10% FBS and additives as described [41]. NCI/ADR-RES, an adriamycin-resistant human ovarian cancer cell line, was provided by Drs. M.E. Goldsmith, NCI, Bethesda, MD, and K.H. Cowan, University of Nebraska Medical Center, Omaha, NE. These cells were maintained in RPMI 1640 medium with 10% FBS. 2780AD cells were maintained in medium containing 1.0 μg/ml doxorubicin, which was removed for experiments.
Cell Supplements
[14C]C6-ceramide and unlabeled C6-ceramide were dissolved in ethanol and added to culture media. The ethanol concentration was kept constant in all cultures within experiments and ranged between 0.05 – 0.2%. Ethanol was also vehicle for ethylenedioxy-P4, tamoxifen, VX-710, verapamil, and cyclosporin A. FB1 and SP600125 were dissolved in DMSO.
Western Blot
P-glycoprotein was detected by Western blot using C219 monoclonal antibody as previously described [42].
Cell-free GCS Assay
GCS activity was measured as previously described [37] using the 100,000 × g membrane fraction isolated from the various cell lines. This method is a modification of the procedure of Shulka and Radin [43]. The enzyme assay containing 50 μg of microsomal protein, in a final volume of 0.2 ml, was performed in a shaking water bath at 37 °C for 60 min. The reaction contained liposomal substrate composed of C6-ceramide (1.0 mM), phosphatidylcholine (3.6 mM; molecular weight, 786.15), and brain sulfatides (0.9 mM; molecular weight, 563). The liposomal substrate was prepared by mixing the components, evaporating the solvents under a stream of nitrogen, and sonicating in water over ice for 1 min using a microtip at 50% output (Kontes, Micro Ultrasonic Cell Disrupter). Other reaction components included sodium phosphate buffer (0.1 M), pH 7.8, EDTA (2.0 mM), MgCl2 (10 mM), dithiothreitol (1.0 mM), β-nicotinamide adenine dinucleotide (2.0 mM), and [3H]UDP-glucose (0.5 mM). Radiolabeled and unlabeled UDP-glucose were diluted to achieve the desired radiospecific activity (4700 dpm/nmol). To terminate the reaction, tubes were placed on ice, and 0.5 isopropanol and 0.4 ml Na2SO4 were added. After brief vortex missing, 3 ml t-butyl methyl ether was added, and tubes were mixed for 30 s. After centrifugation, 0.5 ml of the upper phase, which contained GC, was withdrawn and mixed with 4.5 ml of EcoLume for analysis of radioactivity by LSC.
Lipid Analysis
Total cellular lipids were extracted as previously described [44]. After nitrogen evaporation of the chloroform lower phase of the biphasic extraction, radiolabeled lipids were taken up in 200 μl chloroform/methanol (2:1, v/v) and a 5 μl aliquot was sampled for radioactivity by liquid scintillation counting. This sampling was used to calculate uptake and recoveries. The solvent was again evaporated, 50 μl chloroform/methanol was added, and of this, 10 μl was applied to the origin of a TLC plate. Lipids were resolved using a solvent system containing chloroform/methanol/acetic acid/water (65:25:2:2, v/v) or a slight variation there of, in filter paper-lined tanks. Two other solvent systems were employed to verify results: chloroform/methanol/ammonium hydroxide (70:30:4, v/v) and chloroform/methanol/water (60:40:8, v/v). These chromatography systems resolved C6-ceramide, C6-GC, and C6-lactosylceramide from corresponding natural counterparts. After separation, lipids were visualized in iodine vapor and identified by co-migration with commercial standards. Areas of interest were scrapped and radioactivity quantitated by LSC [45].
Cell Viability, Drug-Induced Cytotoxic Synergy, MAPK Inhibition
Cell viability was determined using the Cell Titer 96 Aqueous One Solution kit from Promega (Madison, WI). NCI/ADR-RES cells were seeded at a density of 3,000/well and A2780 and 2780AD cells were seeded at 5,000/well. Agents were added the following day for 72 hr. Absorbance at 490 nm was recorded with a Microplate Fluorescent Reader FL600, BIO-TEK Instruments, Inc (Winooski, VT). Drug-induced cytotoxic synergy was analyzed by CalcuSyn® software from Biosoft (Great Shelford, Cambridge, United Kingdom). Each condition (single agent and combination) was tested in replicates of six and repeated at least twice. The mean proliferative index for each compound at the indicated concentrations was entered into the CalcuSyn program for dose-effect analysis. By this method, a combination index (CI) is determined based on the Chou-Talalay method [46] where a CI of 1 indicates an additive effect, and CI’s of 0.7–0.85 and 0.3–0.7 indicate moderate synergism and synergism, respectively. The involvement of MAPK cascades in cytotoxicity elicited by combination C6-ceramide/cyclosporin A treatment in NCI/ADR-RES cells was assessed by introduction of SP600125, SB20358, and PD98059, inhibitors of c-JUN N-terminal kinase (JNK), p38, and MEK, respectively. Inhibitors were dissolved in DMSO and introduced for 60 min prior to treatment.
Caspase Activity
For caspase determinations, NCI/ADR-RES cells were seeded in 96-well plates at a density of 3,000 cells/well and cultured overnight. Following treatment for 24 hr, caspase 3/7 activity was determined using the Caspase 3/7 luminescence assay (Promega, Madison, WI) according to the manufacturer’s instructions. Data were recorded using a Promega GLOMAX multidetection system.
Microscopy
Photomicrographs were obtained on an Olympus IX70 microscope equipped with a mercury lamp. Images were collected on A Nikon DS-2Mv-UI and processed using NIS elements F 3.0 software.
Results
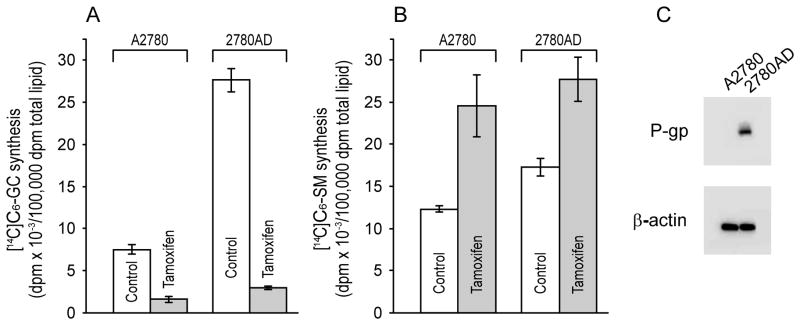
Initial experiments were designed to assess the metabolic fate of C6-ceramide in wild-type and multidrug resistant ovarian cancer cells and determine whether tamoxifen, which inhibits P-gp regulated transport [47] would influence metabolic fate. As shown in Fig. 1A (white bars), when challenged with equal amounts of C6-ceramide, multidrug resistant 2780AD cells generated 3.7-fold more C6-GC compared to the parental cell line, A2780. The difference in C6-SM synthesis in 2780AD cells was not as pronounced (40% higher than in A2780) (Fig. 1B, white bars). The addition of tamoxifen effectively blocked the large increase in C6-GC synthesis in 2780AD and unexpectedly inhibited C6-GC synthesis as well in wild-type A2780 cells (Fig. 1A, gray bars). Blocking C6-GC synthesis with tamoxifen resulted in compensatory increases in C6-SM synthesis in both cell lines (Fig. 1B). The levels of P-gp expression in A2780 and 2780AD cells is shown by Western blot (Fig. 1C); under our conditions, P-gp was undetectable in A2780 cells. Although synthesis of C6-GC was more pronounced in 2780AD cells compared to A2780 cells, cell-free GCS activity was nearly equal, 280 ± 21 and 252 ± 11 nmol C6-GC/mg protein/hr, respectively. Using the drug-resistant cell line as a model we sought to determine whether C6-ceramide cytotoxicity could be enhanced via the introduction of P-gp antagonists.
Fig. 1.
Metabolism of C6-ceramide in A2780 and 2780AD cells and the effect of tamoxifen. Cells seeded in 6-well plates (200,000/well) were supplemented with [14C]C6-ceramide (5 μg/ml medium) the following day in medium without and with tamoxifen (5.0 μM), and cultured for 24 hr before lipid extraction and analysis by TLC. A. C6-GC synthesis. B. C6-SM synthesis. C. P-gp expression detected by Western blot (25 μg protein). Data are the mean ± S.D. from triplicate cultures. Experiments, repeated 2–3 times, yielded similar results.
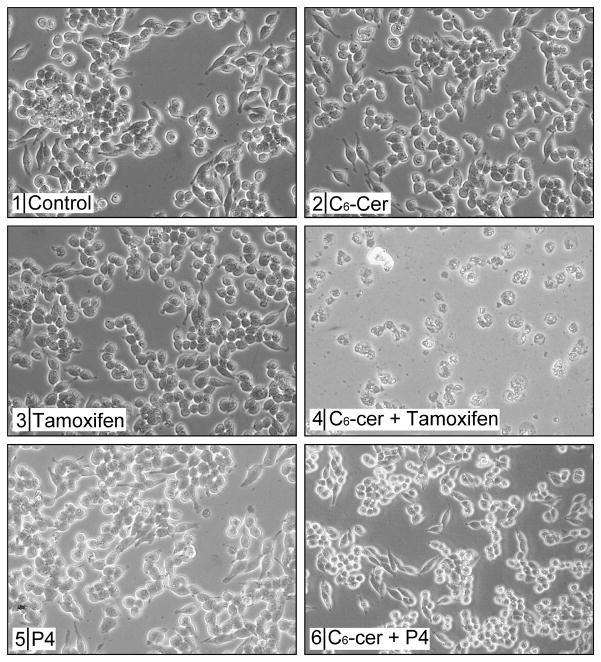
The capacity of P-gp antagonists to inhibit long-chain GC synthesis in intact multidrug resistant cancer cells [39, 48] suggests that targeting P-gp could work to enhance C6-ceramide cytotoxicity, an alternative strategy to targeting GCS [49]. The photomicrographs in Fig. 2 show that whereas 2780AD cells were largely refractory to single agent C6-ceramide and tamoxifen, co-administration was cytotoxic, and decreased cell number and produced cells with granular, vacuolated morphology (Fig. 2, panels 1–4). In contrast, co-administration of C6-ceramide and the GCS inhibitor, ethylenedioxy-P4, produced only slight cell rounding, whereas cell number was not greatly affected (Fig. 2, panels 1, 5, 6). These data demonstrate the superiority of a P-gp antagonist compared to a GCS inhibitor for amplifying C6-ceramide cytotoxicity.
Fig. 2.
Effect of tamoxifen, ethylenedioxy-P4, and C6-ceramide on 2780AD cell growth and morphology. Cells (200,000/well) were seeded in 6-well plates and exposed the following day to C6-ceramide (10 μg/ml), tamoxifen (5 μM), ethylenedioxy-P4 (0.2 μM), or the combinations designated, for 24 hr. Photomicrographs were taken to document growth and morphological characteristics. Ethanol (0.2%) was present in control cultures. C6-cer, C6-ceramide; P4, ethylenedioxy-P4.
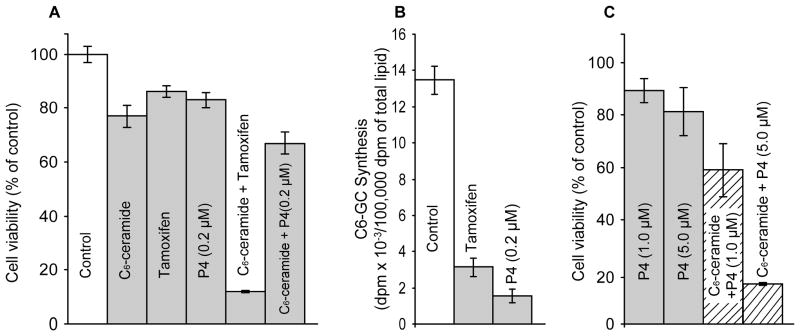
We next assessed the effect of C6-ceramide and partnering agents on 2780AD cell viability. As shown in Fig. 3A, single agent C6-ceramide, tamoxifen, and ethylenedioxy-P4 elicited only moderate decreases in cell viability (78–85% viable); however, co-administration of C6-ceramide and tamoxifen decreased viability to 12% of control. In contrast, partnering C6-ceramide with ethylenedioxy-P4 decreased cell viability to only 67% of control. Figure 3B shows that tamoxifen at a concentration of 5.0 μM and ethylenedioxy-P4 at a concentration of 0.2 μM inhibited C6-GC synthesis by 75 and 90%, respectively. The work of Sietsma et al [50] showed that 1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP), a GCS inhibitor of the same family as ethylenedioxy-P4, decreased chemotherapy efflux in cultured tumor cells, suggesting that GCS inhibitors also antagonize P-gp. Interestingly, increasing the concentration of ethylenedioxy-P4 from 0.2 to 5.0 μM, the concentration routinely used for the less potent but commonly employed analogs, PDMP and 1-phenyl-2-hexadecanoylamino-3-morpholino-1-propanol (PPMP), sensitized 2780AD cells to C6-ceramide (Fig. 3C). For example, although high dose ethylenedioxy-P4 (1.0 and 5.0 μM) decreased cell viability only slightly, similar with the 0.2 μM dose of ethylenedioxy-P4 (Fig. 3A), co-administration of high dose ethylenedioxy-P4 and C6-ceramide yielded cell viabilities of 58 and 15% of control, respectively (Fig. 3C, cross-hatch). Of note, the results of a similar experiment conducted in P-gp-poor A2780 cells showed that increasing the concentration of ethylenedioxy-P4 failed to enhance C6-ceramide cytotoxicity (data not shown).
Fig. 3.
Effect of C6-ceramide, tamoxifen, and ethylenedioxy-P4 on 2780AD cell viability. (A) Viability. Cells in 96-well plates were exposed to the agents shown (C6-ceramide, 10 μg/ml; tamoxifen 5 μM) for 24 hr, after which viability was measured by MTS assay. (B) Effect of tamoxifen and GCS inhibitor ethylenedioxy-P4 on C6-GC synthesis from C6-ceramide. 2780AD cells in 6-well plates were exposed to the agents indicated (tamoxifen, 5 μM) for 24 hr in medium containing [14C]C6-ceramide (1.0 μg/ml medium). [14C]C6-GC synthesis was measured by TLC of total lipid extracts. (C) Effect of high-dose ethylenedioxy-P4 on C6-ceramide cytotoxicity in 2780AD cells. Cells were exposed to 10 μg/ml C6-ceramide and the concentrations of ethylenedioxy-P4 indicated, for 24 hr. Viability was determined by MTS. Results are the mean ± S.D. of six replications for viability determinations. Repeated experiments yielded similar results. C6-GC synthesis results are the mean ± S.D., n = 3.
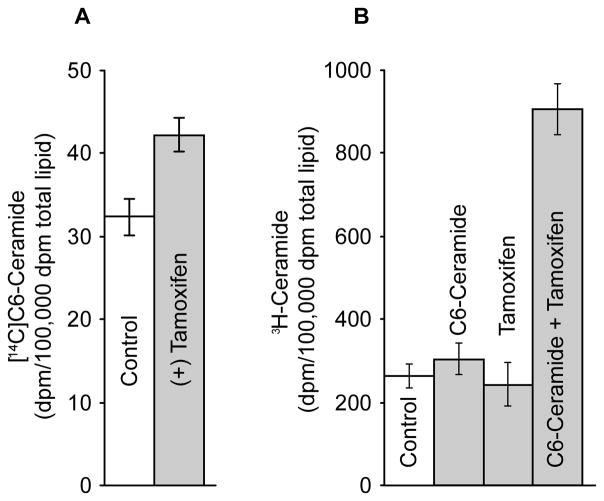
As shown in the above experiments, tamoxifen enhanced cytotoxicity of C6-ceramide in 2780AD cells. Although this was accompanied by a large decrease in the synthesis of C6-GC when tamoxifen was present (see Fig. 3B), only small increases in free, intracellular C6-ceramide (30%) resulted in response to tamoxifen inclusion (Fig. 4A). This in part is may be due to a channeling of C6-ceramide into C6-SM synthesis when tamoxifen was present (see Fig. 1B). Although it is well known that short-chain ceramides are cytotoxic in a number of systems [51, 52], Takeda et al [53] and Ogretmen et al [54] described a pathway whereby apoptosis occurred via a ceramide recycling pathway wherein sphingosine generated by hydrolysis of C6-ceramide was reacylated to form long-chain ceramide. We determined whether this pathway was functional in 2780AD cells by assaying the amount of 3H-palmitate-labeled long-chain ceramide generated in the absence and presence of unlabeled C6-ceramide. Figure 4B shows that whereas neither unlabeled C6-ceramide nor tamoxifen affected an appreciable change in synthesis of long-chain ceramide, co-administration of these agents produced a 3-fold increase over control in long-chain ceramide synthesis. Notably, C6-ceramide in combination with ethylenedioxy-P4 did not affect an increase in the synthesis of long-chain ceramide (data not shown). These data suggest that the cytotoxic effect of C6-ceramide/tamoxifen is in part related to synthesis of long-chain ceramides. To test this possibility, we assessed the effect of FB1, an inhibitor of ceramide synthase; however, the inclusion of FB1 failed to rescue cells from the cytotoxic effects of the drug combination.
Fig. 4.
Influence of tamoxifen on intracellular levels of C6-ceramide and on synthesis on long-chain ceramide from C6-ceramide in 2780AD cells. (A) Influence of tamoxifen on intracellular C6-ceramide levels. Cells in 6-well plates were exposed to [14C]C6-ceramide (5 μg/ml medium) in the absence and presence of tamoxifen (5 μM) for 24 hr. [14C]C6-ceramide was isolated from the total cellular lipid extracts by TLC and quantified by LSC. (B) Influence of tamoxifen on synthesis of long-chain ceramide from C6-ceramide. Cells in 6-well plates were cultured as indicated (unlabeled C6-ceramide, 5 μg/ml; tamoxifen 5 μM) in medium containing 1.0 μCi/ml [3H]palmitic acid for 24 hr. 3H-Ceramide was isolated and quantitated from total cellular lipid extracts by TLC and LSC. Results represent mean ± S.D., n = 3 cultures/condition.
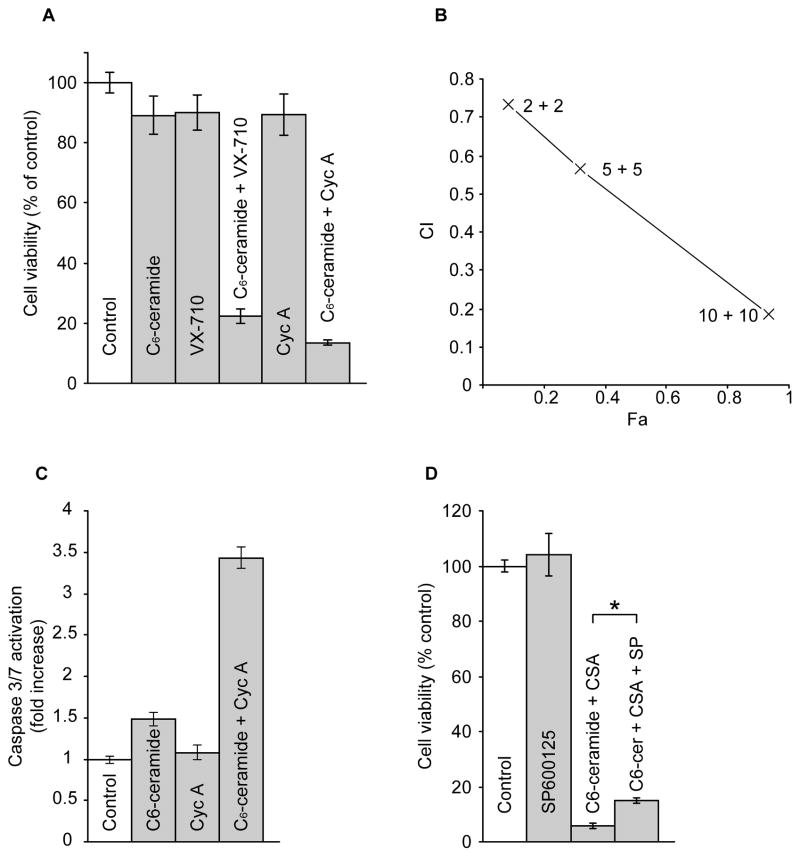
Lastly, we determined whether administration of C6-ceramide with P-gp antagonists would be cytotoxic in other multidrug resistant ovarian cancer cell lines. Both VX-710, a broad-spectrum modulator that interacts with P-gp, multidrug resistance protein (MRP1), and breast cancer resistance protein (BCRP) [55], and cyclosporin A, a P-gp and MRP antagonist [56], were effective in enhancing C6-ceramide cytotoxicity in NCI/ADR-RES cells [57](Fig. 5A). This is shown by marked decreases in cell viability with combination treatment compared to treatment with single agents. For example, C6-ceramide and VX-710 each reduced cell viability to 90% of control, whereas co-administration decreased cell viability to 22% of control. Combinations of C6-ceramide with cyclosporin A at 2, 5, and 10 μM synergistically enhanced cell killing, as shown by marked decreases in viability that resulted in Chou-Talalay derived CI’s of 0.73, 0.56, and 0.19, respectively as illustrated by the Fa (fraction affected)-CI plot (Fig. 5B). Using this same drug combination, we determined whether apoptosis was involved in the cytotoxic response. As shown in Fig. 5C, C6-ceramide elicited only a 1.5-fold enhancement in caspase 3/7 activation and cyclosporin A was essentially devoid of influence. However, the combination increased caspase activity 3.4-fold over control values. Lastly, by the employ of inhibitors, we investigated the contribution of stress kinases such as p38 and JNK in cytotoxicity elicited by combination C6-ceramide/cyclosporin A. Although there was a strong caspase component, the specific JNK inhibitor SP600125 afforded only modest protection from C6-ceramide/cyclosporin A cytotoxicity (Fig. 5D). We additionally assessed the influence of SB203580, a selective p38 MAPK inhibitor, and PD98059, a specific inhibitor of MEK. SB203580 exposure (1.0 and 10 μM) failed to rescue cells as tested after 72 hr exposure to C6-ceramide/cyclosporin A, and PD98059 tested at concentrations of 5.0 and 10 μM also did not effect NCI/ADR-RES cell viabilities in response to combination C6-ceramide/cyclosporin A (data not shown).
Fig. 5.
Effect of P-gp antagonists on C6-ceramide cytotoxicity and caspase activity in NCI/ADR-RES cells, and the effect of JNK inhibition on viability. (A) Cell viability. Cells in 96-well plates were grown with the agents indicated (C6-ceramide, 4 μg/ml; VX-710 and cyclosporin A, 5 μM) for 72 hr. Viability was measured by MTS assay. Results given as mean ± S.D., n = 6 cultures/condition. (B) C6-ceramide/cyclosporin A synergy. CI (Combination Index) vs. Fa (Fraction affected) plot obtained from 72 hr exposure of cells to increasing concentrations of C6-ceramide and cyclosporin A combinations. CI of 0.73 indicates moderate to slight synergism; CI of 0.56 indicates synergism; CI of 0.2 indicates strong synergism. Fa calculated from cell viability assays (MTS), n = 6 cultures/condition. (C) Caspase 3/7 activity. Cells in 96-well plates were exposed to C6-ceramide (4 μg/ml), cyclosporin A (10 μM) or the combinations, for 24 hr, after which caspase activation was measured by the Promega caspase 3/7 assay. Cyc A, cyclosporin A. Results represent the mean ± S.D., n = 3 cultures/condition. (D) Effect of SP600125 (SP) on C6-ceramide/Cyc A cytotoxicity. Cells in 96-well plates were cultured for 72 hr with C6-ceramide (4 μg/ml)/Cyc A (10 μM) in the absence and presence of JNK inhibitor SP600125 (10 μM). *p<0.01. Data are the mean ± S.D., n = 6 cultures/condition. Repeat experiments yielded identical results.
Discussion
The objective of this study was to investigate avenues for enhancing cytotoxicity of short-chain ceramides, analogs of natural long-chain ceramides and candidates for clinical investigation [58, 59]. The majority of studies on augmenting ceramide-induced apoptosis have focused on GCS as a control point [49, 60], either at the level of gene expression or by using chemical inhibitors of the enzyme. However, with the discovery that P-gp antagonists such as verapamil, cyclosporin A, tamoxifen, and toremifene inhibited GC synthesis in multidrug resistant cancer cells [39, 61, 62] came the idea that ceramide metabolism could be regulated by targeting multidrug resistance transport proteins. Subsequent studies by Shabbits and Mayer [63] showed that P-gp tempered ceramide-mediated sensitivity in breast cancer cells in response to paclitaxel, the tubulin-binding anticancer drug that induces de novo ceramide formation in breast cancer cells [35]. Later investigations by De Rosa et al [6] showed that drug resistance proteins played a role in neutral glycosphingolipid biosynthesis.
The present study demonstrates that multidrug resistant ovarian cancer cells display enhanced capacity for conversion of C6-ceramide to C6-GC compared to wild-type, even though the levels of GCS enzyme activity, as measured by cell-free assays, were nearly equal. This is characteristic to a number of multidrug resistant cancer cell lines and in some instances is accompanied by coordinate overexpression of GCS and P-gp [41, 44, 64, 65]. However, as pointed out by Veldman et al [66], increased GC levels in 2780AD cells result in part from decreased conversion of GC to LC.
Data from cell viability/proliferation assays and morphological assessment showed that C6-ceramide cytotoxicity could be enhanced in both 2780AD and NCI/ADR-RES cells by co-administration with antagonists of multidrug resistance transport proteins but not by co-administering the specific GCS inhibitor, ethylenedioxy-P4. P-gp translocates GC from cytosol to Golgi lumen [3–5, 67]. This property has been shown to enhance GC production by eliminating product inhibition of GCS [6, 63, 67]. In our study, low-dose ethylenedioxy-P4 (0.2 μM) inhibited C6-GC formation by 90% but had little effect on C6-ceramide cytotoxicity (see Fig. 3A, B). This demonstrated that inhibition of GCS failed to sensitize 2780AD cells to C6-ceramide. However, increasing the concentration of ethylenedioxy-P4 to 5.0 μM sensitized 2780AD cells to C6-ceramide. These data suggest that high dose ethylenedioxy-P4 interacts with P-gp in much the same fashion as tamoxifen to sensitize 2780AD cells to C6-ceramide. Thus, whereas low-dose ethylenedioxy-P4 acts as a potent inhibitor of GCS, at higher concentrations it may behave as a P-gp antagonist. This idea is supported by the work of Sietsma et al [50] who showed that the GCS inhibitor and chemical cousin of ethylenedioxy-P4, PDMP, decreased paclitaxel and vincristine efflux in neuroblastoma cells. Although 2780AD cells overexpress P-gp, Veldman et al [66], using the Golgi marker ManII in co-localization assays, did not detect P-gp in the Golgi apparatus of 2780AD cells. This argues against our premise that targeting intracellular P-gp is one means of regulating ceramide metabolism, at least in 2780AD cells. However, the process of cellular selection for doxorubicin resistance is complex, and the resultant clones used by Veldman et al [66] and in the present study could vary with regard to P-gp localization. Molinari et al [68] however demonstrated that a significant level of P-gp was expressed in the Golgi apparatus of NCI/ADR-RES cells, cells also used in the present study.
To determine whether the effect of P-gp antagonists on C6-ceramide cytotoxicity was limited to tamoxifen, which has been shown to reverse vinblastine resistance in multidrug resistant ovarian cancer cells [69], we evaluated other drug resistance modulators. Although tamoxifen was not as effective in NCI/ADR-RES cells, VX-710 and cyclosporin A enhanced C6-ceramide cytotoxicity in NCI/ADR-RES cells in a synergistic fashion. Although VX-710 and cyclosporin A demonstrate broad affinity for multidrug resistance proteins [55, 56], it is noteworthy that C6-ceramide-enhancing activity was not limited to tamoxifen, thus widening the applicability of our findings. Based on biochemical analysis of caspase 3/7 activity, combination C6-ceramide/cyclosporin A induced significant apoptotic cell death, and effect dependent on co-administration. Curiously, both the formation of long-chain ceramides (see Fig. 4B) and the stimulation of caspase 3/7 activity (Fig. 5C) were dependent on the presence of P-gp antagonists; however, addition of FB1, which effectively inhibits long-chain ceramide, failed to rescue 2780AD cells and A2780 cells from C6-ceramide/tamoxifen and C6-ceramide/cyclosporin A combinations, respectively. This suggests that long-chain ceramide generated in response to the binary regimens, is not contributing to the cytotoxic response.
In summary, this work demonstrates that partnering C6-ceramide with P-gp antagonists enhances cytotoxic response. NCI/ADR-RES cell rescue by the JNK inhibitor SP600125, although meager, suggests that this MAPK pathway is involved, in part, in apoptotic responses to C6-ceramide/cyclosporin A. The introduction of either SB203580 or PD98059, p38 and MEK inhibitors, respectively, did not alter viability status of NCI/ADR-RES cells exposed to the C6-ceramide/cyclosporin A regimen. Previous studies have shown that elevation of ceramide by treatment of cells with ceramide analogs activated JNK/SAPK cascade but not ERK [70]. Nica et al [71] in K562, a chronic myelogenous leukemia-derived cell line, showed that whereas cytotoxic C6-ceramide did not activate p38 kinase, it did promote JNK activation, and treatment of cells with SP600215 afforded partial rescue. In PC-3 prostate cancer cells, apoptosis induced by curcumin exposure, which was accompanied by ceramide accumulation, was not prevented by p38 MAPK or JNK inhibition. Mabuchi et al [72] demonstrated that tamoxifen induced apoptosis in cultured ovarian cancer cells via JNK and/or p38 cascades independently of estrogen receptor expression. Deciphering the mechanism of action of combination agents like C6-ceramide and tamoxifen can be arduous as tamoxifen can activate phospholipase C and D and elicit protein kinase C translocation [73], and as pointed out in a review by Mandlekar and Kong [74], induction of apoptosis by tamoxifen can as well enlist calmodulin, ceramide, JNK or p38, and mitochondrial/caspase cascades. Further, it is possible that the inclusion of C6-ceramide amplifies tamoxifen’s induction of ceramide-driven cell death pathways. Targeting multidrug transporters to control ceramide metabolism and apoptosis is a novel concept that has not been previously evaluated for therapeutic benefit. Because P-gp and MRP are constituents of intracellular organelles, this approach is independent of the multidrug resistant phenotype that is often a hallmark of later-stage disease. It is possible that P-gp can be more effective as a target to amplify short-chain ceramide cytotoxicity than as a target for attenuating chemotherapy efflux in vivo.
Acknowledgments
This study was supported by the National Institute of General Medical Sciences (grant no. GM77391). We thank Matthew Bush for compiling the typescript and creating the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abe A, Wu D, Shayman JA, Radin NS. Metabolic effects of short-chain ceramide and glucosylceramide on sphingolipids and protein kinase C. Eur J Biochem. 1992;210:765–773. doi: 10.1111/j.1432-1033.1992.tb17479.x. [DOI] [PubMed] [Google Scholar]
- 2.Modrak DE, Gold DV, Goldenberg DM. Sphingolipid targets in cancer therapy. Mol Cancer Ther. 2006;5:200–208. doi: 10.1158/1535-7163.MCT-05-0420. [DOI] [PubMed] [Google Scholar]
- 3.Eckford PD, Sharom FJ. The reconstituted P-glycoprotein multidrug transporter is a flippase for glucosylceramide and other simple glycosphingolipids. Biochem J. 2005;389:517–526. doi: 10.1042/BJ20050047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borst P, Zelcer N, van Helvoort A. ABC transporters in lipid transport. Biochim Biophys Acta. 2000;1486:128–144. doi: 10.1016/s1388-1981(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 5.van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, van Meer G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 6.De Rosa MF, Sillence D, Ackerley C, Lingwood C. Role of multiple drug resistance protein 1 in neutral but not acidic glycosphingolipid biosynthesis. J Biol Chem. 2004;279:7867–7876. doi: 10.1074/jbc.M305645200. [DOI] [PubMed] [Google Scholar]
- 7.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 8.Sikic BI. Modulation of multidrug resistance: a paradigm for translational clinical research. Oncology (Williston Park) 1999;13:183–187. [PubMed] [Google Scholar]
- 9.Bradley G, Ling V. P-glycoprotein, multidrug resistance and tumor progression. Cancer Metastasis Rev. 1994;13:223–233. doi: 10.1007/BF00689638. [DOI] [PubMed] [Google Scholar]
- 10.Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 11.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol. 2006;46:381–410. doi: 10.1146/annurev.pharmtox.46.120604.141238. [DOI] [PubMed] [Google Scholar]
- 13.Yang K, Wu J, Li X. Recent advances in the research of P-glycoprotein inhibitors. Biosci Trends. 2008;2:137–146. [PubMed] [Google Scholar]
- 14.Nobili S, Landini I, Giglioni B, Mini E. Pharmacological strategies for overcoming multidrug resistance. Curr Drug Targets. 2006;7:861–879. doi: 10.2174/138945006777709593. [DOI] [PubMed] [Google Scholar]
- 15.Baumert C, Hilgeroth A. Recent advances in the development of P-gp inhibitors. Anticancer Agents Med Chem. 2009;9:415–436. doi: 10.2174/1871520610909040415. [DOI] [PubMed] [Google Scholar]
- 16.Fracasso PM, Brady MF, Moore DH, Walker JL, Rose PG, Letvak L, Grogan TM, McGuire WP. Phase II study of paclitaxel and valspodar (PSC 833) in refractory ovarian carcinoma: a gynecologic oncology group study. J Clin Oncol. 2001;19:2975–2982. doi: 10.1200/JCO.2001.19.12.2975. [DOI] [PubMed] [Google Scholar]
- 17.Baekelandt M, Lehne G, Trope CG, Szanto I, Pfeiffer P, Gustavssson B, Kristensen GB. Phase I/II trial of the multidrug-resistance modulator valspodar combined with cisplatin and doxorubicin in refractory ovarian cancer. J Clin Oncol. 2001;19:2983–2993. doi: 10.1200/JCO.2001.19.12.2983. [DOI] [PubMed] [Google Scholar]
- 18.Lhomme C, Joly F, Walker JL, Lissoni AA, Nicoletto MO, Manikhas GM, Baekelandt MM, Gordon AN, Fracasso PM, Mietlowski WL, Jones GJ, Dugan MH. Phase III study of valspodar (PSC 833) combined with paclitaxel and carboplatin compared with paclitaxel and carboplatin alone in patients with stage IV or suboptimally debulked stage III epithelial ovarian cancer or primary peritoneal cancer. J Clin Oncol. 2008;26:2674–2682. doi: 10.1200/JCO.2007.14.9807. [DOI] [PubMed] [Google Scholar]
- 19.Hall MD, Handley MD, Gottesman MM. Is resistance useless? Multidrug resistance and collateral sensitivity. Trends Pharmacol Sci. 2009;30:546–556. doi: 10.1016/j.tips.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raspollini MR, Amunni G, Villanucci A, Baroni G, Boddi V, Rossi Degl’innocenti D, Taddei GL. Microvessel density in ovarian carcinoma: computer image analysis in patients with shorter and longer survival. Int J Gynecol Cancer. 2005;15:844–849. doi: 10.1111/j.1525-1438.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- 21.Penson RT, Oliva E, Skates SJ, Glyptis T, Fuller AF, Jr, Goodman A, Seiden MV. Expression of multidrug resistance-1 protein inversely correlates with paclitaxel response and survival in ovarian cancer patients: a study in serial samples. Gynecol Oncol. 2004;93:98–106. doi: 10.1016/j.ygyno.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 22.Baekelandt MM, Holm R, Nesland JM, Trope CG, Kristensen GB. P-glycoprotein expression is a marker for chemotherapy resistance and prognosis in advanced ovarian cancer. Anticancer Res. 2000;20:1061–1067. [PubMed] [Google Scholar]
- 23.Green H, Soderkvist P, Rosenberg P, Horvath G, Peterson C. ABCB1 G1199A polymorphism and ovarian cancer response to paclitaxel. J Pharm Sci. 2008;97:2045–2048. doi: 10.1002/jps.21169. [DOI] [PubMed] [Google Scholar]
- 24.Yakirevich E, Sabo E, Naroditsky I, Sova Y, Lavie O, Resnick MB. Multidrug resistance-related phenotype and apoptosis-related protein expression in ovarian serous carcinomas. Gynecol Oncol. 2006;100:152–159. doi: 10.1016/j.ygyno.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 25.Krasznai ZT, Friedlander E, Nagy A, Szabo G, Vereb G, Goda K, Hernadi Z. Quantitative and functional assay of MDR1/P170-mediated MDR in ascites cells of patients with ovarian cancer. Anticancer Res. 2005;25:1187–1192. [PubMed] [Google Scholar]
- 26.Hendrick AM, Harris AL, Cantwell BM. Verapamil with mitoxantrone for advanced ovarian cancer: a negative phase II trial. Ann Oncol. 1991;2:71–72. doi: 10.1093/oxfordjournals.annonc.a057830. [DOI] [PubMed] [Google Scholar]
- 27.Morgan RJ, Jr, Synold TW, Gandara D, Muggia F, Scudder S, Reed E, Margolin K, Raschko J, Leong L, Shibata S, Tetef M, Vasilev S, McGonigle K, Longmate J, Yen Y, Chow W, Somlo G, Carroll M, Doroshow JH. Phase II trial of carboplatin and infusional cyclosporine in platinum-resistant recurrent ovarian cancer. Cancer Chemother Pharmacol. 2004;54:283–289. doi: 10.1007/s00280-004-0818-x. [DOI] [PubMed] [Google Scholar]
- 28.Gewirtz DA, Holt SE, Grant S. Apoptosis, Senescence, and Cancer. Humana Press Inc; Totowa, NJ: 2007. [Google Scholar]
- 29.Fracasso PM. Overcoming drug resistance in ovarian carcinoma. Curr Oncol Rep. 2001;3:19–26. doi: 10.1007/s11912-001-0038-z. [DOI] [PubMed] [Google Scholar]
- 30.Radin NS. Killing tumours by ceramide-induced apoptosis: a critique of available drugs. Biochem J. 2003;371:243–256. doi: 10.1042/BJ20021878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruvolo PP. Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol Res. 2003;47:383–392. doi: 10.1016/s1043-6618(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 32.Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001;40:4893–4903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- 33.Senchenkov A, Litvak DA, Cabot MC. Targeting ceramide metabolism--a strategy for overcoming drug resistance. J Natl Cancer Inst. 2001;93:347–357. doi: 10.1093/jnci/93.5.347. [DOI] [PubMed] [Google Scholar]
- 34.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- 35.Charles AG, Han TY, Liu YY, Hansen N, Giuliano AE, Cabot MC. Taxol-induced ceramide generation and apoptosis in human breast cancer cells. Cancer Chemother Pharmacol. 2001;47:444–450. doi: 10.1007/s002800000265. [DOI] [PubMed] [Google Scholar]
- 36.Maurer BJ, Melton L, Billups C, Cabot MC, Reynolds CP. Synergistic cytotoxicity in solid tumor cell lines between N-(4-hydroxyphenyl)retinamide and modulators of ceramide metabolism. J Natl Cancer Inst. 2000;92:1897–1909. doi: 10.1093/jnci/92.23.1897. [DOI] [PubMed] [Google Scholar]
- 37.Liu YY, Han TY, Giuliano AE, Cabot MC. Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells. J Biol Chem. 1999;274:1140–1146. doi: 10.1074/jbc.274.2.1140. [DOI] [PubMed] [Google Scholar]
- 38.Liu YY, Han TY, Giuliano AE, Hansen N, Cabot MC. Uncoupling ceramide glycosylation by transfection of glucosylceramide synthase antisense reverses adriamycin resistance. J Biol Chem. 2000;275:7138–7143. doi: 10.1074/jbc.275.10.7138. [DOI] [PubMed] [Google Scholar]
- 39.Lavie Y, Cao H, Volner A, Lucci A, Han TY, Geffen V, Giuliano AE, Cabot MC. Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. J Biol Chem. 1997;272:1682–1687. doi: 10.1074/jbc.272.3.1682. [DOI] [PubMed] [Google Scholar]
- 40.Lee L, Abe A, Shayman JA. Improved inhibitors of glucosylceramide synthase. J Biol Chem. 1999;274:14662–14669. doi: 10.1074/jbc.274.21.14662. [DOI] [PubMed] [Google Scholar]
- 41.Liu YY, Yu JY, Yin D, Patwardhan GA, Gupta V, Hirabayashi Y, Holleran WM, Giuliano AE, Jazwinski SM, Gouaze-Andersson V, Consoli DP, Cabot MC. A role for ceramide in driving cancer cell resistance to doxorubicin. Faseb J. 2008 doi: 10.1096/fj.07-092981. [DOI] [PubMed] [Google Scholar]
- 42.Gouaze V, Liu YY, Prickett CS, Yu JY, Giuliano AE, Cabot MC. Glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Res. 2005;65:3861–3867. doi: 10.1158/0008-5472.CAN-04-2329. [DOI] [PubMed] [Google Scholar]
- 43.Shukla GS, Radin NS. Glucosyceramide synthase of mouse kidney: further characterization with an improved assay method. Arch Biochem Biophys. 1990;283:372–378. doi: 10.1016/0003-9861(90)90657-k. [DOI] [PubMed] [Google Scholar]
- 44.Gouaze V, Yu JY, Bleicher RJ, Han TY, Liu YY, Wang H, Gottesman MM, Bitterman A, Giuliano AE, Cabot MC. Overexpression of glucosylceramide synthase and P-glycoprotein in cancer cells selected for resistance to natural product chemotherapy. Mol Cancer Ther. 2004;3:633–639. [PubMed] [Google Scholar]
- 45.Gouaze-Andersson V, Yu JY, Kreitenberg AJ, Bielawska A, Giuliano AE, Cabot MC. Ceramide and glucosylceramide upregulate expression of the multidrug resistance gene MDR1 in cancer cells. Biochim Biophys Acta. 2007;1771:1407–1417. doi: 10.1016/j.bbalip.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 47.Callaghan R, Higgins CF. Interaction of tamoxifen with the multidrug resistance P-glycoprotein. Br J Cancer. 1995;71:294–299. doi: 10.1038/bjc.1995.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabot MC, Giuliano AE, Volner A, Han TY. Tamoxifen retards glycosphingolipid metabolism in human cancer cells. FEBS Lett. 1996;394:129–131. doi: 10.1016/0014-5793(96)00942-8. [DOI] [PubMed] [Google Scholar]
- 49.Cabot M. Ceramide Glycosylation and Chemotherapy Resistance. In: Futerman AH, editor. Ceramide Signaling. Vol. 21. Landes Bioscience; Georgetown, Texas: 2002. pp. 133–139. [Google Scholar]
- 50.Sietsma H, Veldman RJ, Kolk D, Ausema B, Nijhof W, Kamps W, Vellenga E, Kok JW. 1-phenyl-2-decanoylamino-3-morpholino-1-propanol chemosensitizes neuroblastoma cells for taxol and vincristine. Clin Cancer Res. 2000;6:942–948. [PubMed] [Google Scholar]
- 51.Auzenne E, Leroux ME, Hu M, Pollock RE, Feig B, Klostergaard J. Cytotoxic effects of sphingolipids as single or multi-modality agents on human melanoma and soft tissue sarcoma in vitro. Melanoma Res. 1998;8:227–239. doi: 10.1097/00008390-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Fillet M, Bentires-Alj M, Deregowski V, Greimers R, Gielen J, Piette J, Bours V, Merville MP. Mechanisms involved in exogenous C2- and C6-ceramide-induced cancer cell toxicity. Biochem Pharmacol. 2003;65:1633–1642. doi: 10.1016/s0006-2952(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 53.Takeda S, Mitsutake S, Tsuji K, Igarashi Y. Apoptosis occurs via the ceramide recycling pathway in human HaCaT keratinocytes. J Biochem. 2006;139:255–262. doi: 10.1093/jb/mvj026. [DOI] [PubMed] [Google Scholar]
- 54.Ogretmen B, Pettus BJ, Rossi MJ, Wood R, Usta J, Szulc Z, Bielawska A, Obeid LM, Hannun YA. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J Biol Chem. 2002;277:12960–12969. doi: 10.1074/jbc.M110699200. [DOI] [PubMed] [Google Scholar]
- 55.Minderman H, O’Loughlin KL, Pendyala L, Baer MR. VX-710 (biricodar) increases drug retention and enhances chemosensitivity in resistant cells overexpressing P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Clin Cancer Res. 2004;10:1826–1834. doi: 10.1158/1078-0432.ccr-0914-3. [DOI] [PubMed] [Google Scholar]
- 56.Germann UA, Shlyakhter D, Mason VS, Zelle RE, Duffy JP, Galullo V, Armistead DM, Saunders JO, Boger J, Harding MW. Cellular and biochemical characterization of VX-710 as a chemosensitizer: reversal of P-glycoprotein-mediated multidrug resistance in vitro. Anticancer Drugs. 1997;8:125–140. doi: 10.1097/00001813-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Liscovitch M, Ravid D. A case study in misidentification of cancer cell lines: MCF-7/AdrR cells (re-designated NCI/ADR-RES) are derived from OVCAR-8 human ovarian carcinoma cells. Cancer Lett. 2007;245:350–352. doi: 10.1016/j.canlet.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Tran MA, Smith CD, Kester M, Robertson GP. Combining nanoliposomal ceramide with sorafenib synergistically inhibits melanoma and breast cancer cell survival to decrease tumor development. Clin Cancer Res. 2008;14:3571–3581. doi: 10.1158/1078-0432.CCR-07-4881. [DOI] [PubMed] [Google Scholar]
- 59.Stover TC, Kim YS, Lowe TL, Kester M. Thermoresponsive and biodegradable linear-dendritic nanoparticles for targeted and sustained release of a pro-apoptotic drug. Biomaterials. 2008;29:359–369. doi: 10.1016/j.biomaterials.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 60.Bleicher RJ, Cabot MC. Glucosylceramide synthase and apoptosis. Biochim Biophys Acta. 2002;1585:172–178. doi: 10.1016/s1388-1981(02)00338-4. [DOI] [PubMed] [Google Scholar]
- 61.Lucci A, Giuliano AE, Han TY, Dinur T, Liu YY, Senchenkov A, Cabot MC. Ceramide toxicity and metabolism differ in wild-type and multidrug-resistant cancer cells. Int J Oncol. 1999;15:535–540. doi: 10.3892/ijo.15.3.535. [DOI] [PubMed] [Google Scholar]
- 62.Lucci A, Han TY, Liu YY, Giuliano AE, Cabot MC. Modification of ceramide metabolism increases cancer cell sensitivity to cytotoxics. Int J Oncol. 1999;15:541–546. doi: 10.3892/ijo.15.3.541. [DOI] [PubMed] [Google Scholar]
- 63.Shabbits JA, Mayer LD. P-glycoprotein modulates ceramide-mediated sensitivity of human breast cancer cells to tubulin-binding anticancer drugs. Mol Cancer Ther. 2002;1:205–213. [PubMed] [Google Scholar]
- 64.Lavie Y, Cao H, Bursten SL, Giuliano AE, Cabot MC. Accumulation of glucosylceramides in multidrug-resistant cancer cells. J Biol Chem. 1996;271:19530–19536. doi: 10.1074/jbc.271.32.19530. [DOI] [PubMed] [Google Scholar]
- 65.Lucci A, Cho WI, Han TY, Giuliano AE, Morton DL, Cabot MC. Glucosylceramide: a marker for multiple-drug resistant cancers. Anticancer Res. 1998;18:475–480. [PubMed] [Google Scholar]
- 66.Veldman RJ, Klappe K, Hinrichs J, Hummel I, van der Schaaf G, Sietsma H, Kok JW. Altered sphingolipid metabolism in multidrug-resistant ovarian cancer cells is due to uncoupling of glycolipid biosynthesis in the Golgi apparatus. Faseb J. 2002;16:1111–1113. doi: 10.1096/fj.01-0863fje. [DOI] [PubMed] [Google Scholar]
- 67.Lala P, Ito S, Lingwood CA. Retroviral transfection of Madin-Darby canine kidney cells with human MDR1 results in a major increase in globotriaosylceramide and 10(5)- to 10(6)-fold increased cell sensitivity to verocytotoxin. Role of p-glycoprotein in glycolipid synthesis. J Biol Chem. 2000;275:6246–6251. doi: 10.1074/jbc.275.9.6246. [DOI] [PubMed] [Google Scholar]
- 68.Molinari A, Calcabrini A, Meschini S, Stringaro A, Crateri P, Toccacieli L, Marra M, Colone M, Cianfriglia M, Arancia G. Subcellular detection and localization of the drug transporter P-glycoprotein in cultured tumor cells. Curr Protein Pept Sci. 2002;3:653–670. doi: 10.2174/1389203023380413. [DOI] [PubMed] [Google Scholar]
- 69.Kirk J, Houlbrook S, Stuart NS, Stratford IJ, Harris AL, Carmichael J. Selective reversal of vinblastine resistance in multidrug-resistant cell lines by tamoxifen, toremifene and their metabolites. Eur J Cancer. 1993;29A:1152–1157. doi: 10.1016/s0959-8049(05)80306-5. [DOI] [PubMed] [Google Scholar]
- 70.Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 71.Nica AF, Tsao CC, Watt JC, Jiffar T, Kurinna S, Jurasz P, Konopleva M, Andreeff M, Radomski MW, Ruvolo PP. Ceramide promotes apoptosis in chronic myelogenous leukemia-derived K562 cells by a mechanism involving caspase-8 and JNK. Cell Cycle. 2008;7:3362–3370. doi: 10.4161/cc.7.21.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mabuchi S, Ohmichi M, Kimura A, Ikebuchi Y, Hisamoto K, Arimoto-Ishida E, Nishio Y, Takahashi K, Tasaka K, Murata Y. Tamoxifen inhibits cell proliferation via mitogen-activated protein kinase cascades in human ovarian cancer cell lines in a manner not dependent on the expression of estrogen receptor or the sensitivity to cisplatin. Endocrinology. 2004;145:1302–1313. doi: 10.1210/en.2003-0709. [DOI] [PubMed] [Google Scholar]
- 73.Cabot MC, Zhang Z, Cao H, Lavie Y, Giuliano AE, Han TY, Jones RC. Tamoxifen activates cellular phospholipase C and D and elicits protein kinase C translocation. Int J Cancer. 1997;70:567–574. doi: 10.1002/(sici)1097-0215(19970304)70:5<567::aid-ijc13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 74.Mandlekar S, Kong AN. Mechanisms of tamoxifen-induced apoptosis. Apoptosis. 2001;6:469–477. doi: 10.1023/a:1012437607881. [DOI] [PubMed] [Google Scholar]