Abstract
The notion that dietary flavonoids exert beneficial health effects in humans is often based on in vitro studies using the glycoside or aglycone forms of these flavonoids. However, flavonoids are extensively metabolized in humans, resulting in formation of glucuronide, methyl and sulphate derivatives, which may have different properties than their parent compounds. The goal of this study was to investigate whether different chemical modifications of the same flavonoid molecule affect its biological and antioxidant activities. Hence, we studied the anti-inflammatory effects of several major human metabolites of quercetin and (−)-epigallocatechin-3-O-gallate (EGCG) by assessing their inhibitory effects on tumor necrosis factor α (TNFα)-induced protein expression of cellular adhesion molecules in human aortic endothelial cells (HAEC). HAEC were incubated with 1–30 μM quercetin, 3′- or 4′-O-methyl-quercetin, quercetin-3-O-glucuronide and quercetin-3′-sulphate; or 20–100 μM EGCG, 4″-O-methyl-EGCG and 4′,4″-di-O-methyl-EGCG, prior to co-incubation with 100 U/ml of TNFα. 3′-O-Methyl-quercetin, 4′-O-methyl-quercetin and their parent aglycone compound, quercetin, all effectively inhibited expression of intercellular adhesion molecule-1 (ICAM-1) with IC50 values (concentration required for 50% inhibition) of 8.0, 5.0 and 4.4 μM, respectively; E-selectin expression was suppressed to a somewhat lesser but still significant degree by all three compounds, whereas vascular cell adhesion molecule-1 (VCAM-1) was not affected. In contrast, quercetin-3-O-glucuronide (20–100 μM), quercetin-3′-O-sulphate (10–30 μM) and phenolic acid metabolites of quercetin (20–100 μM) did not inhibit adhesion molecule expression. 4′,4″-di-O-methyl-EGCG selectively inhibited ICAM-1 expression with an IC50 value of 94 μM, whereas EGCG (20–60 μM) and 4″-O-methyl-EGCG (20–100 μM) had no effect. The inhibitory effects of 3′-O-methyl-quercetin and 4′,4″-di-O-methyl-EGCG on adhesion molecule expression were not related to either inhibition of NF-κB activation or their antioxidant reducing capacity. Our data indicate that flavonoid metabolites have different biological and antioxidant properties than their parent compounds, and suggest that data from in vitro studies using non-metabolites of flavonoids are of limited relevance in vivo.
INTRODUCTION
The health benefits of consuming fruits and vegetables are often attributed, in part, to their high content of polyphenolic compounds [1, 2]. The consumption of polyphenols in a plant-derived diet can be several times higher than the consumption of other phytochemicals and vitamins, including ascorbic acid (vitamin C), α-tocopherol (vitamin E) or carotenoids [3, 4]. Among the polyphenols, flavonoids have attracted considerable attention. Epidemiological studies have found that an increased intake of dietary flavonoids is associated with a decreased risk of chronic diseases, including certain cancers and cardiovascular diseases [5–8]. Dietary flavonoids have many interesting in vitro properties, including antioxidant and anti-inflammatory effects, which have been postulated to underlie their beneficial health effects [9–12].
Flavonoids undergo extensive first-pass metabolism, and the chemical forms of flavonoids present in fruits and vegetables, usually glycosides or aglycones, are quite different from their in vivo metabolites. In the intestinal mucosa and the liver, flavonoids are subjected to extensive glucuronidation, methylation and sulphation [4, 13]. Thus, after intake of flavonoid-rich foods, these flavonoid metabolites are the main forms found in the circulatory system, where they are present for up to 4–6 hours (e.g., catechins) [14, 15] or may remain longer, probably as a result of enterohepatic circulation (e.g., quercetin) [16–18]. As with pharmaceutical drugs and other xenobiotics [19], biotransformation greatly affects the physical and chemical properties of flavonoids, making them more water-soluble and readily excreted in bile and urine.
Therefore, in this study we investigated whether different chemical modifications of the same flavonoid molecule alter its biological activity and antioxidant capacity. To this end, we compared the effects of different derivatives of two representative flavonoids, the flavonol, quercetin, and the flavan-3-ol, (−)-epigallocatechin-3-O-gallate (EGCG), to their respective parent aglycones. Specifically, we studied whether O-methylation of the flavonoid B ring, glucuronidation or sulphation of quercetin and O-methylation of EGCG affect the antioxidant properties of quercetin and EGCG and their anti-inflammatory effects on tumor necrosis factor α (TNFα)-induced activation of human aortic endothelial cells (HAEC). Endothelial activation was characterized by surface protein expression of cellular adhesion molecules and release of monocyte chemotactic protein-1 (MCP-1), which is known to critically contribute to monocyte recruitment to the vascular wall and, hence, initiation of vascular inflammation and atherosclerotic lesion formation [20, 21]. Our results show that biotransformation of dietary flavonoids can result in loss or gain of biological or antioxidant activity, which cannot be predicted from the chemical nature of the flavonoid.
MATERIAL AND METHODS
Materials
3′-O-Methyl-quercetin (isorhamnetin) and 4′-O-methyl-quercetin (tamarixetin) were obtained from Indofine (Hillsborough, NJ). Quercetin and EGCG were purchased from Sigma-Aldrich (St. Louis, MO). TNFα was purchased from Roche (Mannheim, Germany). All other chemicals were of the highest grade available.
Metabolites of quercetin
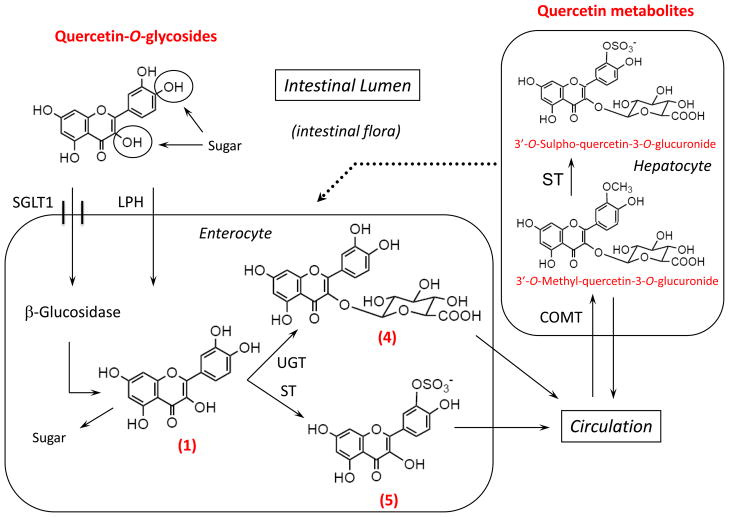
Quercetin-3′-O-sulphate was synthesized according to the method described by Day et al. [22], while quercetin-3-O-glucuronide extracted from developing seeds of French bean (Phaseolus vulgaris) with methanol was purified by liquid-liquid partitioning and preparative HPLC. The phenolic acids 3,4-dihydroxyphenylacetic acid, 3,4-dihydroxybenzoic acid, 3-hydroxyphenylacetic acid and 3-methoxy-4-hydroxyphenylacetic acid were obtained from Sigma-Aldrich. The 3-(3′-hydroxyphenyl)propionic acid was obtained from Fluorochem, (Derby, UK). The structures of quercetin and related compounds are shown in Scheme I.
Scheme I.
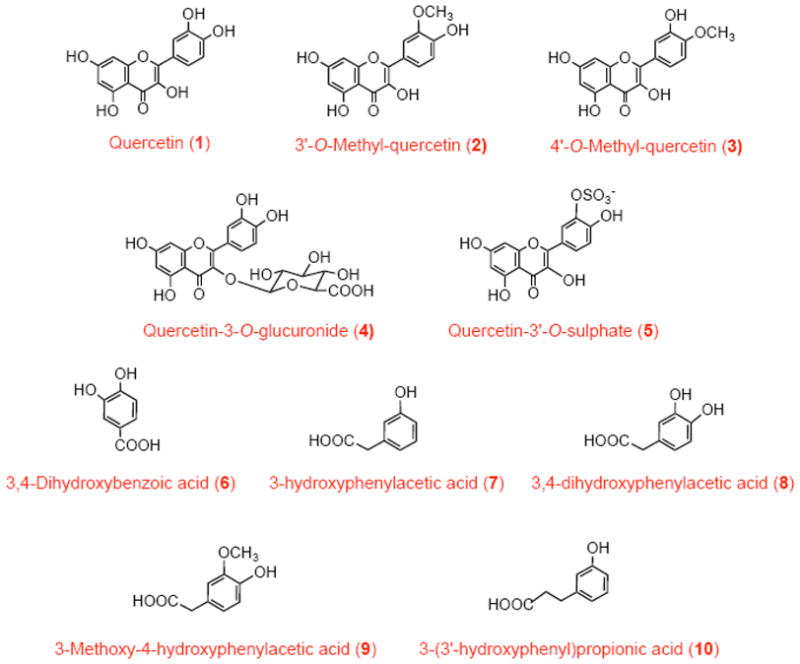
Structures of quercetin and its in vivo methyl, sulphate, glucuronide and phenolic acid metabolites.
Metabolites of (−)-epigallocatechin-3-O-gallate
The metabolites 4″-O-methyl-EGCG and 4′,4″-di-O-methyl-EGCG were prepared by methylating EGCG with methyl iodide, as described previously [23]. The structures of EGCG and related compounds studied in this work are shown in Scheme II.
Scheme II.
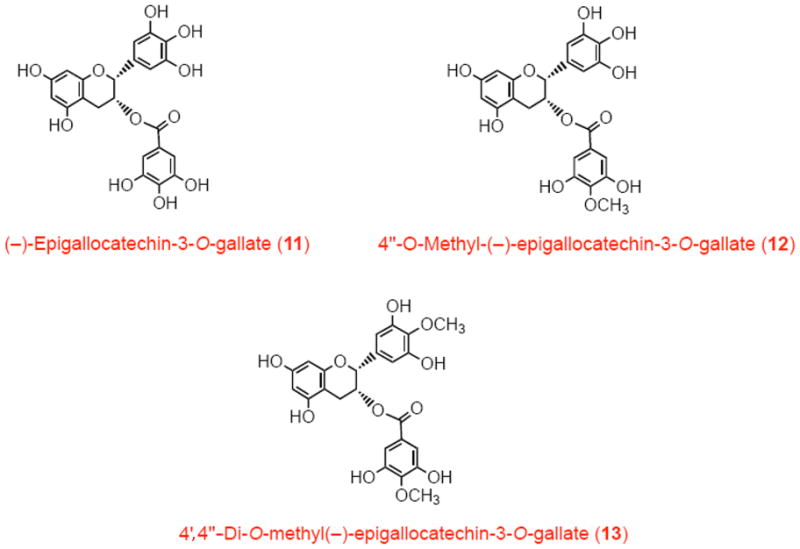
Structures of EGCG and its in vivo methyl metabolites.
Endothelial cells
Human aortic endothelial cells were obtained from Lonza (Walkersville, MD) at third passage. Upon receipt, the cells were seeded at a ratio of 1:3 in 75-cm2 flasks (pre-coated with 1% bovine gelatin; Sigma-Aldrich) and grown at 37°C, under 95% air–5% CO2 and in a humidified atmosphere in endothelial cell growth medium (Lonza) containing bovine brain extract, human epithelial growth factor, hydrocortisone, amphotericin B, gentamicin sulphate and 2% fetal bovine serum (FBS). The medium was periodically renewed until the cells reached 70–90% confluence, at which point they were treated with 0.05% Trypsin–0.02% EDTA (Sigma-Aldrich). Subsequently, the cells were expanded in 75-cm2 pre-coated flasks at a ratio of 1:5 until passages 5–6, when they were plated and the experiments were carried out.
Experiments
Human aortic endothelial cells were plated in 96-well plates (pre-coated with 1% gelatin) at an average density of 5 × 104 cells/ml medium. The medium consisted of Medium 199 (Sigma-Aldrich) supplemented with 20% FBS (GIBCO Invitrogen, Grand Island, NY), 1 mM glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 0.1 μg/ml amphotericin B (Sigma-Aldrich) and 1 ng/ml human basic fibroblast growth factor (Roche). The cells were allowed to attach to the plates overnight (18 h), after which they were washed with Hanks’ balanced salt solution (Sigma-Aldrich) and the medium was renewed. The cells were incubated at 37°C, under 95% air–5% CO2 and in a humidified atmosphere until they reached confluence, typically 24–48 h after seeding.
For experiments, HAEC were incubated for 18 h with medium (100 μl) containing different concentrations of quercetin or its derivatives, 3′-O-methyl-quercetin, 4′-O-methyl-quercetin, quercetin-3-O-glucuronide and quercetin-3′-O-sulphate, or phenolic acid metabolites of quercetin; or for 1 h with EGCG or its O-methyl derivatives, 4″-O-methyl-EGCG or 4′,4″-di-O-methyl-EGCG, prior to the addition of 100 U/ml of TNFα. We chose longer incubation times for quercetin and its derivatives to mimic enterohepatic circulation. In addition, preliminary experiments indicated that 18-h pre-incubation was required for maximal inhibition of adhesion molecule expression. The solutions were freshly prepared by dissolving the flavonoids in either DMSO or deionized water and subsequent dilution in culture medium containing 20% serum. The final concentration of DMSO in the medium did not exceed 0.1 %. Proper controls with the vehicle DMSO or water were carried out.
The cells were examined regularly using an inverted optical microscope. No changes in cell morphology were observed with any of the treatments. Cell viability was evaluated by the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay, using the Cell Proliferation kit I, according to the manufacturer’s instructions (Roche). In addition, to test for potential effects of artificially generated reactive oxygen species, catalase (0.1 μM) and/or superoxide dismutase (3 μM) was added and/or phenol-red free medium was used.
Cellular adhesion molecule expression
Surface protein levels of the cellular adhesion molecules, E-selectin, vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), were determined by ELISA performed on HAEC monolayers in flat-bottom 96-well plates. Following treatment, the cells were fixed in PBS containing 0.1% glutaraldehyde. For cell ELISA, plates were blocked at 37°C for 1 h with 5% skim milk powder in PBS and then incubated overnight at 4°C with a primary antibody to either E-selectin, ICAM-1 (R&D Systems, Minneapolis, MN) or VCAM-1 (Dako, Carpinteria, CA). The plates were then incubated for 1 h with a horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody (Amersham, Piscataway, NJ) at 37°C. The expression of E-selectin, ICAM-1 and VCAM-1 was assessed by the addition of o-phenylendiaminehydrochloride (Sigma-Aldrich). The absorbance at 492 nm was recorded in a plate-reader spectrophotometer (Spectromax 190, Molecular Devices, Sunnyvale, CA).
Monocyte chemotactic protein-1 expression
Monocyte chemotactic protein-1 (MCP-1) was measured in the conditioned medium of cell cultures by the quantitative sandwich enzyme immunoassay technique (R&D Systems, Minneapolis, MN). A monoclonal antibody specific for MCP-1 was pre-coated onto a microplate. Standards and conditioned medium of HAEC (diluted 1/10) were loaded into the wells and incubated for 2 h. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for MCP-1 was added to the wells and incubated for 1 h. Following a wash to remove any unbound antibody-enzyme reagent, a chromogen-substrate (tetramethylbenzidine) was added to the wells. Color developed in proportion to the amount of MCP-1 bound in the initial step. The absorbance at 450 nm was recorded in a plate-reader spectrophotometer (Spectromax 190).
NF-κB activation
Activation of NF-κB was assessed by measuring the p65 protein–DNA binding activity in nuclear extracts of HAEC. HAEC were grown in 10-cm Petri dishes in endothelial cell growth medium (passage 5). When the cells were 70–90% confluent, the medium was changed to 15 ml of complete M199 (20% FBS) and incubated for 18 h in the absence or the presence of 3′-O-methyl-quercetin or for 1 h with 4′,4″-di-O-methyl-EGCG, after which HAEC were challenged with 100 U/ml TNFα. For comparison purposes, HAEC were also incubated for 1 h with pyrrolidine dithiocarbamate (PDTC), a known inhibitor of NF-κB activation [24, 25]. After 1 h of incubation with TNFα, the reaction was stopped by washing the cells with cold PBS. Nuclear extracts were prepared according the manufacturer’s instructions, and the DNA binding activity of p65 was measured by ELISA (Active Motif, Carlsbad, CA). Briefly, nuclear extracts were added to wells previously coated with DNA containing specific sequences for the binding of p65. After incubation at room temperature for 1 h, wells were washed and sequentially incubated for 1 h with a primary antibody raised against p65 and a secondary enzyme-linked antibody. The plate was developed by addition of chromogen, and the absorbance at 450 nm was recorded in a plate-reader spectrophotometer (Spectromax 190).
Ferric reducing antioxidant parameter
The antioxidant capacity of flavonoid metabolites was evaluated by their iron-reducing capacity, using the ferric reducing antioxidant parameter (FRAP) assay [26]. In this assay, antioxidants act as reductants of Fe3+ to Fe2+, which is chelated by tripyridyltriazine to form a colored complex. Briefly, 40 μl of flavonoid-containing solutions or culture medium were mixed in a 96-well plate with 300 μl of reagent solution containing 1.7 mM FeCl3 and 0.8 mM tripyridyltriazine in 300 mM sodium acetate, pH 3.6. The samples were incubated for 15 min at 37°C, and the absorbance at 593 nm was recorded in a plate-reader spectrophotometer (Spectromax 190). Results were compared to a standard curve prepared with different concentrations of the antioxidant Trolox and are expressed as “Trolox equivalents”.
Statistical analysis
Results shown are the mean ± SEM of at least three independent experiments (involving three to five observations for each treatment), using HAEC from at least three different donors. Statistical analysis was performed by one-way analysis of variance (ANOVA). If statistical significance was reached for ANOVA (p<0.05), Tukey-Kramer was applied as post-hoc test.
RESULTS
Quercetin and its B-ring methylated derivatives are equally effective inhibitors of cellular adhesion molecule expression
The biotransformation of quercetin is complex but has been studied extensively and is well understood (Scheme III). A major reaction is methylation of the catechol group in the B ring of the quercetin molecule by catechol-O-methyl transferase (COMT), resulting in formation of 3′- and 4′-O-methyl-quercetin derivatives. A major in vivo metabolite of quercetin containing an O-methylated catechol group is 3′-O-methyl-quercetin-3-O-glucuronide. To investigate whether O-methylation affects the biological activity of quercetin, we first studied the effect of 3′-O-methyl-quercetin and 4′-O-methyl-quercetin on cellular adhesion molecule expression.
Scheme III. Major metabolic pathways for the conversion of dietary quercetin.
Quercetin, present in food as glycosides, is absorbed intact by the sodium-glucose transporter 1 (SGLT1) and hydrolyzed by β-glucosidase in enterocytes. In addition, dietary glycosides of quercetin can be hydrolyzed before absorption by membrane-bound lactose-phloretin hydrolase (LPH). In the enterocyte, quercetin aglycone (“free” quercetin) (1) is mostly metabolized to glucuronides (4) by UDP-glucuronosyltransferase (UGT) or to sulphate derivatives (5) by sulphate transferase (ST) and exported into the circulation. In the liver, the glucuronides undergo further metabolism, mainly by ST and catechol O-methyl transferase (COMT). These processes and enzymes combined are responsible for the metabolites detected in plasma and urine. In addition, quercetin may undergo enterohepatic circulation by its excretion through bile and subsequent intestinal re-absorption. For structure names and reference numbers, see Scheme I.
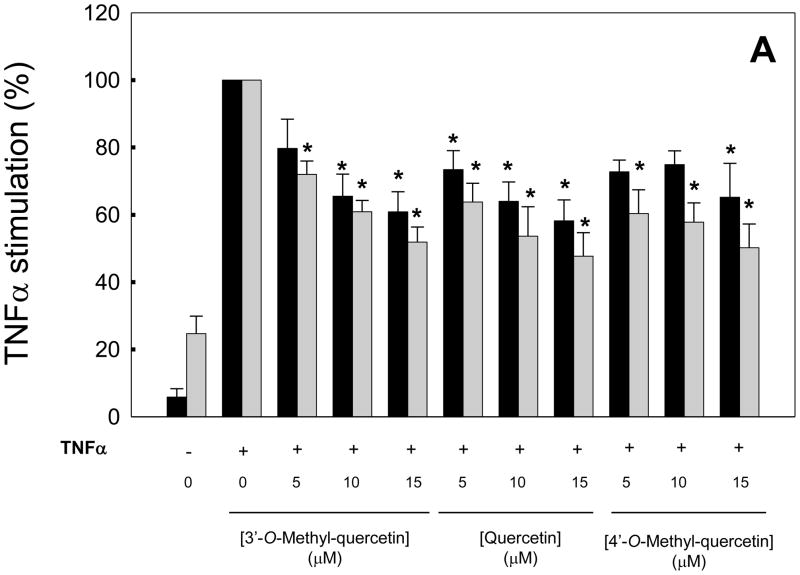
HAEC were incubated for 18 h with quercetin, 3′-O-methyl-quercetin or 4′-O-methyl-quercetin and then co-incubated for 7 h with 100 U/ml TNFα. All three compounds dose-dependently inhibited TNFα-induced expression of E-selectin and ICAM-1 with similar efficacy (Figure 1A), but did not significantly inhibit VCAM-1 expression (data not shown). The dose-response curves for the inhibitory effects of 3′-O-methyl-quercetin on expression of E-selectin and ICAM-1 as well as the chemokine, MCP-1, followed a hyperbolic dependency (Figure 1B). Very similar dose-response curves were obtained with 4′-O-methyl-quercetin (data not shown) and quercetin (as previously reported by us, ref. [25]). The concentrations required for 50% inhibition (IC50) of ICAM-1 expression were 8.0, 5.0 and 4.4 μM, respectively, for 3′-O-methyl-quercetin, 4′-O-methyl-quercetin and quercetin. Neither quercetin nor 3′- or 4′-O-methyl-quercetin were cytotoxic at the concentrations used, as evaluated by the MTT assay. Furthermore, the addition of catalase and/or superoxide dismutase to the cell culture medium did not affect the inhibitory effects of quercetin and its methylated derivatives on adhesion molecule expression, suggesting that these effects were not mediated by reactive oxygen species generated by autoxidation of the flavonoids. Taken together, our results indicate that methylation of the catechol group in the B ring of quercetin does not alter the anti-inflammatory activity of this dietary flavonoid.
Figure 1. Dose-response effects of 3′-O-methyl-quercetin and 4′-O-methyl-quercetin compared to quercetin on adhesion molecule expression in human aortic endothelial cells.
HAEC were incubated for 18 h in the absence (0 μM) or presence of 5–15 μM quercetin, 3′-O-methyl-quercetin or 4′-O-methyl-quercetin followed by the addition of 100 U/ml TNFα and incubation for 7 h. Panel A shows protein levels of E-selectin (black bars) and ICAM-1 (gray bars) as % of protein levels in HAEC incubated with TNFα in the absence of flavonoids. Results from control cells incubated in the absence of flavonoids and TNFα are also shown. Panel B shows % inhibition of E-selectin (black circles), ICAM-1 (gray circles) and MCP-1 (open circles) expression by 3′-O-methyl-quercetin. Data shown are means ± SEM of at least three independent experiments. One-way ANOVA was used to analyze dose-response trends; *significantly different from 0 μM (Tukey-Kramer, post-hoc analysis).
Quercetin-3-O-glucuronide and quercetin-3′-O-sulphate do not inhibit cellular adhesion molecule expression
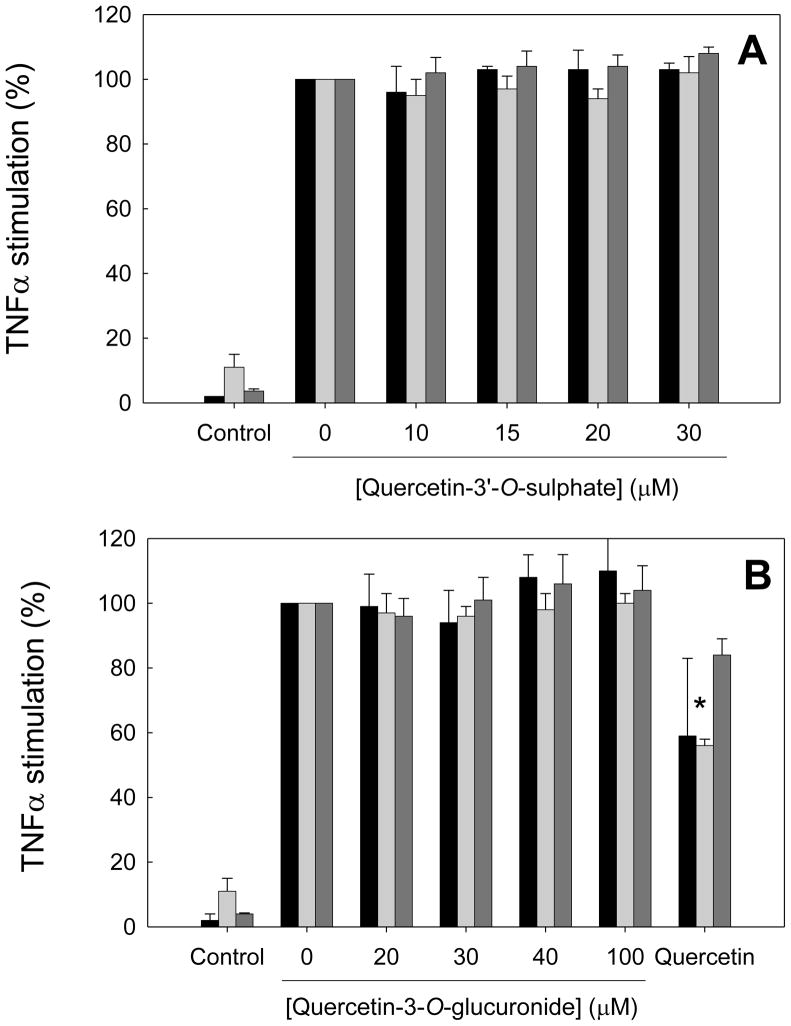
After first-pass metabolism of quercetin by intestine and liver, the main circulating metabolites in humans are methylated or non-methylated glucuronides and sulphates (Scheme III). Therefore, we next studied whether glucuronide and sulphate conjugates of quercetin retain its anti-inflammatory activity. HAEC were incubated for 18 h with 10–30 μM quercetin-3′-O-sulphate, 20–100 μM quercetin-3-O-glucuronide or 30 μM quercetin and then co-incubated for 7 h with 100 U/ml TNFα. Interestingly, no inhibition of adhesion molecule expression was observed for either metabolite, in contrast to the significant inhibition of E-selectin and ICAM-1 expression by quercetin (Figure 2, c.f. Figure 1). Neither quercetin nor quercetin-3-O-glucuronide and quercetin-3′-O-sulphate were cytotoxic at the concentrations tested, as evaluated by the MTT assay. Taken together, these results suggest that glucuronidation and sulphation of quercetin abolish its anti-inflammatory activity.
Figure 2. Dose-response effects of quercetin-3′-O-sulphate and quercetin-3-O-glucuronide on adhesion molecule expression in human aortic endothelial cells.
HAEC were incubated for 18 h in the absence (0 μM) or presence of 10–30 μM quercetin-3′-O-sulphate (panel A), 20–100 μM quercetin-3-O-glucuronide (panel B) or 30 μM quercetin (panel B), followed by the addition of 100 U/ml TNFα and incubation for 7 h. Protein levels of E-selectin (black bars), ICAM-1 (light grey bars) and VCAM-1 (dark gray bars) are shown as % of protein levels in HAEC incubated with TNFα in the absence of flavonoids. Results from control cells incubated in the absence of flavonoids and TNFα are also shown. Data shown are means ± SEM of at least three independent experiments. One-way ANOVA showed no significant dose-response trends; *significantly different from 0 μM.
Phenolic acid metabolites of quercetin do not inhibit adhesion molecule expression
We also tested the effect of different phenolic acids (see Scheme I), which have been described as in vivo metabolites of dietary quercetin [12]. HAEC were incubated overnight with increasing concentrations (20–100 μM) of these phenolic compounds and then co-incubated with TNFα for another 7 h, as described above for quercetin and its derivatives. However, none of the phenolic acid metabolites examined, even at supra-physiological concentrations, exerted any significant inhibitory effects on adhesion molecule expression (data not shown).
EGCG and its 4″-O-methylated metabolite do not affect cellular adhesion molecule and MCP-1 expression, in contrast to 4′,4″-di-O-methyl-EGCG
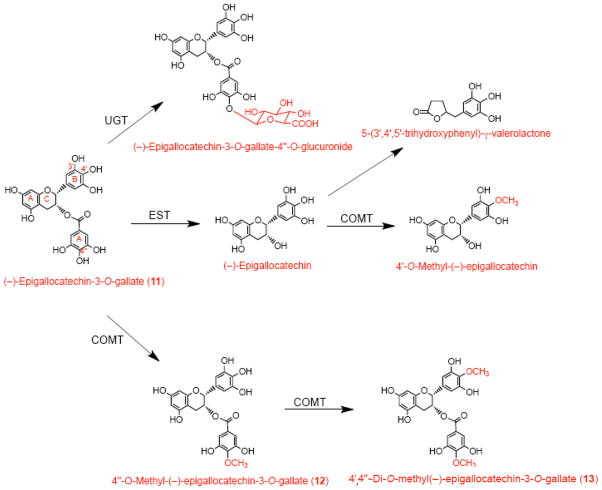
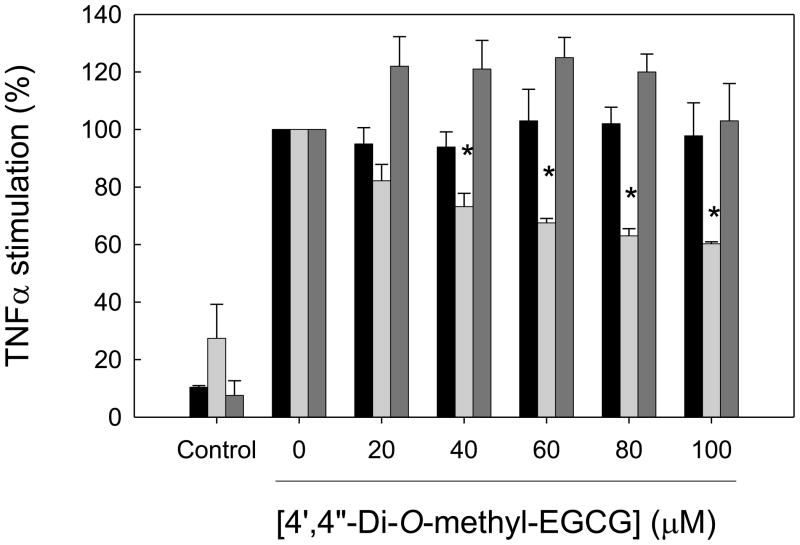
After consumption, EGCG can be detected unmetabolized in the circulation. However, it also undergoes extensive metabolism, and glucuronidated, methylated and ring-fission metabolites of EGCG have been identified (Scheme IV) [23]. In particular, about 15% of EGCG is present in human plasma as 4′,4″-di-O-methyl-EGCG, which is formed from its precursor, 4″-O-methyl EGCG, by the action of COMT (Scheme IV). Hence, we studied the biological effects of these two methyl derivatives compared to their parent compound, EGCG. HAEC were incubated for 1 h with 20–60 μM EGCG or 20–100 μM 4″-O-methyl-EGCG or 4′,4″-di-O-methyl-EGCG and then co-incubated for 4 h with 100 U/ml TNFα. While EGCG and 4″-O-methyl-EGCG failed to attenuate the expression of any of the adhesion molecules measured (data not shown), 4′,4″-di-O-methyl-EGCG significantly inhibited ICAM-1, but not E-selectin or VCAM-1, expression in a dose-dependent manner (Figure 3). In fact, VCAM-1 expression was slightly, but non-significantly, increased by 4′,4″-di-O-methyl-EGCG (Figure 3). MCP-1 expression was also slightly increased by 4′, 4″-di-O-methyl-EGCG (data not shown).
Scheme IV. Major metabolic pathways for the conversion of dietary EGCG.
EGCG is one of the very few flavonoids that can be detected ‘intact’ in plasma, without further metabolism. However, its concentration in circulation is very low, and considerable glucuronidation and methylation also take place, resulting in a complex profile of metabolites. After absorption, EGCG undergoes methylation in the small intestine, liver, kidney and other organs. Metabolites of EGCG may also undergo enterohepatic circulation. (−)-Epigallocatechin (EGC) may be an intermediate during in vivo degradation of EGCG by microbial esterases (EST). Other enzymes involved in EGCG metabolism include catechol O-methyl transferase (COMT) and UDP-glucuronosyltransferase (UGT).
Figure 3. Dose-response effects of 4′,4″-di-O-methyl-EGCG on adhesion molecule expression in human aortic endothelial cells.
HAEC were incubated for 1 h in the absence (0 μM) or presence of 20–100 μM 4′,4″-di-O-methyl-EGCG, followed by the addition of 100 U/ml TNFα and incubation for 4 h. Protein levels of E-selectin (black bars), ICAM-1 (light grey bars) and VCAM-1 (dark gray bars) are shown as % of protein levels in HAEC incubated with TNFα in the absence of 4′,4″-di-O-methyl-EGCG. Results from control cells incubated in the absence of 4′,4″-di-O-methyl-EGCG and TNFα are also shown. Data shown are means ± SEM of at least three independent experiments. One-way ANOVA was used to analyze dose-response trends; *significantly different from 0 μM (Tukey-Kramer, post-hoc analysis).
3′-O-Methyl-quercetin has greater reducing activity than 4′,4″-di-O-methyl-EGCG, but neither compound inhibits NF-κB activation
Gene expression of adhesion molecules in endothelial cells is regulated primarily by the transcription factor, NF-κB, which is thought to be activated in a reduction/oxidation (redox)-sensitive manner [27]. Therefore, we investigated whether flavonoid derivatives or metabolites that inhibited protein expression of cellular adhesion molecules, i.e., 3′-O-methyl-quercetin and 4′,4″-di-O-methyl-EGCG (Figures 1 and 3), also inhibited NF-κB activation and whether such an effect was related to their reducing activity.
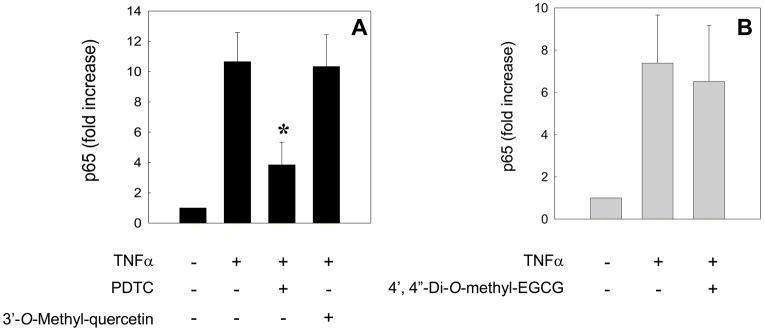
To investigate NF-κB activation, HAEC were incubated for 18 h with 30 μM 3-O-methyl-quercetin or for 1 h with 100 μM 4′,4″-di-O-methyl-EGCG and then co-incubated for 1 h with 100 U/ml TNFα. Activation of NF-κB, as indicated by nuclear translocation and DNA binding of its p65 subunit, was not inhibited by either flavonoid metabolite, whereas 10 μM pyrrolidine dithiocarbamate–used as a positive control–exerted strong inhibition (Figure 4).
Figure 4. Effects of 3′-O-methyl-quercetin and 4′,4″-di-O-methyl-EGCG on NF-κB activation in human aortic endothelial cells.
HAEC were incubated for 18 h in the absence or presence of 30 μM 3′-O-methyl-quercetin (panel A) or for 1 h with 100 μM 4′,4″-di-O-methyl-EGCG (panel B), followed by the addition of 100 U/ml TNFα and incubation for 1 h. As positive control, HAEC were also incubated for 1 h with 10 μM pyrrolidine dithiocarbamate (PDTC) before incubation with TNFα (panel A). Nuclear p65 DNA binding activity was measured by ELISA. Data shown are means ± SEM of at least three independent experiments. One-way ANOVA was used to analyze the data; *significantly different from TNFα–only treatment (Tukey-Kramer, post-hoc analysis).
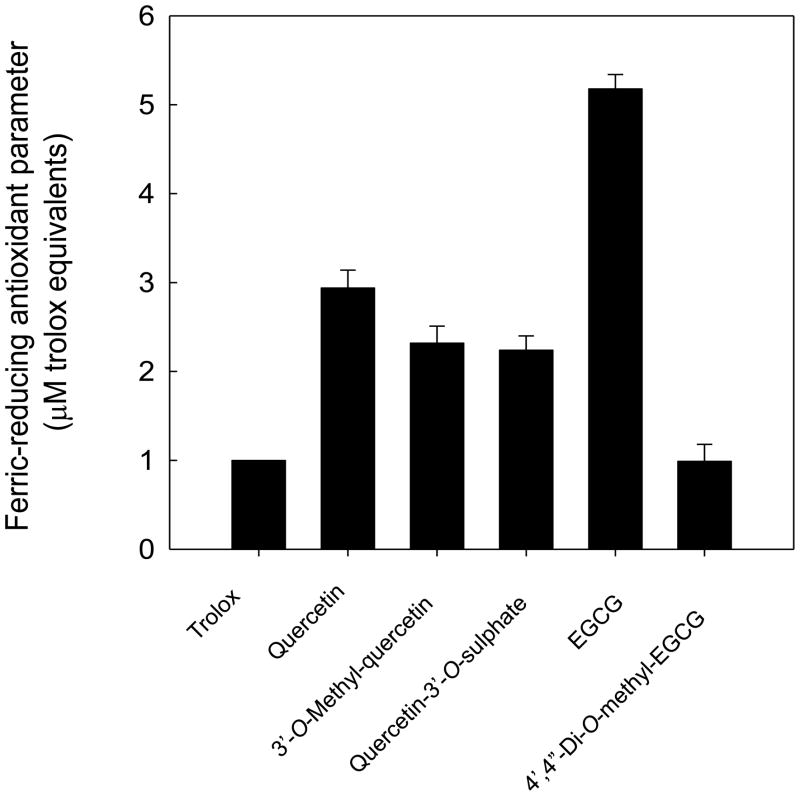
Furthermore, the antioxidant capacity of the various flavonoids was evaluated by their iron-reducing activity, using the FRAP assay (Figure 5). The reducing activity of quercetin was almost 3 times higher than that of Trolox, a water-soluble vitamin E analogue used as standard antioxidant in the assay. Both 3′-O-methyl-quercetin and quercetin-3′-O-sulphate had somewhat lower reducing activity than quercetin. EGCG had a FRAP value more than 5 times higher than that of Trolox; in contrast, FRAP of 4′,4″-di-O-methyl-EGCG was similar to Trolox. These data suggest that dimethylation of EGCG results in a dramatic loss of its reducing activity and that the reducing activity of quercetin, EGCG and their metabolites is not related to the capacity of these compounds to inhibit NF-κB activation or adhesion molecule expression in HAEC.
Figure 5. Ferric reducing antioxidant parameter of quercetin, EGCG and selected metabolites.
FRAP was measured for quercetin and its metabolites, 3′-O-methyl-quercetin and quercetin-3′-O-sulphate, and for EGCG and its metabolite, 4′,4″-di-O-methyl-EGCG. Values are expressed as μM Trolox equivalents. Data shown are means ± SEM of at least three independent determinations.
DISCUSSION
Quercetin intake from vegetables has been associated with a lower risk of cardiovascular diseases, although the underlying mechanisms are incompletely understood [28]. Quercetin is rapidly metabolized in the human intestinal mucosa and liver into glucuronide and sulphate conjugates with or without methylation (Scheme III). After consumption of quercetin-rich foods, quercetin metabolites are largely associated with the albumin-containing fraction of human plasma [29]. Interestingly, quercetin was detected (after deconjugation) in the aortas of cholesterol-fed rabbits supplemented with quercetin glucosides [30]. In line with this observation, immunohistochemical studies with monoclonal antibodies raised against quercetin conjugates demonstrated that quercetin glucuronides specifically accumulate in human atherosclerotic lesions, but not in non-lesion aortic areas, and the staining was associated with macrophage-derived foam cells [31]. These studies suggest that quercetin metabolites can penetrate into the vascular wall and accumulate in atherosclerotic plaque and, hence, may interact with the vascular endothelium.
In this work, we hypothesized that the in vivo effects of dietary quercetin on endothelial cells are modulated by chemical modifications of the quercetin molecule. Therefore, we studied the effects of different O-methylated isomers of quercetin, 3′- and 4′-O-methyl-quercetin, and two additional conjugates with in vivo relevance, quercetin-3-O-glucuronide and quercetin-3′-O-sulphate, on the expression of inflammatory proteins in HAEC. Because quercetin and its metabolites are likely to associate with albumin and other plasma proteins and, furthermore, may undergo enterohepatic circulation, we performed our experiments in high-serum medium (20% FBS) and exposed HAEC to the quercetin metabolites for 18 h prior to the addition of TNFα.
We first studied 3′-O-methyl-quercetin, commonly known as isorhamnetin, and 4′-O-methyl-quercetin, to evaluate whether O-methylation affects the biological activity of quercetin. We found that these methylated quercetin derivatives inhibited TNFα-induced E-selectin and ICAM-1 expression as effectively as the quercetin aglycone. Therefore, it can be speculated that O-methylation of quercetin by COMT would not affect the potential of dietary quercetin to attenuate vascular endothelial inflammation in vivo. We have shown previously that the basic A and C-ring flavone structure (see Scheme I) is critical for the inhibition of adhesion molecule expression in HAEC, but not the B-ring catechol group [25], consistent with the results presented here for quercetin and its methyl derivatives. In contrast, the B and C-ring hydroxyl groups were critical for the reducing activity of the molecule [25], again in agreement with the data presented here. Hence, O-methyl substitutions in the catechol group of quercetin did not affect its anti-inflammatory activity but lowered its antioxidant capacity.
In contrast to 3′- and 4′-O-methyl-quercetin, quercetin-3-O-glucuronide and quercetin-3′-O-sulphate did not inhibit TNFα-mediated inflammatory responses in HAEC, even when tested at supra-physiological concentrations. Hence, a novel observation of our work is that the biological activity of quercetin with respect to adhesion molecule expression is dependent on the chemical nature of the substituent, not just the position in the molecule that is modified. For example, we found that 3′-O-methylation did not affect the biological activity of quercetin, while 3′-O-sulphation abolished it. Both molecules, however, exhibited similar antioxidant activity, which was lower than the antioxidant activity of quercetin due to lack of the catechol group.
Our data also indicate that HAEC do not have the ability to hydrolyze the metabolites to the active quercetin aglycone, as has been suggested for other cell types [32]. The introduction of bulky or charged groups and the resulting increased hydrophilicity may impair the ability of quercetin-3-O-glucuronide and quercetin-3′-O-sulphate to reach the appropriate intracellular target(s) in HAEC. This notion is supported by the observation that quercetin-3-O-glucuronide does not seem to be taken up to a significant degree by PC-12 cells [33]. In contrast, murine macrophages have been shown to accumulate quercetin-3-O-glucuronide and metabolize it to the much more active aglycone and its methylated form [31]. In vitro studies using large unilaminar vesicles have shown that quercetin-3-O-glucuronide can interact with phospholipid bilayers with low, but significant, affinity, suggesting that an interaction with cell membranes may also be possible [34].
While our data indicate that quercetin-3-O-glucuronide and quercetin-3′-O-sulphate cannot inhibit inflammatory responses in HAEC, previous work has suggested that these quercetin metabolites may exert relevant biological effects in cells or endothelium. For example, Tribolo et al. [35] reported that quercetin-3′-O-sulphate, quercetin-3-O-glucuronide and 3′-O-methyl-quercetin-3-O-glucuronide inhibited VCAM-1 cell surface expression at concentrations as low as 2 μM. Furthermore, quercetin-3-O-glucuronide was effective in preventing endothelial dysfunction induced by endothelin-1, and both quercetin-3-O-glucuronide and quercetin-3′-O-sulphate inhibited NADPH oxidase-dependent superoxide production in thoracic aortic rings from rats [36]. Quercetin-3-O-glucuronide also inhibited angiotensin II-induced hypertrophy in vascular smooth muscle cells, which was attributed in part to its inhibitory effect on the JNK/AP-1 signaling pathway [37]. The use of different cell types and experimental conditions may account, in part, for these discrepant results.
Our results are, however, in agreement with those reported by Mochizuki et al. [38] showing that quercetin-3-O-glucuronide did not inhibit TNFα-induced expression of ICAM in HAEC. Interestingly, these authors also found that inflammation triggered by interleukin-1α resulted in increased cell permeability of quercetin-3-O-glucuronide, suggesting that it could pass through the endothelium to reach the underlying vascular smooth muscle cells. Taking all our results into account, it can be speculated that the major in vivo metabolite, 3′-O-methyl-quercetin-3-O-glucuronide, would be inactive in our model, not because of the biotransformation of the catechol group but because of the glucuronidation in position C3. If intracellular hydrolysis occurred in HAEC in vivo, the resulting 3′-O-methyl-quercetin would be an intracellular ‘active’ metabolite. However, non-glucuronidated, O-methylated quercetin cannot be detected in human plasma, and there is no evidence for hydrolysis of quercetin glucuronides in endothelial cells.
We previously reported that flavonols and flavones were able to inhibit E-selectin and ICAM-1 expression in HAEC, whereas flavanones or flavan-3-ols were not, due to lack of the basic A and C-ring flavone structure [25]. Accordingly, caffeic acid, which contains a catechol group but lacks a basic flavonoid structure, and–as reported here–phenolic acid metabolites of quercetin were unable to inhibit adhesion molecule expression in HAEC. However, in-vitro anti-inflammatory effects of phenolic acids have been recently reported. Differences in cell types and inflammatory challenges may account for some of these seemingly discrepant results. For instance, it has been reported that phenolics such as hydrocaffeic, dihydroxyphenyl acetic and hydroferulic acids were able to inhibit interleukin-1β-induced prostaglandin E(2) production by CCD-18 colon fibroblast cells [39]. The hydroxylated phenolic acids, 3,4-dihydroxyphenylpropionic acid and 3,4-dihydroxyphenylacetic acid, were also able to inhibit lipopolysaccharide-stimulated cytokine release from isolated peripheral blood mononuclear cells [40], in contrast to our observations in TNFα-exposed HAEC. In general, studies using endothelial cells are scarce. However, Moon et al. [41] reported that caffeic acid reduced TNFα-induced adhesion molecule expression in human umbilical vein endothelial cells (HUVEC) by inhibiting NF-κB activation. While some of their experimental conditions were similar to ours, e.g., 18-h pre-incubation with caffeic acid followed by 6-h incubation with TNFα, other conditions differed significantly and may have accounted for the discrepancies in results, e.g., use of HUVEC vs. HAEC and 0.2% vs. 20% FBS in cell culture media. Our work has consistently found that none of the phenolic acids tested inhibited TNFα-mediated adhesion molecule expression in HAEC, supporting the notion that a basic hydroxyflavone structure is required for activity in this experimental system.
Catechins are widely distributed in the human diet, in particular tea, wine, grapes and cocoa. Considering that tea is the second most consumed beverage in the world after water [42, 43], catechin consumption by humans can be significant. One catechin, EGCG, is of particular interest because of its numerous biological effects. Although the metabolic transformation of catechins in humans is well understood, relatively little is known about the biological effects of catechin metabolites. It has been reported, for instance, that O-methylated derivatives of (−)-epicatechin are good inhibitors of peroxynitrite-mediated nitrotyrosine formation [44]. In intact cells, 3′-O-methyl-epicatechin inhibited cell death induced by hydrogen peroxide through inhibition of caspases [45]. 3′-O-methyl-epicatechin also was shown to attenuate UVA-induced oxidative damage in human skin fibroblasts [46]. Interestingly, it was recently reported that HUVEC have the capacity to convert (−)-epicatechin into methyl derivatives, which inhibited NADPH oxidase activity [47]. In this work, we studied the effect of EGCG–the main catechin in green tea–and two of its in vivo metabolites, 4″-O-methyl-EGCG and 4′,4″-di-O-methyl-EGCG. EGCG is one of the few dietary flavonoids that have been detected in plasma without further modification, but it is also known that EGCG undergoes extensive biotransformation (see Scheme IV). Both EGCG and their methylated metabolites are substrates and potent inhibitors of hepatic COMT, suggesting they might have biological activity [48].
To investigate EGCG and its metabolites in vitro, we applied a protocol compatible with EGCG’s in vivo pharmacokinetic behavior. Because of the much shorter half-life in human plasma of EGCG than quercetin derivatives, HAEC were exposed to EGCG and its metabolites for only 1 h prior to the addition of TNFα. Neither EGCG nor its metabolite 4″-O-methyl-EGCG inhibited adhesion molecule or MCP-1 expression. In contrast, 4′,4″-di-O-methyl-EGCG was effective in selectively inhibiting ICAM-1 expression. However, 4′,4″-di-O-methyl-EGCG was far less effective than quercetin or 3′-O-methyl-quercetin in our experimental model. Due to the high concentrations required for inhibition of ICAM-1 expression by 4′,4″-di-O-methyl-EGCG, the physiological relevance of these findings is doubtful, unless high local concentrations accumulate in target cells and tissues, as has been described for quercetin metabolites [31, 49].
In addition, further intracellular biotransformation of flavonoid metabolites is possible. Kawai et al. [31] showed that isolated macrophages can metabolize quercetin glucuronides to quercetin aglycone and methylated quercetin, even in the absence of an inflammatory challenge. Notably, methylated quercetin was the active metabolite, as inhibition of COMT blocked the biological effect of the quercetin glucuronides [31]. Similarly, Steffen et al. [47] showed that HUVEC can metabolize (−)-epicatechin to mono-O-methylated epicatechin, which was the metabolite responsible for NADPH oxidase inhibition. Interestingly, demethylation of flavonoids in human cells also may occur, as has been reported for biochanin A (4′-O-methyl genistein) in cancer cells [50]. In this work, we didn’t pursue the identification of intracellular metabolites, but it is possible that the combined effect of selective cellular uptake and intracellular metabolism contributed to our results.
NF-κB activation is the main transcription factor mediating TNFα-induced expression of inflammatory genes [51]. Our previous results indicated that pharmacological inhibitors of NF-κB completely abolished expression of adhesion molecules in HAEC. However, the active flavonoid metabolites studied here were unable to inhibit NF-κB activation, suggesting that they act through (an) alternative mechanism(s), for example, stimulation of the Nrf2 pathway [52].
We have previously shown that the reducing activity of flavonoids is not related to their capacity to inhibit adhesion molecule expression in HAEC [25]. In this paper, we also measured the ferric reducing activity of the individual compounds and metabolites. As expected, 3′-O-methyl-quercetin showed lower reducing activity than quercetin, confirming that the catechol group is as an important structural feature. Interestingly, quercetin-3′-O-sulphate also exhibited good reducing activity, despite its lack of an effect on adhesion molecule expression, confirming that the antioxidant capacity of an individual compound is not relevant for its anti-inflammatory effect. Other authors have also shown that metabolites of quercetin can retain, at least in part, the antioxidant capacity of the quercetin aglycone. For instance, the conjugated metabolite, quercetin-3-O-glucuronide, effectively prevented peroxynitrite-induced depletion of lipophilic antioxidants in isolated human low-density lipoproteins [53]. Quercetin-3-O-glucuronide also inhibited lipid peroxidation of liposome membranes induced by iron, peroxynitrite or lipoxygenase, although the metabolite was less effective than quercetin [34]. We also compared the reducing activity of EGCG with that of 4′,4″-di-O-methyl-EGCG and found that the latter was much less active than EGCG, despite being more effective at inhibiting adhesion molecule expression. These results indicate a significant role of the 4′- and 4″-hydroxyl groups for the antioxidant capacity of EGCG, but also highlight that the antioxidant capacity cannot predict the biological effect in this model.
In summary, our work shows that chemical modifications of dietary flavonoids – resulting in formation of different in vivo metabolites – can significantly alter their biological and antioxidant activities. While glucuronidation and sulphation abolished the inhibitory effect of quercetin on adhesion molecule expression, methylation preserved its anti-inflammatory activity. In contrast, 4′,4″-di-O-methyl-EGCG dose-dependently inhibited TNFα-induced expression of ICAM-1 but not other adhesion molecules, while EGCG was ineffective. Thus, the study of the biological responses to physiologically relevant forms and concentrations of circulating flavonoids is pivotal for the proper evaluation of the potential health benefits of dietary flavonoids, considering that the biological activities of the metabolites can be neither predicted nor extrapolated from their dietary forms. In vitro studies of flavonoid glycosides or aglycones are unlikely to be relevant to biological or health effects of flavonoids in humans, with the possible exception of effects in the gastrointestinal tract.
Acknowledgments
This work was supported by a grant from USANA Health Sciences Inc., Salt Lake City, UT (BF/SBL) and by Grant Number P01 AT002034 (BF/WJZ) from the National Center for Complementary and Alternative Medicine (NCCAM). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCAM or the National Institutes of Health.
ABREVIATIONS
- COMT
catechol-O-methyl transferase
- EGCG
(−)-epigallocatechin3-O-gallate
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- FRAP
ferric reducing antioxidant parameter
- HAEC
human aortic endothelial cells
- HUVEC
human umbilical vein endothelial cells
- ICAM-1
intercellular adhesion molecule-1
- IC50
concentration required for 50% inhibition
- NF-κB
nuclear factor-kappa B
- MCP-1
monocyte chemotactic protein-1
- TNFα
tumor necrosis factor α
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113(Suppl 9B):71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 2.Ullah MF, Khan MW. Food as medicine: potential therapeutic tendencies of plant derived polyphenolic compounds. Asian Pac J Cancer Prev. 2008;9:187–195. [PubMed] [Google Scholar]
- 3.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 4.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 5.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 6.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary flavonoids and cancer risk in the Zutphen Elderly Study. Nutr Cancer. 1994;22:175–184. doi: 10.1080/01635589409514342. [DOI] [PubMed] [Google Scholar]
- 7.Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 8.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 9.Croft KD. The chemistry and biological effects of flavonoids and phenolic acids. Ann N Y Acad Sci. 1998;854:435–442. doi: 10.1111/j.1749-6632.1998.tb09922.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004;96:229–245. doi: 10.1254/jphs.crj04003x. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Lafuente A, Guillamon E, Villares A, Rostagno MA, Martinez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 12.Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26:1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 13.Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52(Suppl 1):S139–151. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- 14.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 15.Rein D, Lotito S, Holt RR, Keen CL, Schmitz HH, Fraga CG. Epicatechin in human plasma: in vivo determination and effect of chocolate consumption on plasma oxidation status. J Nutr. 2000;130:2109S–2114S. doi: 10.1093/jn/130.8.2109S. [DOI] [PubMed] [Google Scholar]
- 16.Hollman PC, vd Gaag M, Mengelers MJ, van Trijp JM, de Vries JH, Katan MB. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic Biol Med. 1996;21:703–707. doi: 10.1016/0891-5849(96)00129-3. [DOI] [PubMed] [Google Scholar]
- 17.Graefe EU, Wittig J, Mueller S, Riethling AK, Uehleke B, Drewelow B, Pforte H, Jacobasch G, Derendorf H, Veit M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J Clin Pharmacol. 2001;41:492–499. doi: 10.1177/00912700122010366. [DOI] [PubMed] [Google Scholar]
- 18.Moon YJ, Wang L, DiCenzo R, Morris ME. Quercetin pharmacokinetics in humans. Biopharm Drug Dispos. 2008;29:205–217. doi: 10.1002/bdd.605. [DOI] [PubMed] [Google Scholar]
- 19.Alavijeh MS, Chishty M, Qaiser MZ, Palmer AM. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx. 2005;2:554–571. doi: 10.1602/neurorx.2.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 21.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 22.Day AJ, Mellon F, Barron D, Sarrazin G, Morgan MR, Williamson G. Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin. Free Radic Res. 2001;35:941–952. doi: 10.1080/10715760100301441. [DOI] [PubMed] [Google Scholar]
- 23.Meng X, Sang S, Zhu N, Lu H, Sheng S, Lee MJ, Ho CT, Yang CS. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chem Res Toxicol. 2002;15:1042–1050. doi: 10.1021/tx010184a. [DOI] [PubMed] [Google Scholar]
- 24.Wolle J, Ferguson E, Keshava C, Devall LJ, Boschelli DH, Newton RS, Saxena U. Inhibition of tumor necrosis factor induced human aortic endothelial cell adhesion molecule gene expression by an alkoxybenzo[b]thiophene-2-carboxamide. Biochem Biophys Res Commun. 1995;214:6–10. doi: 10.1006/bbrc.1995.2249. [DOI] [PubMed] [Google Scholar]
- 25.Lotito SB, Frei B. Dietary flavonoids attenuate tumor necrosis factor alpha-induced adhesion molecule expression in human aortic endothelial cells. Structure-function relationships and activity after first pass metabolism. J Biol Chem. 2006;281:37102–37110. doi: 10.1074/jbc.M606804200. [DOI] [PubMed] [Google Scholar]
- 26.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 27.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Huxley RR, Neil HA. The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2003;57:904–908. doi: 10.1038/sj.ejcn.1601624. [DOI] [PubMed] [Google Scholar]
- 29.Kaldas MI, Walle UK, van der Woude H, McMillan JM, Walle T. Covalent binding of the flavonoid quercetin to human serum albumin. J Agric Food Chem. 2005;53:4194–4197. doi: 10.1021/jf050061m. [DOI] [PubMed] [Google Scholar]
- 30.Kamada C, da Silva EL, Ohnishi-Kameyama M, Moon JH, Terao J. Attenuation of lipid peroxidation and hyperlipidemia by quercetin glucoside in the aorta of high cholesterol-fed rabbit. Free Radic Res. 2005;39:185–194. doi: 10.1080/10715760400019638. [DOI] [PubMed] [Google Scholar]
- 31.Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, Kobayashi M, Kanayama M, Uchida K, Terao J. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids. J Biol Chem. 2008;283:9424–9434. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- 32.Bartholome R, Haenen G, Hollman CH, Bast A, Dagnelie PC, Roos D, Keijer J, Kroon PA, Needs PW, Arts CW. Deconjugation Kinetics of Glucuronidated Phase II Flavonoid Metabolites by beta-glucuronidase from Neutrophils. Drug Metab Pharmacokinet. 25:379–387. doi: 10.2133/dmpk.dmpk-10-rg-002. [DOI] [PubMed] [Google Scholar]
- 33.Shirai M, Kawai Y, Yamanishi R, Kinoshita T, Chuman H, Terao J. Effect of a conjugated quercetin metabolite, quercetin 3-glucuronide, on lipid hydroperoxide-dependent formation of reactive oxygen species in differentiated PC-12 cells. Free Radic Res. 2006;40:1047–1053. doi: 10.1080/10715760600794287. [DOI] [PubMed] [Google Scholar]
- 34.Shirai M, Moon JH, Tsushida T, Terao J. Inhibitory effect of a quercetin metabolite, quercetin 3-O-beta-D-glucuronide, on lipid peroxidation in liposomal membranes. J Agric Food Chem. 2001;49:5602–5608. doi: 10.1021/jf010713g. [DOI] [PubMed] [Google Scholar]
- 35.Tribolo S, Lodi F, Connor C, Suri S, Wilson VG, Taylor MA, Needs PW, Kroon PA, Hughes DA. Comparative effects of quercetin and its predominant human metabolites on adhesion molecule expression in activated human vascular endothelial cells. Atherosclerosis. 2008;197:50–56. doi: 10.1016/j.atherosclerosis.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 36.Lodi F, Jimenez R, Moreno L, Kroon PA, Needs PW, Hughes DA, Santos-Buelga C, Gonzalez-Paramas A, Cogolludo A, Lopez-Sepulveda R, Duarte J, Perez-Vizcaino F. Glucuronidated and sulfated metabolites of the flavonoid quercetin prevent endothelial dysfunction but lack direct vasorelaxant effects in rat aorta. Atherosclerosis. 2009;204:34–39. doi: 10.1016/j.atherosclerosis.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Yoshizumi M, Tsuchiya K, Suzaki Y, Kirima K, Kyaw M, Moon JH, Terao J, Tamaki T. Quercetin glucuronide prevents VSMC hypertrophy by angiotensin II via the inhibition of JNK and AP-1 signaling pathway. Biochem Biophys Res Commun. 2002;293:1458–1465. doi: 10.1016/S0006-291X(02)00407-2. [DOI] [PubMed] [Google Scholar]
- 38.Mochizuki M, Kajiya K, Terao J, Kaji K, Kumazawa S, Nakayama T, Shimoi K. Effect of quercetin conjugates on vascular permeability and expression of adhesion molecules. Biofactors. 2004;22:201–204. doi: 10.1002/biof.5520220142. [DOI] [PubMed] [Google Scholar]
- 39.Larrosa M, Luceri C, Vivoli E, Pagliuca C, Lodovici M, Moneti G, Dolara P. Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Mol Nutr Food Res. 2009;53:1044–1054. doi: 10.1002/mnfr.200800446. [DOI] [PubMed] [Google Scholar]
- 40.Monagas M, Khan N, Andres-Lacueva C, Urpi-Sarda M, Vazquez-Agell M, Lamuela-Raventos RM, Estruch R. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br J Nutr. 2009;102:201–206. doi: 10.1017/S0007114508162110. [DOI] [PubMed] [Google Scholar]
- 41.Moon MK, Lee YJ, Kim JS, Kang DG, Lee HS. Effect of caffeic acid on tumor necrosis factor-alpha-induced vascular inflammation in human umbilical vein endothelial cells. Biol Pharm Bull. 2009;32:1371–1377. doi: 10.1248/bpb.32.1371. [DOI] [PubMed] [Google Scholar]
- 42.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 43.Weisburger JH. Tea and health: a historical perspective. Cancer Lett. 1997;114:315–317. doi: 10.1016/s0304-3835(97)04691-0. [DOI] [PubMed] [Google Scholar]
- 44.Natsume M, Osakabe N, Yasuda A, Osawa T, Terao J. Inhibitory Effects of Conjugated Epicatechin Metabolites on Peroxynitrite-mediated Nitrotyrosine Formation. J Clin Biochem Nutr. 2008;42:50–53. doi: 10.3164/jcbn.2008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spencer JP, Schroeter H, Kuhnle G, Srai SK, Tyrrell RM, Hahn U, Rice-Evans C. Epicatechin and its in vivo metabolite, 3′-O-methyl epicatechin, protect human fibroblasts from oxidative-stress-induced cell death involving caspase-3 activation. Biochem J. 2001;354:493–500. doi: 10.1042/0264-6021:3540493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basu-Modak S, Gordon MJ, Dobson LH, Spencer JP, Rice-Evans C, Tyrrell RM. Epicatechin and its methylated metabolite attenuate UVA-induced oxidative damage to human skin fibroblasts. Free Radic Biol Med. 2003;35:910–921. doi: 10.1016/s0891-5849(03)00436-2. [DOI] [PubMed] [Google Scholar]
- 47.Steffen Y, Gruber C, Schewe T, Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch Biochem Biophys. 2008;469:209–219. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Chen D, Wang CY, Lambert JD, Ai N, Welsh WJ, Yang CS. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: structure-activity relationship and molecular-modeling studies. Biochem Pharmacol. 2005;69:1523–1531. doi: 10.1016/j.bcp.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 49.Kawai Y, Tanaka H, Murota K, Naito M, Terao J. (−)-Epicatechin gallate accumulates in foamy macrophages in human atherosclerotic aorta: implication in the anti-atherosclerotic actions of tea catechins. Biochem Biophys Res Commun. 2008;374:527–532. doi: 10.1016/j.bbrc.2008.07.086. [DOI] [PubMed] [Google Scholar]
- 50.Peterson TG, Ji GP, Kirk M, Coward L, Falany CN, Barnes S. Metabolism of the isoflavones genistein and biochanin A in human breast cancer cell lines. Am J Clin Nutr. 1998;68:1505S–1511S. doi: 10.1093/ajcn/68.6.1505S. [DOI] [PubMed] [Google Scholar]
- 51.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. Faseb J. 1995;9:899–909. [PubMed] [Google Scholar]
- 52.Sahin K, Tuzcu M, Gencoglu H, Dogukan A, Timurkan M, Sahin N, Aslan A, Kucuk O. Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci. 87:240–245. doi: 10.1016/j.lfs.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Terao J, Yamaguchi S, Shirai M, Miyoshi M, Moon JH, Oshima S, Inakuma T, Tsushida T, Kato Y. Protection by quercetin and quercetin 3-O-beta-D-glucuronide of peroxynitrite-induced antioxidant consumption in human plasma low-density lipoprotein. Free Radic Res. 2001;35:925–931. doi: 10.1080/10715760100301421. [DOI] [PubMed] [Google Scholar]