Abstract
In this report we provide evidence that neuronal nicotinic acetylcholine receptors (nAChRs) are present on hippocampal astrocytes and their activation produces rapid currents and calcium transients. Our data indicate that these responses obtained from astrocytes are primarily mediated by an AChR subtype that is functionally blocked by α-bungarotoxin (αBgt) and contains the α7 subunit (αBgt-AChRs). Furthermore, their action is unusual in that they effectively increase intracellular free calcium concentrations by activating calcium-induced calcium release from intracellular stores, triggered by influx through the receptor channels. These results reveal a mechanism by which αBgt-AChRs on astrocytes can efficiently modulate calcium signaling in the central nervous system in a manner distinct from that observed with these receptors on neurons.
Alpha-bungarotoxin acetylcholine receptors (αBgt-AChRs) are abundant in the central nervous system (1, 2). They are thought to participate in brain functions such as modulation of synaptic transmission and memory formation (3). In addition, it has been proposed that they play an important role in the reinforcement of smoking behavior (4) and in disease states such as schizophrenia (5).
A key mediator of events triggered by the activation of αBgt-AChRs is calcium. These receptors exhibit a high permeability to calcium relative to sodium (6) and effectively raise intracellular calcium concentrations by depolarizing neurons and causing calcium influx through voltage-gated calcium channels (VGCCs) (7, 8).
Activation of αBgt-AChRs can influence a variety of calcium-dependent events in the central nervous system. For example, undifferentiated hippocampal progenitor cells express low levels of the receptor and their activation can lead to apoptotic cell death (9). Mice expressing a mutant form of the α7 gene, one that reduces receptor desensitization, are susceptible to abnormal apoptosis, possibly because of increased calcium influx through the receptor channels (10). Furthermore, αBgt-AChRs can increase the frequency of spontaneous events in the hippocampus, most likely because of their ability to increase transmitter release by altering presynaptic calcium levels (11, 12).
The existence of hyperpolarizing and depolarizing responses to nicotine reported from astrocytes (13) prompted us to examine nicotinic acetylcholine receptor (nAChR) signaling in astrocytes. Astrocytes have elaborate calcium signaling mechanisms (14). Recent studies have demonstrated that these cells respond to neuronal activity and modulate synaptic transmission (15). Bidirectional calcium signaling between astrocytes and neurons has been demonstrated (16). In addition, astrocytes have been shown to participate in calcium-mediated vesicular release of glutamate that can modulate neuronal activity (17, 18).
In this paper we describe an unusual cholinergic signaling cascade in hippocampal astrocytes that is mediated by αBgt-AChRs. We show that astrocytes exhibit currents sensitive to α7-specific antagonists. Activation of these receptors generates calcium transients in the cells resulting mainly from calcium-induced calcium release (CICR) triggered by ion flux through the receptor channels. Our results suggest that αBgt-AChRs activate a calcium-signaling cascade in astrocytes that is distinct from that activated by these receptors on neurons, suggesting that astrocytes could be important contributors to cholinergic signaling.
Methods
Cell Culture.
Primary astrocytes were cultured by one of the following two protocols. (i) Purified astrocytes were obtained according to Levison and McCarthy (19). Briefly, the hippocampus was dissected from newborn rats. Cells were dissociated with papain and plated on uncoated 7.5-cm flasks (Falcon) at 1 to 3 × 105 cells per flask. Cultures were grown in MEM/GLUTAMAX medium (Life Technologies, Rockville, MD) containing 20 mM glucose, penicillin (1000 IU/ml), streptomycin (1 mg/ml), and 10% FBS. No mitotic inhibitors were added. After a week in culture, the flask was shaken at 250 rpm overnight (12–16 h). The cells were then trypsinized and replated on coverslips coated with polylysine (100 μg/ml) and collagen (500 μg/ml), at the required density and used 36 to 48 h later. With this method, >99% of the cells were positive for glial fibrillary acidic protein (GFAP), indicating that they were astrocytic in nature. No neuronal contamination was observed. (ii) Hippocampi were dissociated and cells plated on coated coverslips at a density of 5–10 × 103 cells per 16-mm well. After 5–7 days in culture, >75% of the cells were GFAP-positive and could be morphologically identified. GFAP expression was determined by using an anti-GFAP antibody (Sigma) followed by a fluorescein-conjugated secondary antibody (Jackson Immunochemicals, West Grove, PA). All experiments in this study were performed on purified astrocytes and results were confirmed by using primary mixed cultures.
Agonist Application.
For both calcium imaging and electrophysiology, agonist was applied with a homemade piezo-based application systems. Tri-theta tubing (Vitrocom, Mountain Lakes, NJ) was mounted on a piezo-bending element (Morgan Matroc, Bedford, OH) and moved by applying a voltage across the piezo element. Tip diameter of each flow pipe was 250 μm, and the applicator was placed 150 to 200 μm away from the cell. Inflow was controlled by three-way solenoid valves (Cole–Parmer, Vernon Hills, IL) and the speed of solution exchange was routinely monitored by measuring junction currents across an open patch pipette. On an average, complete solution exchange was achieved within a millisecond. The rate of solution exchange at the astrocytes, some of which are extremely flat cells with ruffled membranes, is likely to be slower. The valves and the piezo element were controlled by software written in visual basic or by pclamp 7 (Axon Instruments, Foster City, CA). In all experiments with acetylcholine (ACh), atropine was always included to block muscarinic receptors.
Calcium Imaging.
The 35-mm dishes with glass bottoms were coated and cells were plated as described above. External recording solutions contained 140 mM NaCl, 2.5 mM KCl, 0.5 mM MgCl2, 2 mM CaCl2, 10 mM Hepes (pH 7.4), 10 mM glucose, and 0.0005 mM atropine. Specific modifications to this composition are described in the text. Osmolarity of all solutions was adjusted to between 310 and 315 milliosmolar. Cells were incubated with 10 μM fluo-3 acetoxymethyl ester (Molecular Probes) for 30 min.
Imaging experiments were performed with a Nikon Eclipse 300 microscope equipped for epifluorescence. A Nikon PlanApo ×60 (numerical aperture 1.4) oil immersion lens was used for all experiments. Excitation light was provided by a 150-W xenon arc lamp (Optiquip, Highland Mills, NY) and was attenuated to 3.125% by using neutral density filters to avoid photobleaching. Excitation and emission wavelengths were obtained by using a standard fluorescein filter set. Images were collected with a Cooke Sensicam peltier-cooled camera (PCO, Kelheim, Germany). Exposure times were usually 100 ms and images were collected at various frequencies as indicated. Acquisition and analyses were performed with slidebook software (Intelligent Imaging Innovations, Denver).
Fluorescence was measured over the entire cell body and responses are presented as % ΔF/F0, where F0 is the resting fluorescence of the cells and ΔF is the change in fluorescence over resting levels. The mean background autofluorescence, determined from 100 cells, was subtracted from all fluorescence traces. For normalizing fluo-3 signals, the following approaches were used: (i) In experiments where multiple ACh responses were obtained from the same cell, F0 was taken as the baseline fluorescence at the beginning of image acquisition and data were presented as percent increase in fluorescence. (ii) For direct comparisons among dishes, at the end of an experiment, the external solution was changed to one containing 10 mM MnCl2 and 10 μM ionomycin and no added calcium. The manganese-saturated fluorescence (FMn) was measured 5 to 10 min later, once the fluorescence had equilibrated. Fluorescence of each cell was then normalized to its respective FMn and compared. In some cells, FMax was also calculated with 10 mM CaCl2 and 10 μM ionomycin. The mean FMn/FMax was 0.205 ± 0.014 (mean ± SEM; n = 21) consistent to that reported for this dye in other systems (20). Manganese-saturated fluorescence should correspond to an intracellular calcium concentration of 100 nM (20). The average baseline fluorescence normalized to FMn was 0.99 ± 0.03 (n = 93 cells). For all experiments using manganese calibration, only one field per dish was imaged.
For calibrating the relative fluorescence of the dye to other divalent cations, 50 μM fluo-3 pentapotassium salt solutions with 10 mM chloride salts of Ca2+, Ba2+, or Sr2+ were loaded onto 50-μm-thick capillary tubes (Dynamic, NJ) and imaged as above. This procedure was necessitated because both ionomycin and calcium ionophore A23187 show poor selectivity for these ions (21, 22). Based on these experiments, the mean FSr/FCa was 0.27, whereas FBa/FCa was 0.14.
For rapid image acquisition, the cells were under constant illumination. The size of the acquired image was reduced so that the camera was able to capture images at an average rate of one frame per 59 ms. All images were corrected for photobleaching.
Electrophysiology.
Astrocytes in culture were used 4–7 days after initial plating or 36–48 h after replating. As far as possible, cells that were isolated in the field were used for the recordings. Recording conditions were as described for the measurement of rapid currents from astrocytes (23). Briefly, the external solution was the same as that used for the imaging experiments. Internal solution contained 130 mM potassium gluconate, 10 mM NaCl, 10 mM Hepes (pH 7.2), 10 mM EGTA, 2 mM ATP, and 0.2 mM GTP and were adjusted to pH 7.2 and 306 milliosmolar. Patch pipettes were made from borosilicate glass and had resistances between 3 and 5 MΩ. Series resistances varied between 5 and 7 MΩ after compensation. Cell capacitances ranged from 20 to 60 pF with a mean ± SEM of 40.8 ± 5.5 pF, consistent with what has been reported for these cells (24). Cells were voltage-clamped at −60 mV. Currents were recorded with an Axopatch 200B patch amplifier (Axon Instruments) and an MIO-16-E2 A/D interface (National Instruments, Austin, TX). Acquisition and analysis were performed with software written in visual basic.
Current–voltage relationships for the slow component of the current were generated by applying 100 μM ACh for 1.3 s. The voltage was ramped (1 mV/ms) from −60 mV to +35 mV, 300 ms after the onset of drug application. A second ramp without the drug was applied, and the currents were subtracted to arrive at the final current–voltage relationship.
Results
Astrocytes Have Functional nAChRs.
We examined the presence of functional nAChRs on hippocampal astrocytes by two different techniques: whole cell voltage-clamp and calcium measurements.
Whole-cell voltage-clamp measurements were performed on cultured hippocampal astrocytes. ACh was applied rapidly for 300 ms in the presence of 100 nM atropine to block any muscarinic effects. Fig. 1A shows a representative current trace from such experiment. Rapid application of 1 mM ACh elicits fast desensitizing responses. Under our experimental conditions, all astrocytes responded to 1 mM ACh, although the amplitude of the response was variable (range, 100 pA to 1.5 nA) with an average amplitude of 490 ± 142 pA (mean ± SEM, from 11 cells).
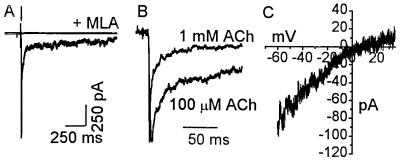
Figure 1.
Astrocytes exhibit nicotinic cholinergic current responses. (A) A 300-ms application of 1 mM ACh generated a fast desensitizing response (lower trace). This response is absent when the agonist was applied with 10 nM MLA, an αBgt-AChR-specific antagonist (upper trace). (B) Current responses to 1 mM and 100 μM ACh in the presence of atropine from different cells were normalized (peak current = 1) and averaged. Data from 11 cells (1 mM ACh) and 15 cells (100 μM ACh) are shown. ACh responses decay faster at the higher agonist concentration. (C) Current–voltage relationship from a single cell, obtained in the presence of 100 μM ACh, exhibits an inward rectification. Similar results were obtained from 4 cells. All experiments were done in presence of 100 nM atropine to block muscarinic receptors.
Data obtained from application of 1 mM ACh (n = 11 cells) and 100 μM ACh (n = 15 cells) are presented in Fig. 1B. Currents were normalized to their peak and averaged. A double exponential fit, imposed on the responses for the period of the agonist application, indicated that upon application of 1 mM ACh currents decayed with time constants of 2 ms and 25 ms (r2 = 0.98). Currents were slower when agonist concentration was reduced to 100 μM and decayed with time constants of 6.17 ms and 49.5 ms (r2 = 0.97). Applying buffer alone did not elicit discernable currents (data not shown). As expected, current–voltage relationships obtained in the presence of 100 μM ACh showed an inward rectification (n = 4 cells; Fig. 1C).
The response to ACh was nicotinic in nature. Application of ACh in the presence of 10 nM methyllycaconitine (MLA), an agent selective for αBgt-AChRs (25), completely blocked the response (n = 6 cells, Fig. 1A). Washout for 5 min resulted in the recovery of 69 ± 8% of control. Similarly, treating cells with 100 nM αBgt also resulted in a complete block of ACh-induced currents (n = 4 cells). These results indicate that the currents elicited in astrocytes by ACh are due to the activation of αBgt-AChRs. Current densities calculated with the measured peak current on activation by 1 mM ACh and cell capacitance were 11.2 ± 4 pA/pF at −60 mV. This suggests that only 2.5 or 5 functional channels are present per 100 μm2 (assuming uniform distribution and a single channel conductance of 73 pS (26) or 35 pS (27). This is about an order of magnitude lower than that seen in hippocampal neurons (28).
A second set of experiments made use of the ability of αBgt-AChRs to increase the intracellular free calcium concentration. To determine whether application of ACh to astrocytes would lead to detectable calcium transients, we loaded the cells with 10 μM fluo-3 acetoxymethyl ester and imaged them at 0.3 Hz. ACh was applied for 2 s in the presence of 500 nM atropine. This procedure resulted in a rapid and transient increase in cytosolic calcium as indicated by changes in fluo-3 fluorescence (Fig. 2A). Calcium rise occurred immediately upon application of the agonist and decayed to baseline over the next 2 min (Fig. 2B).
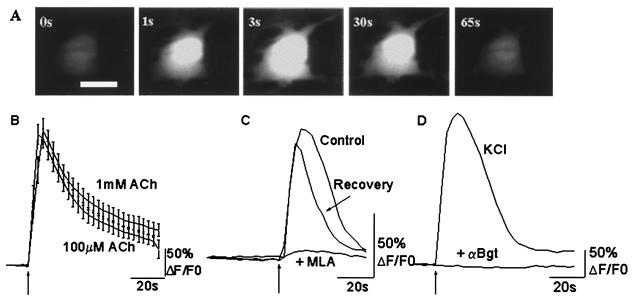
Figure 2.
Calcium responses in astrocytes on activation of nAChRs. (A) Fluo-3 fluorescence of a single astrocyte after a 2-s application of 100 μM ACh. Images shown were acquired before (0 s), and 1 s, 3 s, 30 s, and 65s after ACh application. (Bar = 10 μm.) (B) Averaged calcium transients from 31 cells (1 mM ACh) and 42 (100 μM ACh) fluo-3-loaded astrocytes. The averages are not normalized and represent actual increase in fluorescence above baseline. Similar calcium transients were obtained with both agonist concentrations. (C) Calcium response of an astrocyte in the absence (Control) and presence (+MLA) of 10 nM MLA. The third trace (Recovery) shows the ACh response after a 5-min wash to remove the antagonist. MLA reversibly blocked the ACh response. (D) Changes in calcium transients in presence of 100 nM α-Bgt. Cells were preincubated for 30 min with the toxin before application of 100 μM ACh. The lack of response of an astrocyte to ACh application is shown (lower trace). The upper trace (KCl) shows the response of the same cell to a 2-s application of 75 mM KCl, indicating that calcium responses to other stimuli were unperturbed. Cells were loaded with Fluo-3 acetoxymethyl ester for 30 min before being challenged by the agonist. ACh was applied for 2 s as indicated by the arrows, and the cell was imaged at 0.3 Hz. Atropine (500 nM) was present at all times.
Results obtained from application of 1 mM ACh (n = 31 cells) and 100 μM ACh (n = 42 cells) are presented in Fig. 2B. On an average, 1 mM ACh increased the fluorescence by 245 ± 15%. Interestingly, application of 100 μM ACh showed a similar calcium rise. Fluorescence increased 237 ± 13% over baseline (n = 42 cells). A second ACh response, elicited 5 min later from the same cells, was 66 ± 8% of the first response (mean ± SEM, n = 45 cells, eight experiments). Application of buffer without the agonist produced no response (67 of 72 cells), whereas all of the same cells responded to subsequent application of ACh (data not shown), thus ruling out the activation of mechanosensitive channels. Among the 5 cells that responded to buffer application the mean change in fluorescence was 50 ± 33% (mean ± SEM).
Confirming evidence that the response was mediated by αBgt-AChRs came from the finding that the calcium transients, in response to 100 μM ACh, can be reversibly blocked by 10 nM MLA. In the presence of the antagonist, the calcium response was blocked by 99 ± 0.6% (n = 21 cells). Washout of the drug for 5 min resulted in a recovery to 48 ± 15% of control responses (Fig. 2C).
In addition, preincubation of astrocytes with 100 nM αBgt for 30 min completely abolished ACh-induced calcium transients in a manner that was not reversible over a 30-min washout period (n = 61 cells). In the same cells, depolarization by 75 mM KCl produced a large calcium transient, indicating that the lack of response was indeed caused by the block of αBgt-AChRs. Data from a representative cell are presented in Fig. 2D.
αBgt-AChRs Activation Can Trigger CICR.
We then examined the nature and the source of calcium transients observed on activation of αBgt-AChRs on astrocytes. We found that removing calcium from extracellular solution resulted in no calcium transients in response to ACh application (0 ± 1% increase in fluorescence, n = 17 cells; Fig. 3A). This result suggested that influx of calcium from the outside was essential, consistent with signaling through ligand-gated ion channels.
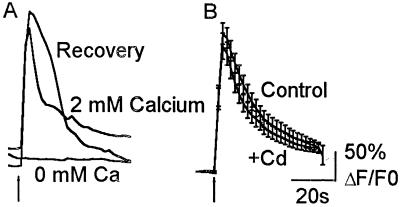
Figure 3.
Dependence of the ACh response on extracellular calcium. (A) Response of a single astrocyte to a 2-s application of 100 μM ACh in the presence of normal calcium (2 mM Calcium), where external calcium was substituted with 0.5 mM EGTA; (0 mM Ca) and a second challenge with ACh in 2 mM calcium (Recovery). Removing extracellular calcium abolished the ACh-induced calcium response. Similar results were obtained from 17 cells. (B) Averaged responses to a 2-s application of 100 μM ACh in the presence of 100 μM CdCl2. Robust ACh responses were observed in the presence of the VGCC blocker (+Cd). These responses were similar to those observed from untreated cells (Control). Data are the mean ± SEM from 40 cells (+Cd) and 42 cells (Control). ACh was applied in the presence of 500 nM atropine.
In neurons, increases in the intracellular calcium concentration on activation of αBgt-AChRs are mainly due to calcium influx through VGCCs (7). To determine whether αBgt-AChR activation resulted in influx through these channels, we examined the responses to 100 μM ACh in the presence of 100 μM CdCl2. This concentration of cadmium was sufficient to block KCl-evoked responses from astrocytes (data not shown). Blocking calcium channels had no effect on calcium influx (215 ± 22% increase in fluorescence, n = 40 cells; Fig. 3B). These results suggest VGCCs do not contribute significantly to the nicotinic responses and that the influx necessary for calcium response comes from ion flux through αBgt-AChR channels.
Is calcium influx through αBgt-AChR channels further amplified by release from intracellular calcium stores? To answer this question, we blocked microsomal calcium pumps by incubating cells with 1 μM thapsigargin (TG) for 30 min. At the end of the incubation period, astrocytes were challenged with 20 mM caffeine to test for store depletion. Results were expressed as ΔF/FMn calculated for each cell. As expected, caffeine gave a very small response (increase in fluorescence of 13 ± 1.1%, n = 28 cells) indicating that intracellular stores had indeed been depleted (Fig. 4A). Under these conditions, rapid application of 100 μM ACh with 500 nM atropine resulted in a very small increase in fluorescence (Fig. 4A, increase in fluorescence of 4 ± 0.2%).
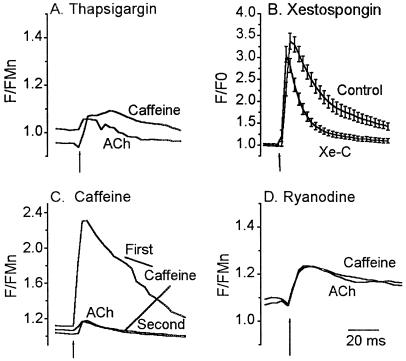
Figure 4.
αBgt-AChR-mediated calcium response is primarily caused by CICR. (A) Depletion of all intracellular calcium stores by TG abolished response to ACh. Cells were incubated in 1 μM TG for 30 min, washed, and then challenged with 20 mM caffeine (Caffeine) to deplete any residual store calcium. Subsequent application of ACh lead to a small increase in calcium, on average about 2% of control, indicating that most of the rise in cytosolic calcium is due to release from intracellular stores. Data are the mean response from 28 cells. (B) InsP3 stores contribute to ACh-induced calcium transient. Untreated astrocytes (Control) or astrocytes treated with 20 μM Xe-C for 30 min (Xe-C) were challenged with 100 μM ACh for 2 s. ACh elicited a large calcium transient in Xe-C-treated cells that decayed faster than that obtained from untreated cells. Data are the mean ± SEM from 38 cells (Xe-C) and 42 cells (Control). (C) Caffeine stores are necessary for αBgt-AChR-mediated calcium response. Application of 20 mM caffeine for 2 s depleted calcium stores. Application of ACh 90 s later produced a small response as did a second application of 20 mM caffeine, suggesting that αBgt-AChRs and caffeine share a common pool of intracellular calcium. Data are the mean transient from 38 cells. (D) ACh response can be blocked by ryanodine. Cells were incubated with 100 μM ryanodine for 30 min to block ryanodine receptors. Cells were challenged with 20 mM caffeine for 2 s, followed 5–7 min later by 100 μM ACh for 2 s. Responses to both ACh (lower trace) and caffeine (upper trace) were blocked, suggesting that the rise in cytosolic calcium resulting from activation of αBgt-AChRs is indeed amplified by CICR. Data are the mean response from 40 cells. Basal F/FMn levels after incubation with TG and ryanodine were 1.02 ± 0.04 and 1.1 ± 0.05, respectively. All responses were in the presence of 500 nM atropine.
This lack of response was not caused by elevated resting calcium levels as showed by resting F/FMn values in Fig. 4. We also ruled out direct action of TG or changes in resting calcium on nAChRs by measuring currents under these conditions. No significant changes in ACh-evoked currents were detected (n = 8 cells; data not shown).
The data indicated that activation of αBgt-AChRs leads to release of calcium from intracellular stores. Previous studies have demonstrated the presence of functionally distinct inositol trisphosphate (InsP3) and ryanodine-sensitive stores in astrocytes (29). We examined the contributions from both these sources.
Involvement of InsP3-sensitive stores was investigated by incubating cells in 20 μM Xestospongin C (Xe-C), a membrane-permeant noncompetitive inhibitor of InsP3 receptors (30). After a 30-min incubation, the mean change in fluorescence in response to 100 μM ACh was 204 ± 18%, not significantly different from control responses (n = 38 cells, Fig. 4B). Decay rate of the calcium transient was, however, faster. Mean time constant of decay for Xe-C-treated cells was 11.8 s (r2 = 0.99) compared with 24.8 s (r2 = 0.99) in untreated astrocytes. In addition, from the integral of the transient, we calculated the total calcium change after Xe-C treatment to be ≈25% less than in control cells. This suggests that InsP3 stores are activated later in the calcium response and are minor contributors to peak of the calcium transients resulting from the activation of αBgt-AChRs.
Our data implied that most of the αBgt-AChR-induced increase in calcium is caused by CICR from ryanodine-sensitive stores. If this were true, we would expect that a second response under conditions where the store had not had time to refill would be decreased. We demonstrated this response by depleting the stores by addition of 20 mM caffeine. As shown in Fig. 4C, the response elicited by 100 μM ACh, 90 s after challenging the cells with caffeine was 6.5 ± 3% of control responses (n = 38 cells). As, expected, a second caffeine response after the ACh application was also reduced (12.02 ± 4% of the control caffeine response), indicating that both caffeine and αBgt-AChRs use the same pool of intracellular calcium.
We then tested the effects on the ACh response of blocking caffeine-sensitive stores with 100 μM ryanodine. In the presence of this concentration of ryanodine, application of 100 μM ACh gave a very small increase in fluo-3 fluorescence (13.1 ± 1%; n = 40 cells; Fig. 4D), as did an application of caffeine (15.5 ± 1.3%), leading us to conclude that most of the calcium rise seen in control cells was caused by release from ryanodine-sensitive stores.
One concern with pharmacological manipulations of intracellular stores is that they perturb calcium homeostasis in cells. We, therefore, confirmed our findings by using a different approach taking advantage of two previous observations. (i) αBgt-AChRs have a broad permeability to divalent cations (31). (ii) Although strontium can substitute for calcium in triggering CICR (32), this effect is not mimicked by barium (33). A further advantage with this approach is that fluo-3 has a much reduced fluorescence when bound to both barium and strontium than that seen with the calcium-bound dye. Thus, if most of the calcium rise were caused by calcium released from stores, one would see a rise in fluorescence with strontium but not with barium.
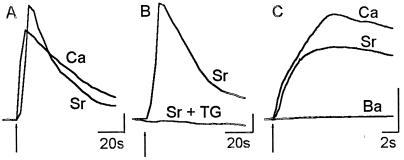
Data from ion-substitution experiments are shown in Fig. 5. Application of ACh, in the presence of 10 mM extracellular strontium, resulted in a robust rise in fluorescence. A second application of ACh 5 min later, this time with extracellular calcium, elicited responses that were 79 ± 8.5% of the first strontium response (Fig. 5A; n = 25 cells; compare with 66 ± 8% for the second of two calcium responses from these cells). Pretreatment of astrocytes with TG abolished the response (Fig. 5B). Further, no calcium transients were seen when barium was used as the divalent cation (Fig. 5C), even though the average current response was the same as control (n = 6 cells; data not shown).
Figure 5.
ACh response in the presence of other divalent cations. (A) Fluorescence response of an astrocyte to a 2-s application of 100 μM ACh in external medium containing no added calcium and 10 mM SrCl2 (Sr). Response of the same cell to ACh application in the presence of 2 mM calcium (Ca). A robust response to ACh was observed in the presence of strontium comparable to that seen with calcium. (B) No transient was observed when ACh in 10 mM SrCl2 was applied in the presence of 1 μM TG [compare the control response (Sr) with the response from a treated cell (Sr + TG)]. (C) Rapid imaging in the presence of various divalent cations. Agonist (100 μM ACh) was applied 1 s into the acquisition, for 25 s. Three hundred images were acquired on an average at 17 Hz. At the end of each experiment, a second acquisition was done at the same rate to determine the rate of photobleaching. All data were corrected for photobleaching. Data are the averaged response from 21 cells (Sr), 16 cells (Ca), and 25 cells (Ba). (y-axis bar = 50% ΔF/F0.)
A requirement for CICR mediated by αBgt-AChR activation in astrocytes would be calcium influx through the receptor channels (because VGCCs are not involved). Our results with TG suggest that this trigger calcium is at best a minor component of the response. It is, however, possible that we were unable to detect a small and rapid influx component because of slow rate of imaging (1 Hz). We, therefore, increased our acquisition rate to one frame every 59 ms. Fig. 5C shows averaged results from 16 to 25 cells. As expected, ACh application in the presence of both strontium and calcium elicited a transient response, whereas application in the presence of barium did not. These data imply that the small influx component is not a discrete, temporally segregated event.
Discussion
In this study, we describe nicotinic cholinergic responses from hippocampal astrocytes that shed light on the unique properties and signaling mechanisms used by αBgt-AChRs. Our data allow us to draw the following conclusions: (i) Astrocytes express functional αBgt-AChRs. Their activation leads to discernible currents and a large rise in cytosolic calcium. (ii) Unlike nAChRs on neurons and ligand-gated ion channels in general, αBgt-AChRs on astrocytes increase intracellular calcium concentration mainly by triggering calcium release from intracellular caffeine-sensitive stores.
αBgt-AChRs on astrocytes exhibit several significant differences from those present on neurons. One difference is in the number of active receptors. From our calculations, the density of functional receptors on astrocytes is lower than that seen on hippocampal neurons. The actual numbers, however, are likely to be underestimates. Because αBgt-AChR channels open very briefly and desensitize rapidly (34) and because receptor activation will be asynchronous, it is probable that number of functional receptors calculated from the peak currents represent only a small fraction of the total. Further, this estimate assumes uniform distribution of αBgt-AChRs, which has been shown not be true in systems tested thus far.
We expect that αBgt-AChRs, described in this study from cultured astrocytes, are present in situ. There is considerable evidence that a number of receptors and signaling mechanisms described from astrocytes in culture also exist in brain slices. These include receptors for glutamate, γ-aminobutyric acid, glycine, and ATP (for review, see ref. 35). For example, ionotropic glutamate receptors seen on astrocytic in culture have recently been identified from the cells in slice preparations (36). Furthermore, glutamate release mediated by rises in intra-astrocytic calcium levels, described in detail from cultures, has been shown to occur in slices (17). These studies establish the reliability of astrocyte analyses in culture for predicting behavior in situ, at least in terms of neurotransmitter receptor expression and function.
A second important difference between αBgt-AChRs on neurons and astrocytes is that, in hippocampal neurons, most of the changes in bulk calcium induced by receptor activation result from influx through VGCCs. Activation of αBgt-AChRs on astrocytes, however, leads to an increase in cytosolic calcium mainly via CICR from caffeine-sensitive calcium stores. Despite the reported presence of VGCCs on cultured astrocytes and our data showing calcium transients in response to rapidly applied KCl, they do not seem to play a role in calcium entry upon activation of αBgt-AChRs.
Our experiments show that the influx component at best accounts for about 6% of the bulk calcium response. This result is not surprising and is consistent with what is known about αBgt-AChRs and CICR in other systems. In the absence of contributions from VGCCs, influx through αBgt-AChRs on hippocampal neurons accounts for about 10% of the peak bulk calcium response (7). Examination of CICR from dendrites in hippocampal slices triggered by synaptic stimulation has shown that the trigger calcium is undetectable (37), suggesting a spatial coupling between N-methyl-d-aspartate receptors and intracellular stores. Our results also suggest that the small influx component is not a discrete, temporally segregated event, at least at our current temporal resolution. Thus, it is possible that astrocytes show spatial coupling between αBgt-AChRs and caffeine-sensitive channels. Our study and studies on N-methyl-d-aspartate receptors (37) and a recent report on the α9-AChRs (38) suggest that functional coupling of ligand-gated ion channels with high calcium permeability to CICR may be more widespread than has been recognized.
Preliminary studies examining the involvement of InsP3 receptors raise the interesting possibility of sequential amplification of intracellular calcium levels. The data suggest that release from InsP3 stores is minor and secondary to release from ryanodine stores. As CICR seems to be necessary for the AChR response (based on the finding that ryanodine blocks most of the response), it follows that calcium release from CICR channels in turn elicits further release from InsP3 stores. This report, along with previous studies (29, 38, 39), also provides evidence for the existence of two separate and spatially resolved endoplasmic reticulum stores in astrocytes.
Such sequential amplification could be a mechanism by which fast desensitizing receptors such as αBgt-AChRs can elicit large and long lasting changes in bulk cytosolic calcium. This interplay would also allow for greater flexibility in modulating calcium levels in these cells.
The concept of astrocytes as active modulators of synaptic transmission is receiving considerable attention. Both functional (40) and anatomical studies (41, 42) show that astrocytic processes have intimate contacts with individual synapses and that synaptically released neurotransmitters can have access to receptors on astrocytic membranes (42). Activation of astrocytic receptors surrounding a single synapse causes local increases in calcium (40). Complex calcium signaling cascades involving CICR and release from InsP3 stores will allow these local signals to be transmitted from one part of the astrocyte to another (16). As the processes of one astrocyte contact many synapses, signals received by the cell at one synapse can be transmitted to processes surrounding other synapses. Calcium transients at these astrocytic processes could result in the vesicular release of glutamate (17), which would, in turn, leads to an increase in the frequency of glutamatergic and GABAergic spontaneous postsynaptic currents (43).
We propose that activation of αBgt-AChRs on astrocytes by synaptically released ACh at a few sites could alter the frequency of excitatory and inhibitory miniature postsynaptic potentials at a large number of synapses. Thus it is possible that the reported presynaptic nicotinic modulation of transmitter release (11, 44, 45) might, in some cases, represent astrocyte-mediated glutamate release. Such a mechanism would provide means by which nAChRs regulate synaptic efficacy more globally in the central nervous system than has been recognized.
Acknowledgments
We thank Dr. Emily Huang at the Cold Spring Harbor Press for assistance with the visual basic software and Dr. Kamran Khodakhah for helpful discussions. This work was funded by a grant from the National Institute on Drug Abuse, RO1 DA 10266 (S.V.). G.S. is a recipient of the National Research Service Award postdoctoral award from National Institutes of Health, a postdoctoral fellowship from American Heart Association, Mountain Affiliate, and a National Science Foundation POWRE grant.
Abbreviations
- αBgt
α-bungarotoxin
- ACh
acetylcholine
- AChR
ACh receptor
- nAChR
neuronal AChR
- CICR
calcium-induced calcium release
- MLA
methyllycaconitine
- TG
thapsigargin
- Xe-C
Xestospongin C
- VGCC
voltage-gated calcium channel
- InsP3
inositol trisphosphate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 3631.
References
- 1.Clarke P B, Schwartz R D, Paul S M, Pert C B, Pert A. J Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Role L W, Berg D K. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 3.MacDermott A B, Role L W, Siegelbaum S A. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 4.Mansvelder H D, McGehee D S. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 5.Leonard S, Breese C, Adams C, Benhammou K, Gault J, Stevens K, Lee M, Adler L, Olincy A, Ross R, et al. Eur J Pharmacol. 2000;393:237–242. doi: 10.1016/s0014-2999(00)00035-2. [DOI] [PubMed] [Google Scholar]
- 6.Castro N G, Albuquerque E X. Biophys J. 1995;68:516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrantes G E, Murphy C T, Westwick J, Wonnacott S. Neurosci Lett. 1995;196:101–104. doi: 10.1016/0304-3940(95)11859-u. [DOI] [PubMed] [Google Scholar]
- 8.Vijayaraghavan S, Pugh P C, Zhang Z W, Rathouz M M, Berg D K. Neuron. 1992;8:353–362. doi: 10.1016/0896-6273(92)90301-s. [DOI] [PubMed] [Google Scholar]
- 9.Berger F, Gage F H, Vijayaraghavan S. J Neurosci. 1998;18:6871–6881. doi: 10.1523/JNEUROSCI.18-17-06871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orr-Urtreger A, Broide R S, Kasten M R, Dang H, Dani J A, Beaudet A L, Patrick J W. J Neurochem. 2000;74:2154–2166. doi: 10.1046/j.1471-4159.2000.0742154.x. [DOI] [PubMed] [Google Scholar]
- 11.Gray R, Rajan A S, Radcliffe K A, Yakehiro M, Dani J A. Nature (London) 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 12.McGehee D S, Heath M J, Gelber S, Devay P, Role L W. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 13.Hosli L, Hosli E, Della B G, Quadri L, Heuss L. Neurosci Lett. 1988;92:165–170. doi: 10.1016/0304-3940(88)90054-7. [DOI] [PubMed] [Google Scholar]
- 14.Verkhratsky A, Kettenmann H. Trends Neurosci. 1996;19:346–352. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]
- 15.Araque A, Parpura V, Sanzgiri R P, Haydon P G. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 16.Pasti L, Volterra A, Pozzan T, Carmignoto G. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini B L, Pozzan T, Volterra A. Nature (London) 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 18.Parpura V, Haydon P G. Proc Natl Acad Sci USA. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levison S W, McCarthy K D. In: Culturing Nerve Cells. Banker G, Goslin K, editors. Cambridge, MA: MIT Press; 2000. pp. 309–336. [Google Scholar]
- 20.Minta A, Kao J P, Tsien R Y. J Biol Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- 21.Liu C, Hermann T E. J Biol Chem. 1978;253:5892–5894. [PubMed] [Google Scholar]
- 22.Pfeiffer D R, Reed P W, Lardy H A. Biochemistry. 1974;13:4007–4014. doi: 10.1021/bi00716a029. [DOI] [PubMed] [Google Scholar]
- 23.Cull-Candy S G, Wyllie D J. Ann NY Acad Sci. 1991;633:458–474. doi: 10.1111/j.1749-6632.1991.tb15636.x. [DOI] [PubMed] [Google Scholar]
- 24.Wyllie D J, Cull-Candy S G. J Physiol (London) 1994;475:95–114. doi: 10.1113/jphysiol.1994.sp020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward J M, Cockcroft V B, Lunt G G, Smillie F S, Wonnacott S. FEBS Lett. 1990;270:45–48. doi: 10.1016/0014-5793(90)81231-c. [DOI] [PubMed] [Google Scholar]
- 26.Castro N G, Albuquerque E X. Neurosci Lett. 1993;164:137–140. doi: 10.1016/0304-3940(93)90876-m. [DOI] [PubMed] [Google Scholar]
- 27.Yu C R, Role L W. J Physiol (London) 1998;509:651–665. doi: 10.1111/j.1469-7793.1998.651bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alkondon M, Albuquerque E X. J Pharmacol Exp Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- 29.Golovina V A, Bambrick L L, Yarowsky P J, Krueger B K, Blaustein M P. Glia. 1996;16:296–305. doi: 10.1002/(SICI)1098-1136(199604)16:4<296::AID-GLIA2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 30.Gafni J, Munsch J A, Lam T H, Catlin M C, Costa L G, Molinski T F, Pessah I N. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- 31.Liu Q S, Berg D K. J Neurophysiol. 1999;82:1124–1132. doi: 10.1152/jn.1999.82.3.1124. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez-Cruz A, Escobar A L, Jimenez N. J Gen Physiol. 1997;109:147–167. doi: 10.1085/jgp.109.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee H C. J Biol Chem. 1993;268:293–299. [PubMed] [Google Scholar]
- 34.McNerney M E, Pardi D, Pugh P C, Nai Q, Margiotta J F. J Neurophysiol. 2000;84:1314–1329. doi: 10.1152/jn.2000.84.3.1314. [DOI] [PubMed] [Google Scholar]
- 35.Verkhratsky A, Steinhauser C. Brain Res Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 36.Shelton M K, McCarthy K D. Glia. 1999;26:1–11. doi: 10.1002/(sici)1098-1136(199903)26:1<1::aid-glia1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 37.Emptage N, Bliss T V, Fine A. Neuron. 1999;22:115–124. doi: 10.1016/s0896-6273(00)80683-2. [DOI] [PubMed] [Google Scholar]
- 38.Jagger D J, Griesinger C B, Rivolta M N, Holley M C, Ashmore J F. J Physiol (London) 2000;527:49–54. doi: 10.1111/j.1469-7793.2000.t01-1-00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Golovina V A, Blaustein M P. Glia. 2000;31:15–28. doi: 10.1002/(sici)1098-1136(200007)31:1<15::aid-glia20>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 40.Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Nat Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- 41.Ventura R, Harris K M. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aoki C. J Neurosci. 1992;12:781–792. doi: 10.1523/JNEUROSCI.12-03-00781.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Araque A, Sanzgiri R P, Parpura V, Haydon P G. J Neurosci. 1998;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alkondon M, Pereira E F, Barbosa C T, Albuquerque E X. J Pharmacol Exp Ther. 1997;283:1396–1411. [PubMed] [Google Scholar]
- 45.Radcliffe K A, Fisher J L, Gray R, Dani J A. Ann NY Acad Sci. 1999;868:591–610. doi: 10.1111/j.1749-6632.1999.tb11332.x. [DOI] [PubMed] [Google Scholar]