Abstract
Spring waters from alpine karst aquifers are important drinking water resources. To investigate in situ prokaryotic heterotrophic production (HP) and its controlling factors, two alpine karst springs of contrasting hydrogeology but of nearby catchments were studied over two annual cycles. Heterotrophic production in spring water, as determined by [3H]leucine incorporation, was low but revealed strong seasonal variations ranging from 0.06 to 6.83 pmol C l−1 h−1 (DKAS1, dolomitic karst-spring) and from 0.50 to 75.6 pmol C l−1 h−1 (LKAS2, limestone karst-spring). Microautoradiography combined with catalyzed reporter deposition - fluorescence in situ hybridization (MAR-CARD-FISH) showed that only about 7 % of the picoplankton community took up [3H]leucine resulting in generation times of 3 to 684 days. Principal component analysis, applying hydrological, chemical and biological parameters demonstrated that planktonic heterotrophic production in LKAS2 was strongly governed by hydrogeographical components (e.g. discharge), whereas variations in DKAS1 are also strongly influenced by changes within the aquifer itself. Measurements in sediments recovered from LKAS2, DKAS1 and similar alpine karst aquifers (n=12) revealed an 106-fold higher heterotrophic production (average 19 μmol C dm−3 h−1) with significantly lower generation times as compared to the planktonic fraction, highlighting the metabolic potential of surface associated endokarst communities to add to self-purification processes. Estimates of microbially mediated CO2 in this compartment indicated a possible contribution to karstification.
Keywords: groundwater, heterotrophic prokaryotic production, karstic spring water, clastic sediments
INTRODUCTION
Groundwater resources from alpine or mountainous karstic aquifers are of fundamental importance for public water supply in many regions throughout the world. Hydrogeology is an important factor to consider in such systems, as rainfall events in the catchment area can lead to immediate surface runoff into karst conduits, probably not only altering spring water quality but also influencing biogeochemical cycles and indigenous (micro)organisms within the aquifer (Gibert et al. 1994, Mahler et al. 2000, Farnleitner et al. 2005).
During the past two decades it has become obvious that groundwater resources should not only be viewed as drinking water reservoirs but also as distinct aquatic ecosystems (Gibert 2001). Diverse microbial communities were found in the deep subsurface, at depths believed to be sterile hitherto (Griebler 2001, Goldscheider et al. 2006, Griebler & Lueders 2008, Pronk et al. 2008). These microbes are involved in many subterranean geochemical processes, such as diagenesis, weathering, precipitation, and in oxidation or reduction reactions of metals, carbon, nitrogen and sulphur (Lauritzen & Bottrell 1994, Hirsch et al. 1995). However, except for a few reports, mostly dealing with (low-) mountain karstic systems and caves, accessible information on microbial communities in karst aquifers is still sparse (Ghiorse & Wilson 1988, Gounot 1994, Rusterholtz & Mallory 1994, Menne 1999, Simon et al. 2001, Pronk et al. 2006, Pronk et al. 2008).Consequently, studies are needed that lead to a better understanding of the natural structure and function of subterranean systems and allow for effective protection of groundwater aquifers and its inhabiting biota (Mösslacher et al. 2001, Danielopol et al. 2003). Knowledge on the prokaryotic communities inhabiting such ecosystems is of interest for both, basic and applied research (water suppliers), because it (1) contributes to the understanding of self-purification processes in the aquifer, regarding allochthonous chemical or microbial contamination, (2) elucidates potential microbially mediated biogeochemical cycles, e.g., the possible impact of microbes on the karstification-process and (3) helps to judge microbial spring-water quality, such as the biostability of the abstracted water.
Recently, we could demonstrate the presence of stable autochthonous microbial endokarst communities (AMEC) in alpine spring-water (Farnleitner et al. 2005), which form an indigenous part of the endokarst system. Two contrasting springs, having a nearby catchment area but revealing different hydrogeological conditions (i.e., contrasting water storage capacity) were investigated. The LKAS2 spring represents a system with well developed karst conduits (Stadler & Strobl 1997) allowing for high flow velocities (resulting in detectable microbial surface input during summer) with an average water residence time of 1.5 years, whereas DKAS1 is dominated by fissured and porous media and an average water residence time of around 22 years (Stadler & Strobl 1997). However, one should keep in mind that some water will spend much less time in the aquifer than average and some much more, e.g. during rainfall events, surface water can pass through the LKAS2 system within hours. Evidence was presented that prokaryotic numbers (PN), biomass and community composition in the spring water was directly related to the respective hydrogeology of the studied aquifers (Farnleitner et al. 2005), as was also shown for other ecosystems (Lindström & Bergström 2004). Although oligotrophic environments are generally known to have low rates of activity (Church et al. 2000, Church et al. 2004), measurements of bacterial biomass production are central to infer the role of heterotrophic prokaryotes in food webs and their effect in biogeochemical cycles (Kirchman et al. 1985, Kirschner et al. 1999, Buesing & Gessner 2003, Cottrell & Kirchman 2003).
The aim of this study was 1) to determine the variation and control of prokaryotic heterotrophic in situ activity in spring-water of alpine karst aquifers as important drinking water resources and 2) to elucidate the potential relevance of planktonic versus sessile endokarst communities on processes within karst groundwater systems (e.g. self purification, karstification). [3H]leucine incorporation (Kirchman et al. 1985, Simon & Azam 1989) was used to estimate the prokaryotic heterotrophic activity within the plankton community. Furthermore, a combination of catalyzed reporter deposition-fluorescence in situ hybridization (CARD-FISH) (Wilhartitz et al. 2007) and microautoradiography (MAR) was applied to examine assimilation of [3H]leucine at the single-cell level, in order to determine the contribution of Bacteria and Archaea to planktonic heterotrophic production (Teira et al. 2004). For porous aquifers it has been concluded that the attached populations reveal higher metabolic activity than planktonic cells (Pedersen 1993, Alfreider et al. 1997). Sediments in karstic aquifers attracted much attention during the last years (Vesper & White 2004b, Toran et al. 2006, Herman et al. 2007, Herman et al. 2008). However, they might not only be interesting in terms of transport mechanisms, e.g. bacteria or certain metallic elements (Mahler et al. 2000, Vesper & White 2004a), but also because of their huge surface abetting biofilm formation. There are speculations that CO2 from aerobic microorganisms could be an important factor when discussing karstification, especially in bare karst areas (Gabrovsek et al. 2000). Therefore, we measured prokaryotic numbers and heterotrophic production ([14C]leucine) in karst aquifer sediments recovered from LKAS2, DKAS1 and other locations in order to estimate the metabolic potential of attached autochthonous endokarst communities. To our knowledge this is the first study determining prokaryotic heterotrophic in situ activity in alpine karst spring water and its respective aquifer sediments, allowing for leadoff speculations about possible ecological implications of the microbial compartment in such groundwater systems.
MATERIAL AND METHODS
Study site and basic microbiological parameters
The two springs (LKAS2 and DKAS1) are located in the Northern Calcareous Alps in Austria (detailed description in Farnleitner et al, 2005). Samples for microbiological parameters were collected from December 2003 to December 2005 every three to four weeks directly at the spring outlet. Additional MAR-CARD-FISH analysis was performed from January 2004 to June 2004. Samples for all parallel analysis (unless stated otherwise) were taken aseptically in a sterilized sampling device (20 L), stored at 4°C (in situ temperature) during the transport and processed within 24 h. Sediment samples and the respective overlying water (OW) were taken inside the mountain from sediment depositions at 12 different locations within the aquifer area (800 km2, Northern Calcareous Alps in Austria) and at one sediment trap installed in LKAS2. The OW was taken first to avoid suspended material, then sediment was sampled into a sterile bottle. The bottle was filled with water to avoid drying of the sediment during transport. All analysis was performed from the same homogenised sediment sample and sub-samples for production measurements were processed within two hours.
For determination of prokaryotic numbers (PN) in the planktonic fraction (OW), a slightly modified version of the acridine-orange direct count method after Hobbie et al. (1977) was applied, as described in Kirschner and Velimirov (1997). Filters were examined under a Leitz Diaplan epifluorescence microscope equipped with a HBO 50 W mercury lamp (excitation wavelength 450 to 490 nm, cut off filter 515 nm). Prokaryotic cells were sized by an ocular micrometer. Cell volume estimations were based on the assumption, that all bacteria are spheres or cylinders with two hemispherical caps. At least 10 microscopic fields per sample were counted and 100-150 cells were measured. Cellular carbon content in fg C cell−1 (C) was calculated from estimated cell volumes (V; μm3) assuming the allometric relation C = 120 × V0.72 (Norland 1993).
Planktonic prokaryotic heterotrophic production
The [3H]leucine incorporation method (Kirchman et al. 1985, Simon & Azam 1989) following a modified protocol according to Kirschner and Velimirov (1999) with several additional adaptations to this ultra-oligotrophic environment was used. Five samples and three blanks were collected in sterile 50 ml Falcon tubes directly at the spring outlet. Each sample and blank was amended with [3H]leucine (final concentration 7 nM; 120 Ci mmol−1; ARC, USA) before or after the incubation time respectively, and stopped with trichloro-acetic acid (TCA, final concentration 5% (v/v); Sigma, Austria). Sample volume and incubation time varied from 40 ml and 48 h in winter to 10 ml and 24 h during summer months. Incubation was done at 4°C (approx. in situ temperature) in the dark. Saturation experiments and time course analysis proved that a 7 nM leucine solution was sufficient to prevent extra cellular isotope dilution and that leucine uptake was still in a linear range after 48 h. The radioactivity incorporated into heterotrophic cells was measured with a Canberra Packard scintillation counter (1900 TR). Incorporation rates were converted to carbon production using a conversion factor of 1.55 kg C mol−1 leucine (Simon & Azam 1989) assuming no isotope dilution. The overlying water samples from the corresponding sediment samples were treated in the same way.
Microautoradiography
Two replicates of 40 ml and 20 ml were taken from DKAS1 and LKAS2, respectively, spiked with L-[3H] leucine (120 Ci mmol−1; final concentration 20 nM) and incubated at 4°C in the dark for 8 h. We experimentally estimated that under these conditions the percentage of cells taking up L-[3H]leucine reached saturation after 6 to 8 h. Incubations were terminated by adding paraformaldehyde (final concentration 2 %) and samples were fixed at 4°C in the dark for 14–18 h. Subsequently, the samples were filtered through polycarbonate filters (0.2 μm pore-size; Millipore Corp. Bedford, MA, USA), supported by cellulose acetate filters (0.45 μm pore-size; Millipore), washed twice with 5 ml of 0.2 μm filtered Milli-Q water and air dried. Filters were embedded in low-gelling-point agarose (0.1% [wt/vol] Biozym, USA; in Milli-Q water) dried upside down on a glass petri dish at 37°C, dehydrated in 96 % (vol/vol) ethanol (Pernthaler et al. 2002a), air dried and stored at −20°C. Fixation times of more than 3 h and immediate embedding in agarose before the first freezing step minimize the amount of tritium-labelled compounds leaking from the cells (Nielsen et al. 2003a). CARD-FISH was performed as described previously (Wilhartitz et al. 2007) using probes EUB338, EUB338-II, EUB338-III and Non-EUB for bacteria, probe EURY806 for Euryarchaea and probe CREN537 for Crenarchaea. At the last step, dried filter sections were not mounted in DAPI-mix, but air dried and stored at −20°C until further processed. The CARD-FISH approach was conscientiously tested and adapted for this specific ultra-oligotrophic environment (Wilhartitz et al. 2007). Microautoradiography was performed after the protocol of Teira et.al. (2004). The influence of exposure time on the percentage of cells taking up [3H]leucine was evaluated by developing slides every 8 h for 3 days, resulting in an optimal exposure time of 36 - 48 h. Formaldehyde-killed samples were used as negative control. Size of silver grain clusters did not further increase after 32 h of exposure and no additional MAR-positive cells could be detected.
Sediment samples
For heterotrophic production six samples and four TCA killed controls were measured for each sediment. 1 g of sediment was incubated with [14C]leucine (306 mCi mmol−1, ARC Research Products; final concentration 2 nM) for 2 h. Measurements were done following the protocol of Tietz et.al. (2007). HP in the corresponding overlying water was measured as described above.
Sediment for determination of bacterial numbers was split into three aliquots and fixed with formaldehyde (final conc. 2 % vol/vol). The samples were stored at 4°C and processed within three days. Prokaryotic abundance, sediment bulk density, dry mass (DM) and pore water (PW) were determined as described previously (Farnleitner et al. 2003). Organic content of sediment samples was calculated by subtracting the sample weight after combusting (500°C, 4 h) from DM. Subsamples for grain size distribution and lithological description were taken at every sampling site. The mineralogical compositions of the samples were analysed by infrared spectroscopy (Perkin Elmer FTIR Spectrum 100) and in the case of five samples semi- quantitatively by x-ray diffraction (Phillips PW 1800, Co). The chemical composition of the samples was measured by x-ray fluorescence (Phillips PW2404). The grain size distribution of one sample (DKAS1 coarse) was determined by dry sieving from 12.5 mm to 0.63 mm.
About 2 g of the other samples were wet sieved to separate the grain size fractions < 63μm and < 40 μm. The grain size distribution in the fraction < 40 μm down to 0.1 μm was measured by a combined gravitative and centrifugal sedimentation analyses using a Shimadzu particle size analyzer (SA-CP2). The specific surface area of the samples was geometrically estimated by the respective grain size distribution data under the assumption that all particles have shapes of rhombic calcite.
Hydrological and chemical parameters
All hydrological and chemo-physical data were recovered by in-field on-line sensors. Conductivity, water temperature and discharge related parameters (water pressure, current meters, inductive discharge measurements) were registered with the data collecting system GEALOG-S from Logotronic (Vienna, Austria). The conductivity and pressure probes were Tetracon 96A (WTW, Weilheim, Germany) and PDCR 1830 (Druck, London, Great Britain). Impeller flow sensors were Peek 400 models (Houston, USA) and ELIN Water Technology (Vienna, Austria) current meters were used. Signals from these sensors were converted with algorithms provided by the manufacturers and from discharge stage relations (Stadler & Strobl 1998a). Data were stored every 15 minutes. Turbidity, spectral absorbance coefficient at 254 nm and pH were measured with a Sigrist and a HL2200 device.
All chemical analyses were performed after Legler (1988). All photometric measurements were performed on a Hitachi U2000+ spectrophotometer in a 5 cm light path (1 cm for higher concentrations) cuvette.
Ion concentrations in the samples were measured by ion chromatography. All columns and chemicals were supplied by Dionex (Sunnyvale, CA). AS-14 columns (DX-120) with AG-14 precolumns were used for anions (SO4, NO3; EN ISO 10304-1 (D19)) and CS-12A columns and CG-12A precolumns were used for cations (Ca2+, Mg2+; EN ISO 14911 (E34)). Total phosphorus was determined photometrical after dissolution of the unfiltered sample with potassium peroxydisulfate. The fractions were measured as PO4-P using the molybdenium-blue method. Soluble reactive phosphorus was determined as PO4-P in the filtered sample (EN 1189 (D11)). Total nitrogen was determined photometrical after dissolution of the unfiltered and filtered sample with conc. H2SO4 and H2O2. The fractions were measured using the indophenol-blue method (λ=655nm). Total nitrogen can be calculated by addition of nitrite (NO2-N determined photometrical from filtered sample with sulphanilamide and N-(1-naphthyl)-ethylene-diamine dihydrochloride solution after Griess-reaction (λ=543 nm)) and nitrate (NO3) (EN 26 777 / ISO 6777).
Sampling for acid neutralizing capacity, −p/+p – value and free CO2 was done in 1 L - PET, wide mouth screw-capped bottles (DIN 38409 section 7 (H7)). The bottles were filled avoiding any headspace. Alkalinity is used interchangeably with acid neutralizing capacity (Wetzel & Likens 1991) and refers to the capacity to neutralize strong acids.
Total organic carbon was measured via UV-oxidation after filtration (DIN EN 1484) and dissolved organic carbon was measured via UV-oxidation of C (DIN EN 1484). For the determination of the biodegradable dissolved organic carbon the protocol of Servais et. al. (1992) was used.
Statistical Analysis
Potential relationships among variables were tested by linear pair-wise correlations (Spearman correlation analysis). Data were log (+1) transformed to satisfy the requirements of normality and homogeneity of variance necessary for parametric statistics (principal component analysis). All statistical analyses were performed with SPSS software for Windows (Release 11).
RESULTS
Spring characterisation
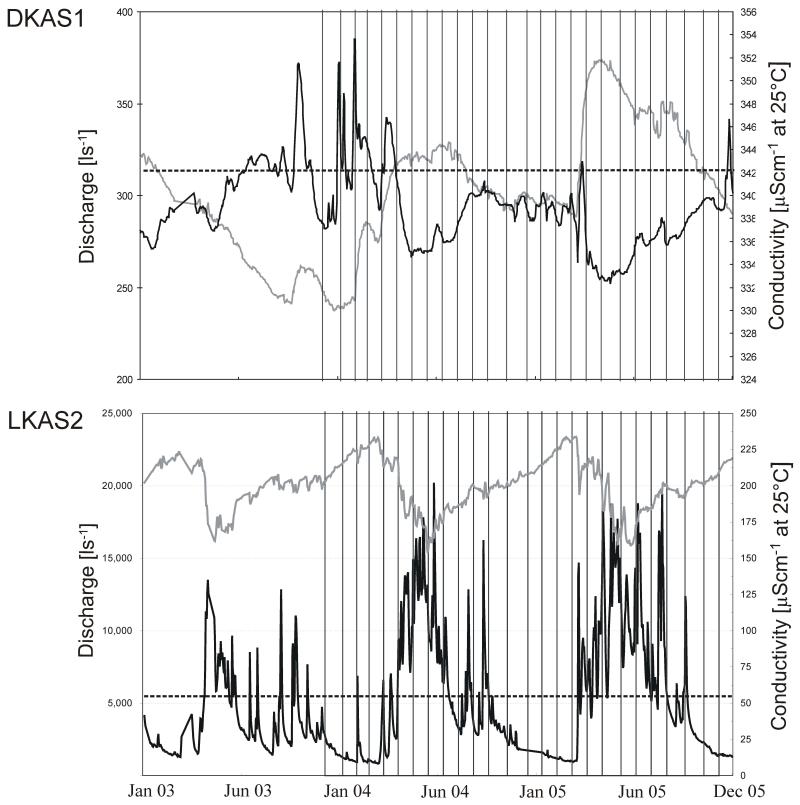
The dynamics of discharge and conductivity during the sampling period reflect the contrasting hydrological regimes in DKAS1 and LKAS2 (Fig. 1, Table 4a and 4b supplements) and were in accordance with previous studies (Stadler & Strobl 1997, 1998b, Farnleitner et al. 2005). The chosen sampling dates covered the full range of discharge variability, as an indicator of hydraulic reactions, and of conductivity, as an indicator of mass transport. NH4+ and K+ concentrations were generally below the detection limit in both springs. Cl− concentrations were not detectable in LKAS2. More details on chemical parameters are given in Table 1.
Figure 1.
Hydrological dynamics in the two investigated alpine karst springs. Vertical lines indicate sampling dates during the period from Dec 2003/Dec 2005. The grey lines show the conductivity at 25°C (right axes); the black lines depict the daily mean discharge (left axes). The mean discharges throughout the investigation period are indicated by broken lines. Note that different scales between aquifers are used.
Table 1.
Biogeochemical and microbial characterization of two different karst spring waters (n = 19 - 21)a.
| parameters | unit | LKAS2 |
DKAS1 |
|||
|---|---|---|---|---|---|---|
| median | range min - max |
median | range min - max |
|||
| Hydro-graphical parameters |
Q | [l s−1] | 5340 | 902 - 15479 | 319 | 290 - 373 |
| EC | [μS cm−1] | 195 | 156 - 222 | 338 | 333 - 345 | |
| TEMP | [°C] | 5.3 | 4.9 - 5.8 | 6.7 | 6.7 - 6.7 | |
| pHc | [] | 8.1 | 7.8 - 8.3 | 8.0 | 7.4 – 8.7 | |
| SAC | [m−1] | 1.7 | 0.44 - 4.10 | n.d. | n.d. | |
| TUR | [NTU] | 0.16 | 0.03 - 0.96 | n.d. | n.d. | |
|
| ||||||
| Geochemical parameters |
total hardness | [mval l−1] | 2.08 | 1.68 - 2.43 | 3.63 | 3.50 – 3.73 |
| Ca2+ | [μM] | 875.8 | 722.6 – 976.4 | 1200.2 | 1162.4 – 1252.1 | |
| Mg2+ | [μM] | 160.3 | 117.3 – 240.5 | 612 | 584 - 638 | |
| Cl− | [μM] | n.d. | n.d. | 64.6 | 42.0 – 120.7 | |
| SO4-S | [μM] | 11.1 | 5.9 – 19.9 | 29.0 | 21.7 – 41.6 | |
| NO3-N | [μM] | 9.0 | 6.5 – 17.6 | 16.5 | 15.4 – 17.1 | |
| TN | [μM] | 42.3 | 32.0 – 81.4 | 74.4 | 69.0 – 77.3 | |
| SRP | [μM] | 0.15 | 0.13 – 0.17 | n.d. | n.d. | |
| TP | [μM] | 0.18 | 0.13 – 0.48 | n.d. | n.d. | |
|
| ||||||
| Biological parameters |
PN | [106cells l−1] | 44.4 | 27.0 - 69.7 | 13.1 | 11.2 – 19.0 |
| PB | [nmol C l−1] | 58.28 | 28.31 – 94.08 | 13.32 | 9.16 – 19.15 | |
| HP | [pmol C l−1h−1] | 12.9 | 2.3 – 75.6 | 0.7 | 0.1 – 6.8 | |
| HP cell−1b | [amol C cell−1h−1] | 0.29 | 0.06 – 0.96 | 0.06 | 0.01 – 0.61 | |
|
| ||||||
| Carbon- associated parameters |
DOC | [μM C] | 46.6 | 16.7 – 99.1 | 25.0 | 18.3 – 45.0 |
| BDOC | [μM C] | 4.16 | 0.83 – 11.66 | 4.16 | 0.83 – 18.32 | |
| CO2b | [μM] | 32.5 | 15.7 - 55.5 | 254 | 97 – 637 | |
| −p - Value | [mmol l−1] | 0.04 | 0.01 - 0.21 | 0.14 | 0.01 – 0.59 | |
| ANC | [mval l−1] | 2.04 | 1.63 - 2.43 | 3.4 | 3.32 – 3.51 | |
Samples were taken monthly over a two years period
calculated parameter
measured in laboratory
The abbreviations are: Q = discharge, EC = electrical conductivity, SAC = spectral absorbance coefficient at 254nm, TUR = turbidity, NTU = nephalometric turbidity unite, TN = total nitrogen, SRP = soluble reactive phosphorus, TP = total phosphorus; PN = prokaryotic number, PB = prokaryotic biomass, HP = heterotrophic (secondary) production, (B)DOC = (biodegradable) dissolved organic carbon, CO2 = free CO2, −p-Value = amount of OH− to reach pH 8.3, ANC = acid neutralization capacity (≡ alkalinity), TEMP = temperature; n.d. = not detectable.
Prokaryotic numbers, biomass and heterotrophic production were significantly higher in the dynamic spring type LKAS2. The determined cellular carbon content of the prokaryotic cells resulted in 15 fg C cell−1 (1.25 fmol C cell−1) in LKAS2 and 12 fg C cell−1 (0.99 fmol C cell−1) in DKAS1. Dissolved organic carbon (DOC) and biodegradable dissolved organic carbon (BDOC) were similar in both systems during base flow conditions. During storm-flow events, dissolved organic carbon concentrations in LKAS2 increased due to enhanced surface runoff (Table 1).
Heterotrophic production and microautoradiography in the planktonic compartment
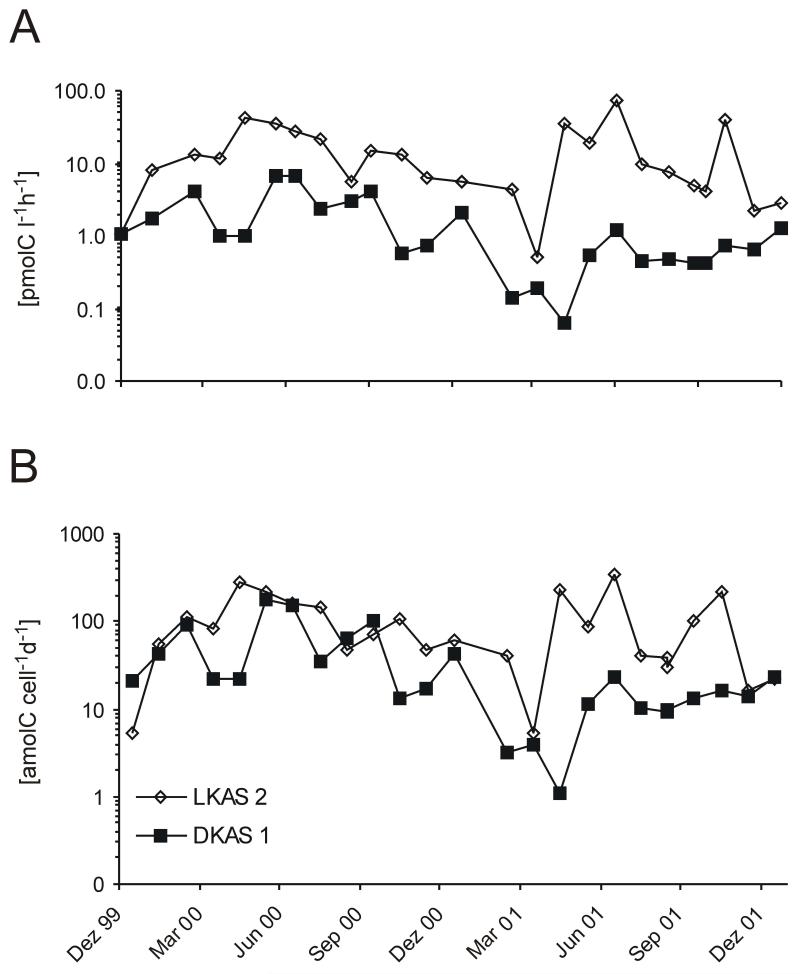
Heterotrophic production ranged from 0.1 to 75.6 pmol C l−1 h−1. The lowest production rates in both springs were observed during the winter months (November to February). The highest [3H]leucine incorporation was detected during storm-flow events in summer and autumn. Seasonal variations were more pronounced in LKAS2 but, in general, congruent with fluctuations in DKAS1 (Fig. 2A). In LKAS2 variations could be explained by a high correlation between discharge and prokaryotic numbers (r = 0.74; p < 0.01) and discharge and heterotrophic production (r = 0.78; p < 0.01) (Table 4a, supplements). No correlation could be observed between discharge and prokaryotic numbers in DKAS1 (r = −0.2; p > 0.05) (Table 4b). Depending on the hydrogeological situation, [3H]leucine uptake in LKAS2 spring water was 10- to 100-fold higher than in DKAS1 during the entire investigation period. In contrast, cell-specific uptake rates were similar during base flow conditions (Fig. 2B), only differing during snow melt or storm-flow events.
Figure 2.
Dynamics of bulk prokaryotic production rates (A) and cell-specific production (B) of the planktonic microbial communities of two alpine karst springs. Y-axes in log-scale.
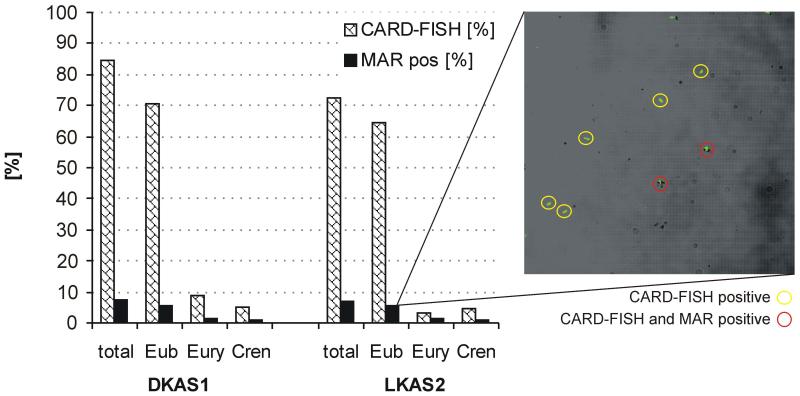
The contribution of organisms targeted by probes used for Bacteria and Archaea to the assimilation of [3H]leucine was examined over a period of 6 months (January to June 2004). The recovery efficiency (sum of Bacteria, Crenarchaea and Euryarchaea) averaged 83 % (range, 74 – 91 %) of DAPI-stainable cells. Microautoradiography revealed that on average only about 7 % (range, 3 – 14 %) of all DAPI-stainable cells visibly assimilated [3H]leucine (Fig. 3). Organisms targeted by the three EUB-probes (Bacteria) were the dominating group taking up leucine (76 % Bacteria, 24 % Archaea; related to the active fraction). Among Archaea, Euryarchaea showed a higher percentage of active cells than Crenarchaea (Fig. 3) with an average of 16 % and 8 % for LKAS2 and DKAS1, respectively (related to the active fraction). No cells with associated silver grains were observed in the formaldehyde-killed controls. Based on the bulk heterotrophic production and the prokaryotic biomass, the average bulk generation time of prokaryotes in LKAS2 and DKAS1 was 202 days and 712 days, respectively. Considering, however, only the leucine-assimilating cells, as determined by MAR-FISH, the average generation time was 14 days for LKAS2 and 55 days for DKAS1. The average cell-specific uptake rates varied from 1.23 to 195 amol C cell−1 day−1 in DKAS1 and from 6.18 to 401 amol C cell−1 day−1 in LKAS2 (Fig.2).
Figure 3.
CARD-FISH and MAR results for the two alpine karst springs revealed that Bacteria (Eub; probe-mix of EUB338, EUB338II and EUB338III) comprise the largest fraction of the microbial community in both springs. About 10 % of the community were identified as Euryarchaea (probe EURY806) and Crenarchaea (probe CREN537). MAR-active cells were found in both prokaryotic fractions. The picture depicts CARD-FISH positive cells showing green fluorescence, and two cells that are additionally surrounded by black silver grains indicating 3H-leucine uptake. All counts were made in relation to DAPI counts.
Integrated view of data: principal component analysis (PCA)
In order to elucidate the main environmental factors describing the spring water quality, the key parameters for both systems were used for PCA (Table 2). The component matrix revealed fundamental differences for LKAS2 and DKAS1. Three significant components with an eigenvalue >1 were extracted for LKAS2 explaining 93.5 % of the total variation. The main variations in spring-water from the limestone spring type (LKAS2) were characterized by the first component (comprising discharge, heterotrophic production, dissolved organic carbon, and prokaryotic numbers) responsible for 60.8 % of the total variation. In combination with the second component (comprising discharge, conductivity and SO4) they revealed that the main forces determining LKAS2 were the dynamic hydrological components, explaining 78.9 % of the system’s variation (discharge was represented in both components). Only one parameter (BDOC) showed a correlation higher than r = 0.5 to the third component, describing 13.1 % of total variation.
Table 2.
Component matrix for two different spring types. Data set for principal component analysis was reduced to the key parameters that were available for both springs (n = 19 - 21).
| LKAS 2a |
DKAS 1a |
||||||
|---|---|---|---|---|---|---|---|
| parameter | 1 | 2 | 3 | parameter | 1 | 2 | 3 |
|
|
|
||||||
| HP | 0.92 | −0.32 | −0.01 | SO4 | 0.97 | 0.14 | 0.06 |
| DOC | 0.87 | 0.07 | 0.37 | Q | −0.96 | −0.13 | −0.10 |
| Q | 0.83 | −0.51 | −0.11 | EC | 0.90 | −0.12 | 0.16 |
| PN | 0.77 | −0.29 | 0.37 | PN | −0.18 | −0.91 | 0.10 |
| EC | −0.17 | 0.98 | −0.05 | BDOC | −0.07 | 0.88 | 0.21 |
| SO4 | −0.27 | 0.90 | −0.27 | DOC | 0.12 | −0.14 | 0.90 |
| BDOC | 0.15 | −0.17 | 0.97 | HP | 0.12 | 0.29 | 0.85 |
|
| |||||||
| % of variance | 60.8 | 18.0 | 14.7 | 42.8 | 24.4 | 20.0 | |
|
| |||||||
| Cumulative | 93.5 % | 87.2 % | |||||
Correlations >0.5 are printed bold. Extraction method: Principal component analysis. Abbreviations as given in Tab. 1.
3 components extracted
The three significant components extracted for DKAS 2 explained 87.2 % of the total variation, but revealed completely different coherences compared to LKAS2. The first, the hydrographical component comprising SO4, discharge and conductivity, explained 42.8 % of the system’s variation but showed no direct relationship with the microbiological and biological parameters included in the second and third components. The second component including prokaryotic numbers and BDOC, and the third component including DOC and heterotrophic production, together were responsible for 44.4 % of the total variation.
Prokaryotic numbers and heterotrophic production in the sediment
Average prokaryotic numbers (42 × 107 cells l−1) and heterotrophic production (41 pmol C l−1 h−1) in the overlaying water (OW) from the 12 sediment locations were comparable to the values obtained in the planktonic fraction of LKAS2 and DKAS1. In contrast, values in the sediment were dramatically higher than in the planktonic fraction. Prokaryotic numbers averaged 4.03 × 1011 cells dm−3 (range, 1.25 × 1011 to 7.91 × 1011 cells dm−3, n = 12×6) and heterotrophic production averaged 19 μmol C dm−3 h−1 (range, 3 to 71 μmol C dm−3 h−1, n = 12×6). On a volumetric base this depicts a 106 - fold increase when comparing heterotrophic production in the planktonic and the attached autochthonous endokarst community. Heterotrophic production in LKAS2 averaged 25 μmol C dm−3 h−1 (n = 18) resulting in generation times of 24 hours (Table 3). Unlike uptake rates in the planktonic fraction, [14C]leucine incorporation in DKAS1 was higher than in LKAS2 revealing lower generation times in the range of some hours (Table 3). Cell specific uptake rates calculated for the sediment bulk community were 75.9 pmol C cell−1 h−1 for LKAS2 and 218.2 pmol C cell−1 h−1 for DKAS1. In comparison the cell specific uptake rates for the planktonic community was 0.29 × 10−6 pmol C cell−1 h−1 for LKAS2 and 0.06 × 10−6 pmol C cell−1 h−1 for DKAS1 (Table 1).
Table 3.
Prokaryotic numbers (PN) and heterotrophic production (HP) measured in sediments and the respective overlaying water (OW) in LKAS2 and DKAS1 (n = 18).
Sediment analysis
Lithological analysis of the recovered aquifer sediments showed almost the same composition for all samples. Sediments were composed of dolomite, calcite and quartz with dolomite being the quantitatively prevalent component. The dominant grain size distributions varied between 2 μm and 60 μm in diameter except for one coarse sediment sample, from DKAS1, ranging from 0.3 mm to 100 mm. The averaged specific surface area for the sediment fraction < 63μm (the dominant size fraction) was 188 m2 dm−3. The pore volume, as determined from water content, was 288 cm3 dm−3. The correlation between heterotrophic production and surface area (fraction <63μm) was high (r = 0.81) but not significant. No significant correlations were detectable between heterotrophic production and the dominant grain size distribution.
DISCUSSION
Heterotrophic production in spring water
In situ measurements of heterotrophic production in different aquatic habitats ranges from 738 μg C l−1 h−1 (61.5 μmol C l−1 h−1) in hypertrophic shallow soda lakes (Eiler et al. 2003) to 0.01 - 0.05 μg C l−1 h−1 (0.83 – 4.12 nmol C l−1 h−1) in an ultra-oligotrophic Antarctic lake (Laybourn-Parry et al. 2001). To our knowledge, this is the first study directly measuring in situ heterotrophic production in groundwater from an alpine karst aquifer. Considering the values ranging from 0.1 to 75.6 pmol C l−1 h−1, heterotrophic production is extremely low, pointing towards a high biostability of the abstracted water, used for water supply (low in situ cell activity), especially during base flow conditions. However, high variations were observed during two seasonal cycles (Fig. 2). Peaks in heterotrophic production in LKAS2 coincide with peaks in discharge (r = 0.78, p < 0.01), prokaryotic numbers (r = 0.53, p > 0.05), DOC (r = 0.64, p < 0.01) and total phosphorus (r = 0.74, p < 0.01). As phosphorus concentrations were below the detection limit in DKAS1 these results indicate a phosphorus-limitation in alpine karst aquifers as was also described for other freshwater ecosystems (Wilhelm & Suttle 1999). Higher cell abundance and biomass in LKAS2 during the summer months can be explained by (1) detachment of cells from biofilms like it has been shown for streams and rivers (Blenkinsopp & Lock 1994) and (2) an increased input of allochthonous cells by surface runoff into the aquifer also providing (3) enhanced substrate supply (DOC, total phosphorus) stimulating the growth of the indigenous prokaryotes. These factors were probably also responsible for the higher heterotrophic production in LKAS2 during periods of increased discharge. In contrast to LKAS2, heterotrophic production in DKAS1 was only correlated with the −p-value (r = 0.74, p < 0.01), indicating a minor role of the input of transient microorganisms and allochthonous nutrients, and pointing towards seasonal variations within the indigenous microbial community (e.g. interplay with viruses and protozoan as known from other habitats (Weinbauer & Höfle 1998)). The high NO3 concentrations in DKAS1 arise from differences in the vegetation of the two catchment areas (Dirnböck & Greimler 1999b) and are not necessarily related to biogeochemical cycles within the aquifer. Isotopic analyses would be required to decipher the origin of both nitrate and sulphate in this study (Einsiedl & Mayer 2005, 2006).
A small fraction of active cells in karstic spring water
The combined technique of CARD-FISH (or FISH) and MAR (microautoradiography) allows to link key physiological features to the identity of microorganisms and has been applied in various systems (Ouverney & Fuhrman 1999, 2000, Ito et al. 2002, Nielsen et al. 2003b, Teira et al. 2004, Teira et al. 2006). In highly productive shallow soda lakes in Austria the percentage of active cells ranged from 40 % to 90 % (Eiler et al. 2003). In contrast a recent study showed that in the North Atlantic deep water the fraction of active cells can be lower than 5 % (Teira et al. 2006). This is in accordance with our results revealing an average cell fraction of about 7 % of the total cells taking up leucine. Although new findings suggest that the fraction of active Archaea can be larger than that of Bacteria in some oligotrophic marine environments (Teira et al. 2004), the average fraction of Eury- and Crenarchaea taking up leucine in alpine spring water comprised less than one fourth of the total active community.
As bacterial activities in nutrient poor environments are low, doubling times for the microbial community of decades and centuries have been calculated (Phelps et al. 1994). However, bulk calculations of doubling times, including all microorganisms are overestimates. Taking into account that only 7 % of the cells took up [3H]leucine, growth rates averaged 0.07 day−1 in LKAS2 and 0.02 day−1 (ranging from 0.13 to 0.001 day−1) in DKAS1. These values are comparable with results obtained in the deep ocean (0.01 to 0.008 day−1) (Bendtsen et al. 2002, Reinthaler et al. 2006). The average cell-specific uptake rates, except for measurements during storm-flow events, were in the range of uptake rates determined for sea water (0.53 – 47.4 amol C cell−1 day−1) (Sintes & Herndl 2006).
Principal component analysis
PCA can be used for dimensionality reduction in a data set by retaining those characteristics of the data set that contribute most to its variance. In this study it was used to elaborate differences between the two spring systems, especially with respect to key parameters influencing heterotrophic production. Therewith, we tried to shed light on the ecological drivers in such systems.
To a certain degree the nature of the sediment matrix in porous aquifers determines the heterogeneity in the distribution and activity of microorganisms in the subsurface (Griebler 2001). In agreement with these observations from porous aquifers, the component matrix for DKAS1 and LKAS2 revealed fundamental differences when evaluating the factors dominating the microbial fraction within these karstic systems. All parameters in LKAS2, except BDOC, were directly related to variations in discharge (Table 2). The first component constituted the “input factor”, describing an enhanced surface influence within the spring-water during increased discharge, represented by positive correlations with prokaryotic numbers, DOC and heterotrophic production. The second component constituted the “dilution factor” and comprised parameters that typically decrease as surface water enters the system, like conductivity and SO4 concentration. This obvious dependence on discharge corresponds with the morphology of this spring type, showing well-developed karst conduits (Stadler & Strobl 1997) that allow for a very quick discharge response after precipitation and hence, for a pronounced surface influence. A different situation is given in DKAS1 where the component matrix revealed microbiological characteristics within the system that are not related to hydrographical parameters. Biotic factors are more important in describing the variance of the system, whereas the influence of discharge related components halved compared to LKAS2 (Table 2). Porous and fissured rock (such as in DKAS1) does not allow for high velocity fluxes and is characterized by high water residence times. These conditions possibly allow for the development of a microbiologically dominated compartment within the aquifer and boost the potential impact of biofilms.
Heterotrophic production of surface associated endokarst communities
For porous aquifers it has already been concluded that well-water prokaryotes do not accurately reflect attached microbial communities and planktonic cells might be subsets of biofilms (Hazen et al. 1991, Pedersen 1993, Alfreider et al. 1997). In many cases, the attached microbial communities showed distinctive morphological and physiological patterns compared to the planktonic cells in the interstitial groundwater (e.g. higher portion of gram-positive cells, higher morphologic diversity, sessile forms, higher activity rates, higher degradation potential for complex compounds) (Kölbel-Boelke & Hirsch 1989, Griebler et al. 1999, Griebler et al. 2002). Heterotrophic production in alpine karst aquifer sediments as determined in this study, exceeded measurements in the respective planktonic fraction by orders of magnitudes, revealing generation times that were surprisingly low (hours).
As it was impossible to recover samples from the matrix surface within the aquifer, the sediments in this study constituted of material that was flushed out of the system and was either deposited at the spring outlet or collected in a sediment trap. Nevertheless, sediment analysis confirmed that the sediment composition was comparable at all sampling sites (dolomite) and in accordance with the lithological composition described for this area (Bryda 2001). Therefore, heterotrophic production data obtained in this study are likely to reflect the general situation for clastic sediments within the aquifer matrix.
When estimating the carbon demand needed to sustain the measured production rates, the data suggest that one dm3 of sediment would hypothetically consume the DOC content in one litre of spring water within hours (0.5 h for DKAS1 and 1.4 h for LKAS2). This result implicates that attached AMEC are probably effective in degrading allochthonous DOC entering the spring system and thus significantly enhance the resulting spring water quality. The depletion in degradable carbon is likely to be stronger in systems where water percolates slowly through pores and fractures in the bedrock, like in DKAS1. The rock matrix in natural karst aquifers provides surface areas that range from 30 m2 m−3 (limestone) to 300 m2 m−3 (dolomitic limestone) (Decker et al. 1998) and thus the bedrock itself can act as a “trickling filter”. Clearly, these surface areas are low when compared to the loose sediments collected in this study. Nevertheless, the estimated time span needed for degrading the measured DOC ranges from 10 days in a bare limestone bedrock (assuming 300 m2 m−3, low DOC (17 μM C)) to 369 days in a bare dolomitic bedrock (assuming 30 m2 m−3, high DOC (99 μM C)) and is therefore even shorter than the estimated average transit time for the dynamic spring type LKAS2 (1.5 years). The degree of DOC consumption will therefore depend on the bedrock and the amount of clastic sediment on the passage floor and walls. It should be mentioned that for the given scenarios only DOC concentration in the spring outlets were considered. Real input DOC concentrations may be higher but are currently not available for calculations. However, DOC concentrations as determined during the seasonal cycles, including summer events probably showing the maximum DOC input possible in this catchment, should be a first good approximation. Furthermore, DOC concentrations measured in the spring water during base flow might also contain fractions that are not degradable by prokaryotes, suggesting the need for other sources to meet the carbon demand of the autochthonous endokarst communities (e.g. internal turnover).
It is a long known fact that in some karst systems the CO2 introduced from external sources, namely soil or atmosphere, is not sufficient to explain for the extent of karstification observed. There are some studies suggesting that there has to be a CO2 source, other than the soil compartment or the atmosphere, especially in the deeper regions of the vadose and phreatic zone (Atkinson 1977, Wood 1985, Gabrovsek et al. 2000). Based on our heterotrophic production measurements in alpine karst groundwater aquifers a possible influence on geological processes (e.g., karstification) by the microbial community was evaluated by estimating their CO2 production. The theoretical time needed to reach CO2 levels measured in the respective spring water was calculated. CO2 input from the surface and CO2 consumption within the aquifer where not included in the calculation, as equilibrium chemistry and kinetics of dissolution reactions are very complex and vary over space and time (White 1988, Vesper & White 2004b, Groves & Meiman 2005). The estimated time needed for the planktonic fraction to produce the prevailing CO2 level was 2 years for LKAS2 and 81 years for DKAS1, assuming a bacterial growth efficiency of 1 %. Estimates with higher bacterial growth efficiencies lead to results of up to 1174 years for DKAS1. When considering the estimated average water residence times of 1.5 years for the LKAS2 and 22 years for the DKAS1 system (Stadler & Strobl 1998b), these findings show that plankton microbial communities are very unlikely to influence geomorphological processes in the aquifer. Considering the high heterotrophic production in aquifer sediments microbially mediated CO2 would reach CO2-levels measured in DKAS1 and LKAS2 within hours in the case of an equivalent available surface per volume ratio of respective aquifer locations. Considering the prevailing natural rock matrix, CO2 levels would approximately be reached within less than one year in a bare limestone bedrock (30 m2 m−3) and within about one month in a bare dolomitic bedrock (300 m2 m−3). Given the fact that there are areas where water moves only slowly through fractures and pores and has a longer transit time, these results indicate a considerable potential for microbes to contribute to the prevalent CO2 level and with that to geomorphological processes. Gabrovsek et.al. (2000) already speculated that this influence would increase in a bare catchment and in deeper aquifers where external CO2-supply decreases. DOC supply is crucial in this context, because little CO2-input in a bare area normally also denotes a reduced DOC input due to the absence of a soil layer. One effect that could enhance microbially mediated CO2 level, in this case, is that viral lysis products including phages turn over relatively rapidly, especially in oligotrophic, P-limited environments (Noble & Fuhrman 1999). This “viral loop” could help to replenish the nutrient pool in deeper aquifers and provide DOC for prokaryotic growth. Lysis products are available to bacteria at the expense of a reduced growth efficiency, which should enhance prokaryote-mediated CO2 production (Weinbauer 2004). In a recent study 105-107 VLP ml−1 (virus like particles) were found in granitic groundwater (Kyle et al. 2008), giving room for speculations. However, it is likely that the microbially mediated CO2 fraction is not linked to the CO2 measured at the spring outlet, as it is produced in micro-zones very close to the rock surface (boundary layer) and is hence immediately involved in redox-reactions to re-establish an equilibrium-state.
Conclusion
In situ activity of planktonic autochthonous endokarst communities were extremely low indicating a high biostability of the abstracted water, especially during base flow conditions. Most of the planktonic fraction is in an inactive or dormant state. One could speculate that this fraction mainly constitutes of swarming cells released from the biofilm to colonize new surfaces. These findings are relevant for public water supply as biostability is an important factor considering the regrowth potential of water in a distribution network. The crucial compartment regarding prokaryotic heterotrophic production is the surface associated community. As shown for dolomite sediments these attached communities could contribute to important biogeochemical processes taking place in alpine groundwater aquifers (e.g. energy or matter fluxes, karstification).
ACKNOWLEDGMENTS
The study was funded by the FWF (project P18247-B06) granted to Priv. Doz. Dr. A.H.Farnleitner and supported by the MA 31 by providing background information and technical assistance during the sampling procedure. DI G. Lindner and Dr. K. Donabaum helped with chemical analyses. IW was supported also by a Marie Curie grant (HPMT-CT-2001-00213) of the 5FWP of the EU. Radioactive measurements were performed at the Department of Anatomy and Cell Biology (Prof. Dr. B. Velimirov), Medical University Vienna.
REFERENCES
- Alfreider A, Krössbacher M, Psenner R. Groundwater samples do not reflect bacterial densities and activity in subsurface systems. Water Res. 1997;32:832–840. [Google Scholar]
- Atkinson TC. Carbon dioxide in the atmosphere of the unsaturated zone: an important control of groundwater hardness in limestones. J. Hydrol. 1977;35:111–123. [Google Scholar]
- Bendtsen J, Lundsgaard C, Middelboe M, Archer D. Influence of bacterial uptake on deep-ocean dissolved organic carbon. Global Biogeochemical Cycles. 2002;16 [Google Scholar]
- Blenkinsopp SA, Lock MA. The impact of storm flow on river biofilm architecture. J Phycol. 1994;30:807–818. [Google Scholar]
- Bryda G. Geologische Kartierung im Hochschwabgebiet – Entscheidungshilfe zur Abgrenzung von Quelleinzugsgebieten. In: Mandl G, editor. Arbeitstagung 2001. Wien (Geol. B.-A.); Vienna: 2001. pp. 220–231. [Google Scholar]
- Buesing N, Gessner MO. Incorporation of radiolabeled Leucine into protein to estimate bacterial production in plant litter, sediment, epiphytic biofilms and water samples. Microb Ecol. 2003;45:291–301. doi: 10.1007/s00248-002-2036-6. [DOI] [PubMed] [Google Scholar]
- Church MJ, Ducklow HW, Karl DA. Light dependence of [H-3]leucine incorporation in the oligotrophic North Pacific ocean. Applied and Environmental Microbiology. 2004;70:4079–4087. doi: 10.1128/AEM.70.7.4079-4087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church MJ, Hutchins DA, Ducklow H. Limitation of bacterial growth by dissolved organic matter and iron in the southern ocean. Appl Environ Microbiol. 2000;66:455–466. doi: 10.1128/aem.66.2.455-466.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell MT, Kirchman DL. Contribution of major bacterial groups to bacterial biomass production (thymidine and leucine incorporation) in the Delaware estuary. Limnol. Oceanogr. 2003;48:168–178. [Google Scholar]
- Danielopol DL, Griebler C, Gunatilaka A, Notenboom J. Present state and future prospects for groundwater ecosystems. Environmental Conservation. 2003;30:104–130. [Google Scholar]
- Decker K, Heinrich M, Klein P, Kociu A, Lipiarski P, Pirkl H, Rank D, Wimmer H. Karst springs, groundwater and surface runoff in the calcareous Alps: assessing quality and relience of long term water supply. Vol. 248. IAHS Publ; 1998. [Google Scholar]
- Dirnböck T, Greimler J. Vegetationskartierung in den Einzugsgebieten der Wiener Hochquellwasserleitungan (Schneeberg, Rax und Hochschwab) und ihre Anwendung aus hydrologisch-ökologischer Sicht. Biotopkartierung im Alpenraum. 1999b;10:201–218. [Google Scholar]
- Eiler A, Farnleitner AH, Zechmeister TC, Herzig A, Hurban C, Wesner W, Krachler R, Velimirov B, Kirschner AKT. Factors controlling extremely productive heterotrophic bacterial communities in shallow soda pools. Microb Ecol. 2003;46:43–54. doi: 10.1007/s00248-002-2041-9. [DOI] [PubMed] [Google Scholar]
- Einsiedl F, Mayer B. Sources and processes affecting sulfate in a karstic groundwater system of the Franconian Alb, southern Germany. Environ Sci Technol. 2005;39:7118–7125. doi: 10.1021/es050426j. [DOI] [PubMed] [Google Scholar]
- Einsiedl F, Mayer B. Hydrodynamic and microbial processes controlling nitrate in a fissured-porous karst aquifer of the Franconian Alb, Southern Germany. Environ. Sci. Technol. 2006;40:6697–6702. doi: 10.1021/es061129x. [DOI] [PubMed] [Google Scholar]
- Farnleitner AH, Kasimir GD, Kavka GG, Zechmeister TC, Mach RL, KIrschner AKT. The choice of standardisation reveales a significant influence on the dynamics of bacterial abundance in newly deposited river sediments. Internat. Rev. Hydrobiol. 2003;88:284–289. [Google Scholar]
- Farnleitner AH, Wilhartitz I, Kirschner AKT, Stadler H, Burtscher MM, Hornek R, Szewzyk U, Herndl G, Mach R. Bacterial dynamics in spring water of alpine karst aquifers indicates the pesence of stable autochthonous microbial endokarst communities. Environmental Microbiology. 2005;7:1248–1259. doi: 10.1111/j.1462-2920.2005.00810.x. [DOI] [PubMed] [Google Scholar]
- Gabrovsek F, Menne B, Dreybrodt W. A model of early evolution of karst conduits affected by subterranean CO2 sources. Environmental Geology. 2000;39:531–543. [Google Scholar]
- Ghiorse WC, Wilson JT. Microbial ecology of the terrestrial subsurface. Adv Appl Microbiol. 1988;33:107–172. doi: 10.1016/s0065-2164(08)70206-5. [DOI] [PubMed] [Google Scholar]
- Gibert J. Basic Attributes of Groundwater Ecosystems. In: Griebler C, Danielopol DL, Gibert L, Nachtnebel HP, Notenboom J, editors. Groundwater Ecology. Vienna: 2001. pp. 39–52. [Google Scholar]
- Gibert J, Vervier P, Malard F, Laurent R, Reygrobellet J-L. Dynamics of Communities and Ecology of Karst Ecosystems: Example of three Karsts in Eastern and South France. In: Gibert J, Danielopol DL, Stanford JA, editors. Groundwater Ecology. Academic Press; San Diego: 1994. pp. 425–450. [Google Scholar]
- Goldscheider N, Hunkeler D, Rossi P. Review: Microbial biocenoses in pristine aquifers and an assessment of investigative methods. Hydrogeology Journal. 2006;14:941. [Google Scholar]
- Gounot AM. Microbial oxidation and reduction of manganese: consequences in groundwater and applications. FEMS Microbiol Rev. 1994;14:339–349. doi: 10.1111/j.1574-6976.1994.tb00108.x. [DOI] [PubMed] [Google Scholar]
- Griebler C. Microbial Ecology of the subsurface. In: Griebler C, Danielopol DL, Gilbert L, Nachtnebel HP, Notenboom J, editors. Groundwater Ecology. Vienna: 2001. pp. 81–108. [Google Scholar]
- Griebler C, Lueders T. Microbial biodiversity in groundwater ecosystems. Freshwater Biology. 2008 online: doi:10.1111/j.1365-2427.2008.02013.x. [Google Scholar]
- Griebler C, Mindl B, Danielopol DL. Biofilme in Grundwasser-Ökosystemen. Biofilme. 1999;127:23–51. [Google Scholar]
- Griebler C, Mindl B, Slezak D, Geiger-Kaiser M. Distribution patterns of attached and suspended bacteria in pristine and contaminated shallow aquifers studied with an in situ sediment exposure microcosm. Aquatic Microbial Ecology. 2002;28:117–129. [Google Scholar]
- Groves C, Meiman J. Weathering, geomorphic work, and karst landscape evolution in the Cave City groundwater basin, Mammoth Cave, Kentucky. Geomorphology. 2005;67:115–126. [Google Scholar]
- Hazen TC, Jiménez L, López de Victoria G, Fliermans CB. Comparison of bacteria from deep subsurface sediment and adjacent groundwater. Microb Ecol. 1991;22:293–304. doi: 10.1007/BF02540231. [DOI] [PubMed] [Google Scholar]
- Herman EK, Tancredi JH, Toran L, White WB. Mineralogy of suspended sediment in three karst springs. Hydrogeology Journal. 2007;15:255–266. [Google Scholar]
- Herman EK, Toran L, White WB. Threshold events in spring discharge: Evidence from sediment and continuous water level measurement. Journal of Hydrology. 2008;351:98–106. [Google Scholar]
- Hirsch P, Eckhardt FEW, Palmer RJ., Jr. Methods for the study of rock-inhabiting microorganisms-A mini review. Journal of Microbiological Methods. 1995;23:143–167. [Google Scholar]
- Hobbie JE, Daley RJ, Jasper S. Use of Nucleopore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Nielsen JL, Okabe S, Watanabe Y, Nielsen PH. Phylogenetic identification and sustrate uptake patterns of sulfate-reducing bacteria inhabitating an oxic-anoxic sewer biofilm determined by combining microautoradiography and fluorescent in situ hybridization. Appl Environ Microbiol. 2002;68:356–364. doi: 10.1128/AEM.68.1.356-364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D, K’Nees E, Hodson R. Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl Environ Microbiol. 1985;49:599–607. doi: 10.1128/aem.49.3.599-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner A, Ulbricht T, Steitz A, Velimirov B. Material fluxes through the procaryotic compartment in a eutrophic backwater branch of the River Danube. Aquat Microb Ecol. 1999;17:211–230. [Google Scholar]
- Kirschner AKT, Velimirov B. A seasonal study od bacterial community succession in a temperate backwater system, indicated by variation in morphotype numbers, biomass, and secondary production. MICROBIAL ECOLOGY. 1997;34:27–38. doi: 10.1007/s002489900031. [DOI] [PubMed] [Google Scholar]
- Kirschner AKT, Velimirov B. Benthic bacterial secondary production measured via simultaneous H-3-thymidine and C-14-leucine incorporation, and its implication for the carbon cycle of a shallow macrophyte-dominated backwater system. Limnology and Oceanography. 1999;44:1871–1881. [Google Scholar]
- Kölbel-Boelke JM, Hirsch P. Comparative physiology og biofilm and suspended organisms in the groundwater environment. In: Characklis WG, Wilderer PA, editors. Structure and Function of Biofilms. John Wiley & Sons Ltd; New York: 1989. pp. 221–238. [Google Scholar]
- Kyle JE, Eydal HSC, Ferris FG, Pedersen K. Viruses in granitic groundwater from 69 to 450m depth of the Äspö hard rock laboratory, Sweden. The ISME Journal. 2008;2:571–574. doi: 10.1038/ismej.2008.18. [DOI] [PubMed] [Google Scholar]
- Lauritzen S-E, Bottrell S. Microbiological activity in thermoglacial karst springs, South Spitsbergen. Geomicrobiology Journal. 1994;12:161–173. [Google Scholar]
- Laybourn-Parry J, Quayle W, Henshaw T, Ruddell A, Marchant HJ. Life on the edge: the plankton and chemistry of Beaver Lake, an ultra-oligotrophic epishelf lake, Antarktica. Freshwater Biology. 2001;46:1205–1217. [Google Scholar]
- Legler C. Ausgewählte Methoden der Wasseruntersuchung. 1988. Vol. [Google Scholar]
- Lindström ES, Bergström A-K. Influence of inlet bacteria on bacterioplankton assemblage composition in lakes of different hydraulic retention time. Limnol. Oceanogr. 2004;449:125–136. [Google Scholar]
- Mahler BJ, Personne J-C, Lods GF, Drogue C. Transport of free and particulate-associated bacteria in karst. Journal of Hydrology. 2000;238:179–193. [Google Scholar]
- Menne B. Myxobacteria in cave sediments of the French Jura Mountains. Microbiol. Res. 1999;154:1–8. [Google Scholar]
- Mösslacher F, Griebler C, Notenboom J. Biomonitoring of groundwater systems: Methods, applications and possible indicators among the groundwater biota. In: Griebler C, Danielopol DL, Gilbert L, Nachtnebel HP, Notenboom J, editors. Groundwater Ecology. Vienna: 2001. pp. 174–182. [Google Scholar]
- Nielsen JL, Christensen D, Kloppenborg M, Nielsen PH. Quantification of cell-specific substrate uptake by probe-defined bacteria under in situ conditions by microautoradiography and fluorescence in situ hybridization. Environ Microbiol. 2003a;5:202–211. doi: 10.1046/j.1462-2920.2003.00402.x. [DOI] [PubMed] [Google Scholar]
- Nielsen JL, Wagner M, Nielsen PH. Use of microautoradiography to study in situ physiology of bacteria in biofilms. Reviews in Environmental Science and Biotechnology. 2003b;2:261–268. [Google Scholar]
- Noble R, Fuhrman JA. Breakdown and microbial uptake of marine viruses and other lysis products. Aquat Microb Ecol. 1999;20:1–11. [Google Scholar]
- Norland . The relationship between biomass and volume of bacteria. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ, editors. Handbook of Aquatic Microbial Ecology. Lewis Publishers; Boca Raton: 1993. pp. 303–308. [Google Scholar]
- Ouverney CC, Fuhrman JA. Combined microautoradiography-16S rRNA probe technique for determination of radioisotope uptake by specific microbial cell types in situ. Appl Environ Microbiol. 1999;65:1746–1752. doi: 10.1128/aem.65.4.1746-1752.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouverney CC, Fuhrman JA. Marine planktonic archaea take up amino acids. Appl Environ Microbiol. 2000;66:4829–4833. doi: 10.1128/aem.66.11.4829-4833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K. The deep subterranean biosphere. Earth-Science Rev. 1993;34:243–260. [Google Scholar]
- Pernthaler A, Pernthaler J, Amann R. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl Environ Microbiol. 2002a;68:3094–3101. doi: 10.1128/AEM.68.6.3094-3101.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps TJ, Murphy EM, Pfiffner SM, White DC. Comparison between geochemical and biological estimates of subsurface microbial activities. Microb Ecol. 1994;28:335–349. doi: 10.1007/BF00662027. [DOI] [PubMed] [Google Scholar]
- Pronk M, Goldscheider N, Zopfi J. Dynamics and interaction of organic carbon, turbidity and bacteria in a karst aquifer system. Hydrogeology Journal. 2006;14:473–484. [Google Scholar]
- Pronk M, Goldscheider N, Zopfi J. Microbial communities in karst groundwater and their potential use for biomonitoring. Hydrogeology Journal. 2008 online: DOI 10.1007/s10040-10008-10350-x. [Google Scholar]
- Reinthaler T, van Aken H, Veth C, Aristegui J, Robinson C, Williams P, Lebaron P, Herndl G. Prokaryotic respiration and production in the meso- and bathypelagic realm of the eastern and western North Atlantic basin. Limnol. Oceanogr. 2006;51:1262–1273. [Google Scholar]
- Rusterholtz KL, Mallory LM. Density, activity and diversity of bacteria indigenous to a karstic aquifer. Microbial Ecology. 1994;28:79–99. doi: 10.1007/BF00170249. [DOI] [PubMed] [Google Scholar]
- Servais P, Billen G, Laurent P, Lévi Y, Randon G. Studies of BDOC and bacterial dynamics in the drinking water distribution system of the Northern Parisian suburbs. Sci. Eau. 1992;5:69–89. [Google Scholar]
- Simon KS, Ginert J, Petitot P, Laurent R. Spatial and temporal patterns of bacterial densitiy and metabolic activity in karst. Arch. Hydrobiol. 2001;151:67–82. [Google Scholar]
- Simon M, Azam F. Protein content and protein synthesis rates of planctonic marine bacteria. Mar. Ecol. Prog. Ser. 1989;51:201–213. [Google Scholar]
- Sintes E, Herndl GJ. Quantifying substrate uptake by individual cells of marine bacterioplankton by catalyzed reporter deposition fluorescence in situ hybridization combined with microautoradiography. Appl Environ Microbiol. 2006;72:7022–7028. doi: 10.1128/AEM.00763-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler H, Strobl E. Karstwasserdynamik Zeller Staritzen. Endbericht Joanneum für MA31 Stadt Wien. 1997:171. [Google Scholar]
- Stadler H, Strobl E. Karstwasserdynamik und Karstwasserschutz Hochschwab STA 28K/96. Enbericht 2. Arbeitsjahr im Auftrag MA31 Stadt Wien. 1998a [Google Scholar]
- Stadler H, Strobl E. Karstwasserdynamik und Karstwasserschutz Hochschwab STA 28K/96. Endbericht 2. Arbeitsjahr im Auftrag MA31 Stadt Wien. 1998b [Google Scholar]
- Teira E, Reinthaler T, Pernthaler A, Pernthaler J, Herndl GJ. Combining catalyzed reporter deposition-fluorescence in situ hybridization and microautoradiography to detect substrate utilization by bacteria and archaea in the deep ocean. Appl Environ Microbiol. 2004;70:4411–4414. doi: 10.1128/AEM.70.7.4411-4414.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teira E, van Aken H, Veth C, Herndl GJ. Archaeal uptake of enantiomeric amino acids in the meso- and bathypelagic waters of the North Atlantic. Limnol. Oceanogr. 2006;51:60–69. [Google Scholar]
- Tietz A, Langergraber G, Watzinger A, Haberl R, Kirschner AKT. Bacterial carbon utilization in vertical subsurface flow constructed wetlands. Water Research. 2007 doi: 10.1016/j.watres.2007.10.011. PMID: 17991505:ahead of print. [DOI] [PubMed] [Google Scholar]
- Toran L, Tancredi JH, Herman EK, White WB. Conductivity and sediment variation during storms as evidence of pathways to karst springs. Geological Society of America, Special Paper. 2006;404:169–176. [Google Scholar]
- Vesper DJ, White WB. Spring and conduit sediments as storage reservoirs for heavy metals in karst aquifers. Environmental Geology. 2004a;45:481–493. [Google Scholar]
- Vesper DJ, White WB. Storm pulse chemographs of saturation index and carbon dioxide pressure: implications for shifting recharge sources during storm events in the karst aquifer at Fort Campbell, Kentucky/Tennessee, USA. Hydrogeology Journal. 2004b;12:135–143. [Google Scholar]
- Weinbauer MG. Ecology of prokaryotic viruses. FEMS Microbiol Rev. 2004;28:127–181. doi: 10.1016/j.femsre.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Weinbauer MG, Höfle MG. Significance of viral lysis and flagellate grazing as factors controlling bacterioplankton production an a eutrophic lake. Appl.Environ.Microbiol. 1998;64:431–438. doi: 10.1128/aem.64.2.431-438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel RG, Likens GE. The inorganic carbon complex. In: Wetzel RG, Likens GE, editors. Limnological Analyses. Vol. 2. Springer; New York: 1991. pp. 107–128. [Google Scholar]
- White WB. Geomorphology and Hydrology of Karst terrains. J. Hydrol. 1988;61:45–67. [Google Scholar]
- Wilhartitz IC, Mach RL, Teira E, Reinthaler T, Herndl GJ, Farnleitner AH. Prokaryotic community analysis with CARD-FISH in comparison to FISH in ultra-oligotrophic ground- and drinking water. Journal of Applied Microbiology. 2007;103:871–881. doi: 10.1111/j.1365-2672.2007.03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm SW, Suttle CA. Viruses and Nutrient Cycles in the Sea. BioScience. 1999;49:781–788. [Google Scholar]
- Wood WW. Origin of caves and other slolution openings in the unsaturated (vadose) zone of carbonate rocks: A model for CO2 generation. Geology. 1985;13:822–824. [Google Scholar]