Abstract
Both aging and loss of sex steroids have adverse effects on skeletal homeostasis, but whether and how they may influence each others negative impact on bone remains unknown. We report herein that both female and male C57BL/6 mice progressively lost strength (as determined by load-to-failure measurements) and bone mineral density in the spine and femur between the ages of 4 and 31 months. These changes were temporally associated with decreased rate of remodeling as evidenced by decreased osteoblast and osteoclast numbers and decreased bone formation rate; as well as increased osteoblast and osteocyte apoptosis, increased reactive oxygen species levels, and decreased glutathione reductase activity and a corresponding increase in the phosphorylation of p53 and p66shc, two key components of a signaling cascade that are activated by reactive oxygen species and influences apoptosis and lifespan. Exactly the same changes in oxidative stress were acutely reproduced by gonadectomy in 5-month-old females or males and reversed by estrogens or androgens in vivo as well as in vitro.We conclude that the oxidative stress that underlies physiologic organismal aging in mice may be a pivotal pathogenetic mechanism of the age-related bone loss and strength. Loss of estrogens or androgens accelerates the effects of aging on bone by decreasing defense against oxidative stress.
Age-related loss of bone mass and strength is an invariable feature of human biology, affecting women and men alike. Moreover, population-based studies demonstrate that substantial bone loss begins as early as the 20s in young adult women and men, long before any hormonal changes (1).3 The extent to which estrogen deficiency contributes to age-related bone loss and the slower rate of decline of bone mass and strength during the late postmenopausal years, and the molecular and cellular mechanisms of such putative interactions, are unknown.
The universality of age-associated bone loss irrespective of sex steroid status notwithstanding, age is by far a more critical determinant of fracture risk than bone mass in humans indicating that age-related increase in fracture risk reflects a loss of bone strength that is only partly accounted for by loss of bone mass (2). Whereas an increased propensity to fall due to age-related decline in neuromuscular function is a factor, there are also age-related changes in the bone itself. Such changes include disrupted architecture, altered composition of the bone mineral and matrix, delayed repair of fatigue micro-damage, excessive turnover, and inadequate bone size (3–7). The most recently appreciated qualitative factor is loss of osteocytes (8, 9), former osteoblasts entombed into the mineralized matrix. Osteocyte death may influence the signals necessary for mechanical adaptation and repair and also lead to long term changes in bone hydration. The anti-apoptotic effect of sex steroids on osteocytes, which has been well documented in mice, rats, and humans (10 –12), may contribute to their anti-fracture efficacy independently of their effect on bone mineral density (BMD)4 (8).
We and others had shown earlier that estrogens and androgens protect the adult skeleton against bone loss by suppressing the rate of bone turnover and maintaining a focal balance between bone formation and resorption (13–15). Suppression of bone turnover results from attenuating effects of sex steroids on the birth rate of osteoblast and osteoclast progenitors. Maintenance of a focal balance between formation and resorption results from opposite effects on the lifespan of osteoblasts/osteocytes and osteoclasts: an anti-apoptotic effect on the former and a pro-apoptotic effect on the latter cell type. Conversely, loss of sex steroids increases the rate of remodeling by up-regulating osteoblastogenesis and osteoclastogenesis. Specifically, we have shown that estrogen loss rapidly up-regulates osteoblastogenesis in mice, and this effect is at least in part cell autonomous, and independent of the increased osteoclastic resorption, as it is manifested in ovariectomized (OVX) mice treated with bisphosphonates, known inhibitors of osteoclastic bone resorption (16). Moreover, the adverse effects of estrogen or androgen withdrawal on bone seem to be mediated, at least in part, by cells of the osteoblastic lineage and are obviated by constraints on osteoblastogenesis, whether genetic (17) or acquired (18), most likely because of the critical dependence of osteoclastogenesis on osteoblastogenesis (19, 20). Nonetheless, the effect of the loss of estrogens on the rate of remodeling and the up-regulation of osteoblastogenesis and bone formation in animals and humans wanes with time (5–10 years in women and 6–8 weeks in mice) to about the same rate as in elderly eugonadal males, raising the possibility that aging might be overriding the acute effects of estrogen deficiency.
Increased levels of reactive oxygen species (ROS) influence numerous cellular processes, including the timing of death by apoptosis; and have been linked to aging and the development of age-related diseases. Moreover, oxidative stress has been strongly correlated with longevity in flies, nematodes, and mammals (21, 22). The p53 tumor suppressor and the adapter protein p66shc represent key components of a signal transduction pathway that not only is activated by increased intracellular ROS and converts oxidative signals into apoptosis but also generates ROS in the mitochondria (23–25). Strikingly, an activating mutation of p53 causes early onset of aging-associated phenotypes in mice (26). Conversely, deletion of p66shc increases resistance to oxidative stress, as well as lifespan, by as much as 30% (23). One of the mechanisms used by cells to defend against oxidative damage involves the reduction of peroxides to harmless alcohols in a reaction in which glutathione peroxidase oxidizes glutathione (GSH) to the disulfide GSSH, and glutathione reductase (GSR) converts it back into GSH (27).
The above lines of evidence have strongly suggested to us the possibility that organismal aging per se, rather than an age-associated failure of other organs and tissues, may be the predominant mechanism of the bone fragility disease, which has become synonymous with osteoporosis, just one of the many features and risk factors underlying the problem of fractures. And, that loss of estrogens or androgens may exaggerate the adverse effects of organismal aging on bone. We report several previously unappreciated age-related changes in both female and male C57BL/6 mice that may provide critical clues into the mechanisms of the age-related decline of bone strength and mass, and the influence of sex steroid deficiency in the process.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents
l-Buthionine-(S,R)-sulfoximine (BSO), diethyl maleate (DEM), N-acetyl-l-cysteine (NAC), etoposide, flutamide, H2O2, dihydrotestosterone (DHT), and E2 were purchased from Sigma. PD98059 was purchased from Cell Signaling Technology, Inc. (Danvers, MA). ICI 182,780 was purchased from Tocris Cookson Inc. (Ellisville, MO), PP1 from Biomol International P.A. (Plymouth Meeting, PA), and U0126 from Promega (Madison, WI). Tumor necrosis factor (TNF) α recombinant protein was purchased from R&D Systems (Minneapolis, MN). Sixty-day slow-release pellets containing DHT were purchased from Innovative Research of America (Sarasota, FL). Estradiol was assayed with a kit from DiaSorin (Stillwater, MN) and testosterone with a kit from MP Biomedicals (Costa Mesa, CA). Glutathione reductase was assayed with a kit from Cayman Chemical Co. (Ann Arbor, MI). Intracellular ROS were quantified using dichlorodihydrofluorescein diacetate dye (28), using bone marrow cells flushed from femurs and washed with phosphate-buffered saline.
Animal Experimentation
Male and female C57BL/6 mice 4–31 months old were purchased from Harlan Inc. from a cohort maintained with support from the National Institute of Aging. The age-associated changes of intact animals were studied in three separate experiments: two with females (the first at 8, 16, 25, and 31 months old; and the second at 4, 8, 16, and 25 months old) and one with males (4, 8, 16, 25, and 31 months old). For the studies examining the effects of sex steroid deficiency, 5 month-old C57BL/6 mice were purchased from Harlan Sprague-Dawley Inc. Two to 3 days before surgery, all animals were electronically tagged (Biomedic Data System Inc., Maywood, NJ), BMD measurements were performed on each, and they were then allocated to various experimental groups to achieve equivalent mean femur BMD values. Animals were then sham-operated, OVX, or orchidectomized (ORX). Sham-operated animals were administered vehicle or BSO (2 mmol/kg intraperitoneally) twice a day (n = 12 per group). BSO was also included in the drinking water (20mm).OVX and ORX animals were subcutaneously injected with vehicle or with replacement doses of E2 (30 ng/g) or NAC (100 mg/kg/day) twice a day or were implanted with 60-day slow-release pellets containing DHT (10 mg) (n = 12 per group). After 6 weeks of treatment, animals were sacrificed and the tissues dissected for further analyses. BMD, bone geometry measurements, histomorphometry, and osteoblast/osteocyte apoptosis were performed as previously described (29–31).
Biomechanical Testing
The load bearing properties of L6 were measured using a single column material testing machine and a calibrated tension/compression load cell (model 5542, Instron Corp., Canton, MA). Load cell calibration was verified in accordance with American Society for Testing and Materials E74-02 standards and traceable to the National Institute of Standards and Technology. Data were recorded and analyzed using the Merlin IX software package (Instron Corp.). The L6 specimens were cleaned of surrounding soft tissue, wrapped in gauze soaked in 37 ± 0.5 °C normal saline, and tested on the day of sacrifice. The length, width, and depth of the bones were recorded with a digital caliper at a resolution of 0.01 mm (Mitutoyo number 500-196, Ace Tools, Ft. Smith, AR). The cross-sectional area was assumed to be an ellipse and calculated as A = 0.25 π (width)(depth). Articular and spinous processes that would interfere with compression were excised using an iris scissors. After pre-seating with less than 0.5 newtons (N) of applied load, vertebrae were compressed between screw-driven loading platens using a lower-platen, customized miniature spherical seat that minimizes shear by adjusting to irregularities in the end plates of the specimens. Best seating was obtained with the load applied along the caudocephalad axis at a speed of 0.5 mm/min until failure. Standard materials for compression were run before each set of determinations. Three-point bending of the femur was also performed at 37 ± 0.5 °C using a miniature bending apparatus with the posterior femoral surface lying on lower supports (7 mm apart) and the left support immediately proximal to the distal condyles. Load was applied to the anterior femoral surface by an actuator midway between the two supports moving at a constant rate of 3 mm/min to produce a physiological in vivo stain rate of 1% for the average murine femur. The external measurements (length, width, and thickness) of the femora were recorded with a digital caliper. Measurements of the internal marrow cavity (greater and lesser diameters) were obtained with a hand-held microscope at ×100 magnification using a calibrated linear reticule eyepiece (Klarmann Rulings, Manchester, NH). Maximum load (N) and displacement (mm) were recorded. The mechanical properties were normalized for bone size and ultimate strength or stress (N/mm2; in megapascals) was calculated. Standard precision steel piano wire with stiffness in the same range as murine femoral bone was evaluated before each set of determinations.
Western Blot Analysis
The phosphorylation status of p53, p66shc, and ERK1/2 was analyzed by immunoblotting in fifth lumbar vertebra lysates, as previously described (32). The antibodies used were: a rabbit polyclonal antibody recognizing Ser15-phosphorylated p53 (Cell Signaling Technology, Inc., Danvers, MA), a mouse monoclonal antibody recognizing Ser36-phosphorylated p66shc (Calbiochem, San Diego, CA), and a mouse monoclonal antibody recognizing tyrosine-phosphorylated ERK1/2 (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Protein levels of p-53, p66shc, and ERK1/2 were analyzed using a mouse monoclonal antibody recognizing p53 (Cell Signaling), a rabbit polyclonal antibody recognizing p66shc (BD Biosciences, Palo Alto, CA), and a rabbit polyclonal antibody recognizing total ERK1/2 (Santa Cruz).
Cell Culture
OB-6 cells, an osteoblastic cell line derived in our laboratory from the murine bone marrow (33), were cultured in α-minimal essential medium (Invitrogen) supplemented with 10% fetal calf serum (Hyclone, Logan, UT), penicillin (100 units/ml), streptomycin (100 µg/ml), and glutamine (292 µg/ml). C2C12 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, antibiotics as above, and 1% sodium pyruvate. Osteoclasts were derived from bone marrow cells cultured in α-minimal essential medium supplemented with 30 ng/ml macrophage- colony stimulations factor and 30 ng/ml soluble RANK ligand. For the quantification of osteoblast apoptosis, OB-6 cells were treated for 1 h with BSO or DEM followed by the steroids for 1 and 6 h with the pro-apoptotic agent etoposide (5 × 10−5 m), TNFα (10−9 m), or H2O2 (5 × 10−5 m). Osteoclast apoptosis was assayed in cells treated with BSO or DEM for 1 h followed by the steroids for 24 h. Apoptotic cells were quantified by measuring caspase 3 activity as described previously (32). For quantification of osteoclasts, after 4 days in culture, cells were treated for 1 h with BSO or DEM followed by the steroids for 24 h. Osteoclasts were enumerated after staining for TRAPase; both mononucleated and multinucleated cells were counted.
Quantitative PCR
Total RNA was extracted from tibia or calvaria using Ultraspec RNA (Biotecx Laboratories, Houston, TX) and reverse-transcribed using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Primers and probes for estrogen receptor (ER) α, ERβ, androgen receptor (AR), and glyceraldehyde-3-phosphate dehydrogenase were manufactured by the TaqMan® Gene Expression Assays service (Applied Biosystems).
Transient Transfections
C2C12 cells were transfected with 0.1 µg of green fluorescent protein, 0.1 µg of empty vector or wild-type p66shc plasmid, and 0.2 µg of pcDNA using Lipofectamine Plus (Invitrogen).
Statistical Analysis
Effects of age and gender on BMD and strength of the spine and the femur were evaluated by two-way ANOVA. The time of onset of the changes was determined by linear regression followed by a test for significant lack of fit or departure from linearity (34). Whenever there was significant departure from linearity for the entire data set, data from one or two earlier time points (4 month or both 4 and 8 months) were excluded from the analysis so that linearity could be established within the remaining set of data. Subsequently, the earliest time point that was used to establish linearity, i.e. 8 or 16 months, was compared with 4 or 4 and 8 months by Student’s t test or ANOVA. If no different from the preceding time points, the earliest time point of the linearity plot was declared the time after which a particular change was manifested and the change was continuous from that time point on; if, on the other hand, differences were detected, we concluded that the change began at the earliest time point, but it was discontinuous.
ANOVA was used to detect other age-related changes, and effects of various in vivo and in vitro treatments, after establishing that the data were normally distributed and equivalency of variances. Bonferroni’s method was used to perform appropriate pairwise comparisons of treatment groups. In cases when one or both of the requirements for performing ANOVA were not met, Kruskal-Wallis ANOVA on Ranks test was used followed by Dunn’s method to perform pairwise comparisons of treatment groups.
RESULTS
Similar Age-associated Changes in BMD and Strength in Female and Male Mice
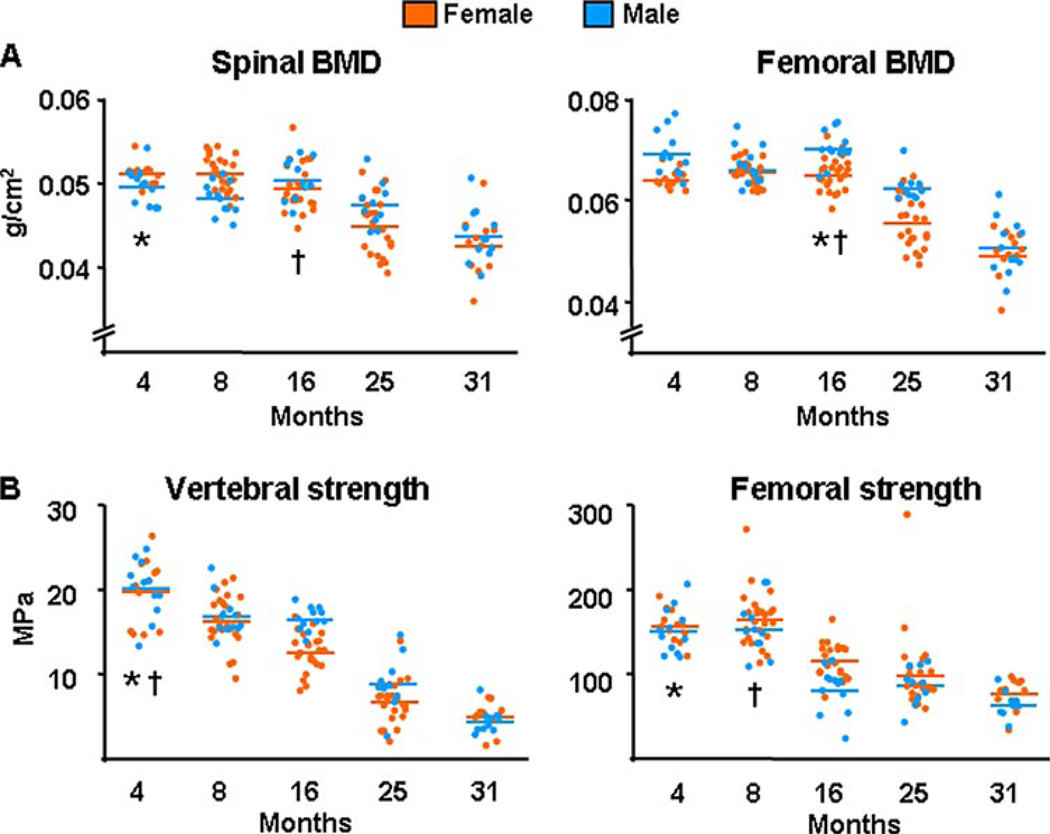
Both female and male C57BL/6 mice exhibited loss of bone mass, as determined by the surrogate measurement of BMD, and loss of strength in the spine and femur between ages of 4 and 31 months (Fig. 1, A and B). Strength in these experiments was assessed by obtaining load-to-failure estimates of the 6th lumbar vertebra (L6) and the left femur, using compression and 3-point bending, respectively. The decline in bone strength occurred earlier as compared with the loss of BMD, with the exception of the spine in females in which both changes began simultaneously. Furthermore, the decline of BMD and strength was progressive (i.e. the data fit linear regression) in all instances other than vertebral and femoral strength in males, which were discontinuous. The fall in spinal BMD and femoral strength occurred earlier in females than in males.
FIGURE 1. BMD and strength decrease with age in sex steroid sufficient female or male C57BL/6 mice.
A, BMD at the spine and femur was assessed by dual energy x-ray absorptiometry in two experiments with female and one experiment with male mice. The n was 12 animals per age group in each experiment with females, and 10 – 12 in the experiment with males. In the two experiments with females the age-dependent changes were statistically indistinguishable, hence the data were combined. B, load-to-failure, a measure of strength, was determined by compression testing of the 6th lumbar vertebra (L6) and by 3-point bending of the left femur. Colored horizontal lines in A and B indicate the mean values for each sex. * and † indicate the age after which a time-dependent decline began in females and males, respectively.
Age-associated Changes in Osteoblast and Osteoclast Number, Bone Formation, and Osteoblast and Osteocyte Apoptosis
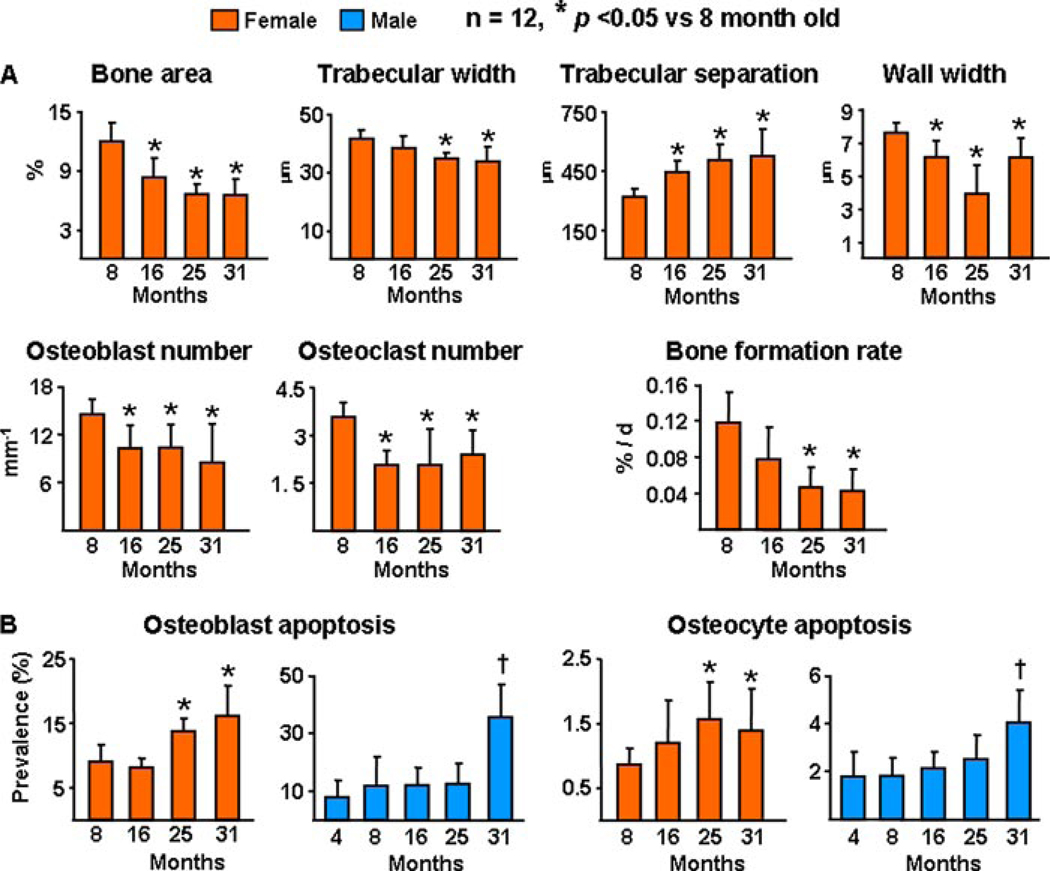
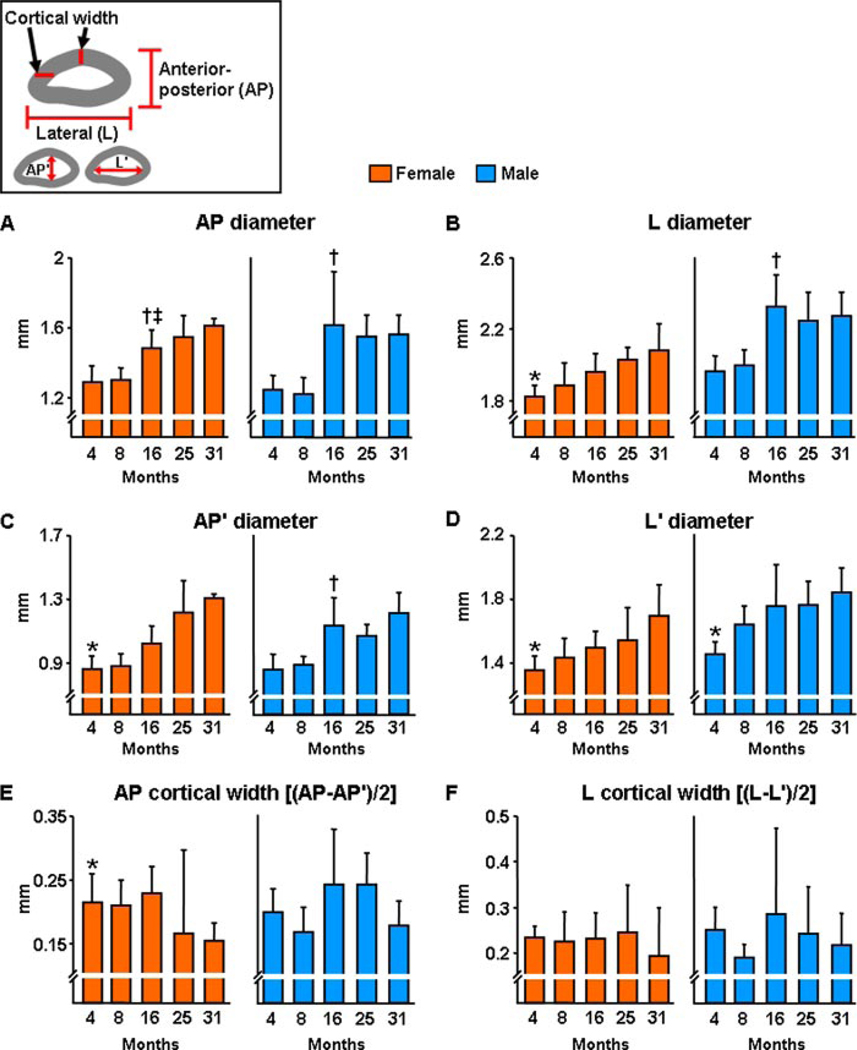
As evidenced from histomorphometric analysis (Fig. 2) of the vertebrae of the first of the two experiments with female C57BL/6 mice, advancing age from 8 to 31 months was associated with decreased cancellous bone area and trabecular width and increased trabecular separation (Fig. 2A). Furthermore, advancing age was associated with decreased wall width, decreased osteoblast and osteoclast number, as well as a decrease in bone formation rate. All these changes are unmistakable indications of a low turnover state, with resorption exceeding formation; and are clearly distinct from the high turnover state caused by loss of estrogens or androgens in rodents and humans. Furthermore, advancing age was associated with an increase in osteoblast and osteocyte apoptosis (Fig. 2B). The increase in osteoblast and osteocyte apoptosis with age in females was confirmed in the experiment with the males between the ages of 4 and 31 months. As is the case in humans (35, 36), both female and male mice exhibited an age-dependent increase in both the external and internal diameter of the femoral diaphysis, indicating ongoing periosteal apposition and endocortical resorption, respectively (Fig. 3).
FIGURE 2. Bone remodeling and bone formation rate decrease with age in sex steroid sufficient female or male C57BL/6 mice, whereas osteoblast and osteocyte apoptosis increases.
A, static and dynamic histomorphometric analysis of longitudinal undecalcified sections of L1–L4 vertebrae. Osteoblasts were enumerated on sections from the same specimens stained with toluidine blue and bone formation rate was determined from tetracycline-labeled surfaces. B, osteoblast and osteocyte apoptosis were determined by in situ end-labeling. Bars indicate mean ± S.D.; * or † indicate p < 0.05 versus 8-month-old animals in females and 4-month-old in males. In A, only mice from the first experiment with females were analyzed.
FIGURE 3. The external and internal diameters of the femoral diaphysis increase with age in both female and male C57BL/6 mice.
The geometry of the left femurs from the two experiments with females and the single experiment with males was determined after performance of the 3-point bending test at the diaphyseal breakpoint by measuring the diameters depicted in the inset. The anterior-posterior external diameter (AP) and lateral exterior diameter (L) were measured using a digital caliper; and the corresponding internal diameters, (AP′) and (L′), were measured with a hand-held microscope, as described under “Experimental Procedures.” † indicates significant difference only between 6 and 18 months by ANOVA. ‡ indicates a progressive increase after 16 months of age. * indicates progressive increase after 4 months of age without significant departure from linearity for the entire data set.
Age-related Increase of Oxidative Stress in the Bone of Females and Males
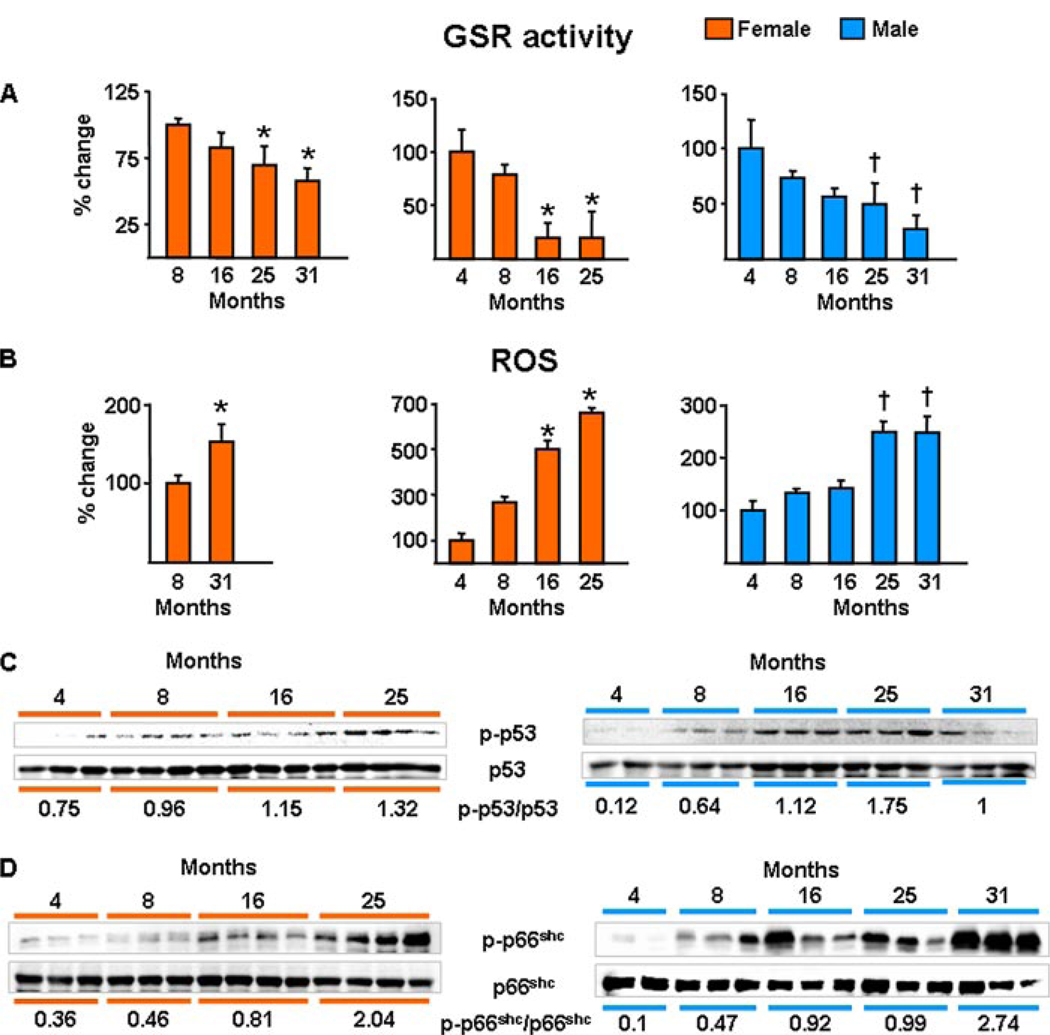
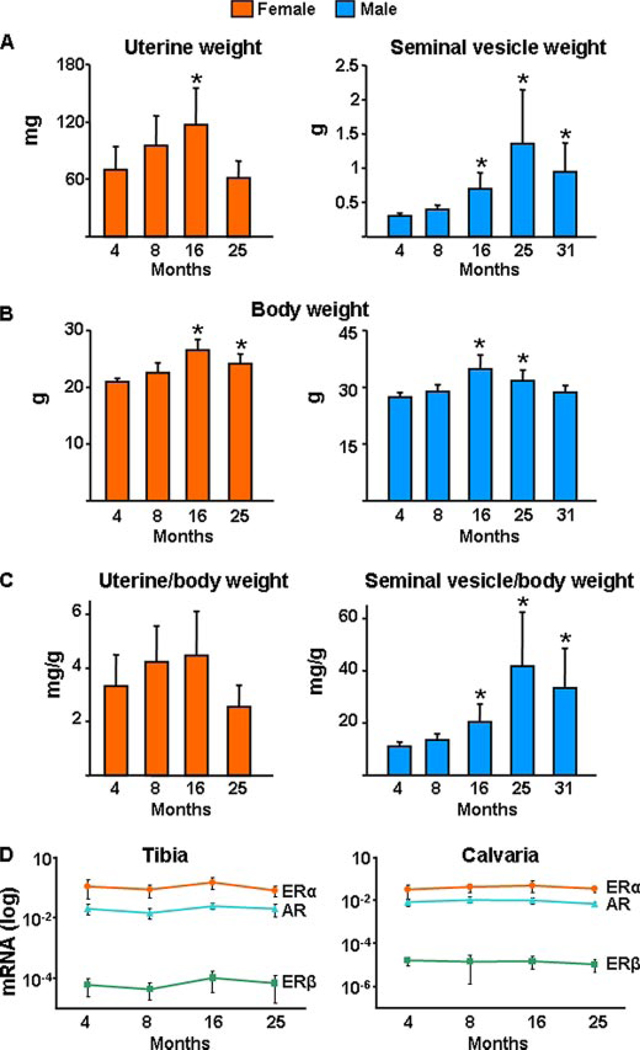
Consistent with the evidence for a critical role of oxidative damage in age-related diseases in general, and of the p53/p66shc signaling cascade in apoptosis, the changes in bone mass, strength, osteoblast/osteocyte apoptosis, osteoblast number, and bone formation rate with age in the aging murine model were associated with age-dependent decreased activity of GSR (Fig. 4A) and increased levels of ROS in the bone marrow (Fig. 4B). Furthermore, the pattern of all these changes was temporally associated with a progressive increase in the phosphorylation of p53 (Fig. 4C) and p66shc (Fig. 4D) in lysates from vertebrae. In agreement with published studies by others showing no change in circulating estrogen or androgen levels with age in C57BL/6 mice (37, 38), we found no evidence of a decline in estrogen or androgen status between 4 and 31 months of age, as measured by the sensitive estrogen and androgen status indicators, uterine and seminal vesicle weight, respectively (Fig. 5, A–C). Likewise, transcript levels of the ERα or -β or the androgen receptor in bone were not altered with age (Fig. 5D). In addition, using commercially available radioimmunoassays, we attempted to measure serum levels of testosterone and 17β-estradiol. Detectable levels of testosterone were found in all males from which we have available serum and at all ages, and there was no difference among ages (4 months = 0.09 ng/ml ± 0.11, 8 months = 0.04 ng/ml ± 0.03, 16 months = 0.06 ng/ml ± 0.04, 25 months = 0.17 ng/ml ± 0.34, and 31 months = 0.10 ng/ml ± 0.06; n = 3–9 per group). On the other hand, serum estradiol was at or below the detection limit of the radioimmunoassay (2 pg/ml) in all the animals we assayed (n = 45) at all ages (4, 8, 16, 25, and 31; n = 9 per group).
FIGURE 4. Oxidative stress increases with age in the bone of female or male C57BL/6 mice.
GSR activity (A) and ROS levels (B) were determined in bone marrow aspirates. The results from the two experiments with females and the single experiment with males are shown separately (n = 4 animals per group per experiment). Bars indicate mean ± S.D.; * or † indicate p < 0.05 versus 8-month-old animals in females and 4-month-old in males. C, the levels of phosphorylated p53; and D, p66shc were determined by Western blot analyses in vertebral lysates; each lane represents one animal. The mean ratio of phosphorylated to total protein is depicted numerically at the bottom of the corresponding blots.
FIGURE 5. Uterine or seminal vesicle weight or the estrogen and androgen receptor mRNA levels do not change with age in C57BL/6 mice.
A, wet uterine or seminal vesicle weight; total body weight (B) and uterine or seminal vesicle (C) corrected for body weight of mice from the second experiment with females and the single experiment with males are shown. Bars represent mean ± S.D.; and n = 10–12 animals per group. * indicates p < 0.05 versus 4-month-old animals. D, mRNA levels of ERα, ERβ, or AR were determined by quantitative PCR in calvaria and tibia obtained from the second experiment with females (n = 5–9), as described under “Experimental Procedures.”
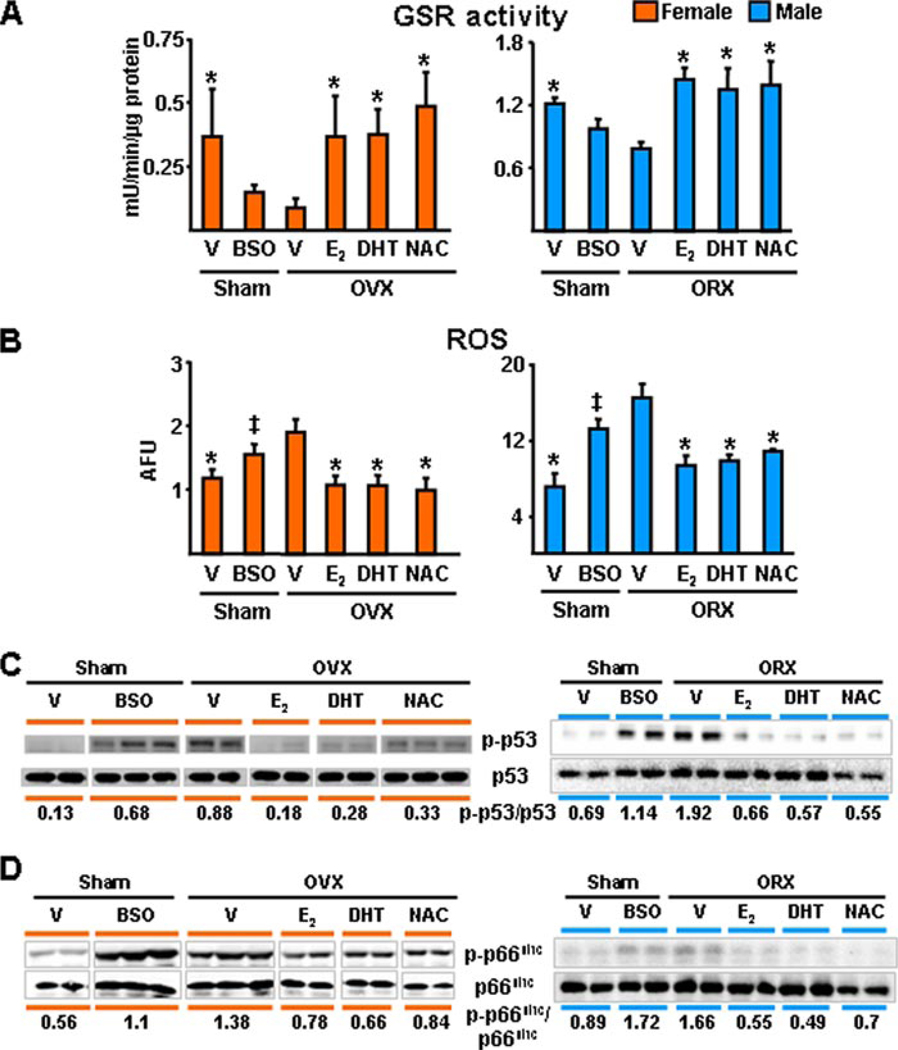
Gonadectomy Acutely Recapitulates the Effect of Aging on Oxidative Stress, Osteoblast and Osteocyte Apoptosis, and BMD: Protection by an Antioxidant
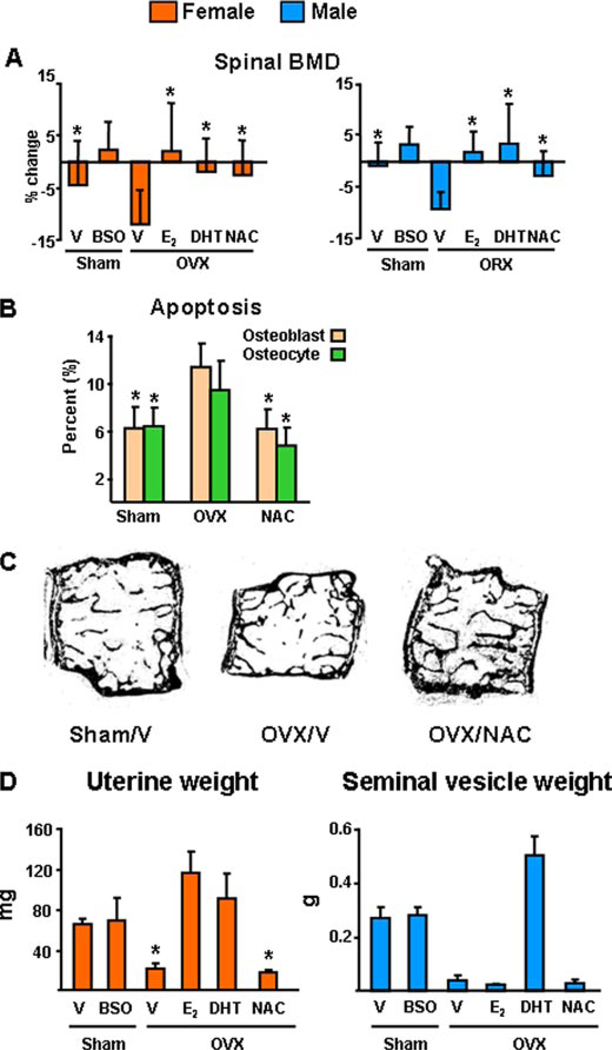
Prompted by evidence that ROS, such as H2O2, may be responsible for the increased loss of bone caused by OVX in mice (39), we proceeded to test the hypothesis that sex steroid deficiency accelerates the effects of aging on bone, by examining whether sex steroid deficiency in younger mice will acutely reproduce the effects of advancing age on oxidative stress (Fig. 6). To this end, we sham-operated or gonadectomized 5-month-old C57BL/6 females or males. Immediately after surgery, the sham-operated animals were treated with either saline or BSO, a specific inhibitor of GSH synthesis. Gonadectomized animals received vehicle, E2, DHT, or the antioxidant NAC. Six weeks later, animals were sacrificed and uterine or seminal vesicle weight, ROS, and GSR activity in the bone marrow, as well as the phosphorylation of p53 and p66shc in vertebral lysates was determined. In either sex of animals, E2, DHT, or NAC prevented the effects of gonadectomy on all measures of oxidative stress tested here, including GSR activity, ROS levels, as well as p53 and p66shc phosphorylation (Fig. 6, A–D). Moreover, NAC was as effective as E2 or DHT in preventing the decrease of spinal BMD caused by either OVX or ORX (Fig. 7A). Furthermore, NAC prevented the increase in osteoblast and osteocyte apoptosis induced by OVX (Fig. 7B); and, in agreement with Lean et al. (39), NAC prevented OVX-induced loss of cancellous bone (Fig. 7C). As expected, OVX decreased the uterine weight, and estrogen replacement reversed this effect (Fig. 7D). DHT also restored uterine weight as noted previously by us and others. Likewise, ORX decreased seminal vesicle weight, and DHT replacement prevented it. BSO or NAC had no effect on uterine weight; and BSO, E2, or NAC had no effect on seminal vesicle weight.
FIGURE 6. The antioxidant NAC, as well as estrogens or androgens, prevent gonadectomy-induced increase in oxidative stress in females and males.
A–D, 5-month-old mice were sham-operated, OVX, or ORX. Sham-operated animals were administered vehicle (V) or BSO twice a day. One day after surgery, OVX and ORX animals were injected daily with E2 (30 ng/g) or NAC (100 mg/kg) or were implanted with 60-day slow-release pellets containing DHT (10 mg). Animals were sacrificed 6 weeks later. In A and B, n = 4 animals per group. The results depicted for males in D were reproduced in a second blot, in which lysates from two more animals were assayed. The mean ratio of phosphorylated to total protein is depicted numerically in the bottom of the corresponding blots; and, in the case of the male data, represents the results from all four animals. * indicates p < 0.05 versus vehicle-treated OVX or ORX animals; and ‡ indicates p < 0.05 versus sham operated animals treated with vehicle.
FIGURE 7. The antioxidant NAC, as well as estrogens or androgens, prevent gonadectomy-induced bone loss and osteoblast and osteocyte apoptosis in females and males.
A, spinal BMD was determined by dual energy x-ray absorptiometry 1 to 3 days before and 6 weeks after surgery in the mice of the experiments shown in Fig. 4. The mean ± S.D. of the percent change from the pre-surgery measurement is shown (n = 10–12 per group). B, osteoblast and osteocyte apoptosis were determined in longitudinal undecalcified sections of L1–L4 by in situ end labeling, n = 7–13 per group. * indicates p < 0.05 versus vehicle-treated OVX or ORX animals; and ‡ indicates p < 0.05 versus sham operated animals treated with vehicle. C, representative photomicrographs of lumbar vertebrae from the female mice of the experiment. Note that the loss of central cancellous bone in the OVX/vehicle (OVX/V), as compared with the Sham/vehicle control (Sham/V), has been prevented in the OVX animals that received NAC (OVX/NAC). Unstained and viewed at ×25 with no coverslip. D, wet uterine or seminal vesicle weight of female and male mice. Bars represent mean ± S.D.; and n = 10 –12 animals per group. * indicates p < 0.05 versus vehicle treated sham operated animals.
The Effects of Both Estrogens and Androgens on Osteoclasts and Osteoblasts Result from Antioxidant Actions
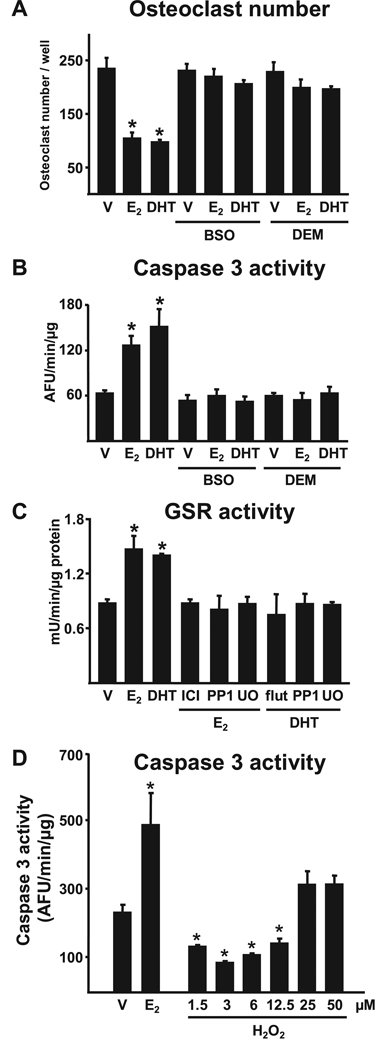
Finally, we investigated the possibility that the bone protective effects of estrogens or androgens result from direct antioxidant actions on bone cells. BSO or the electrophilic agent DEM abrogated the suppressive effects of E2 or DHT on osteoclastogenesis (Fig. 8A). BSO or DEM also abrogated E2-or DHT-induced osteoclast apoptosis (Fig. 8B). In addition, E2 or DHT stimulated the activity of GSR in osteoclasts (Fig. 8C); and the effect of E2 or DHT on GSR was abrogated by the ER antagonist ICI 182,780 or the AR antagonist flutamide, respectively. Furthermore, the effects of E2 or DHT on GSR were abrogated by the specific inhibitors of Src kinase PP1 and MEK kinase U0126. Consistent with the idea that low doses of H2O2 promote osteoclast survival, H2O2 at concentrations reported to cause NF-κB activation (1.5 to 12.5µm), attenuated osteoclast apoptosis; however, at higher concentrations (25 to 50 µm) it was ineffective (Fig. 8D).
FIGURE 8. Estrogens or androgens regulate osteoclastogenesis and the survival of osteoclasts via antioxidant actions.
A and B, bone marrow-derived osteoclasts were treated for 1 h with BSO (10−6 m) or DEM (10−4 m), followed by E2 or DHT (10−8 m) for 24 h. Osteoclasts were enumerated after staining for TRAPase and apoptosis was quantified by determining caspase 3 activity. C, GSR activity in osteoclasts treated with ICI 182,780 (10−7 m), flutamide (10−7 m), PP1 (10−6 m), or U0126 (10−6 m) for 1 h followed by the indicated steroids for 24 h. D, apoptosis was quantified by determining caspase 3 activity in bone marrow-derived osteoclasts treated for 24 h with 10−8 m E2 or the indicated doses of H2O2. Bars indicate mean ± S.D. of triplicate determinations; * p < 0.05 versus vehicle.
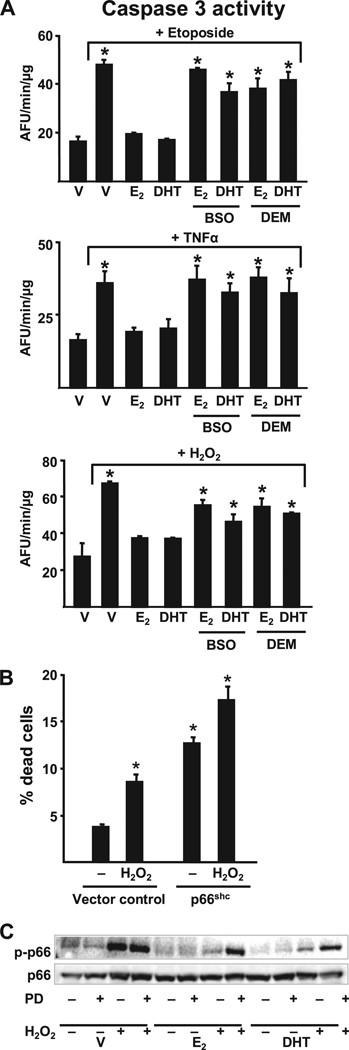
BSO or DEM also abrogated the anti-apoptotic effects of E2 or DHT on bone marrow-derived osteoblastic cells, OB-6 (Fig. 9A), as well as in primary cultures of calvaria cells and the C2C12 osteoblast progenitor cell line (data not shown), irrespective of whether apoptosis was induced by the topoisomerase inhibitor etoposide,TNFα, or H2O2. BSO or DEM, at higher concentrations than those that blocked the anti-apoptotic effect of E2, induced apoptosis of calvaria cells, presumably by depleting GSH (data not shown). This observation raises the possibility that a threshold level of GSH production by osteoblasts may be an inhibitor of endogenous ROS production and, thereby, apoptosis.
FIGURE 9. Estrogens or androgens regulate the survival of osteoblasts via antioxidant actions.
A, apoptosis was quantified by determining caspase 3 activity in OB-6 cells treated for 1 h with BSO or DEM followed by the steroids for 1 and 6 h with the pro-apoptotic agent etoposide (5 × 10−5 m), TNFα (10−9 m), or H2O2 (5 × 10−5 m). Bars indicate mean ± S.D. of triplicate determinations; * indicates p < 0.05 versus vehicle. B, C2C12 cells transfected with a vector control or wild-type p66shc plasmid, along with green fluorescent protein, were treated with or without H2O2 (5 × 10−5 m). The number of apoptotic cells was determined by examining the nuclear morphology of fluorescent cells 6 h later. Bars indicate mean ± S.D. of triplicate determinations; * p < 0.05 versus vector control untreated. C, OB-6 cells were treated for 1 h with the MEK inhibitor PD98059 (5 × 10−5 m), then the indicated steroids (10−8 m) were added, and 1 h later the cultures were exposed for 15 min to H2O2 (5 × 10−5 m). Phosphorylated p66shc was determined by Western blot analyses in cell lysates.
Phosphorylation of serine 36 (Ser36) of p66shc is required for transduction of oxidant stress signals leading to apoptosis. Consistent with this, overexpression of p66shc in C2C12 cells induced apoptosis both under basal conditions and in the presence of H2O2 (Fig. 9B). Therefore, we examined whether E2 or DHT affects p66shc phosphorylation. H2O2 stimulated the phosphorylation of p66shc as early as 2 min and for at least 1 h following its addition to cultures of OB-6 osteoblastic cells but had no effect on the expression of the protein (Fig. 9C). E2 or DHT suppressed the H2O2-induced p66shc phosphorylation as early as 15 min following treatment of OB-6 cells; and the effect of either steroid was reversed in the presence of the MEK inhibitor PD98059. These results suggest that, not only attenuation of osteoclastogenesis, but also the stimulation of osteoclast and attenuation of osteoblast apoptosis by estrogens involve non-protein thiol metabolism and are the result of actions mediated via cytoplasmic kinases and probably downstream transcriptional control (32).
DISCUSSION
The results reported herein demonstrate that both female and male C57BL/6 mice exhibit an age-related decrease in strength, BMD, bone remodeling, and bone formation rate together with an increase in osteoblast and osteocyte apoptosis. These changes are temporally associated with increased ROS levels, decreased GSR activity, and increased phosphorylation of p53 and p66shc. The exact same changes in ROS, GSR, and the phosphorylation of p53 and p66shc were acutely reproduced by gonadectomy in 5-month-old females or males; and prevented by the antioxidant N-acetyl-l-cysteine. Moreover, estradiol or DHT acted directly in vitro to attenuate osteoclastogenesis and osteoblast apoptosis, stimulate osteoclast apoptosis, and suppress p66shc phosphorylation by a kinase-dependent mechanism that involves non-protein thiol metabolism. This evidence strongly suggests that increased ROS is a pivotal mechanism of age-related bone loss and strength; and that estrogen or androgen deficiency accelerates the adverse effects of aging on bone by decreasing defense against ROS. To the best of our knowledge, the in-depth characterization of the mice used in our studies, including the demonstration of increased ROS and p66shc phosphorylation with physiologic aging and acute loss of estrogens or androgens, demonstrates for the first time that aging and sex steroid deficiency exert their adverse effects by similar mechanisms in an animal model at the molecular, cellular, and tissue level. A model summarizing the results of the present report and findings from earlier studies by us and others on the effects of ROS and sex steroids on bone cells is provided in Fig. 10.
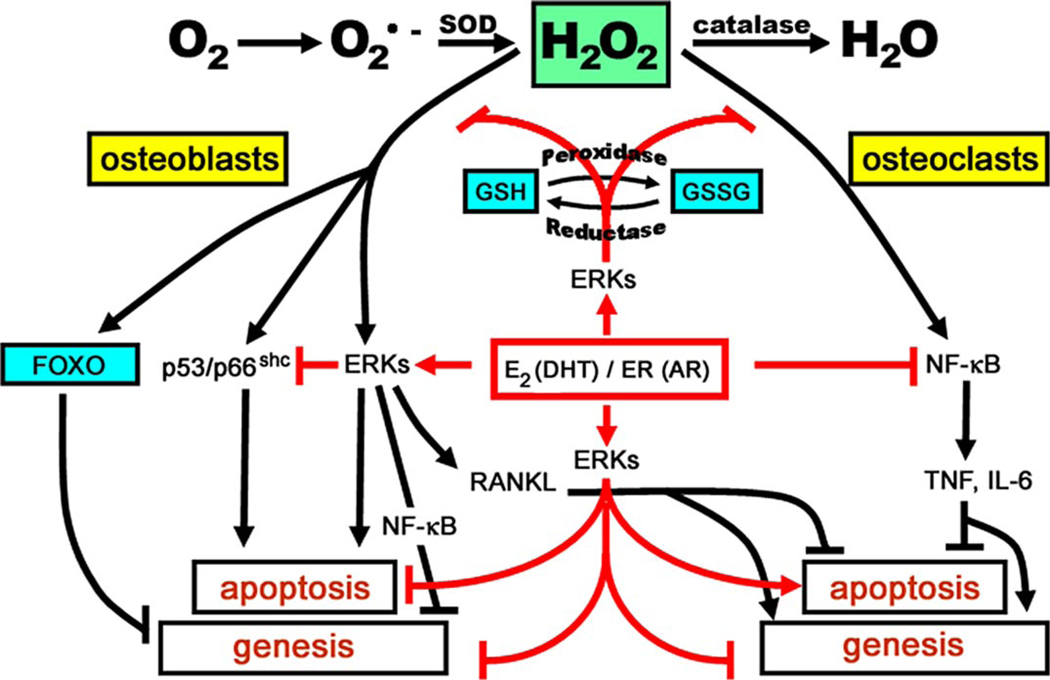
FIGURE 10. ROS-activated signals affecting the genesis and lifespan of osteoblasts and osteoclasts and the counter-regulatory actions of sex steroids.
Effects of ROS, exemplified here by H2O2, on the genesis and survival of both osteoblasts and osteoclasts are depicted in black, and as shown require the same signaling cascades and factors, i.e. ERKs, NF-κB, and osteoclastogenic cytokines like Rankl, Tnf, and interleukin (IL) 6, used by estrogens to regulate the birth and death of osteoblasts and osteoclasts, albeit in the exactly opposite manner (13, 14). Like most other cell types, bone cells attempt to counteract the adverse effects of ROS by several defense mechanisms (depicted in blue). Such mechanisms include the up-regulation of ROS scavenging enzymes (superoxide dismutases and catalases) as well as DNA-damage repair genes by Forkhead transcription factors (FoxO; see accompanying article, Almeida et al. (82)). Additionally, enzymes like glutathione peroxidase use glutathione to reduce ROS to alcohols. Glutathione reductase is a key partner in this cycle because it converts the disulfide (GSSG) back into glutathione (GSH). The pro-apoptotic effects of H2O2 on osteoblasts (and probably their mesenchymal stem cell progenitors) are associated with phosphorylation of p53 and p66shc. ROS decreases osteoblastogenesis by at least two mechanisms: 1) antagonism of Wnt signaling by diversion of β-catenin from Tcf- to FoxO-mediated transcription (see accompanying article, Almeida et al. (82)); and 2) direct and sustained activation of ERKs and NF-κB (78). ROS inhibit osteoclast apoptosis and stimulate osteoclastogenesis by increasing RANKL production in cells of the stromal/osteoblastic lineage (79) as well as an ERK/NF-κB/Tnf/interleukin 6-mediated mechanism(39).E2 or DHT antagonizes the effects of ROS via several mechanisms(depicted in red): (a) up-regulation of GSR(and thioredoxin reductase activity (39, 80); (b) attenuation of p66shc phosphorylation via a Src- and ERK-dependent pathway; and (c) down-regulation of the production of osteoclastogenic cytokines like TNF and interleukin 6 via attenuation of NF-κB. In addition, E2 or DHT antagonizes the effects of ROS by attenuating osteoblast apoptosis and stimulating osteoclast apoptosis via a transient and sustained ERK activation, respectively; and by inhibiting osteoclastogenesis, through a sustained ERK activation (81). For the interplay between ROS and estrogens on osteoblastogenesis, see “Discussion.”
In agreement with the findings of the present report, osteoporosis has been noted by us and others in mouse models of premature aging (17, 26, 40). Moreover, consistent with our observations temporally linking increased oxidative stress with increased osteoblast apoptosis and decreased osteoblast number and bone formation rate in aging C57BL/6 mice, in studies by Chambers and colleagues (41) both osteoblast numbers and bone formation were decreased in 2-month-old B6;129SF2 mice treated with the glutathione inhibitor BSO. Likewise, in agreement with our findings with the 5-month-old ovariectomized C57BL/6 mice, these workers had shown that the antioxidants, NAC and ascorbate, or inhibition of H2O2 by pegylated catalase prevents the increased osteoclastogenesis and loss of bone caused by acute loss of estrogens in 2-month-old MF-1 mice (39, 41).
Beneficial effects of estrogens in several other tissues, such as lens epithelial cells, arteries, central nervous system, fat, liver, and oviducts, are also shown to result from improved defense against oxidative stress (42–53). Importantly, in view of the fact that E2 inhibits oxidized LDL (54) inhibition of 15-lipoxygenase, the enzyme responsible for the generation of oxidized low density lipoprotein, partially prevents OVX-induced bone loss (55). Moreover, bone loss and vascular calcification progress in parallel with advancing age, indicating an age-dependent relationship between atherosclerosis and osteoporosis (56). On the other hand, adverse effects of estrogens on breast cancer, the uterus, and spermatogenesis may be due to increased ROS production or decreased antioxidant defense (57–59).
The results of the present study in mice also suggest that loss of bone strength with age is only partially accounted for by a reduction in bone mass. Moreover, even though we did not study fractures in mice, our observations are in line with clinical evidence from humans that the age-related increase in fracture risk reflects a loss of bone strength that is only partly accounted for by loss of bone mass (2). Indeed, consistent with this contention, at the lowest values for bone mass for humans, a 20-year increase in age is accompanied by a 4-fold increase in fracture risk. Whereas an increased propensity to fall due to age-related decline in neuromuscular function is clearly a factor in humans, there are also age-related changes in the bone itself. The importance of non-mass factors is demonstrated by several lines of evidence. First, a fracture at any site increases the risk of a subsequent fracture at any other site (60). Second, only a small part of the reduction in fracture incidence in response to anticatabolic therapy can be accounted for by the increase in bone mass (61). Third, many of the genetic effects on bone strength are mediated by factors other than bone mass (62, 63). The results reported here support the idea that loss of osteocytes, which make an independent contribution to vertebral bone strength both in patients with vertebral fracture (9) and in mice, may be an additional factor (8, 64). Importantly, the age-dependent decline in murine bone strength in the present study occurred despite the expansion in bone size, exactly as is the case in humans. Therefore, our findings also support the contention that the C57BL/6 mouse is a good model for the study of age-related geometric changes in human bone.
In line with the results of the present report suggesting that sex steroid status was not altered appreciably, at least as far as the skeletal changes concerned, between the age of 4 and 31 months in C57BL/6 mice, Mobbs and Finch (37) had found no difference in estradiol levels in females up to the age of 18 months or androgen in male C57BL/6 mice up to 31 months (38). Nonetheless, in more detailed studies of the estrus cycle in the same mice these workers subsequently reported that estrogen levels on day 3 and the preovulatory rise beginning on day 4 were decreased at 10–12.5 months as compared with 5.5–7.5 months of age (65). Moreover, whereas Nelson et al. (38) did not show declines in testosterone levels in healthy mice, mice with other diseases of aging did have markedly reduced testosterone levels. In addition, in a very recent paper (66) the authors found marked decreases in testosterone levels in male C57BL/6 mice at age 15 months compared with 4 months. We and others have shown here and elsewhere that seminal vesicle weight has less than half the variance of the testosterone measurements and indicates cumulative androgen status in mice better than a single serum sample (18, 67, 68). Be that as it may, we cannot categorically exclude the possibility that the age-related findings we have demonstrated herein may be accentuated by superimposed sex steroid deficiency in aging mice, as they are in humans. That having been said, however, acute loss of sex steroids causes an increase in the rate of bone remodeling, underlined by an increase in osteoclastogenesis and osteoblastogenesis and a corresponding increase in both bone resorption and formation, with the former exceeding the latter. In contrast, our aging C57BL/6 mice (and elderly individuals without vitamin D deficiency and secondary hyperthyroidism) exhibit a low rate of bone remodeling, as well as a decrease in osteoblast number and bone formation, a phenotype reminiscent also of the osteoporosis associated with glucocorticoid excess. In fact, work, from our group and others in several animal models, has strongly suggested that decreased wall width, the hallmark of age-associated osteoporosis and decreased bone formation results from decreased osteoblastogenesis (13, 17). If both aging and loss of sex steroids exert their adverse effects on bone by oxidative damage, how can osteoblast and osteoclast number be low in the former and high in the latter?
In studies reported in the accompanying manuscript by Almeida et al. (82) we have determined that ROS antagonize the skeletal effects of Wnt/β-catenin in vitro by diverting β-catenin from Tcf- to FoxO-mediated transcription. Moreover, consistent with the notion that increased ROS production with age attenuates Wnt/β-catenin signaling, Axin2 and Opg mRNA were decreased in old as compared with young C57BL/6 mice. Because activation of Wnt signaling enables osteoblastogenesis, suppresses osteoblastic cell apoptosis, inhibits osteoclastogenesis and increases bone mass, the cellular changes seen with increased aging and the accompanying oxidative damage may be due in part to attenuation of Wnt signaling.
In work reported elsewhere (69, 70), we have found that the estrogen-liganded ERα inhibits BMP-2-induced osteoblast progenitor differentiation, whereas the unliganded ERα or ERβ, but not the AR, promote the pro-differentiating actions of BMP-2 by enhancing Smad-mediated transcription. In addition, we have developed methods of expanding the self-renewal of mesenchymal stem cell (MSC) progenitors of osteoblasts, while preserving stemness, and found that their replication capacity is significantly lower in old versus young mice (71).
Based on our own findings and parallel developments in the understanding of the role of FoxOs in hematopoietic stem cell resistance to physiologic oxidative stress (72) we hypothesize that estrogens influence osteoblastogenesis by two distinct but overlapping cell autonomous mechanisms: (a) antioxidant effects on MSC osteoblast progenitors and (b) transcriptional repression of BMP-induced differentiation of their progeny. Increased levels of ROS have deleterious effects on MSC self-renewal (by increasing apoptosis), but promote MSCs entry into the cell cycle and terminal differentiation. These effects are counteracted by ROS-enhanced binding ofFOXOtoβ-catenin, leading to increased defense against ROS. Because of their ability to defend against ROS, estrogens favor MSC quiescence and survival and oppose terminal differentiation. Following acute loss of estrogens, the increased ROS levels in MSCs promote their exit from quiescence, replication, and development of transit amplifying osteoblast progenitors. These mechanisms, together with the unleashing of the transcriptional restraint of the liganded ERα on BMP, lead to up-regulation of osteoblast differentiation and thereby increased bone formation. However, this effect is reversed with time because the gradual buildup of ROS production with aging (accelerated by estrogen deficiency) antagonizes Wnt signaling and also leads to a decrease in the size of the MSC compartment by increasing MSC apoptosis.
In addition, we have found that as compared with 4-month-old mice, 25- and 31-month-old mice exhibited a 2-fold increase in serum corticosterone, adrenal weight, and bone mRNA for 11β-Hsd1, the enzyme that amplifies glucocorticoid action.5 The age-dependent decrease in bone formation rate and increased osteoblast and osteocyte apoptosis, cardinal features of the adverse effects of glucocorticoid excess on bone, along with the increase in 11β-Hsd1 mRNA, suggest that local amplification of endogenous glucocorticoids may also contribute to decreased bone formation, the decline in bone strength, and the disparity between bone quantity and quality with aging. Additional mechanism33s could well include increased production of peroxisome proliferator-activated receptor γ-activating oxidized lipids derived from the lipoxygenase Alox15 (55, 73) and the skeletal unloading resulting from reduced physical activity with old age (74). Hence, the effects of aging must override the effects of sex steroid deficiency by several mechanisms, some of which result directly from increased oxidative stress. In agreement with this contention we have shown before that the expected changes in bone cell progenitor numbers, histomorphometry, and BMD that occur after orchidectomy were either absent or greatly attenuated in a mouse model of defective osteoblastogenesis or in Swiss Webster mice treated simultaneously with glucocorticoids (17, 18).
Although much has been learned about age-related bone loss, it remains unknown whether its mechanisms are the same in all aging persons, but recent evidence indicates that the cellular and molecular mechanisms responsible may vary from subject to subject (75–77). One reason for such variation could be that the increase in responsiveness differs among individuals, so that some would lose more bone than others at, for example, the same glucocorticoid level. In closing, we submit that the results presented in this report constitute a major paradigm shift in the understanding of the pathogenesis of osteoporosis from the “estrogen-centric” view that has dominated the last 50 years to a multifactorial one, in which oxidative damage is a pivotal mechanism for females and males alike. Appreciation of the facts that loss of bone mass and strength occur with aging irrespective of sex steroid loss in animals and humans, and that many other factors contribute to the pathogenesis of skeletal involution, necessitates reappraisal of current ideas about the timing of initiation of prevention strategies, meaningful re-classification of osteoporosis based on the prevailing pathogenetic factor(s), and perhaps even the need for individualization of treatment with future pathogenetic factor-tailored therapies.
Acknowledgments
We thank B. L. Riggs, J. Potts, Jr., and R. Bouillon for critical review of the manuscript prior to submission; A. Warren, V. Lowe, R. Shelton, K. Vyas, T. Chambers, S. Berryhill, C. Wicker III, E. Hogan, R. Wynne, J. Crawford, and W. Webb for technical assistance; and R. I. DeWall for assistance in the preparation of the manuscript.
Footnotes
This work was supported by National Institutes of Health Grants P01 AG13918, R01 AR51187, R01 AR49794, and R01 AR46191, Department of Veterans Affairs merit review grants (to R. S. W., R. L. J., and S. C. M.) and a research enhancement award program; and tobacco settlement funds provided by the University of Arkansas for Medical Sciences.
B. L. Riggs, L. J. Melton III, A. L. Oberg, E. K. Atkinson, and S. Khosla personal communication.
The abbreviations used are: BMD, bone mineral density; AFU, arbitrary fluorescence units/mg of protein; BSO, l-buthionine-(S,R)-sulfoximine; DEM, diethyl maleate; DHT, dihydrotestosterone; E2, estradiol; ER, estrogen receptor; NAC, N-acetyl-l-cysteine; ORX, orchidectomy; OVX, ovariectomy; ROS, reactive oxygen species; TNF, tumor necrosis factor; N, newton; ERK, extracellular signal-regulated kinase; AR, androgen receptor; ANOVA, analysis of variance; MSC, mesenchymal stem cell; FOXO, Forkhead box O; MEK, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase.
R. S. Weinstein, R. L. Jilka, and S. C. Manolagas unpublished data.
REFERENCES
- 1.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay R. Osteoporosis Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 2.Hui SL, Slemenda CW, Johnston CC., Jr J. Clin. Investig. 1988;81:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM. Calcif. Tissue Int. 1985;37:594–597. doi: 10.1007/BF02554913. [DOI] [PubMed] [Google Scholar]
- 4.Burr DB, Turner CH, Naick P, Forwood MR, Ambrosius W, Hasan MS, Pidaparti R. J. Biomech. 1998;31:337–345. doi: 10.1016/s0021-9290(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 5.Heaney RP. Bone. 2003;33:457–465. doi: 10.1016/s8756-3282(03)00236-9. [DOI] [PubMed] [Google Scholar]
- 6.Duan Y, Parfitt A, Seeman E. J. Bone Miner. Res. 1999;14:1796–1802. doi: 10.1359/jbmr.1999.14.10.1796. [DOI] [PubMed] [Google Scholar]
- 7.Silva MJ, Gibson LJ. Bone. 1997;21:191–199. doi: 10.1016/s8756-3282(97)00100-2. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS. Endocrinology. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- 9.Qiu S, Rao DS, Palnitkar S, Parfitt AM. J. Bone Miner. Res. 2003;18:1657–1663. doi: 10.1359/jbmr.2003.18.9.1657. [DOI] [PubMed] [Google Scholar]
- 10.Tomkinson A, Reeve J, Shaw RW, Noble BS. J. Clin. Endocrinol. Metab. 1997;82:3128–3135. doi: 10.1210/jcem.82.9.4200. [DOI] [PubMed] [Google Scholar]
- 11.Tomkinson A, Gevers EF, Wit JM, Reeve J, Noble BS. J. Bone Miner. Res. 1998;13:1243–1250. doi: 10.1359/jbmr.1998.13.8.1243. [DOI] [PubMed] [Google Scholar]
- 12.Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han K, DiGregorio G, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 13.Manolagas SC. Endocr. Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 14.Manolagas SC, Kousteni S, Jilka RL. Recent Prog. Horm. Res. 2002;57:385–409. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- 15.Manolagas SC, Kousteni S, Chen JR, Schuller M, Plotkin L, Bellido T. Kidney Int. Suppl. 2004;91:S41–S49. doi: 10.1111/j.1523-1755.2004.09107.x. [DOI] [PubMed] [Google Scholar]
- 16.Jilka RL, Takahashi K, Munshi M, Williams DC, Roberson PK, Manolagas SC. J. Clin. Investig. 1998;101:1942–1950. doi: 10.1172/JCI1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jilka RL, Weinstein RS, Takahashi K, Parfitt AM, Manolagas SC. J. Clin. Investig. 1996;97:1732–1740. doi: 10.1172/JCI118600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinstein RS, Jia D, Powers CC, Stewart SA, Jilka RL, Parfitt AM, Manolagas SC. Endocrinology. 2004;145:1980–1987. doi: 10.1210/en.2003-1133. [DOI] [PubMed] [Google Scholar]
- 19.Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O’Brien CA, Economides AN, Stahl N, Jilka RL, Manolagas SC. J. Bone Miner. Res. 2000;15:663–673. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- 20.Manolagas SC, Jilka RL, Bellido T, O’Brien CA, Parfitt AM. In: Principles of Bone Biology. Bilezikian JP, Raisz LG, Rodan GA, editors. San Diego: Academic Press; 1996. [Google Scholar]
- 21.Quarrie JK, Riabowol KT. Sci. Aging Knowledge Environ. 2004;2004:re5. doi: 10.1126/sageke.2004.31.re5. [DOI] [PubMed] [Google Scholar]
- 22.Finkel T, Holbrook NJ. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 23.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 24.Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E, Milia E, Padura IM, Raker VA, Maccarana M, Petronilli V, Minucci S, Bernardi P, Lanfrancone L, Pelicci PG. Oncogene. 2002;21:3872–3878. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 25.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Hee PS, Thompson T, Karsenty G, Bradley A, Donehower LA. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 27.Dickinson DA, Forman HJ. Ann. N. Y. Acad. Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Frenkel K, Klein CB, Costa M. Toxicol. Appl. Pharmacol. 1993;120:29–36. doi: 10.1006/taap.1993.1083. [DOI] [PubMed] [Google Scholar]
- 29.Kousteni S, Chen J-R, Bellido T, Han L, Ali AA, O’Brien C, Plotkin LI, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfit AM, Weinstein RS, Jilka RL, Manolagas SC. Science. 2002;298:843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. J. Clin. Investig. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinstein RS, Chen JR, Powers CC, Stewart SA, Landes RD, Bellido T, Jilka RL, Parfitt AM, Manolagas SC. J. Clin. Investig. 2002;109:1041–1048. doi: 10.1172/JCI14538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kousteni S, Han L, Chen J-R, Almeida M, Plotkin LI, Bellido T, Manolagas SC. J. Clin. Investig. 2003;111:1651–1664. doi: 10.1172/JCI17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL. J. Cell Biochem. 1999;74:357–371. [PubMed] [Google Scholar]
- 34.Netter J, Wasserman W, Kutner MH. Applied Linear Statistical Models. 3rd ed. Homewood, IL: Richard D. Irwin, Inc.; 1990. [Google Scholar]
- 35.Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. N. Engl. J. Med. 2003;349:327–334. doi: 10.1056/NEJMoa022464. [DOI] [PubMed] [Google Scholar]
- 36.Duan Y, Beck TJ, Wang XF, Seeman E. J. Bone Miner. Res. 2003;18:1766–1774. doi: 10.1359/jbmr.2003.18.10.1766. [DOI] [PubMed] [Google Scholar]
- 37.Mobbs CV, Cheyney D, Sinha YN, Finch CE. Endocrinology. 1985;116:813–820. doi: 10.1210/endo-116-2-813. [DOI] [PubMed] [Google Scholar]
- 38.Nelson JF, Latham KR, Finch CE. Acta Endocrinol. 1975;80:744–752. doi: 10.1530/acta.0.0800744. [DOI] [PubMed] [Google Scholar]
- 39.Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ. J. Clin. Investig. 2003;112:915–923. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, van Steeg H, Weeda G, van der Horst GT, van Leeuwen W, Themmen AP, Meradji M, Hoeijmakers JH. Science. 2002;296:1276–1279. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- 41.Jagger CJ, Lean JM, Davies JT, Chambers TJ. Endocrinology. 2005;146:113–118. doi: 10.1210/en.2004-1058. [DOI] [PubMed] [Google Scholar]
- 42.Moor AN, Gottipati S, Mallet RT, Sun J, Giblin FJ, Roque R, Cammarata PR. Exp. Eye Res. 2004;78:933–944. doi: 10.1016/j.exer.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Chiang K, Parthasarathy S, Santanam N. Life Sci. 2004;75:2425–2438. doi: 10.1016/j.lfs.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 44.Darblade B, Pendaries C, Krust A, Dupont S, Fouque MJ, Rami J, Chambon P, Bayard F, Arnal JF. Circ. Res. 2002;90:413–419. doi: 10.1161/hh0402.105096. [DOI] [PubMed] [Google Scholar]
- 45.Sack MN, Rader DJ, Cannon RO., III Lancet. 1994;343:269–270. doi: 10.1016/s0140-6736(94)91117-7. [DOI] [PubMed] [Google Scholar]
- 46.Quintanilla RA, Munoz FJ, Metcalfe MJ, Hitschfeld M, Olivares G, Godoy JA, Inestrosa NC. J. Biol. Chem. 2005;280:11615–11625. doi: 10.1074/jbc.M411936200. [DOI] [PubMed] [Google Scholar]
- 47.Sudoh N, Toba K, Akishita M, Ako J, Hashimoto M, Iijima K, Kim S, Liang YQ, Ohike Y, Watanabe T, Yamazaki I, Yoshizumi M, Eto M, Ouchi Y. Circulation. 2001;103:724–729. doi: 10.1161/01.cir.103.5.724. [DOI] [PubMed] [Google Scholar]
- 48.Arnal JF, Clamens S, Pechet C, Negre-Salvayre A, Allera C, Girolami JP, Salvayre R, Bayard F. Proc. Natl. Acad. Sci. U. S. A. 1996;93:4108–4113. doi: 10.1073/pnas.93.9.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawada H, Ibi M, Kihara T, Urushitani M, Honda K, Nakanishi M, Akaike A, Shimohama S. FASEB J. 2000;14:1202–1214. doi: 10.1096/fasebj.14.9.1202. [DOI] [PubMed] [Google Scholar]
- 50.Chambliss KL, Simon L, Yuhanna IS, Mineo C, Shaul PW. Mol. Endocrinol. 2005;19:277–289. doi: 10.1210/me.2004-0008. [DOI] [PubMed] [Google Scholar]
- 51.Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17126–17131. doi: 10.1073/pnas.0407492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baba T, Shimizu T, Suzuki YI, Ogawara M, Isono KI, Koseki H, Kurosawa H, Shirasawa T. J. Biol. Chem. 2005;280:16417–16426. doi: 10.1074/jbc.M500924200. [DOI] [PubMed] [Google Scholar]
- 53.Lapointe J, Kimmins S, Maclaren LA, Bilodeau JF. Endocrinology. 2005;146:2583–2592. doi: 10.1210/en.2004-1373. [DOI] [PubMed] [Google Scholar]
- 54.Yen CH, Hsieh CC, Chou SY, Lau YT. Life Sci. 2001;70:403–413. doi: 10.1016/s0024-3205(01)01486-2. [DOI] [PubMed] [Google Scholar]
- 55.Klein RF, Allard J, Avnur Z, Nikolcheva T, Rotstein D, Carlos AS, Shea M, Waters RV, Belknap JK, Peltz G, Orwoll ES. Science. 2004;303:229–232. doi: 10.1126/science.1090985. [DOI] [PubMed] [Google Scholar]
- 56.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. J. Clin. Endo-crinol. Metab. 2004;89:4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 57.Hurh YJ, Chen ZH, Na HK, Han SY, Surh YJ. J. Toxicol. Environ. Health Part A. 2004;67:1939–1953. doi: 10.1080/15287390490514598. [DOI] [PubMed] [Google Scholar]
- 58.Deroo BJ, Hewitt SC, Peddada SD, Korach KS. Endocrinology. 2004;145:5485–5492. doi: 10.1210/en.2004-0471. [DOI] [PubMed] [Google Scholar]
- 59.Mishra DP, Shaha C. J. Biol. Chem. 2005;280:6181–6196. doi: 10.1074/jbc.M405970200. [DOI] [PubMed] [Google Scholar]
- 60.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, III, Berger M. J. Bone Miner. Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 61.Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix AZ, Black DM. Am. J. Med. 2002;112:281–289. doi: 10.1016/s0002-9343(01)01124-x. [DOI] [PubMed] [Google Scholar]
- 62.Li X, Masinde G, Gu W, Wergedal J, Mohan S, Baylink DJ. Genomics. 2002;79:734–740. doi: 10.1006/geno.2002.6760. [DOI] [PubMed] [Google Scholar]
- 63.Wergedal JE, Sheng MH, Ackert-Bicknell CL, Beamer WG, Baylink DJ. Bone. 2005;36:111–122. doi: 10.1016/j.bone.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 64.Manolagas SC. BoneKey-Osteovision. 2006;3:5–14. [Google Scholar]
- 65.Nelson JF, Felicio LS, Osterburg HH, Finch CE. Endocrinology. 1992;130:805–810. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- 66.Lacombe A, Lelievre V, Roselli CE, Salameh W, Lue YH, Lawson G, Muller JM, Waschek JA, Vilain E. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3793–3798. doi: 10.1073/pnas.0505827103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bellido T, Jilka RL, Boyce BF, Girasole G, Broxmeyer H, Dalrymple SA, Murray R, Manolagas SC. J. Clin. Investig. 1995;95:2886–2895. doi: 10.1172/JCI117995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vanderschueren D, Boonen S, Ederveen AG, de Coster R, Van Herck E, Moermans K, Vandenput L, Verstuyf A, Bouillon R. Bone. 2000;27:611–617. doi: 10.1016/s8756-3282(00)00363-x. [DOI] [PubMed] [Google Scholar]
- 69.Kousteni S, Almeida M, Han L, Bellido T, Jilka RL, Manolagas SC. Mol. Cell Biol. 2007;27:1516–1530. doi: 10.1128/MCB.01550-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Almeida M, Chen X, Han L, Martin-Millan M, Lowe V, Warren A, Stewart SA, Kousteni S, Weinstein RS, O’Brien CA, Bellido T, Jilka RL, Manolagas SC. J. Bone Miner. Res. 2006;21:S95. [Google Scholar]
- 71.Chen X-D, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. J. Bone Miner. Res. 2007 doi: 10.1359/jbmr.070725. in press. [DOI] [PubMed] [Google Scholar]
- 72.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. Cell. 2007;128:332–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. J. Clin. Investig. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T. J. Bone Miner. Res. 2006;21:605–615. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- 75.Smith RG, Betancourt L, Sun Y. Endocr. Rev. 2005;26:203–250. doi: 10.1210/er.2002-0017. [DOI] [PubMed] [Google Scholar]
- 76.Sapolsky RM, Romero LM, Munck AU. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 77.Seeman TE, Robbins RJ. Endocr. Rev. 1994;15:233–260. doi: 10.1210/edrv-15-2-233. [DOI] [PubMed] [Google Scholar]
- 78.Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ. Biochem. Biophys. Res. Commun. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 79.Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou ZP, Zeng WS, Cheng BL, Luo SQ. J. Biol. Chem. 2005;280:17497–17506. doi: 10.1074/jbc.M409332200. [DOI] [PubMed] [Google Scholar]
- 80.Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ. Endocrinology. 2005;146:728–735. doi: 10.1210/en.2004-1021. [DOI] [PubMed] [Google Scholar]
- 81.Chen JR, Plotkin LI, Aguirre JI, Han L, Jilka RL, Kousteni S, Bellido T, Manolagas SC. J. Biol. Chem. 2005;28:4632–4638. doi: 10.1074/jbc.M411530200. [DOI] [PubMed] [Google Scholar]
- 82.Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC. J. Biol. Chem. 2007;280:27298–27305. doi: 10.1074/jbc.M702811200. [DOI] [PubMed] [Google Scholar]