Abstract
The Rec12 (Spo11) protein of the fission yeast Schizosaccharomyces pombe is a meiosis-specific ortholog of the catalytic subunit of type VI topoisomerases and is thought to catalyze double-strand DNA breaks that initiate recombination. We tested the hypothesis that the rec12-117 allele affects the choice of pathways by which recombination is resolved. DNA sequence analysis revealed a single missense mutation in the coding region (rec12-G202E). The corresponding glycine-202 residue of Rec12 protein is strictly conserved in proteins of the Rec12/Spo11/Top6A family. It maps to the base of the DNA binding pocket in the crystal structure of the archaeal ortholog, Top6A. The rec12-G202E mutants lacked crossover and non-crossover recombination, demonstrating that rec12-G202E does not affect choice of resolution pathway. Like rec12-D15 null mutants, the rec12-G202E mutants suffered chromosome segregation errors in meiosis I. The Rec12-G202E protein was as stable as wild-type Rec12, demonstrating that glycine-202 is essential for a biochemical activity of Rec12 protein, rather than for its stability. These findings suggest that Rec12 facilitates binding of the meiotic recombinase to its substrate, DNA. Interestingly, the bulk of Rec12 protein persisted until the time of anaphase I, and a portion of Rec12 protein persisted until the time of anaphase II, after which it was undetectable. This suggests that Rec12 protein has additional meiotic functions after completion of recombination in prophase, as inferred previously from genetic studies.
Keywords: Homologous recombination, Genetic recombination, Meiosis, Fission yeast, Schizosaccharomyces pombe, Topoisomerase
1. Introduction
In mitotic cells a single unrepaired dsDNA break can be lethal—homologous recombination is used to repair such DNA damage (Resnick and Martin, 1976). Paradoxically, in meiosis cells intentionally introduce dsDNA breaks into their chromosomes to induce the rate of homologous recombination about three orders of magnitude, relative to the rate in mitotic cells (reviewed in Lichten, 2001). Meiotic recombination increases genetic diversity and produces crossovers which function cooperatively with sister chromatid cohesion to ensure the faithful segregation of chromosomes in the first meiotic division (Krawchuk et al., 1999; Buonomo et al., 2000; Kitajima et al., 2004; Rabitsch et al., 2004).
The fission yeast Rec12 protein (Spo11 in budding yeast) is a meiosis-specific ortholog of the catalytic subunit (Top6A) of a type VI topoisomerase (Bergerat et al., 1997; Keeney et al., 1997; Sharif et al., 2002) and is thought to catalyze the formation of dsDNA breaks that initiate recombination (reviewed in Keeney, 2001). The breaks are processed to form 3VssDNA tails which initiate recombinational repair from a homologous chromatid (Sun et al., 1989; Cao et al., 1990; Schwacha and Kleckner, 1995). These early intermediates are partitioned into different resolution pathways to yield crossover and non-crossover recombinant chromosomes (Paques and Haber, 1999; Allers and Lichten, 2001; Hunter and Kleckner, 2001; Osman et al., 2003; Borner et al., 2004).
Although it is not clear precisely at what points the pathway of meiotic recombination bifurcates, in budding yeast the partitioning of intermediates into crossover and non-crossover resolution pathways can occur very early, before strand exchange and chromosome synapsis (reviewed in Bishop and Zickler, 2004; Liu and West, 2004). Rec12 (Spo11) protein itself may have a role in the choice of which resolution pathway is used: In budding yeast Spo11 affects resection from double-strand DNA breaks and the length of resulting 3′ single-stranded DNA ends, which may influence subsequent pathway choice (Neale et al., 2002). In fission yeast rec12-D15 (null) mutants lack both crossover and non-crossover recombination (Sharif et al., 2002), but the rec12-117 allele was hypothesized to affect differentially (by a factor of ≥25) the choice of recombination pathways (DeVeaux et al., 1992). We sought to test this hypothesis and to reveal the nature of the rec12-117 allele. We report that the rec12-117 mutants produce Rec12 protein with a single amino acid substitution (G202E) in a DNA binding motif; and that the conserved glycine-202 is essential for both crossover and non-crossover recombination and for proper segregation of homologous chromosomes in the first meiotic division. We also report that Rec12 protein persists well beyond the stage at which recombination is completed, suggesting that Rec12 has additional roles in later meiotic processes.
2. Materials and methods
2.1. Schizosaccharomyces pombe culture and genetic methods
Strain genotypes are listed in Table 1. Culture media and genetic methods were as described (Gutz et al., 1974; Kohli et al., 1977; Krawchuk et al., 1999). Media were supplemented as necessary with growth factors (amino acids, bases) at 100 μg/ml. Methods for mating, meiosis, and determination of recombinant frequencies were as described (Wahls and Smith, 1994; Kon et al., 1997). The identification and analysis of disomic and diploid spore colonies were as previously described (Krawchuk et al., 1999; Molnar et al., 2001).
Table 1.
Genotypes of S. pombe strains used in this study
| Strain | Genotype |
|---|---|
| WSP 0005 | h+ ade6-M216 |
| WSP 0016 | h– ade6-M210 |
| WSP 0346 | h+ ade6-M375 rec12-G202E |
| WSP 0578 | h+ ade6-M375 his3-D1 ura4-D18 leu1-32 |
| WSP 1237 | h+ ade6-M216 rec12-G202E |
| WSP 1804 | h– ade6-M210 rec12-G202E |
| WSP 1807 | h+ lys1-131 rec12-G202E |
| WSP 1820 | h+ ade6-M216 ura4-D18 rec12-D15::ura4+ |
| WSP 1828 | h– ade6-M210 ura4-D18 rec12-D15::ura4+ |
| WSP 1829 | h+ ade6-M375 ura-D18 rec12-D15::ura4+ |
| WSP 2142 | h+ ade6-52 rec12-G202E |
| WSP 2144 | h– arg1-14 rec12-G202E |
| WSP 2148 | h+ his4-329 rec12-G202E |
| WSP 2149 | h– ade4-31 rec12-G202E |
| WSP 2151 | h+ ade2-17 tps19-17 rec12-G202E |
| WSP 2154 | h– his1-102 rec12-G202E |
| WSP 2217 | h– ade1-51 rec12-G202E |
| WSP 2364 | h– ade6-M26 ura4-D18 his3-D1 leu1-32 rec12-Y98F (pREP41-NTAP-rec12+) |
| WSP 2365 | h+ ade6-M210 ura4-D18 his3-D1 leu1-32 rec12-Y98F (pREP41-NTAP-rec12+) |
| WSP 2415 | h– ade6-M26 ura4-D18 his3-D1 leu1-32 rec12-Y98F (pREP41-NTAP) |
| WSP 2419 | h+ ade6-M210 ura4-D18 his3-D1 leu1-32 rec12-Y98F (pREP41-NTAP) |
| WSP 2459 | h+ ade6-M26 pat1-114 rad50S rec12-D15 leu1-32 (pREP41-NTAP) |
| WSP 2462 | h+ ade6-M26 pat1-114 rad50S rec12-D15 leu1-32 (pREP41-NTAP-rec12+) |
| WSP 2468 | h+ ade6-M26 pat1-114 rad50S rec12-D15 leu1-32 (pREP41-NTAP-rec12-G202E) |
2.2. Construction and regulation of pREP41-NTAP-Rec12 vectors
The cloning of the rec12+ cDNA was previously described (Sharif et al., 2002; Wu et al., 2004). Quick-Change® site-directed PCR mutagenesis, conducted according to the instructions of the manufacturer (Stratagene), was used to recapitulate the rec12-G202E mutation in the cDNA. Candidate clones were sequenced to confirm the presence of the desired mutation and to eliminate clones with spurious mutations. The rec12+ and rec12-G202E cDNA fragments were excised by digestion with NdeI and BamHI and subcloned into the expression vector pREP41-NTAP (Gould et al., 2004). The plasmids were transformed into S. pombe leu1-32 strains and maintained by selection for leucine prototrophy on minimal medium in the presence of thiamine (2 μM). Induction of gene expression from the nmt (no message in thiamine) promoter was achieved by culturing cells in the absence of thiamine, as described (Maundrell, 1993).
2.3. Preparation and analysis of proteins in meiosis
Induction of synchronous meiosis by thermal inactivation of Pat1-114ts protein (Iino and Yamamoto, 1985) was as described (Wahls and Smith, 1994). Whole-cell protein extracts were prepared by mechanical disruption as described (Wahls et al., 1993) using a lysis buffer that contained 10 mM sodium phosphate (pH 7.5), 1% NP-40, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, 0.1 mM Na3VO4, 1 mM dithiothreitol, 20% glycerol, and 1× protease inhibitors. Protease inhibitors were 1 μg/ml bestatin, 5 μg/ml pepstatin, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 40 μg/ml TPCK, 1 mM benzamidine, and protease inhibitor cocktail (P8215, Sigma). Total cellular lysates (50 μg/lane) were fractionated on SDS–10% polyacrylamide gels and proteins were transferred to Hybond-ECL membrane according to instructions of the manufacturer (Amersham Pharmacia Biotech). The TAP epitope was detected using rabbit IgG conjugated to horseradish peroxidase (Jackson Immunoresearch Laboratory, Inc.) at a titer of 1:1000. The blots were stripped and developed using rabbit polyclonal α-Cdc2 antibodies (Upstate Biotechnology) at a titer of 1:1000, followed by secondary detection with goat α-rabbit IgG conjugated to horseradish peroxidase (Jackson Immunoresearch Laboratory, Inc.) at a titer of 1:10,000.
3. Results
3.1. Glycine-202 of Rec12 resides in a potential DNA binding pocket
Rec12 and its active site tyrosine (Y98) are essential for meiotic recombination (Sharif et al., 2002). The rec12-117 allele was of particular interest because it was hypothesized to differentially affect crossover and non-crossover recombination (DeVeaux et al., 1992). However, the nature of the rec12-117 allele had not been reported.
We sequenced the DNA from the mutant locus and flanking regions and compared that sequence to the sequence of wild-type rec12+. A single base-pair substitution was detected in exon 5 and, upon translation, this missense mutation would replace glycine at residue 202 with glutamate (Fig. 1A,B). For clarity, we renamed the allele rec12-G202E. Although no structural information is available for any eukaryotic Rec12/Spo11 protein, the structure of an archaeal ortholog, Top6A of Methanococcus jannaschii, has been solved (Nichols et al., 1999). The residue corresponding to Rec12-G202 is invariant (i.e., strictly conserved) in members of the Rec12/Spo11/Top6A family (Fig. 1B). This nonpolar residue maps to the base of a DNA binding pocket in between a stretch of β-sheet and α-helix (Fig. 1C,D).
Fig. 1.
Position of Rec12-G202E missense mutation. (A) Sequence of Rec12 protein showing positions of Y98 and G202 (*); residues conserved among Rec12/Spo11/Top6A family members are shown in black, conservative substitutions are highlighted in gray and open boxes. (B) DNA and protein sequences of rec12+ and rec12-G202E flanking the position of the single missense mutation (italic) identified in the rec12-G202E locus. (C) Orthogonal projection of a homodimer of Top6A from Methanococcus jannaschii (Nichols et al., 1999) prepared using Cn3D version 4.0 (National Center for Biotechnology Information). DNA molecules (not shown) are modeled to project on the Z-axis through the DNA binding pocket. Residues corresponding to G202E of Rec12 are highlighted (yellow) (D) Detail of relative position of G202E of S. pombe Rec12 (yellow). Shown are residues located within 11Å of the corresponding residue in Top6A (glycine-221), which is conserved among Rec12/Spo11/Top6A family members. Amino acids with basic side chains, which may interact with the phosphate backbone of DNA, are shown in blue. Replacement of nonpolar glycine with negatively charged glutamate may disrupt protein–DNA hydrogen bond interactions or destabilize structure within the DNA binding pocket. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Because the rec12-G202E mutants encode a full-length Rec12 protein with a single amino acid substitution, it was plausible that rec12-G202E could be a separation-of-function allele. The rec12-G202E and rec12-D15 (null) mutants were equally proficient for mating, meiotic progression, spore formation, and spore viability (~40%) (data not shown; Sharif et al., 2002). Thus, rec12-G202 is not a separation-of-function allele for those processes. The relatively high frequency of viable spores allowed us to determined the effects of rec12-G202E upon crossover and non-crossover recombination and to compare the results directly to those of the rec12-D15 (null) mutants.
3.2. Glycine-202 of Rec12 is essential for crossover and non-crossover recombination
To study crossover recombination in rec12-G202E mutants, we scored intergenic recombinant frequencies. Multiple genetic intervals encompassing more than 400 cM of the genome were analyzed. These intervals were located on all three chromosomes to address the possibility of chromosome specificity; in centromere-proximal, medial, and distal regions to address the possibility of regional specificity; and in multiple contiguous intervals on the same chromosome to address the possibility of interval-to-interval variability. In the rec12-G202E mutants crossover recombination was reduced an average of 585-fold, relative to wild-type levels (Table 2). The defect in recombination extended uniformly to all intervals tested on all three chromosomes and it was as severe as the defect in rec12-D15 (null) and rec12-Y98F (active site) mutants (Sharif et al., 2002). We conclude that glycine-202 of Rec12 is essential for crossover recombination.
Table 2.
Frequencies of intergenic (crossover) recombination at multiple intervals in rec12-G202E mutants
| Strains crossed | Genetic interval (chromosome) | No. of testeda | No. of recombinanta | Recombinant freq. (%) | cMb | cM in wild-typec | Fold reductionc |
|---|---|---|---|---|---|---|---|
| WSP 1807 × WSP 2154 | lys1 – his1 (I) | 500 | 1 | 0.2 | 0.2 | 89 | 445 |
| WSP 1807 × WSP 2149 | lys1 – ade4 (I) | 500 | 1 | 0.2 | 0.2 | 284 | 1420 |
| WSP 2151 × WSP 2154 | his1 – ade2 (I) | 500 | 0 | ≤ 0.2 | ≤ 0.2 | 57 | ≥ 285 |
| WSP 2151 × WSP 2154 | his1 – tps19 (I) | 500 | 0 | ≤ 0.2 | ≤ 0.2 | 143 | ≥ 715 |
| WSP 2151 × WSP 2154 | ade2 – tps19 (I) | 500 | 0 | ≤ 0.2 | ≤ 0.2 | 86 | ≥ 430 |
| WSP 2148 × WSP 2217 | his4 – ade1 (II) | 500 | 0 | ≤ 0.2 | ≤ 0.2 | 63 | ≥ 315 |
| WSP 2142 × WSP 2144 | ade6 – arg1 (III) | 500 | 0 | ≤ 0.2 | ≤ 0.2 | 97 | ≥ 485 |
| Mean | 585 ± 364 |
Spores were plated on non-selective media and spore colonies were subsequently genotyped. Diploid spore colonies, which could exhibit intergenic complementation for markers, were excluded from analysis.
Genetic distance calculated using the mapping function of Haldane (1919).
Fold reduction in crossing over relative to published map distances in wild-type cells (NCBI Entrez Genome) (Munz, 1994).
To determine the effects of the rec12-G202E mutation on gene conversion, we measured intragenic recombination between ade6-M375 and ade6-M210 alleles. At this locus, which has been characterized extensively, the frequency of adenine-prototrophic spore colonies is directly proportional to the frequency of gene conversion (Gutz, 1971; Schuchert et al., 1991; Grimm et al., 1994). Wild-type (rec12+) meioses produced an Ade+ recombinant frequency of 536 × 10–6 (Table 3). In rec12-G202E mutant crosses only 4 × 10–6 of the meiotic products were recombinant (Table 3). This value is statistically indistinguishable from that obtained in meioses that lacked Rec12 protein entirely (rec12-D15) (Table 3) or its active site tyrosine (Sharif et al., 2002). We conclude that glycine-202 of Rec12 is essential for both gene conversion and crossing over.
Table 3.
Effects of rec12-G202E upon intragenic (gene conversion) recombination at the ade6 locus
| Strains crossed | Genotype at rec12 locus | Recombinant frequency (× 106) |
|---|---|---|
| WSP0016 × WSP0578 | +/+ | 536 ± 113 |
| WSP1828 × WSP1829 | D15/D15 | 9.09 ± 3.42 |
| WSP1804 × WSP0346 | G202E/G202E | 4.06 ± 2.99 |
Spores were plated on nonselective and selective media to determine the viable titer and the adenine prototrophic titer, respectively. The recombinant frequency (RF) was calculated as [(recombinant titer)/(total viable titer)]. Data are mean ± standard deviation of RF values from three separate experiments.
3.3. The rec12-G202E mutants suffer mis-segregation of chromosomes in meiosis I
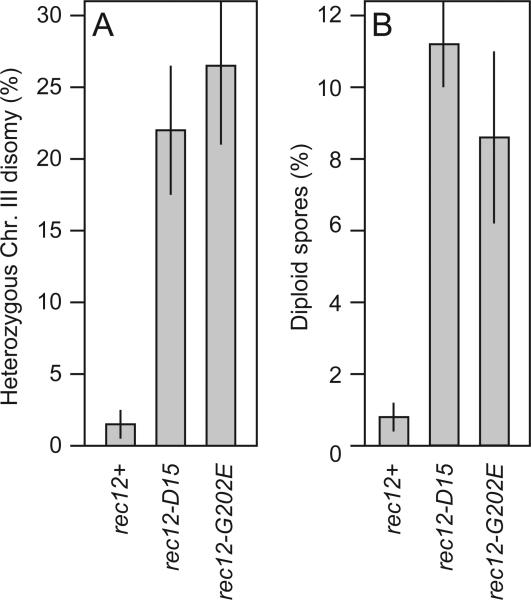
Because crossover recombination structures (chiasmata) help to stabilize paired homologous chromosomes on the metaphase plate of meiosis I (Roeder, 1997), achiasmatic mutants should suffer nondisjunction of homologous chromosomes in meiosis I. Fission yeast cells disomic for chromosome III are viable (Niwa and Yanagida, 1985). In diploids or pseudodiploids the ade6-M210 and ade6-M216 alleles exhibit intragenic complementation (which is quite rare), so heterozygous ade6-M210/ade6-M216 cells form colonies on medium lacking adenine (Gutz, 1971). We crossed strains harboring these markers, plated the spores, and used direct selection for adenine prototrophy to determine the frequency of heterozygous chromosome III disomy.
In wild-type rec12+ crosses, 1.5% of the viable meiotic products were prototrophic for adenine (Ade+) (Fig. 2A). In contrast, 26% of the viable meiotic products from rec12-G202E mutant crosses were Ade+ (Fig. 2A). These Ade+ frequency values are several orders of magnitude higher than the frequency of Ade+ colonies produced by recombination between ade6 alleles (e.g., Table 3). Thus, they indicate the frequency of spore colonies that are disomic and heterozygous for the ade6 markers. Because the ade6+ locus is linked to the centromere of chromo-some III, the heterozygous disomy demonstrates mis-segregation of chromosome III in meiosis I. As expected from the data on chromosome III disomy, the rec12-G202E mutants also produced an elevated frequency of diploid meiotic products (Fig. 2B). The frequencies of disomic and diploid spore colonies from rec12-G202E mutant meioses were similar to those for rec12 null mutants (Fig. 2A,B) (Sharif et al., 2002; Davis and Smith, 2003). We conclude that rec12-G202E mutants are functionally null for gene conversion, crossover recombination, and recombination-dependent chromosome segregation. We infer that the mutants either lack stable Rec12 protein or that glycine-202 is essential for some biochemical function of Rec12.
Fig. 2.
Consequences of aberrant chromosome segregation. (A) Frequency of chromosome III disomic spore colonies heterozygous for centromere-linked ade6-M216 and ade6-M210 alleles. Frequencies were determined by plating efficiency of spores on medium lacking adenine, relative to plating efficiency on medium containing adenine. (B) Frequency of diploid spore colonies. Diploids were scored by differential staining on medium containing phloxin-B. Data are mean±standard deviation of frequencies from three separate experiments.
3.4. Stability and turnover of Rec12-G202E protein is similar to that of wild-type Rec12 protein
The replacement of glycine-202 with glutamate might affect protein stability, so we raised polyclonal antisera in rabbits and chickens for Western blot analysis of protein stability. However, we could not detect Rec12 protein in Western blots of meiotic cell extracts using those antibodies (data not shown). We therefore tested whether Rec12 could be detected by virtue of an epitope tag. The rec12+ and rec12-G202E cDNAs were cloned into pREP plasmid vectors designed to express Rec12 as a fusion protein with a TAP epitope [Rigaut et al., 1999] on its amino terminus (NTAP-Rec12). The TAP epitope allowed us to use the high-affinity interaction between protein A of the TAP epitope and IgG as a basis for immunodetection.
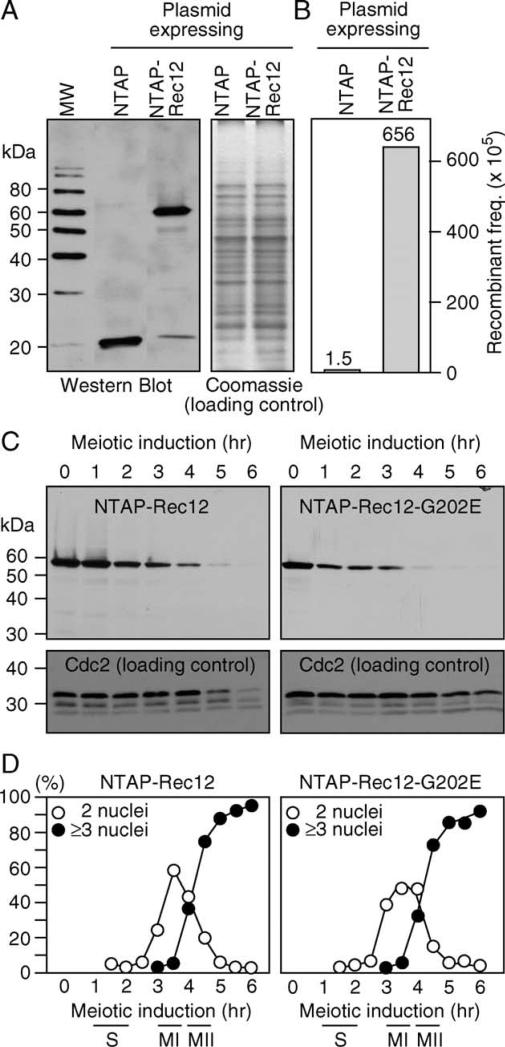
To determine whether NTAP-Rec12 protein was produced, we induced synchronous meioses and harvested the cells in late prophase. Protein extracts were prepared from meiotic cells harboring pREP41-TAP, which encodes the TAP epitope alone, and pREP41-NTAP-rec12+, which encodes a Rec12 protein with a TAP epitope fused to its amino terminus. Western blotting produced bands of the expected sizes (Fig. 3A), demonstrating that the pREP expression vector functions in meiotic cells and that the desired Rec12 fusion protein is produced.
Fig. 3.
Stability and turnover of Rec12+ and Rec12-G202E proteins in synchronous meioses. (A) Expression and detection of epitope-tagged Rec12 protein. Protein extracts were prepared from cells in late meiotic prophase (3 h). Fifty micrograms of total protein from meiotic cell extracts was loaded in each lane. Duplicate gels were stained with Coomassie (loading control) or were subject to Western blotting for presence of the TAP epitope. (B) Complementation of recombination defect by epitope-tagged protein. The frequency of intragenic recombination between ade6-M26 and ade6-M210 was determined in rec12 mutant cells harboring the indicated plasmids. (C) Stability and turnover of Rec12 proteins in meiosis. Protein extracts were prepared from cells at the indicated timepoints of synchronous meioses and subject to Western blotting to detect Rec12. Blots were stripped and reprobed with α-Cdc2 antibodies to serve as a loading control. (D) Timing of Rec12 turnover in synchronous meiosis. The same cultures used in panel “C” were examined by fluorescence microscopy with a DNA-specific stain (DAPI) to determine the timing of the two meiotic divisions. Note that Rec12 is partially degraded at meiosis I and some Rec12 protein persists until the time of the second meiotic division (panel “C”).
To determine whether the NTAP-Rec12 protein was biologically active, the pREP41-TAP and pREP41-NTAP-rec12+ plasmids were introduced into haploid rec12 mutants harboring the ade6-M26 and ade6-M210 heteroalleles. The strains were crossed and the Ade+ recombinant frequencies were determined. The pREP41-TAP construct (empty vector control) failed to complement the hyporecombination defect, whereas the pREP41-NTAP-rec12+ construct restored recombination (Fig. 3B). This demonstrates that the fusion protein was functional in vivo.
Having established that the pREP41-NTAP-rec12+ expresses functional protein, we used Western blotting to compared the steady state levels of NTAP-Rec12 and NTAP-Rec12-G202E proteins at sequential timepoints throughout synchronous meioses. At each timepoint, the NTAP-Rec12-G202E protein was as abundant as the wild-type NTAP-Rec12 protein, indicating that the G202E substitution does not affect the stability of Rec12 (Fig. 3C). The majority of Rec12 protein persisted until, and was degraded at, about the time of the first meiotic division. A smaller fraction of Rec12 protein was present until about the time of the second meiotic division, after which it was undetectable. These data suggest that Rec12 protein is subject to developmentally regulated degradation, and that Rec12 may have additional meiotic functions subsequent to catalysis of recombinogenic dsDNA breaks in meiotic prophase.
4. Discussion
Active site tyrosine-98 of Rec12 is essential for all meiotic recombination (Sharif et al., 2002). This study revealed a second residue of Rec12, glycine-202 that is also essential for recombination and that defines a putative DNA binding domain.
There are several different pathways by which recombination intermediates can be resolved and the decision on resolution choice can occur by the time of strand exchange and synapsis (reviewed by Bishop and Zickler, 2004). If pathway commitment occurs at the time when the meiotic recombinase catalyzes dsDNA breaks, certain non-null mutations of Rec12 (Spo11) may influence pathway choice. It has been hypothesized that the rec12-117 (rec12-G202E) allele differentially affects crossover and non-crossover recombination (DeVeaux et al., 1992). However, this study demonstrated that glycine-202 of Rec12 is essential for all recombination (Tables 2 and 3), so we reject the hypothesis that the rec12-G202E allele affects pathway choice.
The rec12-G202E mutants had phenotypes identical to those of Rec12 null mutants (Sharif et al., 2002; Davis and Smith, 2003). They were proficient for mating and meiosis, the viability frequency of spores was reduced about 60%, they lacked crossover and non-crossover recombination, and they experienced a high frequency of mis-segregation of chromosomes in meiosis I characteristic of achiasmatic mutants (Tables 2 and 3; Fig. 2). Decreased stability of the Rec12-G202E protein could account for the null phenotypes. However, we found that the Rec12-G202E protein is as stable as wild-type Rec12 (Fig. 3), which demonstrates that glycine-202 is essential for some biochemical activity of Rec12 protein, rather than for its stability.
A comparison of the amino acid sequence of Rec12 (Spo11) to the sequence and structure of Top6A (Nichols et al., 1999) allows one to identify specific residues of Rec12 (Spo11) with likely functions and to ascribe potential functions to specific residues altered by mutation (Diaz et al., 2002; Sharif et al., 2002). Glycine-202 of Rec12 is a nonpolar residue that maps to the base of a DNA binding pocket between a stretch of β-sheet and α-helix (Fig. 1C,D). Replacement of nonpolar glycine with acidic glutamate could repel the negatively charged phosphate backbone of DNA. Alternatively, glycine-202 may be required for a transition bend between the β-sheet and α-helix: Insertion of glutamate might destabilize the bend or alter local structure in such a way as to disrupt interactions with DNA. Site-directed mutagenesis of budding yeast Spo11 also suggests the presence of a DNA binding pocket required for function (Diaz et al., 2002). Substitutions at three residues reduced recombination more than 100-fold and one of these (Spo11-Y292=Rec12-H233) also maps to the face of the DNA pocket of Top6A, less than 3.4Å from the corresponding position of Rec12-G202. In addition, mutation of a phenylalanine adjacent to the position of Rec12-G202 (Spo11-F260=Rec12-F203) also reduced recombination as much as to 15-fold (Diaz et al., 2002). Thus in two widely diverged eukaryotes, budding yeast and fission yeast, a conserved region of Rec12 (Spo11) is implicated to interact with DNA in a fashion similar to that of the archaeal ortholog, topoisomerase subunit Top6A (Nichols et al., 1999).
Because Spo11 is removed from protein–DNA cleavage complexes after catalysis of dsDNA breaks (reviewed in Keeney, 2001), it is generally assumed that the protein is degraded at the time of its removal from DNA, before completion of recombination. However, mutants of Sordaria macrospora and S. pombe that lack Rec12 (Spo11) have clear defects at later stages of meiosis, including reduced ability to properly separate sister chromatids at anaphase of meiosis II (Sharif et al., 2002; Storlazzi et al., 2003). These phenotypes could be an indirect, secondary consequence of loss of recombination. Alternatively, Rec12 (Spo11) may have additional, more-direct functions in chiasmatic and distributive segregation at meiosis I and separation of sister chromatids at meiosis II (Sharif et al., 2002). We report here that Rec12 protein persists within meiotic cells well beyond the times at which meiotic recombination is initiated and completed, but it is degraded largely at anaphase I and completely at anaphase II, before completion of meiotic differentiation (Fig. 3C). Thus, Rec12 protein is subject to developmentally regulated turnover and is available to participate in meiotic processes subsequent to initiation of recombination.
Acknowledgments
We thank Audrey Stone for laboratory support. This work was supported by funds from the National Institutes of Health (GM62244). WDS was supported in part by a UNCF-Merck Graduate Science Research Dissertation Fellowship Award.
Abbreviations
- RF
recombinant frequency
- cM
centimorgan
- ss
single-stranded
- ds
double-stranded
References
- Allers T, Lichten M. Differential timing and control of noncross-over and crossover recombination during meiosis. Cell. 2001;106:47–57. doi: 10.1016/s0092-8674(01)00416-0. [DOI] [PubMed] [Google Scholar]
- Bergerat A, de Massy B, Gadelle D, Varoutas P-C, Nicolas A, Forterre P. An atypical topoisomerase II from archaea with implication for meiotic recombination. Nature. 1997;386:414–417. doi: 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- Bishop DK, Zickler D. Early decision; meiotic crossover interference prior to stable strand exchange and synapsis. Cell. 2004;117:9–15. doi: 10.1016/s0092-8674(04)00297-1. [DOI] [PubMed] [Google Scholar]
- Borner GV, Kleckner N, Hunter N. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell. 2004;117:29–45. doi: 10.1016/s0092-8674(04)00292-2. [DOI] [PubMed] [Google Scholar]
- Buonomo SB, Clyne RK, Fuchs J, Loidl J, Uhlmann F, Nasmyth K. Disjunction of homologous chromosomes in meiosis I depends on proteolytic cleavage of the meiotic cohesin Rec8 by separin. Cell. 2000;103:387–398. doi: 10.1016/s0092-8674(00)00131-8. [DOI] [PubMed] [Google Scholar]
- Cao L, Alani E, Kleckner N. A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell. 1990;61:1089–1101. doi: 10.1016/0092-8674(90)90072-m. [DOI] [PubMed] [Google Scholar]
- Davis L, Smith GR. Nonrandom homolog segregation at meiosis I in Schizosaccharomyces pombe mutants lacking recombination. Genetics. 2003;163:857–874. doi: 10.1093/genetics/163.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVeaux LC, Hoagland NA, Smith GR. Seventeen complementation groups of mutations decreasing meiotic recombination in Schizosaccharomyces pombe. Genetics. 1992;130:251–262. doi: 10.1093/genetics/130.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz RL, Alcid AD, Berger JM, Keeney S. Identification of residues in yeast Spo11p critical for meiotic DNA double-strand break formation. Mol. Cell. Biol. 2002;22:1106–1115. doi: 10.1128/MCB.22.4.1106-1115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KL, Ren L, Feoktistova AS, Jennings JL, Link AJ. Tandem affinity purification and identification of protein complex components. Methods. 2004;33:239–244. doi: 10.1016/j.ymeth.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Grimm C, Bahler J, Kohli J. M26 recombinational hotspot and physical conversion tract analysis in the ade6 gene of Schizosaccharomyces pombe. Genetics. 1994;136:41–51. doi: 10.1093/genetics/136.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutz H. Site specific induction of gene conversion in Schizosaccharomyces pombe. Genetics. 1971;69:331–337. doi: 10.1093/genetics/69.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutz H, Heslot H, Leupold U, Loprieno N. Schizosaccharomyces pombe. In: King RC, editor. Handbook of Genetics. Plenum Press; New York: 1974. pp. 395–446. [Google Scholar]
- Haldane JBS. The combination of linkage values, and the calculation of distances between loci of linked factors. J. Genet. 1919;8:299–309. [Google Scholar]
- Hunter N, Kleckner N. The single-end invasion: an asymmetric intermediate at the double-strand break to double-Holliday junction transition of meiotic recombination. Cell. 2001;106:59–70. doi: 10.1016/s0092-8674(01)00430-5. [DOI] [PubMed] [Google Scholar]
- Iino Y, Yamamoto M. Negative control for the initiation of meiosis in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. U. S. A. 1985;82:2447–2451. doi: 10.1073/pnas.82.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney S. Mechanism and control of meiotic recombination initiation. Curr. Top. Dev. Biol. 2001;52:1–53. doi: 10.1016/s0070-2153(01)52008-6. [DOI] [PubMed] [Google Scholar]
- Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- Kitajima TS, Kawashima SA, Watanabe Y. The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature. 2004;427:510–517. doi: 10.1038/nature02312. [DOI] [PubMed] [Google Scholar]
- Kohli J, Hottinger H, Munz P, Strauss A, Thuriaux P. Genetic mapping in Schizosaccharomyces pombe by mitotic and meiotic analysis and induced haploidization. Genetics. 1977;87:471–489. doi: 10.1093/genetics/87.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon N, Krawchuk MD, Warren BG, Smith GR, Wahls WP. Transcription factor Mts1/Mts2 (Atf1/Pcr1, Gad7/Pcr1) activates the M26 meiotic recombination hotspot in S. pombe. Proc. Natl. Acad. Sci. U. S. A. 1997;94:13765–13770. doi: 10.1073/pnas.94.25.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawchuk MD, DeVeaux LC, Wahls WP. Meiotic chromosome dynamics dependent upon the rec8+, rec10+, and rec11+ genes of the fission yeast Schizosaccharomyces pombe. Genetics. 1999;153:57–68. doi: 10.1093/genetics/153.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichten M. Meiotic recombination: breaking the genome to save it. Curr. Biol. 2001;11:R253–R256. doi: 10.1016/s0960-9822(01)00131-2. [DOI] [PubMed] [Google Scholar]
- Liu Y, West SC. Happy Hollidays: 40th anniversary of the Holliday junction. Nat. Rev., Mol. Cell Biol. 2004;5:937–944. doi: 10.1038/nrm1502. [DOI] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- Molnar M, Bahler J, Kohli J, Hiraoka Y. Live observation of fission yeast meiosis in recombination-deficient mutants: a study on achiasmate chromosome segregation. J. Cell. Sci. 2001;114:2843–2853. doi: 10.1242/jcs.114.15.2843. [DOI] [PubMed] [Google Scholar]
- Munz P. An analysis of interference in the fission yeast Schizosaccharomyces pombe. Genetics. 1994;137:701–707. doi: 10.1093/genetics/137.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MJ, Ramachandran M, Trelles-Sticken E, Scherthan H, Goldman AS. Wild-type levels of Spo11-induced DSBs are required for normal single-strand resection during meiosis. Mol. Cell. 2002;9:835–846. doi: 10.1016/s1097-2765(02)00498-7. [DOI] [PubMed] [Google Scholar]
- Nichols MD, DeAngelis K, Keck JL, Berger JM. Structure and function of an archaeal topoisomerase VI subunit with homology to the meiotic recombination factor Spo11. EMBO J. 1999;18:6177–6188. doi: 10.1093/emboj/18.21.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa O, Yanagida M. Triploid meiosis and aneuoploidy in Schizosaccharomyces pombe: an unstable aneuploid disomic for chromosome III. Curr. Genet. 1985;9:463–470. [Google Scholar]
- Osman F, Dixon J, Doe CL, Whitby MC. Generating crossovers by resolution of nicked Holliday junctions: a role for Mus81-Eme1 in meiosis. Mol. Cell. 2003;12:761–774. doi: 10.1016/s1097-2765(03)00343-5. [DOI] [PubMed] [Google Scholar]
- Paques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabitsch KP, Gregan J, Schleiffer A, Javerzat JP, Eisenhaber F, Nasmyth K. Two fission yeast homologs of drosophila Mei-S332 are required for chromosome segregation during meiosis I and II. Curr. Biol. 2004;14:287–301. doi: 10.1016/j.cub.2004.01.051. [DOI] [PubMed] [Google Scholar]
- Resnick MA, Martin P. The repair of double-strand breaks in the nuclear DNA of Saccharomyces cerevisiae and its genetic control. Mol. Gen. Genet. 1976;143:119–129. doi: 10.1007/BF00266917. [DOI] [PubMed] [Google Scholar]
- Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seraphin B. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 1999;17:1030–1032. doi: 10.1038/13732. [DOI] [PubMed] [Google Scholar]
- Roeder GS. Meiotic chromosomes: it takes two to tango. Genes Dev. 1997;11:2600–2621. doi: 10.1101/gad.11.20.2600. [DOI] [PubMed] [Google Scholar]
- Schuchert P, Langsford M, Kaslin E, Kohli J. A specific DNA sequence is required for high frequency of recombination in the ade6 gene of fission yeast. EMBO J. 1991;10:2157–2163. doi: 10.1002/j.1460-2075.1991.tb07750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacha A, Kleckner N. Identification of double Holliday junctions as intermediates in meiotic recombination. Cell. 1995;83:783–791. doi: 10.1016/0092-8674(95)90191-4. [DOI] [PubMed] [Google Scholar]
- Sharif WD, Glick GG, Davidson MK, Wahls WP. Distinct functions of S. pombe Rec12 (Spo11) protein and Rec12-dependent crossover recombination (chiasmata) in meiosis I; and a requirement for Rec12 in meiosis II. Cell Chromosome. 2002;1:1. doi: 10.1186/1475-9268-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storlazzi A, Tesse S, Gargano S, James F, Kleckner N, Zickler D. Meiotic double-strand breaks at the interface of chromosome movement, chromosome remodeling, and reductional division. Genes Dev. 2003;17:2675–2687. doi: 10.1101/gad.275203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Treco D, Schultes NP, Szostak JW. Double-strand breaks at an initiation site for meiotic gene conversion. Nature. 1989;338:87–90. doi: 10.1038/338087a0. [DOI] [PubMed] [Google Scholar]
- Wahls WP, Smith GR. A heteromeric protein that binds to a meiotic homologous recombination hot spot: correlation of binding and hot spot activity. Genes Dev. 1994;8:1693–1702. doi: 10.1101/gad.8.14.1693. [DOI] [PubMed] [Google Scholar]
- Wahls WP, Song JM, Smith GR. Single-stranded DNA binding activity of C1-tetrahydrofolate synthase enzymes. J. Biol. Chem. 1993;268:23792–23798. [PubMed] [Google Scholar]
- Wu H, Gao J, Sharif WD, Davidson MK, Wahls WP. Purification, folding, and characterization of Rec12 (Spo11) meiotic recombinase of fission yeast. Protein Expr. Purif. 2004;38:136–144. doi: 10.1016/j.pep.2004.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]