Abstract
Objective
To estimate completion rate and acceptability of home screening for sexually transmitted infections (STIs) compared to clinic-based screening in a prospective cohort study.
Methods
The first 462 women enrolled in the Contraceptive CHOICE Project were screened at 12 months of follow-up for Chlamydia trachomatis and Neisseria gonorrhoeae using strand displacement analysis of self-collected vaginal swabs. In a telephone interview, participants were given a choice of no-cost screening with swabs mailed to the participant’s home (home-based) or screening that was available at area family planning clinics without an appointment (clinic-based). The clinic-based group also included women who elected to screen with their regular provider according to the clinician’s normal practice. We analyzed the rates of screening, including patient preference and the proportion of completed tests by testing method.
Results
Women were more likely to choose to screen for STIs at home than at a clinic or with their own medical provider (75.7% versus 16.1% versus 8.2%, p < 0.001). Women choosing clinic testing were more likely to be black than those choosing home testing. Black women comprised 42% of the clinic group compared to 28% of the home group (RR 1.63, 95% CI 1.14–2.31). The groups did not differ in other demographic characteristics, STI risk factors, or access to healthcare. Overall, 228 women (56.6%) completed screening. Women who chose home-based testing were more likely to complete a test compared to all clinic-based testers (64.6% versus 31.6%, RR 2.04, 95% CI 1.51–2.76).
Conclusion
Women overwhelmingly preferred to screen for STIs at home. Future interventions to increase screening rates in young women should consider alternative screening strategies such as home-based or patient-controlled testing.
INTRODUCTION
Sexually transmitted infections (STIs) are a major public health problem in the United States. The Centers for Disease Control and Prevention (CDC) estimates that more than 19 million new infections occur each year, with half of the cases occurring among young individuals 15–24 years old (1). Both chlamydia and gonorrhea may lead to pelvic inflammatory disease (PID), infertility, ectopic pregnancy, and chronic pelvic pain (2).
The CDC currently recommends that females age 25 years or younger receive STI screening annually (3); however, only 26–60% of at-risk women in the United States do so (4–7). Screening is an especially important way to diagnose Chlamydia trachomatis and Neisseria gonorrhoeae, because these infections are often asymptomatic, providing little impetus for infected women to seek healthcare. Women who do not regularly seek routine healthcare or who are uninsured are at particularly high risk for repeat infections and the adverse health affects of untreated STIs (8).
Home-based screening for STIs using self-obtained vaginal swabs has the potential to overcome barriers to clinic access. Researchers have offered self-obtained STI screening in a variety of non-clinical settings, including schools, correctional facilities, pharmacies, and through the mail (9–13). Randomized trials of high school students in Denmark (14, 15) and high-risk adolescents in the United States (16) have shown increased rates of screening using home tests. With only one randomized trial to examine screening options in the United States, there is a clear need for additional evidence to support home screening in populations of mixed risk profiles and demographics. In preparation for a large randomized trial, we conducted a prospective cohort study to examine patient preferences and uptake of screening in a diverse population of women participating in the Contraceptive CHOICE Project.
MATERIALS AND METHODS
The Contraceptive CHOICE Project is an ongoing prospective cohort study that seeks to recruit 10,000 women. Recruitment occurs at a university-based clinic, two abortion clinics, and several community-based clinics that provide primary and gynecologic care. Women between 14 and 45 years of age are eligible if they are interested in beginning a new method of reversible contraception, have not had a hysterectomy, speak English or Spanish, and are sexually active with a man or plan to become sexually active within 6 months of joining the study. The study has been approved by the Washington University School of Medicine institutional review board. Informed consent is obtained for all participants. After comprehensive counseling on contraceptive options, the study provides reversible contraception of the woman’s choice for three years at no cost to the participant. All participants are tested for STIs at baseline and 12, 24 and 36 months post-enrollment. Telephone follow-up occurs seven times throughout the three-year study period.
This sub-study compares annual STI screening rates by testing method among the first 471 women who enrolled in the Contraceptive CHOICE Project. Participants were eligible for this sub-study if they had consented to STI testing at enrollment, completed the baseline enrollment survey, and were currently living in the United States. Nine of the 471 were ineligible for the sub-study (six did not provide consent for follow-up STI testing, two did not complete the baseline survey, and one was residing outside of the United States), leaving a sample size of 462. All participants were screened for C. trachomatis and N. gonorrheoae during the enrollment visit. Women were instructed to self-collect vaginal swabs in a clinic bathroom. C. trachomatis and N. gonorrhoeae were detected using the BDProbeTec ET (Becton Dickinson, Sparks, MD) instrument through DNA strand displacement amplification (SDA) technology. These specimens can be stored at room temperature. The specimens must be received by the testing laboratory within 16 days of collection.
During the CHOICE Project 12-month scheduled telephone contact, which occurred between August and December 2008, research assistants offered each participant the choice of home-based or clinic-based testing for the planned 12-month C. trachomatis and N. gonorrhoee screening. Staff varied the order in which the testing options were offered and recorded the order. Participants who chose home-based testing were mailed a collection kit in a plain brown box. The kit could be sent to any address the woman provided (e.g. to a friend’s house) to address potential concerns about privacy. The box contained vaginal swabs and a specimen tube, identical to those used at the enrollment visit. Detailed, step-by-step instructions with photographs explained swab collection, specimen packaging, and mailing. Participants recorded the date of collection on the specimen tube label. A pre-addressed and stamped specimen mailer (Exakt-Pak, Oklahoma City, OK) was provided to return the specimen according to Department of Transportation and United States Postal Service regulations.
Participants who chose clinic-based testing were offered two options. Participants could test with any private physician or clinic according to the normal practice of their health care provider. Reimbursement was provided for the cost of testing and treatment. Medical records were obtained. Secondly, participants could complete testing at four family planning clinics in the St. Louis region. Participants could test without an appointment and at no cost. Specimen collection at the four clinics was the same as that used at enrollment and in the home kits. Identical instructions for swab self-collection were posted in clinic bathrooms. After the participant collected the specimen, clinic staff labeled the specimen with the date collected and placed completed specimens in a bin for later pick-up by study staff.
Home kits were received by mail daily, and specimens were retrieved from the family planning clinics within 5 days of specimen collection. The date of swab collection, the date the specimen was received, and the condition of the specimen were recorded. If the specimen was not in satisfactory condition for testing (e.g. missing preservative fluid or swab), participants were asked to collect another specimen at the study clinic.
All patients with positive tests for C. trachomatis and N. gonorrhoeae were notified by a study nurse by phone. Prescriptions for antibiotic treatment were called to a study-affiliated pharmacy, and were at no cost to the participant. Participants could offer all partners free treatment through the study. All participants with negative tests were notified by letter.
Baseline and 12-month follow-up interviews collected detailed information on demographic characteristics; past and current reproductive history; contraceptive use, satisfaction, and side effects; sexual behavior with male and female partners; and pregnancy and STI occurrences. In the 18-month survey, the next regularly scheduled CHOICE Project telephone survey after completing their annual screening, participants were asked if they had undergone STI testing after the 12-month survey.
A woman was considered to have completed testing if a specimen was received from the home kit or the family planning clinics. Women who chose private provider testing were considered to have completed a test if any of the following occurred: a) reimbursement request for testing was received; b) medical records were obtained documenting testing; c) response on the 18-month survey indicating testing was performed. Medical records were requested for all participants choosing private provider testing to document testing. If there was a discrepancy between self-report and medical records, medical records were used to determine testing completion.
The proportions of women choosing each method of STI testing was compared with the chi-square test, assuming that equal proportions of women would choose each testing method. Characteristics of the home and clinic-based testing groups, and screening completers versus non-completers, were compared using chi-square or Fisher’s exact test for categorical variables and Student’s t-test for continuous variables. The hypothesis that the home-based screening group would have a higher proportion of completed tests was tested using the chi-squared test and quantified with a simple relative risk calculation. Since the outcome (completed tests) occurred frequently, Poisson regression with robust error variance was used, as a more accurate approximation of the relative risk than an odds ratio generated by logistic regression(17, 18). SPSS 17 (SPSS Inc., Chicago IL) and SAS 9.2 (SAS Institute Inc., Cary, NC) were used for statistical analyses. Based on a testing percentage of 25% in the clinic-based group, a RR of 2.0 (testing in home-based testing group of 50%), and alpha of 0.05, we needed 85 patients per group (assuming groups were approximately equal in size) for a total sample of 170 participants to achieve 90% power.
RESULTS
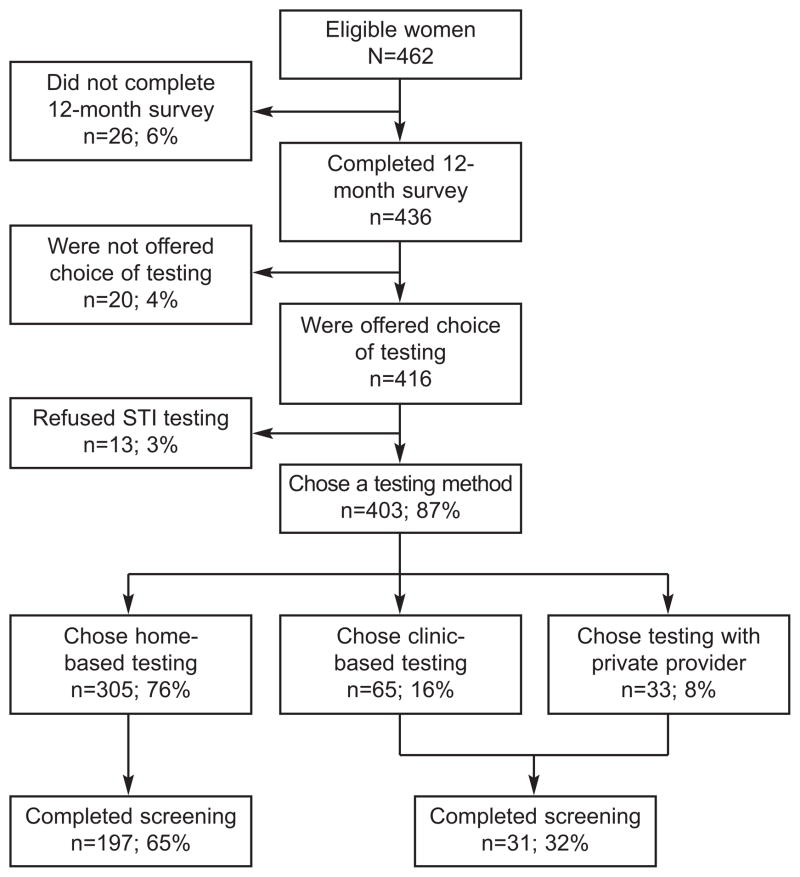
A total of 403 participants chose an STI screening method at 12-month follow-up (Figure 1). Of 462 eligible participants, 26 women did not complete 12-month follow-up, 20 were not offered a choice of STI testing method, and 13 refused testing. Twelve of thirteen participants (92.3%) who refused STI screening reported that they had recently been tested. The order in which the interviewer offered the three methods of STI screening was recorded for 85% of subjects. Of these, 39.3% were offered home screening first, 34.6% family planning clinic screening first, and 26.1% screening by a private medical provider first.
Figure 1.
Illustration of the flow and numbers of study participants.
Women were more likely to choose home-based screening (n = 305, 75.7%) than screening at a clinic (n = 65, 16.1%) or with their own medical provider (n = 33, 8.2%). The proportions of women choosing each screening method were different by the chi-squared test (p < 0.001).
Because of small numbers in each group, women choosing to screen at a family planning clinic or with their own medical provider were grouped together into a “clinic” group for the remainder of analyses. Baseline characteristics of women choosing home- or clinic-based screening were similar (Table 1). The mean age was 25.1 and 24.7 in the home- and clinic-based groups, respectively. Age, education level, income, and marital status were similar between groups. However, the clinic testing group had a larger proportion of black women compared to the home testing group (41.7 vs. 27.6%, RR 1.63, 95% CI 1.14–2.31). Most women in both groups had obtained health care in the last 12 months, though more than a third were uninsured. More than a quarter of women in each group had a previous STI diagnosis. The groups did not differ in other STI risk factors, such as number of lifetime sexual partners or history of an STI diagnosed at enrollment.
Table 1.
Baseline Characteristics of Participants by STI Testing Method Selected.
| Home (n = 305) | Clinic (n = 98) | p value | |
|---|---|---|---|
| Mean age (SD) | 25.1 (5.4) | 24.7 (5.2) | 0.48 |
| Race/Ethnicity | |||
| Black | 83 (27.5) | 41 (42.3) | |
| White | 196 (64.9) | 50 (51.5) | |
| Other | 23 (7.6) | 6 (6.2) | 0.02 |
| Education | |||
| Less than high school diploma | 35 (11.5) | 8 (8.2) | |
| High school diploma or GED | 56 (18.4) | 21 (21.4) | |
| Some college | 118 (38.6) | 43 (43.9) | |
| College or graduate degree | 96 (31.5) | 26 (26.5) | 0.53 |
| Monthly individual income | |||
| $0–800 | 92 (30.2) | 33 (33.7) | |
| $801–1,600 | 75 (24.6) | 27 (27.6) | |
| $1,601+ | 73 (23.9) | 21 (21.4) | |
| Refused or missing | 65 (21.3) | 17 (17.3) | 0.72 |
| Marital Status | |||
| Never Married | 204 (66.9) | 69 (70.4) | |
| Married/Living with a Partner | 85 (27.9) | 23 (23.5) | |
| Separated/Divorced/Widowed | 16 (5.2) | 6 (6.1) | 0.68 |
| Contraceptive Method | |||
| LARC (IUC or Implanon) | 199 (65.3) | 65 (66.3) | |
| DMPA | 8 (2.6) | 4 (4.1) | |
| Short-term refillable contraceptive (OC, patch, vaginal ring) | 97 (31.8) | 29 (29.6) | |
| Barrier (Diaphragm, condom) | 1 (0.3) | 0 (0) | 0.75† |
| Obtained health care in the last 12 months | 271 (88.9) | 88 (89.8) | 0.79 |
| No health insurance | 104 (35.4) | 38 (39.6) | 0.46 |
| History of STI* | 78 (25.7) | 28 (28.6) | 0.58 |
| STI** diagnosed at enrollment | 4 (1.5) | 4 (4.7) | 0.11† |
| Mean number of lifetime male sexual partners (SD) | 8.6 (8.6) | 10.5 (9.2) | 0.07 |
SD, standard deviation; GED, general education development test; LARC, long-acting reversible contraceptive; IUC, intrauterine contraceptive; DMPA, depomedroxyprogesterone; OC, oral contraceptive; STI, sexually transmitted infection. Data are n (%) unless otherwise indicated.
STI includes chlamydia, gonorrhea, trichomoniasis, syphilis, HIV, and genital herpes.
Includes chlamydia, gonorrhea, trichomoniasis, syphilis, and HIV, for which each participant is screened at baseline.
Fisher’s exact test.
Of the 403 women choosing to screen, 228 women completed screening (56.6%). All of the home and clinic kits were returned with an intact tube and swab. Three specimen tubes contained less preservative fluid than expected; two of these were determined to be negative by the laboratory, and one was considered unsatisfactory for testing. Four specimens in apparently good condition were also deemed unsatisfactory for analysis by the laboratory. The five women with unsatisfactory specimens were offered retesting, and three women subsequently tested successfully.
A total of 197 (64.6%) women who chose home-based testing completed a test, compared to 31 (31.6%) clinic-based testers (Figure 1). Thus, women choosing to screen for STIs at home were twice as likely to complete a test (RR 2.04, 95% CI 1.51–2.76). Poisson regression with robust error variance was performed, adjusting for age, race, and order of offering testing options, with a similar result (RR 2.02, 95% CI 1.48 – 2.72).
Medical records were obtained for 20 of the 33 women (60.6%) who planned to complete an STI test with a private provider. Records could not be obtained for 5 women (15%) because there was no medical record release, 5 (15%) because we could not contact the correct provider, 2 (6%) because the provider did not respond to our requests, and 1 (3%) because the participant withdrew from the study before we could obtain records. Excluding the eight women with self-reported tests that could not be confirmed by records, 23 (23.4%) of clinic-based testers completed a test. Using only documented tests, home-testers were nearly three times more likely to complete a test than clinic-based testers (RR 2.75, 95% CI 1.91–3.97). Poisson regression with robust error variance produced similar results (RR 2.75, 95% CI 1.89–4.02).
Women who completed testing had similar baseline characteristics to non-completers, including age, race, marital status, and history of STI (Table 2). The groups had different distribution of contraceptive methods, with the completed testing group having a higher proportion of short-term refillable methods users (36.0% vs. 25.1%). Non-completers included more long-acting reversible contraceptive users (70.3% vs. 61.8%) and DMPA users (4.6% vs. 1.8%). The results were similar using completion status determined by self-report or medical records.
Table 2.
Baseline Characteristics by Screening Status
| Completed Screening (Self-Report, n = 228) | Did Not Complete Screening (Self-Report, n = 175) | p value | |
|---|---|---|---|
| Screening Method | |||
| Home | 197 (64.6) | 108 (35.4) | |
| Clinic | 31 (31.6) | 67 (68.4) | < 0.0001 |
| Mean age (SD) | 24.6 (5.0) | 25.4 (5.8) | 0.13 |
| Race/Ethnicity | |||
| Black | 64 (28.3) | 60 (34.7) | |
| White | 147 (65.0) | 99 (57.2) | |
| Other | 15 (6.6) | 14 (8.1) | 0.28 |
| Education | |||
| Less than high school diploma | 28 (12.3) | 15 (8.6) | |
| High school diploma or GED | 39 (17.1) | 38 (21.7) | |
| Some college | 85 (37.3) | 76 (43.4) | |
| College or graduate degree | 76 (33.3) | 46 (26.3) | 0.18 |
| Monthly individual income | |||
| $0–800 | 65 (28.5) | 60 (34.3) | |
| $801–1,600 | 62 (27.2) | 40 (22.9) | |
| $1,601+ | 49 (21.5) | 45 (25.7) | |
| Refused or missing | 52 (22.8) | 30 (17.1) | 0.25 |
| Marital Status | |||
| Never Married | 162 (71.1) | 111 (63.4) | |
| Married/Living with a Partner | 55 (24.1) | 53 (30.3) | |
| Separated/Divorced/Widowed | 11 (4.8) | 11 (6.3) | 0.27 |
| Contraceptive Method | |||
| LARC (IUC or Implanon) | 141 (61.8) | 123 (70.3) | |
| DMPA | 4 (1.8) | 8 (4.6) | |
| Short-term refillable contraceptive (OC, patch, vaginal ring) | 82 (36.0) | 44 (25.1) | |
| Barrier (Diaphragm, condom) | 1 (0.4) | 0 (0) | 0.028† |
| Obtained health care in the last 12 months | 205 (89.9) | 154 (88.0) | 0.54 |
| No health insurance | 74 (33.8) | 68 (39.8) | 0.22 |
| History of STI* | 57 (25.1) | 49 (28.2) | 0.49 |
| STI** diagnosed at enrollment | 5 (2.5) | 3 (2.0) | 0.76 |
| Mean number of lifetime male sexual partners (SD) | 9.4 (9.4) | 8.5 (7.4) | 0.28 |
SD, standard deviation; GED, general education development test; LARC, long-acting reversible contraceptive; IUC, intrauterine contraceptive; DMPA, depomedroxyprogesterone; OC, oral contraceptive; STI, sexually transmitted infection. Data are n (%) unless otherwise indicated.
Fisher’s exact test.
STI includes chlamydia, gonorrhea, trichomoniasis, syphilis, HIV, and genital herpes.
Includes chlamydia, gonorrhea, trichomoniasis, syphilis, and HIV, for which each participant is screened at baseline.
Six cases of C. trachomatis (3%) and one case of N. gonorrheoae (0.5%) were detected. Four cases were detected in the home-screening group (including one woman with both C. trachomatis and N. gonorrheoae), and two cases were found in the clinic-based screening group.
DISCUSSION
In this analysis of women enrolled in the Contraceptive CHOICE Project, women strongly preferred home-based testing over testing in a clinic or a private physician’s office. Home-based testing had a second advantage; women who chose to be screened at home were nearly twice as likely to complete screening as women who intended to screen at a clinic. Finally, our screening program detected several cases of chlamydia and gonorrhea, which occurred at rates similar to those observed in the Contraceptive CHOICE Project at baseline. Our study demonstrates that home-based screening for STIs using self-obtained vaginal swabs is acceptable to the majority of women, and results in improved screening rates.
Home-based screening became feasible with the introduction of nucleic acid amplification tests (NAAT), such as strand displacement analysis (SDA), polymerase chain reaction (PCR), and transcription-mediated amplification (TMA). These methods have high sensitivity and specificity for both C. trachomatis and N. gonorrhoeae among women. Results from self-obtained vaginal swabs were nearly identical to endocervical samples (4, 19).
Previous studies have shown that many women find self-collection of vaginal swabs an acceptable, even preferable, alternative to traditional screening. Wiesenfeld and colleagues enrolled 228 adolescent female students to complete a self-collected vaginal swab that was tested for gonorrhea, chlamydia, and trichomoniasis. Women reported that self-collection was easy to perform (99%), preferable to a gynecologic examination (84%) and 97% stated that they would undergo testing frequently if self-testing were available (20). Another study enrolled over 1000 women with the average age of 26.6 years, and reported similar results for ease of use (21).
One strength of this study was its diverse population of sexually active women seeking contraception. Compared to previous studies of home- versus clinic-based screening in the United States, our population included nearly equal proportions of black and white women and a wide range of socioeconomic and STI risk factors. Despite this diversity, we found that women choosing home-based screening were very similar to women choosing clinic-based screening. While the clinic-based testing group contained a higher proportion of black women, the majority (66%) of black women in the study chose home screening. Thus, black women prefer home-screening, though not as strongly as white women. While our study population was diverse in many aspects, women enrolled in a contraceptive study may be different from women in the general population and our results may not be fully generalizable.
The study also benefited from low rates of unsatisfactory results (2%) among the home-based testers, suggesting that women understood how to obtain vaginal swabs and properly mail the specimens. There was also a low rate of screening refusal (3%), mostly by women who had recently been screened in other settings. However, given such a low rate of refusal, it is possible that women who did not want to be screened were not equally distributed among the testing groups. A trial in which participants are randomized to screening method would address this limitation.
The primary outcome measure in this study was the number of completed tests. This outcome measure was very reliable for the home-based and clinic-based self-obtained vaginal swab tests, as all tests were counted by the research team before being sent to the laboratory for testing. However, some women who chose to screen for STIs with a private provider may not have reported being tested. To reduce bias, we used several methods to determine the screening status for these 32 women. First, women had a completed screen if they requested reimbursement for screening, using forms we had provided. Secondly, the 18-month phone interview asked whether women had been screened. Finally, we attempted to review medical records for all participants who planned to test with a private provider, using a medical records release which was obtained at enrollment. Using these strategies, we can estimate that the true screening rate for these women in the clinic testing group was between the rate of 23% confirmed by records and 32% by self-report.
This study showed a large increase in screening rates using home-based tests, compared to traditional screening at medical facilities. Despite this, 43.4% of the women in this study still were not screened for sexually transmitted infections. This is comparable to the Centers for Disease Control report showing that 58.4% of women 25 and younger enrolled in health plans did not receive annual screening for chlamydia (22). Innovative methods of screening, including non-invasive and home-based screening, should be expanded. We must emphasize, to both providers and patients, that pelvic exams are not required for chlamydia and gonorrhea screening, opening the door to screening women in non-traditional settings. Home-based testing should be available to women. In particular, women who may be less likely to present for annual exams, such as those using long-acting reversible methods of contraception, could benefit from home-based testing. In Baltimore Gaydos et al. tested 400 women using mailed swabs requested through the internet (11). Recently, Los Angeles County has begun offering women home-based screening, in which women request kits by phone or website. These laudable efforts are unfortunately geographically restricted. United States Food and Drug Administration approval of an over-the-counter chlamydia and gonorrhea testing kit would allow private and convenient self-testing to be available nationwide. We must make screening more available, affordable, and convenient if public health efforts to reduce sexually transmitted infections among women are to be successful.
Acknowledgments
Supported in part by: an anonymous foundation; Midcareer Investigator Award in Women’s Health Research (K24 HD01298); Clinical and Translational Science Awards (UL1RR024992), and Award Numbers TL1 RR024995, KL2RR024994, and K3054628 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research.
Footnotes
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Presented as an oral abstract at Reproductive Health 2009, a joint annual meeting of the Association for Reproductive Health Professionals, Planned Parenthood Federation of America, and the Society for Family Planning.
Financial Disclosure: Anna S. Graseck: received support to travel to ARHP meeting to present research through grant NCRR TL1 RR024995; Gina M. Secura: has received money paid to her by her employer, Washington University School of Medicine. The other authors did not report any potential conflicts of interest.
References
- 1.Weinstock H, Berman S, Cates W., Jr Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004 Jan-Feb;36(1):6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2007. Atlanta: CDC; 2008. [Google Scholar]
- 3.Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006 Aug 4;55(RR–11):1–94. [PubMed] [Google Scholar]
- 4.Cook RL, Hutchison SL, Ostergaard L, Braithwaite RS, Ness RB. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhoeae. Ann Intern Med. 2005 Jun 7;142(11):914–925. doi: 10.7326/0003-4819-142-11-200506070-00010. [DOI] [PubMed] [Google Scholar]
- 5.Levine WC, Dicker LW, Devine O, Mosure DJ. Indirect estimation of Chlamydia screening coverage using public health surveillance data. Am J Epidemiol. 2004 Jul 1;160(1):91–96. doi: 10.1093/aje/kwh162. [DOI] [PubMed] [Google Scholar]
- 6.St Lawrence JS, Montano DE, Kasprzyk D, Phillips WR, Armstrong K, Leichliter JS. STD screening, testing, case reporting, and clinical and partner notification practices: a national survey of US physicians. Am J Public Health. 2002 Nov;92(11):1784–1788. doi: 10.2105/ajph.92.11.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chlamydia screening among sexually active young female enrollees of health plans--United States, 1999–2001. MMWR Morb Mortal Wkly Rep. 2004 Oct 29;53(42):983–985. [PubMed] [Google Scholar]
- 8.Geisler WM, Chyu L, Kusunoki Y, Upchurch DM, Hook EW., 3rd Health insurance coverage, health care-seeking behaviors, and genital chlamydial infection prevalence in sexually active young adults. Sexually Transmitted Diseases. 2006 Jun;33(6):389–396. doi: 10.1097/01.olq.0000194584.80513.4a. [DOI] [PubMed] [Google Scholar]
- 9.Bauer HM, Chartier M, Kessell E, et al. Chlamydia screening of youth and young adults in non-clinical settings throughout California. Sexually Transmitted Diseases. 2004 Jul;31(7):409–414. doi: 10.1097/01.olq.0000130456.03464.ea. [DOI] [PubMed] [Google Scholar]
- 10.Bloomfield PJ, Kent C, Campbell D, Hanbrook L, Klausner JD. Community-based chlamydia and gonorrhea screening through the United States mail, San Francisco. Sex Transm Dis. 2002 May;29(5):294–297. doi: 10.1097/00007435-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Gaydos CA, Dwyer K, Barnes M, et al. Internet-based screening for Chlamydia trachomatis to reach non-clinic populations with mailed self-administered vaginal swabs. Sex Transm Dis. 2006 Jul;33(7):451–457. doi: 10.1097/01.olq.0000200497.14326.fb. [DOI] [PubMed] [Google Scholar]
- 12.Macleod J, Salisbury C, Low N, et al. Coverage and uptake of systematic postal screening for genital Chlamydia trachomatis and prevalence of infection in the United Kingdom general population: cross sectional study. BMJ. 2005 Apr 23;330(7497):940. doi: 10.1136/bmj.38413.663137.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novak DP, Karlsson RB. Simplifying chlamydia testing: an innovative Chlamydia trachomatis testing approach using the internet and a home sampling strategy: population based study. Sexually Transmitted Infections. 2006 Apr;82(2):142–147. doi: 10.1136/sti.2005.016832. discussion 152–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostergaard L, Andersen B, Moller JK, Olesen F. Home sampling versus conventional swab sampling for screening of Chlamydia trachomatis in women: a cluster-randomized 1-year follow-up study. Clin Infect Dis. 2000 Oct;31(4):951–957. doi: 10.1086/318139. [DOI] [PubMed] [Google Scholar]
- 15.Ostergaard L, Andersen B, Olesen F, Moller JK. Efficacy of home sampling for screening of Chlamydia trachomatis: randomised study. BMJ. 1998 Jul 4;317(7150):26–27. doi: 10.1136/bmj.317.7150.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook RL, Ostergaard L, Hillier SL, et al. Home screening for sexually transmitted diseases in high-risk young women: randomised controlled trial. Sex Transm Infect. 2007 Jul;83(4):286–291. doi: 10.1136/sti.2006.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003 May 15;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998 Nov 18;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 19.Hobbs MM, van der Pol B, Totten P, et al. From the NIH: proceedings of a workshop on the importance of self-obtained vaginal specimens for detection of sexually transmitted infections. Sex Transm Dis. 2008 Jan;35(1):8–13. doi: 10.1097/OLQ.0b013e31815d968d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiesenfeld HC, Lowry DL, Heine RP, et al. Self-collection of vaginal swabs for the detection of Chlamydia, gonorrhea, and trichomoniasis: opportunity to encourage sexually transmitted disease testing among adolescents. Sex Transm Dis. 2001 Jun;28(6):321–325. doi: 10.1097/00007435-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Chernesky MA, Hook EW, 3rd, Martin DH, et al. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis or Neisseria gonorrhoeae infections. Sex Transm Dis. 2005 Dec;32(12):729–733. doi: 10.1097/01.olq.0000190057.61633.8d. [DOI] [PubMed] [Google Scholar]
- 22.Chlamydia screening among sexually active young female enrollees of health plans--United States, 2000–2007. MMWR Morb Mortal Wkly Rep. 2009 Apr 17;58(14):362–365. [PubMed] [Google Scholar]