Abstract
Whole body vibration (WBV), consisting of a low-magnitude, high-frequency (LMHF) signal, has been found to be anabolic to bone in vivo, which may act through alteration of the lineage commitment of mesenchymal stromal cells (MSC). Here, we investigated the effect of LMHF vibration on rat bone marrow-derived MSCs (rMSCs) in an in vitro system. We subjected rMSCs to repeated (six) bouts of 1-hour vibration at 0.3g and 60 Hz in the presence of osteogenic induction medium. The osteogenic differentiation of rMSCs under the loaded and non-loaded conditions was assessed by examining cell proliferation, alkaline phosphatase (ALP) activity, mRNA expression of various osteoblast-associated markers (ALP, Runx2, osterix, collagen type I alpha 1, bone sialoprotein, osteopontin, and osteocalcin), as well as matrix mineralization. We observed that LMHF vibration did not enhance the osteogenic differentiation of rMSCs. Surprisingly, we found that the mRNA level of osterix, a transcription factor necessary for osteoblast formation, was decreased, and matrix mineralization was inhibited. Our findings suggest that LMHF vibration may exert its anabolic effects in vivo via mechanosensing of a cell type different from MSCs.
Keywords: mesenchymal stromal cell, low-magnitude, high-frequency vibration, osteogenic differentiation, alkaline phosphatase, osterix
INTRODUCTION
Osteoporosis, a disease characterized by progressive deterioration of bone tissue due to an imbalance in the breakdown and rebuilding of bone, leads to increased bone fragility and susceptibility to fracture. Current measures for the prevention and treatment of osteoporosis are primarily drug-based, which delay disease progression but do not fully restore the balance in bone resorption and formation. Based on the premise that bone is a dynamic and self-regulating organ capable of adapting its mass and morphology according to its mechanical environment, some researchers have turned to a biomechanical approach to treating osteoporosis. Of recent interest is a mechanical signal known as low-magnitude, high-frequency (LMHF) vibrations that, when applied to the entire body of subjects (termed whole-body vibration, or WBV), produces anabolic responses in bone.1,2 Such responses have been attributed to increased bone formation, which was observed in various animal models, including young mice,3 ovariectomized rats,4 and rats subjected to hind limb disuse by tail-suspension.5
The mechanism by which LMHF vibration induces anabolic responses at the cellular level remains largely unknown. Recent in vivo studies suggest that LMHF vibration directs the lineage commitment of bone marrow mesenchymal stromal cells (MSCs) in favor of osteogenesis over adipogenesis.6,7 MSCs can form bone in vitro under appropriate chemical cues,8,9 a process that has been shown to be modulated by mechanical signals. MSCs isolated from various sources, including bone marrow,10–13 adipose tissue,14 and calvariae,15,16 have been tested for their response to a variety of mechanical stimuli in vitro, including tensile strain,10–12 fluid flow-induced shear stress,13–15 and pressure.16,17 Several osteoblast-associated markers of differentiating MSCs were demonstrated to be upregulated by applied mechanical forces such as strain and fluid shear, possibly via activation of MAPK pathways.10,12,18 However, the effect of LMHF vibration applied directly on MSCs has not been studied. Given the complex cellular heterogeneity of the bone marrow compartment, multiple cell type(s) including MSCs may respond to the LMHF signal and account for the observed anabolic responses in vivo. Hence, in this study, we aimed to delineate the effect of LMHF vibration on MSCs in vitro.
We hypothesize that LMHF vibration enhances the osteogenesis of MSCs in vitro in the presence of osteogenic factors, leading to increased expression of osteoblastic markers and matrix mineralization. To test our hypothesis, we subjected rat MSCs to repeated bouts of the mechanical stimulus at a magnitude of 0.3g and a frequency of 60 Hz to mimic the vibration conditions used in various animal and human studies1,5,19. We assessed and compared cell proliferation, alkaline phosphatase (ALP) activity, gene expression of certain transcription factors and matrix molecules, as well as their functional capacity to differentiate and form mineralized bone nodules between MSCs under LMHF stimulation and static controls.
METHODS
Bone marrow cell isolation and culture
Bone marrow cells were isolated from male Wistar rats (~4-week-old) as previously described8 (see Supplementary Materials for details). After 6 days of primary culture, with medium changes every 2–3 days, cells of the first passage were seeded at a density of 5×103 cells/cm2 for all experiments (with the exception of the proliferation study, where cells were seeded at 1×103 cells/cm2 to prevent inhibition of growth due to cell contact) in multi-well plates. 48 hours later, cells were serum-starved overnight in α-MEM containing 10% antibiotics (AB) and 0.1% fetal bovine serum (FBS) to synchronize the cells. After the first bout of vibration, all cultures were maintained in osteogenic (OS) medium (α-MEM containing 10% FBS, 1x AB, 10 nM dexamethasone, 50 μg/ml ascorbic acid, and 5 mM beta-glycerophosphate).
Set-up of LMHF vibration system
A metal vibration plate was custom-made to contain two multi-well tissue culture plates as previously described.20 The vibration plate was attached to a shaker (ET-127, Labworks Inc) that delivered vertical accelerations. The amplitude, waveform, and frequency of the vibration provided by the shaker were controlled with VibeLab computer program (Labworks Inc., Costa Mesa, USA). Peak-to-peak acceleration was measured at the centre of the vibration plate with a piezoelectric accelerometer (8632C5, Kistler, Amherst, USA), which output a voltage signal to the computer for feedback control between the desired and measured waveforms.
Vibration loading of cell cultures
After overnight serum-starvation to synchronize the cells12, each culture well was completely filled with serum-free medium and tightly sealed with gas permeable sealing film (Excel Scientific, Victorville, USA) immediately prior to vibration. This minimized fluid perturbation (and thus fluid shear stress) within the wells when vibrations were applied. Plates were placed securely onto the vibration plate and subjected to 60 Hz of sinusoidal vibrations at 0.3g for 1 hour. Cells in the non-vibration group were placed on the same but stationary plate. After one hour, all cells (both vibrated and non-vibrated groups) received fresh OS medium. Cultures received five more vibration loading bouts over 6 days (Figure 1). Cell samples were collected immediately after the third consecutive bout of vibration (i.e. on days 2 and 6).
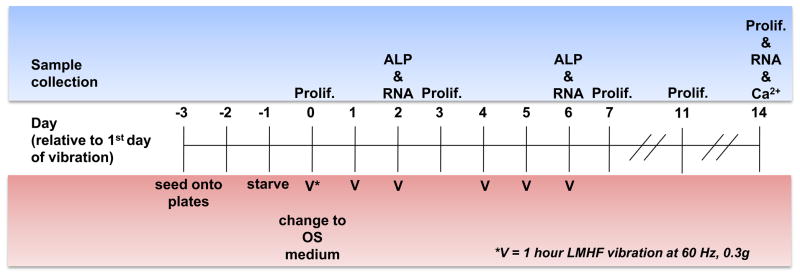
Figure 1. Vibration loading protocol.
Cells were seeded at a density of 5,000 cells/cm2 (or 1,000 cells/cm2 for proliferation study). Two days later, they were serum-starved overnight, and subjected to 1 hour of LMHF vibration at 0.3g and 60 Hz on day 0. After vibration loading, the cultures received and were maintained in OS medium. The vibration loading was repeated on days 1–2 and 4–5. Samples were collected for proliferation study on days 0, 3, 7, 11, and 14; for ALP activity on days 2 and 6 (immediately after vibration); mRNA analysis on days 2, 6, and 14; and for mineralization assay on day 14.
Proliferation assay
The amount of DNA in the cultured cell samples was measured using CyQUANT® Cell Proliferation Assays Kit (Invitrogen, Carlsbad, USA) according to the manufacturer’s protocol. Briefly, cell layers were rinsed 2x with phosphate buffered saline (PBS) and stored at −80°C overnight. Cells were then lysed with the kit’s lysis buffer containing a nucleic acid-binding fluorescence dye. Using a microplate reader with excitation at 485 nm and emission detection at 530 nm, fluorescence measurements were made and compared against a standard curve of known cell number.
mRNA quantification
Quantitative PCR was used to measure the mRNA levels of several early to late osteoblastic markers, including the osteogenic transcription factors runt-related transcription factor 2 Runx2 and osterix (Osx), ALP, and various bone matrix proteins, such as collagen type I alpha 1 (COL1A1), osteopontin (OPN), and bone sialoprotein (BSP). See Supplementary Material for detailed methods and rat-specific primer sequences (S-Table 1).
ALP assay
For quantitative analysis of ALP activity, ALP was extracted and detected with SensoLyte p-nitrophenyl phosphate (pNPP) ALP assay kit (Anaspec, Fremont, USA) according to the manufacturer’s protocol. Briefly, cells were lysed using the kit’s lysis buffer. Proteins were extracted by three rapid freeze-thaw cycles. Cell lysate was centrifuged for 15 minutes at 10,000g at 4°C. The supernatant was collected and combined with pNPP in a colorimetric reaction. Absorbance measurements at 405 nm were normalized to total protein content measured using BCA protein assay (Pierce, Rockford, USA). See Supplementary Material for ALP staining.
Quantification of matrix mineralization
Two weeks after the first bout of vibration (day 14), cell layers were rinsed 2x with calcium-free PBS. To solubilize calcium from the matrix, the samples were incubated overnight at 4°C in 0.6 N HCl. The supernatant was collected, centrifuged to remove cell debris, and reacted with o-cresolphthalein complexone, which produced colorimetric changes proportional to calcium content.21 Absorbance measurements at 570 nm were compared against a standard curve of known calcium concentrations and normalized to DNA content. See Supplementary Material for von Kossa staining.
Statistical Analysis
A two-tailed t-test was used to compare means between two groups. A significance level of p<0.05 was employed. Experiments were repeated twice with cells pooled from 2 rats per experiment (n=6 for all assays). Data are reported as mean ± standard deviation.
RESULTS
MSC proliferation is not affected by LMHF vibration
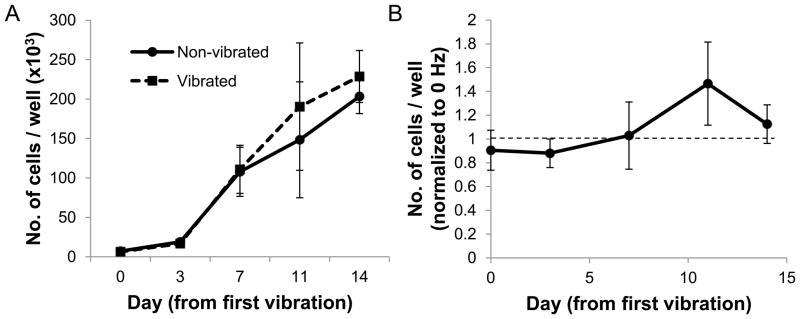
We examined the effect of LMHF vibration on the proliferation of MSCs cultured in OS media. On day 0, cell samples were collected immediately post-vibration to verify that vibration did not promote detachment of cells from the culture plate. Both the vibrated and non-vibrated cultures proliferated over the course of 14 days (Figure 2a), and the proliferation rate did not differ significantly between both groups at all time points (Figure 2b).
Figure 2. Proliferation of MSCs in OS medium.
(A) Both vibrated and non-vibrated groups showed proliferation over the course of 14 days. (B) LMHF vibration did not affect proliferation rate (n=6).
ALP activity is not altered by LMHF vibration
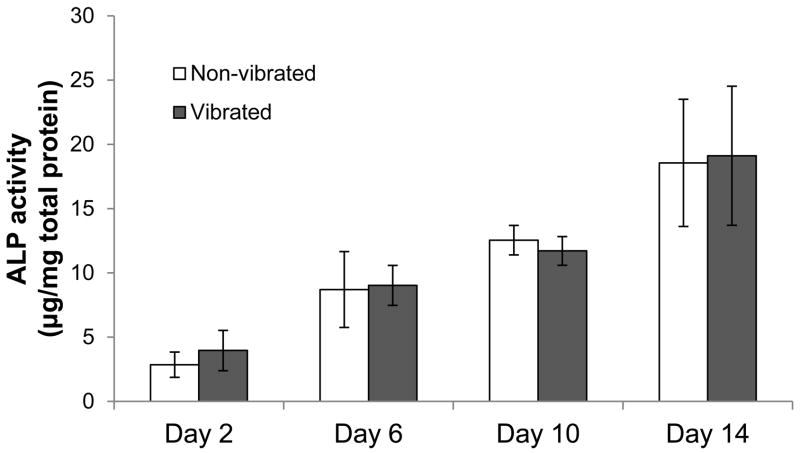
The effect of LMHF on osteogenic differentiation was first assessed by quantifying ALP activity. The ALP levels expressed by rMSCs increased with time in both vibrated and non-vibrated groups in the presence of OS medium (Figure 3). However, at the time points studied, there was not a significant difference in ALP activity due to LMHF vibration.
Figure 3. ALP activity.
ALP activity increased from days 2 to 14. There were no significant differences in ALP activity levels between the vibrated and non-vibrated groups (n=6).
mRNA level of Osx is inhibited by LMHF vibration
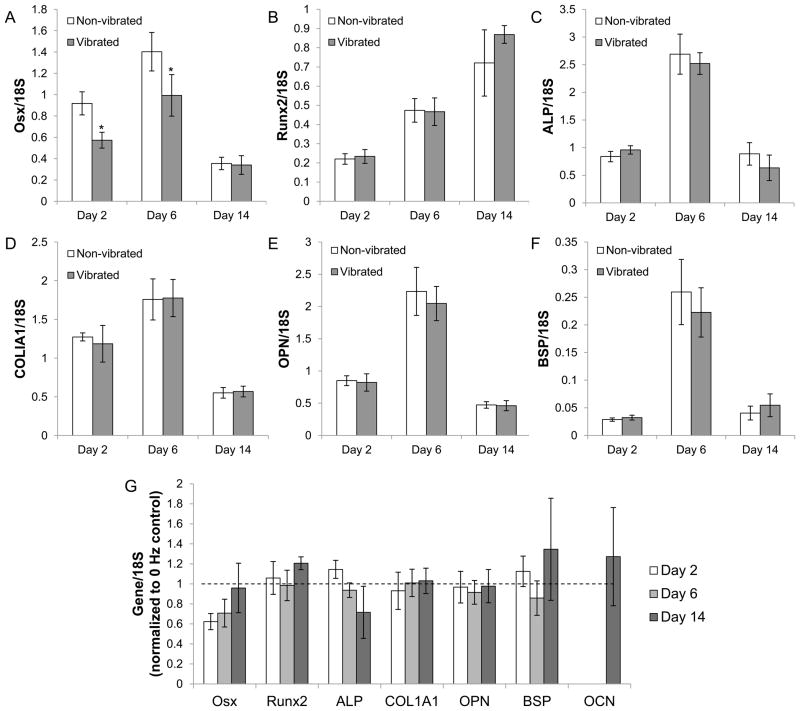
We further characterized the effect of LMHF vibration on osteogenic differentiation by examining the mRNA levels of several early to late bone markers. In both the vibrated and non-vibrated groups, the temporal expression patterns of the examined osteoblastic markers were comparable to those found in previous studies 22,23 (Figure 4A–F). The transcript levels of Osx, ALP, COL1A1, OPN, and BSP peaked at day 6, while that of Runx2 showed an increasing trend from day 2 to day 14. OCN was detectable at days 2 and 6, but could not be accurately quantified by qPCR due to its low expression levels. LMHF did not cause any significant change in the examined genes with the exception of Osx, which was decreased significantly on days 2 and 6 (Figure 4G).
Figure 4. mRNA expression of early to late osteoblastic markers.
Expression levels of Osx (A), ALP (C), COL1A1 (D), OPN (E), and BSP (F) were highest on day 6 while Runx2 expression increased over 14 days (B). (G) Vibrated cultures exhibited a lower mRNA level of Osx on days 2 (−38%) and 6 (−29%). *p<0.01 compared to the non-vibrated control (n=6).
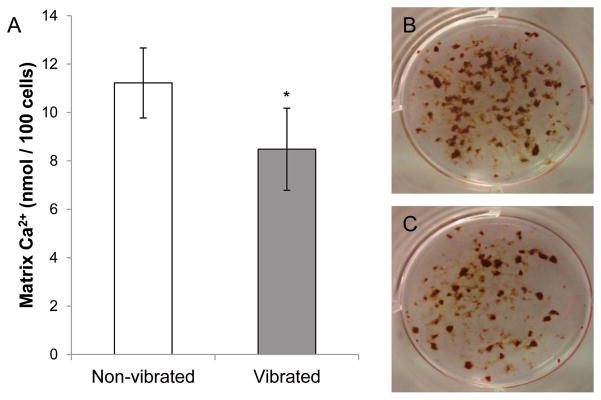
Matrix mineralization is decreased by LMHF vibration
The effect of LMHF vibration on the functionality of the late-stage differentiated cultures was assessed by quantifying the amount of matrix calcium deposition. Cultures that received LMHF vibration loading contained a significantly lower amount of matrix mineralization normalized to the total number of cells (−24%) compared to the non-vibrated controls (Figure 5A). Matrix mineralization in both non-vibrated and vibrated cultures was confirmed by ALP/von Kossa staining (Figures 5B and 5C).
Figure 5. Matrix mineralization on day 14.
(A) Cultures that were subjected to LMHF vibration showed 24% lower matrix calcium deposition.*p<0.05 compared to the non-vibrated control (n=6). Von Kossa/ALP staining of non-vibrated (B) and vibrated (C) cultures.
DISCUSSION
In this study, we investigated the potential osteogenic effect of LMHF vibration applied directly to MSCs. Contrary to our expectation, LMHF vibration did not enhance the osteogenic differentiation of rMSCs, as assessed by cell proliferation, ALP activity, mRNA levels of various early to late markers of osteogenesis, and matrix mineralization. Specifically, we demonstrated that LMHF vibration inhibited the mRNA expression of Osx and decreased the amount of matrix mineralization. Taken together, the results suggest that the direct application of LMHF vibration to MSCs does not produce osteogenic effects.
LMHF vibration did not induce any changes in the proliferation of MSCs in our study. Various proliferation responses of MSCs under other mechanical stimuli were found in previous studies, from having no effect,13 increasing proliferation,24 to inhibiting cell growth.12 Weyts et al. noted that the osteoblastic response to stretching depends on the stage of osteoblast maturation: stretching in early osteoblastic cultures caused apoptosis, while in more differentiated cultures proliferation was stimulated.25 One possible explanation of our observation is that the mechanical stimulation was applied in the early stages of osteogenic differentiation (i.e. on days 0–6 of osteogenic induction).
Similarly, previous studies on the effect of mechanical stimulation on the level of ALP activity, an early osteoblastic differentiation marker, have yielded mixed results. We showed that the amount of ALP activity was not altered by LMHF vibration, which was consistent with certain studies that applied strain to human MSCs12 or fluid flow to rMSCs13 but differed from those that reported an upregulation16,26 or downregulation.27 The differential functional responses of MSCs to various forms of mechanical stimulation underscore the important concept that cellular responses in mechanotransduction are unique to the specific mechanical stimulus.
We continued to assess the effect of LMHF on osteogenic differentiation by measuring the mRNA levels of early to late osteoblast-associated markers. The time course mRNA expressions of the studied markers corresponded well with those reported by Malaval et al. in the same culture system, indicating that the manipulation of the cell cultures during our vibration experimental set-up did not affect the osteogenic differentiation in our in vitro model. We did not observe any differential expression between the vibrated and the non-vibrated groups in the majority of the genes studied. We did, however, observe a decreased mRNA level of Osx on days 2 and 6, which returned to control levels by day 14. Since Osx is crucial for osteoblast formation, the decrease in Osx expression may have contributed to the inhibited matrix mineralization observed in the vibrated cultures. Furthermore, as Osx is known to act downstream of Runx2 in the transcriptional control of bone formation,28 we expected that Runx2 would also exhibit decreased expression under LMHF vibration. Although the observed unaltered level of Runx2 was unexpected, recent studies showed that Osx can be regulated via Runx-independent mechanisms,29 and indicate an incomplete understanding of the molecular mechanisms underlying the action of Osx in osteogenic differentiation.
Our findings that LMHF vibration decreased Osx mRNA levels and matrix calcification but did not alter other indicators of osteoblast differentiation, including ALP activity, suggest that the intermediate signaling molecules mediating these observed effects may be ones other than those studied herein. Indeed, Simmons et al. have shown that increased matrix mineralization as a result of cyclic strain applied to differentiating human MSCs is mediated via extracellular signal-regulated kinase signaling but is independent of ALP stimulation. Furthermore, although widely used as an osteoblastic marker, ALP levels have been found to be independent of matrix calcification.30
Taken together, despite receiving repeated vibration stimulation, rMSCs have shown limited to no response from gene to protein expression levels of early to late osteogenic markers. This lack of response was unlikely due to a transient response that was not captured by the time points selected, as it has been shown that repetitive loading is able to amplify the cellular response elicited by a single bout of loading.13 In addition, it is improbable that potential differential effects between vibrated and non-vibrated groups have been masked by the presence of dexamethasone, a potent osteoinductive agent. We had chosen to supplement cultures with 10 nM dexamethasone beginning from after the first bout of vibration loading to the end of each time point such that the culture protocol is consistent with previous studies.10,12,13 To confirm that dexamethasone had not suppressed any potential osteogenic effect of LMHF vibration on rMSCs, we repeated the same vibration protocol in the absence of any osteoinductive supplements. Quantification of proliferation and mRNA expression of early osteogenic markers showed no significant difference between vibrated and non-vibrated cells (results not shown), suggesting that LMHF vibration does not generate a baseline effect on the osteogenic differentiation of rMSCs even in culture medium deficient of a chemical osteoinductive agent.
The results from this study suggest that in vivo, cells may be responding to secondary mechanical stimuli induced by the vibration signal, such as shear stress due to bone marrow movement,31 as opposed to vibration itself. Another possibility is that there may be alternative mechanisms by which WBV exerts its anabolic effect, possibly via a mechanosensor that is absent in the current in vitro model. Animal studies on the effect of mechanical loading and unloading suggest that alterations in the mechanical environment alter the cell fate of mesenchymal progenitors in the bone marrow.6,7,32 However, the bone marrow is a heterogeneous compartment that houses hierarchical components of hematopoietic and mesenchymal cells. Thus, there may be heterotypic cellular interactions between the mechanosensor cell and the effector cells that are not immediately apparent in previous in vivo studies and in the current in vitro investigation.
A putative mechanosensor for detecting LMHF vibration is the osteocyte. Our laboratory has found that osteocytes under LMHF vibration release soluble factors that inhibit osteoclast formation.20 Furthermore, osteocytes have been found to communicate with other cell types, such as MSCs,33 through gap junctions34 and soluble factors,33 and such communication is mechanically regulated.26,33,35 Future studies are needed to investigate the communication between osteocytes and MSCs and to elucidate the possible role of osteocytes in orchestrating MSC differentiation under vibration stimulation.
Our experimental set-up of LMHF vibration aimed to investigate whether the anabolic effect of LMHF vibration observed in vivo is a direct consequence of MSCs sensing and responding to the vibration signal. However, our system may not be replicating the in vivo environment as it lacks other cell types. Also, a vibration stimulus applied externally to the whole-body may translate to a different signal at the cellular level. However, the precise mechanical consequences of vibratory loading in vivo are currently unknown. Thus, as a first step to understand the cellular basis of vibration effects in bone, our in vitro system allows us to apply a controlled vibratory signal that is isolated from possible confounding cellular responses of other cell types and any secondary mechanical stimuli. Furthermore, although most in vivo studies have typically employed a loading duration of less than 20 minutes, we have chosen to use a 1-hour stimulation for two reasons. Firstly, in our preliminary tests, osteocyte-like MLO-Y4 cells did not respond to brief periods (e.g. 15 minutes) of LMHF vibration, which led us to establish a 1-hour stimulation protocol in our laboratory. Secondly, many in vitro studies testing the effects of other types of mechanical stimulation on bone cells usually employed periods of at least 1 hour,26,36 and have shown that cellular response increased with increasing load duration from 0.5 to 2 hours.36 Thus, in order to strike a balance between existing in vitro and in vivo mechanical loading regimes, which would allow us to compare our results with existing literature, we employed a 1-hour duration of vibration.
In conclusion, under our experimental conditions, we did not detect any osteogenic effect of vibration on rMSCs, but instead observed decreased mRNA level of Osx and inhibited matrix calcification, suggesting that other factors contribute to the anabolic effect of vibration on bone. We speculate that the osteogenic effect of LMHF is elicited through an alternative mechanism, where rMSCs may be activated by secondary mechanical stimuli induced by vibration, or indirectly activated via communication with osteocytes that are more mechanosensitive to LMHF vibration. Our results provide a better understanding of the cellular mechanism that underlies the bone’s adaptive response to vibration. Such information is valuable in identifying the osteogenic components of the physical signal and facilitates the translation of the physical treatment of osteoporosis to clinical settings.
Supplementary Material
Acknowledgments
This research is supported by NSERC DG, CFI LOF, NIH R01AR054385-4, and NIH P20RR016458-09.
References
- 1.Rubin C, Turner AS, Muller R, et al. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17:349–357. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- 2.Rubin C, Turner AS, Mallinckrodt C, et al. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone. 2002;30:445–452. doi: 10.1016/s8756-3282(01)00689-5. [DOI] [PubMed] [Google Scholar]
- 3.Xie L, Jacobson JM, Choi ES, et al. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059–1066. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Oxlund BS, Ortoft G, Andreassen TT, et al. Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone. 2003;32:69–77. doi: 10.1016/s8756-3282(02)00916-x. [DOI] [PubMed] [Google Scholar]
- 5.Garman R, Gaudette G, Donahue L, et al. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res. 2007;25:732–740. doi: 10.1002/jor.20354. [DOI] [PubMed] [Google Scholar]
- 6.Luu YK, Capilla E, Rosen CJ, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24:50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin CT, Capilla E, Luu YK, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A. 2007;104:17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988;254:317–330. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- 9.Peter SJ, Liang CR, Kim DJ, et al. Osteoblastic phenotype of rat marrow stromal cells cultured in the presence of dexamethasone, beta-glycerolphosphate, and l-ascorbic acid. J Cell Biochem. 1998;71:55–62. doi: 10.1002/(sici)1097-4644(19981001)71:1<55::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Thomas G, El Haj A. Bone marrow stromal cells are load responsive in vitro. Calcif Tissue Int. 1996;58:101–108. doi: 10.1007/BF02529731. [DOI] [PubMed] [Google Scholar]
- 11.Rubin J, Murphy TC, Fan X, et al. Activation of extracellular signal-regulated kinase is involved in mechanical strain inhibition of rankl expression in bone stromal cells. J Bone Miner Res. 2002;17:1452–1460. doi: 10.1359/jbmr.2002.17.8.1452. [DOI] [PubMed] [Google Scholar]
- 12.Simmons CA, Matlis S, Thornton AJ, et al. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (erk1/2) signaling pathway. J Biomech. 2003;36:1087–1096. doi: 10.1016/s0021-9290(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 13.Kreke MR, Huckle WR, Goldstein AS. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone. 2005;36:1047–1055. doi: 10.1016/j.bone.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Knippenberg M, Helder MN, Doulabi BZ, et al. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng. 2005;11:1780–1788. doi: 10.1089/ten.2005.11.1780. [DOI] [PubMed] [Google Scholar]
- 15.Klein-Nulend J, Roelofsen J, Semeins CM, et al. Mechanical stimulation of osteopontin mrna expression and synthesis in bone cell cultures. J Cell Physiol. 1997;170:174–181. doi: 10.1002/(SICI)1097-4652(199702)170:2<174::AID-JCP9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 16.Roelofsen J, Klein-Nulend J, Burger EH. Mechanical stimulation by intermittent hydrostatic compression promotes bone-specific gene expression in vitro. J Biomech. 1995;28:1493–1503. doi: 10.1016/0021-9290(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Zhao Z, Zou L, et al. Pressure-loaded mscs during early osteodifferentiation promote osteoclastogenesis by increase of rankl/opg ratio. Ann Biomed Eng. 2009;37:794–802. doi: 10.1007/s10439-009-9638-9. [DOI] [PubMed] [Google Scholar]
- 18.Rubin J, Murphy TC, Zhu L, et al. Mechanical strain differentially regulates endothelial nitric-oxide synthase and receptor activator of nuclear kappa b ligand expression via erk1/2 mapk. J Biol Chem. 2003;278:34018–34025. doi: 10.1074/jbc.M302822200. [DOI] [PubMed] [Google Scholar]
- 19.Judex S, Lei X, Han D, et al. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40:1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Lau E, Al-Dujaili S, Guenther A, et al. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone. 2010;46:1508–1515. doi: 10.1016/j.bone.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar BC, Chauhan UP. A new method for determining micro quantities of calcium in biological materials. Anal Biochem. 1967;20:155–166. doi: 10.1016/0003-2697(67)90273-4. [DOI] [PubMed] [Google Scholar]
- 22.Malaval L, Modrowski D, Gupta AK, et al. Cellular expression of bone-related proteins during in vitro osteogenesis in rat bone marrow stromal cell cultures. J Cell Physiol. 1994;158:555–572. doi: 10.1002/jcp.1041580322. [DOI] [PubMed] [Google Scholar]
- 23.Kasugai S, Todescan R, Nagata T, et al. Expression of bone matrix proteins associated with mineralized tissue formation by adult rat bone marrow cells in vitro: inductive effects of dexamethasone on the osteoblastic phenotype. J Cell Physiol. 1991;147:111–120. doi: 10.1002/jcp.1041470115. [DOI] [PubMed] [Google Scholar]
- 24.Jagodzinski M, Breitbart A, Wehmeier M, et al. Influence of perfusion and cyclic compression on proliferation and differentiation of bone marrow stromal cells in 3-dimensional culture. J Biomech. 2008;41:1885–1891. doi: 10.1016/j.jbiomech.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Weyts FAA, Bosmans B, Niesing R, et al. Mechanical control of human osteoblast apoptosis and proliferation in relation to differentiation. Calcif Tissue Int. 2003;72:505–512. doi: 10.1007/s00223-002-2027-0. [DOI] [PubMed] [Google Scholar]
- 26.You L, Temiyasathit S, Lee P, et al. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42:172–179. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YJ, Batra NN, You L, et al. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J Orthop Res. 2004;22:1283–1289. doi: 10.1016/j.orthres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 29.Matsubara T, Kida K, Yamaguchi A, et al. Bmp2 regulates osterix through msx2 and runx2 during osteoblast differentiation. J Biol Chem. 2008;283:29119–29125. doi: 10.1074/jbc.M801774200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaiswal N, Haynesworth SE, Caplan AI, et al. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 31.Dickerson DA, Sander EA, Nauman EA. Modeling the mechanical consequences of vibratory loading in the vertebral body: microscale effects. Biomech Model Mechanobiol. 2008;7:191–202. doi: 10.1007/s10237-007-0085-y. [DOI] [PubMed] [Google Scholar]
- 32.Keila S, Pitaru S, Grosskopf A, et al. Bone marrow from mechanically unloaded rat bones expresses reduced osteogenic capacity in vitro. J Bone Miner Res. 1994;9:321–327. doi: 10.1002/jbmr.5650090306. [DOI] [PubMed] [Google Scholar]
- 33.Heino TJ, Hentunen TA, Vaananen HK. Conditioned medium from osteocytes stimulates the proliferation of bone marrow mesenchymal stem cells and their differentiation into osteoblasts. Exp Cell Res. 2004;294:458–468. doi: 10.1016/j.yexcr.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Taylor AF, Saunders MM, Shingle DL, et al. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am J Physiol Cell Physiol. 2007;292:C545–552. doi: 10.1152/ajpcell.00611.2005. [DOI] [PubMed] [Google Scholar]
- 35.Tan SD, de Vries TJ, Kuijpers-Jagtman AM, et al. Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone. 2007;41:745–751. doi: 10.1016/j.bone.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Kim CH, You L, Yellowley CE, et al. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through rankl and opg signaling. Bone. 2006;39:1043–1047. doi: 10.1016/j.bone.2006.05.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.