Imaging has become an indispensable tool in cardiovascular research, clinical trials, and medical practice. Traditional imaging modalities provide primarily anatomic as well as some physiological information. The emerging field of molecular imaging aims to expand beyond these traditional targets to visualize specific biochemical structures or biological processes. Platforms under exploration for molecular imaging include ultrasound, single photon emission computed tomography (SPECT), positron emission tomography (PET) computed tomography (CT), magnetic resonance imaging (MRI) and optical techniques such as fluorescence–mediated tomography (FMT) and catheter-based sensors. Ultimately, molecular imaging may allow clinicians to reach beyond anatomy to visualize the expression and activity of particular molecules, cells, or functions that influence disease progression, outcome and/or responsiveness to therapeutics.
The last three decades have seen explosive growth in the application of cardiovascular molecular imaging, as demonstrated by a recent PubMed search (Fig. 1). Despite basic science advances, translation into clinically-available agents and techniques has lagged. In September 2009 the National Heart, Lung, and Blood Institute (NHLBI) convened a Working Group of experts in the fields of molecular imaging and cardiovascular disease to assess the current state of molecular imaging and its application to cardiovascular diseases; to identify areas where cardiovascular molecular imaging was likely to have an impact; to explore barriers to the translation of molecular imaging towards clinical application; and to inform the NHLBI on national priorities for the translation of cardiovascular molecular imaging. Here we summarize state-of-the-art technologies, their challenges, clinical needs, and specific recommendations of the Working group.
Figure 1. Cardiovascular molecular imaging publications from 1987 to 2010.
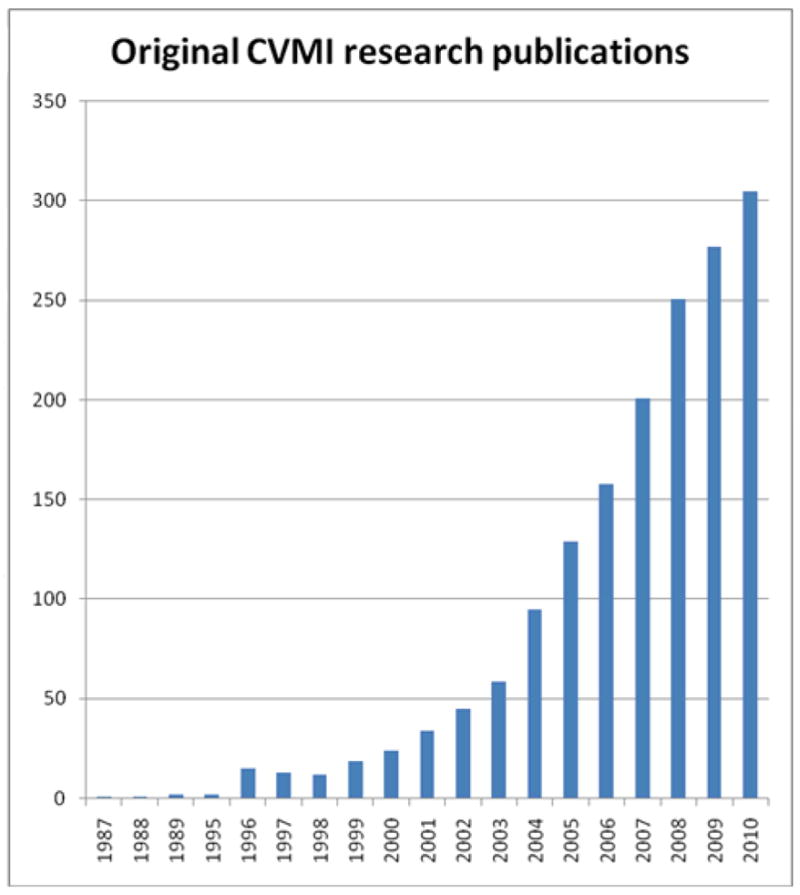
The Scopus database was searched for the terms “molecular” and “imaging” in the title or abstract, and the results obtained were then restricted further by searching on “cardiovascular”. The results obtained from the restricted search were then checked manually to include only original research papers and to ensure relevance to “cardiovascular molecular imaging”
Current State of Molecular Imaging Technology
Optical Imaging
Fluorescence illumination and observation is one of the most rapidly expanding imaging technologies, both in medical and biological sciences 1. Fluorescence refers to the property of molecules termed fluorochromes to absorb light at a specific wavelength and to emit light of longer wavelength after a brief interval. Fluorochrome-containing imaging agents have a number of distinct advantages. They can be administered in trace amounts; they do not decay, simplifying chemical synthesis and obviating the need for onsite radiochemistry; they are not radioactive, avoiding exposure to ionizing radiation and permitting multiple administrations; and they are detectable by a wide variety of other means (e.g. fluorescence activated cell sorting, gel electrophoresis, histochemistry), making them ideally suited for development through high-throughput and/or high-content screening approaches. Further advantages include multichannel capabilities based on spectrally-resolved imaging similar to multi-color microscopy, and the use of activatable probes that report on enzyme activities and improve target-background ratios. A number of fluorescent imaging agents have been developed and are being scaled up for clinical testing 1.
The simplest form of fluorescence detection is by epi-illumination, and a number of experimental imaging platforms exist. Fiber-optical approaches based on epi-illumination are being adapted to intravascular sensing either through miniaturized guide-wires 2 or fluorescence angioscopy 3. The latter are particularly promising and experimental systems have been developed for eventual clinical use. Fluorescence molecular tomography (FMT), using trans-illumination rather than epi-illumination to reconstruct 3D maps of fluorochromes based on sophisticated reconstruction algorithms, is inherently quantitative. Multimodality imaging combines FMT with CT or MRI for improved photon reconstruction and anatomical reference, and is performed in 2-4 channels for concomitant sensing of different biological targets 4. FMT platforms also permit reconstruction of visible fluorescence, permitting tracking of fluorescent proteins or genetically–modified cells irrespective of cell division in vivo 5. Tomographic fluorescent imaging approaches currently serve experimental work primarily, but also have clinical potential, e.g. in conjunction with ultrasound.
Radionuclide Imaging
Noninvasive targeted radiotracer-based SPECT and PET approaches are rapidly evolving through both preclinical imaging studies involving transgenic animals, and advances in imaging technology. Both SPECT and PET have advantages, including high sensitivity and selectivity, that make them particularly suitable for cardiovascular molecular imaging where targets are frequently small and sparse 6. The high sensitivity of nuclear methods is coupled with the favorable resolution of CT and MRI to provide multimodality hybrid imaging systems such as SPECT/CT, PET/CT and PET/MRI 7. Cardiovascular molecular imaging has evolved considerably within the last decade, with the translation of targeted SPECT and PET imaging to clinical practice. SPECT and PET molecular imaging has current clinical use in the evaluation of atherosclerosis and ischemic heart disease. These techniques visualize targets such as myocardial metabolism, the sympathetic nervous system, post-infarction left ventricular remodeling, angiogenesis, and potentially unstable plaque, using both direct imaging of cellular receptors and indirect imaging of reporter gene expression.
Radiotracer approaches for evaluation of myocardial metabolism and sympathetic function of the heart currently are being used in the evaluation and risk stratification of patients with congestive HF (HF), and for selecting patients at risk for sudden cardiac death for implantable defibrillators 8, 9. Imaging arterial uptake of vascular inflammation with fluorodeoxyglucose (FDG)-PET 10 represents another readily clinically applicable approach, but one in need of further optimization and validation.
The development of hybrid or multimodality probes will facilitate validation and advancement of molecular imaging approaches. For example, combining high-sensitivity radiolabeled probes with fluorescent probes allows both in vivo imaging of molecular processes and cellular localization of probes with specific cell markers. This approach will expedite and optimize probe development, and may be best accomplished with development of hybrid nanoparticles or unique small molecule fluorochromes. Optimization of targeted imaging will require registration with physiological and/or anatomical images. Further development of radiotracer-based imaging strategies for direct translation to clinical practice rests on the availability of novel targeted probes, novel hybrid instrumentation, and quantitative algorithms.
MRI and CT
Cardiovascular molecular imaging with MRI has focused primarily on two classes of imaging agents, based on gadolinium or iron oxide 11-13. Gadolinium-based agents include perfluorocarbon nanoemulsions 14 and synthetic high density lipoprotein nanoparticles 11. Imaging techniques such as magnetic nanoparticle enhanced MRI for vascular or macrophage imaging are in clinical trials 15. Ongoing prospective trials will determine whether imaging of vascular or myocardial inflammation with these methods can predict clinical events 16 As CT imaging has an increasing clinical role for noninvasive imaging of the coronary arteries, research efforts are evolving with CT molecular imaging using either iodinated-nanoparticles 17 or gold-nanoparticles 18. CT hardware developments are occurring rapidly, for example, spectrally resolved CT with photon counting detectors, able to detect individual x-ray quanta and measure their energy 19. Finally, the development of platforms that can transport contrast moieties attached to vehicles that can deliver a “payload” to specific biological targets may permit simultaneous visualization of the targeted structure and local delivery of therapeutic agents – an approach dubbed “theranostics.”
Ultrasound Molecular Imaging
Ultrasound molecular imaging was initiated in 1995 with the introduction of targeted nanobeacons, echogenic liposomes and subsequently microbubbles 20. In spite of the large number of ultrasound imaging devices installed worldwide, as well as their familiarity, relative ease of use, portability, safety, and low cost, clinical translation of ultrasound molecular imaging has emerged slowly. Development of new contrast agents has suffered from regulatory pressures on existing diagnostic agents due to safety considerations, underfunding of basic research, lack of open access research platforms for potential developers (in contrast to other platforms such as MRI), and general lack of venture investment in diagnostics. Moving the field forward will require a significant push to develop new, safe, targeted contrast agents operating at the nanoscale to interrogate molecular components of disease beyond the vascular compartment. In concert, new cheaper, portable, programmable, open access/open source ultrasound devices are needed, including handheld or laptop-based systems for point of care use. Interrogation of the anticipated new nanoscale molecular imaging agents will require novel signal processing strategies less susceptible to noise and other artifacts 21. Because ultrasound offers a powerful and controllable method for delivering energy into the body for therapy, the linkage between diagnostic and therapeutic agents should be stressed, such as combining imaging and gene delivery, or ultrasound devices operating in concert with other imaging modalities. Hybrid approaches such as photoacoustic imaging are moving ahead rapidly and can take advantage of the newer molecular imaging contrast agents immediately to demonstrate proof of concept, expanding the field for other applications that extend beyond its depth of penetration limits 22.
Imaging Cardiovascular Disease
Molecular Imaging of Atherosclerosis
Historically, imaging atherosclerosis has been rooted in anatomy, visualizing stenoses and their ischemic consequences or overall plaque burden. While useful as indirect markers of atherosclerotic event risk, the degree of stenosis or calcification does not necessarily predict whether a particular plaque will provoke a clinical event by disruption and thrombosis. Indeed, many fatal coronary artery occlusions arise from lesions that do not cause critical stenoses. Tissue characterization by intravascular ultrasound or CT angiography may refine the structural information but does not report directly on functional aspects of atherosclerotic plaques. In contrast, molecular imaging of atherosclerosis expands imaging beyond anatomy or flow to visualize processes related mechanistically to plaque complication.
A number of molecular imaging targets for atherosclerosis have been identified 23. During atherogenesis, dysfunctional endothelium increases the expression of adhesion molecules that bind blood leukocytes. These surface molecules, notably vascular cell adhesion molecule-1 (VCAM-1), provide imaging targets that can report on the activation state of endothelial cells. Once recruited to the artery wall by adhesion molecules and chemoattractants, blood monocytes become tissue macrophages that can engage in phagocytic activity. Nanoparticles visualized by MRI or by optical techniques undergo phagocytosis by activated macrophages, disclosing the presence and activation state of these phagocytes. Proteolytic enzymes that can degrade plaque’s extracellular matrix critically regulate the ability of plaque to resist rupture and hence thrombosis. Molecular imaging approaches to identifying proteases in plaque include labeling small molecular inhibitors that tag enzyme active sites or quenched fluorescent substrates that are cleaved to give an optical imaging probe to visualize proteinases. Oxidative stress may also promote plaque complication and report on the biological state of the plaque rather than merely its structure. Oxidants produced by inflammatory cells such as myeloperoxidase-generated hypochlorous acid can be visualized experimentally with optical sensors. Ultimately, thrombosis causes most coronary artery events. Subtotal or transient thrombosis often precedes the sustained or occlusive thrombi that lead to myocardial infarction. Agents that visualize fibrin cross-linking in thrombi may reveal sites of thrombosis in situ that do not impede flow and would evade detection by traditional techniques.
Challenges to implementation of molecular imaging of atherosclerosis involve the development of clinically-translatable platforms for imaging optical signals, perhaps targeting superficial arteries such as the carotid and iliofemoral arteries using non-invasive technology. Catheter-based platforms for interrogating deeper arteries such as the coronary arteries would add to the already widely used invasive intravascular ultrasound techniques.
Peripheral Artery Disease
Molecular imaging in peripheral artery disease (PAD) has developed more slowly than in other vascular beds. Clinical investigations include high-resolution MRI to determine plaque burden in the carotid arteries and the aorta 24. In addition, FDG-PET imaging of the carotid artery bifurcation may predict metabolically active plaque 25. However, there has been no significant advancement in molecular imaging to predict symptom progression or development of critical limb ischemia (ischemic rest pain, non-healing ulcerations, gangrene). Micro-SPECT/CT imaging of nitric oxide synthase-deficient mice with experimental hindlimb ischemia using 99mTc-labeled cyclic-Arg-Gly-Asp peptide targeted to αv integrin provided quantitative assessment of peripheral angiogenesis, potentially very helpful in trials of angiogenesis or cell-based therapy in patients with critical limb ischemia 26.
The management of patients with PAD of the lower extremities presents several special clinical needs. As with coronary artery atherosclerosis, assessing the thrombotic potential of atherosclerotic plaque could help to identify those more likely to progress or embolize, resulting in critical limb ischemia. Molecular imaging of the extracranial carotid arteries is critical, as most patients evaluated for carotid artery disease are asymptomatic, with moderate to severe stenosis. Optimal therapy of these asymptomatic patients is debated worldwide, choosing between carotid endarterectomy, carotid artery stent, and comprehensive medical therapy. Yet, given the very low rates of stroke among patients with moderate asymptomatic internal carotid artery stenosis, determination of plaque with high thrombotic potential would be very helpful. The biological properties of certain atherosclerotic plaques in the internal carotid artery could influence their likelihood to progress to artery-to-artery embolus, the most common etiology for ischemic events among these patients.
Aortic Aneurysms
With population aging, aortic aneurysms represent a growing problem. These lesions, which often complicate atherosclerosis, develop and enlarge silently. The current standard for imaging aortic aneurysms involves surveillance by duplex ultrasonography or CT for assessment of increase in aneurysm size or treatment options. Methodology to assess the biological activity of the aneurysm wall, beyond size, might allow more efficient targeting of therapies to prevent aneurysm growth or to promote more effective minimally-invasive intervention to exclude the aneurysm.
The pathophysiology of aneurysm formation suggests several plausible targets for molecular imaging. Most abdominal aortic aneurysms exhibit considerable signs of inflammation, and hence molecular imaging technologies targeting vascular inflammation might provide valuable functional information regarding aneurysms. Uptake of magnetic nanoparticles at the luminal surface of high-risk aortic aneurysms in humans was shown to correlate with the level of thrombus leukocyte infiltration and proteolytic enzyme activity 27.
Moreover, transmural destruction of elastic laminae in the abdominal aorta is characteristic of aneurysm formation. This elastinolysis correlates with overexpression of various elastolytic enzymes, including matrix metalloproteinases (MMPs) 9 and 12, and cathepsins S and K. As optical imaging methodologies exist for these proteinases, their use may provide a valuable tool to probe the biological status of the aortic wall and predict clinical consequences of aneurysms more reliably than dimension alone. Aortic aneurysms provide an attractive target for molecular imaging because of their relatively large size compared with atherosclerotic lesions in smaller arterial systems such as the coronary arteries.
Imaging in Heart Failure (HF)
With aging of the population, HF has become a major public health concern in the U.S. and worldwide. Randomized clinical trials have established the benefits of blockers of the sympathetic nervous system and the renin-angiotensin-aldosterone (RAAS) system in the treatment of HF, but multiple recent trials of other novel therapies have been largely negative. Molecular imaging has the potential to direct development of new drug, gene, and cell-based therapies, and to identify patient sub-populations most likely to respond to such therapies. Molecular targets may also help to identify patients at highest risk for sudden death in whom implantable devices will have the greatest cost effectiveness and clinical impact.
Sympathetic innervation
Imaging the heart with 123I radiolabeled meta-iodo-benzyl-guanidine (mIBG) represents one of the few molecular imaging agents that have endured the arduous scientific journey from bench to bedside. mIBG shares the same reuptake mechanism and endogenous storage with norepinephrine, but is not metabolized and does not interact with postsynaptic receptors. mIBG has received FDA approval for imaging neuro-endocrine tumors, but is not approved for cardiac neuronal imaging. In patients with coronary artery disease and HF, small single-center studies have reported dissociation between myocardial perfusion, metabolism and innervation. Non-invasive characterization of these three features would allow improved disease definition, more intelligent choice of therapeutic intervention, and closer monitoring of response to treatment. The utility of 123I-mIBG imaging in patients with HF was evaluated in the recent ADMIRE-HF trial (AdreView Myocardial Imaging for Risk Evaluation in HF), a multinational prospective open-label, phase 3 trial investigating 123I-mIBG imaging in patients with symptomatic HF and severe left ventricular (LV) systolic dysfunction at risk for cardiac events. Two year event-free survival was 85% in subjects with normal mIBG uptake, compared with 63% in those with abnormal mIBG uptake 8. Additional clinical trials are undoubtedly needed to determine efficacy of HF management strategy with mIBG compared to the standard of care approach without mIBG imaging. In addition, assessment of sympathetic innervations with PET, using 11C hydroxyephedrine, has also demonstrated feasibility in assessing prognosis in patients with LV dysfunction 28.
Post-MI Remodeling
MI results in the activation of RAAS, which in turn results in the activation of MMPs within the heart 9. RAAS tracers radiolabeled for molecular imaging include angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor I antagonists, such as 18F fluorobenzoyl lisinopril and 99mTc –losartan. Another approach to imaging cardiac remodeling uses Cy5.5-RGD imaging peptide (CRIP) labeled with Tc-99m to target αvβ3 integrin associated with supermature focal adhesions on myofibroblasts 29. Tracer uptake is increased almost 3-fold in mouse infarcts 2 weeks post-MI, decreasing towards baseline by 3 months, is inhibited by neurohumoral treatment with captopril, losartan or spironolactone, and correlates with echocardiographic parameters of remodeling 30.
High-sensitivity MMP-targeted molecular imaging approaches has also been employed to evaluate post-MI remodeling 31, 32 Using a near infra-red probe activated by MMP 2 and 9, increased MMP activity associated with leukocytes was shown in mouse myocardial infarction for 4 weeks after injury, with peak activity 1-2 weeks post-infarction. Finally, alterations in collagen have been imaged using a collagen-targeted peptide probe, collagelin; increased uptake of 99mTc-labeled collagelin was found in scar tissue of rat hearts three or more weeks after MI 33.
Apoptosis
Imaging of cardiac apoptosis imaging takes advantage of the binding of annexin V to phosphatidylserine exposed at the cell surface during the apoptotic process. Increased 99mTc-labeled annexin V uptake in the infarct area corresponded to areas of decreased perfusion 34. Apoptosis has also been studied using MRI and annexin-targeted iron oxide nanoparticles35,36.
Complications of Cardiac Transplantation
Cardiac transplantation has become the ultimate therapeutic option for individuals with advanced HF refractory to medical or mechanical management. Despite effective immunosuppressive therapy, many recipients endure one or more episodes of acute parenchymal myocardial rejection, often during the first year following engraftment, and rejection episodes predispose to development of chronic allograft vasculopathy. Because acute rejection episodes often fail to manifest themselves clinically until far advanced, recipients undergo serial surveillance endomyocardial biopsies, entailing discomfort, risk of cardiac perforation, and considerable trouble and expense. Non-invasive imaging modalities to gauge acute myocardial rejection would have a major impact in this patient population. Moreover, principles of imaging cardiac allograft might apply to other organs such as the kidney and liver.
Although T-lymphocytes instigate the alloimmune response and CD8+ cytolytic T cells effect myocardial cytolysis in this condition, mononuclear phagocytes outnumber lymphocytes in the inflammatory cell infiltrate in acutely rejecting allografts. There has therefore been considerable interest in imaging macrophage functions that provide a greater target for molecular imaging than the lymphocytes themselves. Approaches under development include monitoring the phagocytic capacity of the mononuclear phagocytes by uptake of magnetic nanoparticles using MRI 37-39, and detection of myeloperoxidase-rich monocytes using a myeloperoxidase-activated gadolinium chelate 40. Optical techniques may also monitor expression of proteases characteristically abundant in activated mononuclear phagocytes, or adhesion molecules involved in leukocyte recruitment in rejecting allografts. PET applications such as FDG uptake might visualize inflammation, but baseline myocardial uptake could confound this technique.
Imaging Valvular Heart Disease
Valvular heart disease is one of the most common cardiovascular disorders worldwide. Over the last decade, research has shown that certain aspects of the pathophysiology of degenerative aortic stenosis are very similar to that of coronary artery disease. Although initial retrospective studies suggested that statin therapy might reduce the rate of progression in aortic stenosis, larger prospective studies have failed to demonstrate a significant benefit 41. One proposed explanation is that clinically-available imaging techniques that measure calcification, decreased leaflet mobility and reduced valve area only identify the disease at a late stage. The pro-inflammatory processes that lead to leaflet degeneration have been recently demonstrated in explanted animal specimens using molecular imaging probes 42. The translation to in vivo molecular imaging could potentially lead to earlier disease detection and selection of patients who may respond to anti-inflammatory therapy. Molecular imaging tools that may enhance the detection of valve endocarditis, thrombi, and the identification of asymptomatic patients with valvular disease who are susceptible to develop LV dysfunction or sudden death could also aid clinical practice.
Imaging for Cell Therapy Applications
Stem and progenitor cells have the ability to self-renew and potential for multi-lineage differentiation. These properties offer the potential for use in regenerative therapies, to repair the heart after injury caused by myocardial infarction, or to restore blood flow in ischemic limbs. Pre-clinical studies have confirmed that stem and progenitor cells can repair damaged myocardium, but while some clinical studies have also shown promise, results have been inconsistent 43. These mixed results have raised fundamental questions about the application of stem cell therapy for myocardial regeneration. For instance, what are the intrinsic and extrinsic molecular and cellular factors that affect myocardial improvement? What is the optimal cell type, time of delivery, delivery technique, and cell dosage for therapy? Do these transplanted cells survive, integrate, and proliferate in the short or long term? Similar questions also remain for stem cell therapies for peripheral artery disease, which again show promise in animal studies but uncertain clinical benefit 44.
Given these controversies, imaging cell fate after transplantation has become a high priority in both basic research and clinical translation. To optimize and evaluate the success of cell based therapy, we must be able to track the location(s) of delivered cells, the duration of cell survival, and any potential adverse effects. Molecular imaging uses cell labeling to provide real-time imaging of the following processes: 1) stem cell localization and mobilization, 2) short and long-term survival and proliferation kinetics, 3) differentiation into cardiac cells or fusion with host cardiomyocytes, and 4) activation or regulation of genes or cytokines. Undoubtedly, eventual clinical implementation will require better understanding of the fate and efficacy of stem cell transplantation, which can be achieved quantitatively and qualitatively by various conventional and molecular imaging technologies.
Challenges in Translation of Molecular Imaging Research to Humans
Cardiovascular molecular imaging has an established and growing role in elucidating the underlying pathophysiologic mechanisms responsible for diseases of the myocardium and vasculature using small animals. Insights gained from molecular imaging research can identify targets for new drug development. Another goal is the development of molecular imaging agents for novel diagnostic purposes that ultimately can translate to clinical applications. Thus, there is great interest in expansion of molecular imaging probes beyond small animals to large animals and eventually to humans. Yet important challenges limit this progression to clinical reality related to scalability, cost, and regulatory burden.
Development of a new diagnostic imaging agent will undergo the same degree of regulatory oversight by the FDA as the development of a new drug. Bringing a new probe to patients will require millions of dollars and multiple years spent in pre-clinical work and Phase I and II clinical trials to obtain FDA approval. The incremental costs and delays related to development of GMP facilities and GMP-grade products, and the associated toxicology testing, represent additional barriers to clinical application. Further impediments relate to determination of pharmacokinetics and dosimetry. These challenges may be insurmountable for academic laboratories in which funding depends on peer-reviewed extramural grant support, as existing grant mechanisms are unlikely to support sophisticated GMP facilities or toxicology testing, whether performed internally or contracted through a third party. This impasse might be overcome with novel academic-industry partnerships, but the industry partner in turn would need to be convinced that the potential market for either the diagnostic agent or the resulting new therapeutic agent is sufficient to justify the investment in time and resources. Such partnerships could be enhanced if the NHLBI stimulated the research agenda and if the FDA were involved as a partner in the development and evaluation of new agents and technologies, as well as in their approval and regulation. Innovative mechanisms would need to be created to facilitate faster and more cost effective translation from small animals to large animals and humans, with support for development of GMP facilities, GMP-grade products and toxicology testing.
Another barrier to clinical application is the small numbers of centers with current expertise and requisite facilities to perform high quality molecular imaging work. There needs to be expansion beyond the luminary centers. Leveraging existing facilities and programs at other institutions has the potential to develop synergies and reduce costs. This effort could lead to development of molecular imaging networks, which could then have a self-sustaining effect on the field by creation of interdisciplinary training, conferences, and workshops. Such networks could also combine sites with expertise in different levels of the translational paradigm, from small animal to large animal to early phase clinical trials. An imaging network or consortium could also have a leveraging effect to enhance NHLBI-academic-industry relationships.
The NHLBI could also stimulate the field by encouraging molecular imaging sub-studies in new institute-sponsored clinical trials. Outreach and partnering with cardiovascular associations and professional societies to increase awareness, through journals, young investigator conferences, and national meetings, of the potential of molecular imaging to advance the diagnosis, risk assessment, and treatment of patients with cardiovascular disease is another mechanism by which NHLBI could spur the translation of the field toward clinical applications.
Recommendations
To address the challenges identified, the Working Group identified a series of national needs and priorities for the the translation of cardiovascular molecular imaging:
a mechanism to facilitate faster and more cost effective translation from small animals to large animals and humans, with support for development of GMP facilities, GMP-grade products, and toxicology testing
molecular imaging networks or centers that combine sites with expertise in different aspects of translation, e.g. small animal, large animal, and phase 1/2 clinical trials; foster industry collaborations; and promote interdisciplinary training
mechanisms to stimulate discovery and validation of novel imaging targets and biomarkers, including development of appropriate animal models
molecular imaging sub-studies in new NHLBI-sponsored clinical trials in areas such as peripheral arterial disease, stem cell therapies, HF, arrhythmias, and acute coronary syndromes
Acknowledgments
Source of Funding This work was supported by the NHLBI, National Institutes of Health.
Footnotes
Disclosures Dr. Bonow received an honorarium from Ohio State University for a lecture on cardiovascular molecular imaging. Dr. Dilsizian reports receiving consulting fees and a research grant from GE Healthcare, and owns stock in GE Healthcare. Dr. Fayad has received research grants from Roche, Novartis, GlaxoSmithKline, Bristol Myers Squibb, VBL Therapeutics, and Merck, and has received consulting fees from Roche. Dr. Garcia owns stock in Pfizer and has advisory/consultancy relationships with MD Imaging, theheart.org, BG Medicine and the Intersocietal Accreditation Commission. Dr. Klimas is an employee of Merck. Dr. Libby has received in-kind research support from Astra Zeneca and Rigel Pharmaceuticals, and served on Advisory Boards for Interleukin Genetics, Novartis, VIA Pharmaceuticals, BIND Biosciences and Carolus Therapeutics. Dr. Sinusas has received research grants from Lantheus Medical Imaging, Astellas Pharma, GE Healthcare and Molecular Targeting Technologies, and has received consulting fees from Lantheus Medical Imaging and CDL Nuclear Technologies. Dr. Wickline serves on the advisory board and owns stock in Kereos. The remaining authors report no conflicts.
References
- 1.Jaffer FA, Libby P, Weissleder R. Optical and multimodality molecular imaging: Insights into atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1017–1024. doi: 10.1161/ATVBAHA.108.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaffer FA, Vinegoni C, John MC, Aikawa E, Gold HK, Finn AV, Ntziachristos V, Libby P, Weissleder R. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation. 2008;118:1802–1809. doi: 10.1161/CIRCULATIONAHA.108.785881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Upadhyay R, Sheth RA, Weissleder R, Mahmood U. Quantitative real-time catheter-based fluorescence molecular imaging in mice. Radiology. 2007;245:523–531. doi: 10.1148/radiol.2452061613. [DOI] [PubMed] [Google Scholar]
- 4.Nahrendorf M, Sosnovik DE, French BA, Swirski FK, Bengel F, Sadeghi MM, Lindner JR, Wu JC, Kraitchman DL, Fayad ZA, Sinusas AJ. Multimodality cardiovascular molecular imaging, Part II. Circ Cardiovasc Imaging. 2009;2:56–70. doi: 10.1161/CIRCIMAGING.108.839092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zacharakis G, Kambara H, Shih H, Ripoll J, Grimm J, Saeki Y, Weissleder R, Ntziachristos V. Volumetric tomography of fluorescent proteins through small animals in vivo. Proc Natl Acad Sci U S A. 2005;102:18252–18257. doi: 10.1073/pnas.0504628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobrucki LW, Sinusas AJ. Pet and spect in cardiovascular molecular imaging. Nat Rev Cardiol. 2010;7:38–47. doi: 10.1038/nrcardio.2009.201. [DOI] [PubMed] [Google Scholar]
- 7.von Schulthess GK, Schlemmer HP. A look ahead: PET/MR versus PET/CT. Eur J Nucl Med Mol Imaging. 2009;36(Suppl 1):S3–9. doi: 10.1007/s00259-008-0940-9. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, Agostini D, Weiland F, Chandna H, Narula J. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure: Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. Journal of the American College of Cardiology. 2010;55:2212–2221. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Kramer CM, Sinusas AJ, Sosnovik DE, French BA, Bengel FM. Multimodality imaging of myocardial injury and remodeling. J Nucl Med. 2010;51:107S–121. doi: 10.2967/jnumed.109.068221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudd JH, Hyafil F, Fayad ZA. Inflammation imaging in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1009–1016. doi: 10.1161/ATVBAHA.108.165563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cormode DP, Skajaa T, Fayad ZA, Mulder WJ. Nanotechnology in medical imaging: Probe design and applications. Arterioscler Thromb Vasc Biol. 2009;29:992–1000. doi: 10.1161/ATVBAHA.108.165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulder WJ, Strijkers GJ, van Tilborg GA, Cormode DP, Fayad ZA, Nicolay K. Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging. Acc Chem Res. 2009;42:904–914. doi: 10.1021/ar800223c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sosnovik DE, Nahrendorf M, Weissleder R. Magnetic nanoparticles for mr imaging: Agents, techniques and cardiovascular applications. Basic Res Cardiol. 2008;103:122–130. doi: 10.1007/s00395-008-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaneda M, Caruthers S, Lanza G, Wickline S. Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Annals of Biomedical Engineering. 2009;37:1922–1933. doi: 10.1007/s10439-009-9643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang TY, Howarth SP, Miller SR, Graves MJ, Patterson AJ, JM UK-I, Li ZY, Walsh SR, Brown AP, Kirkpatrick PJ, Warburton EA, Hayes PD, Varty K, Boyle JR, Gaunt ME, Zalewski A, Gillard JH. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53:2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Plump AS, Lum PY. Genomics and cardiovascular drug development. J Am Coll Cardiol. 2009;53:1089–1100. doi: 10.1016/j.jacc.2008.11.050. [DOI] [PubMed] [Google Scholar]
- 17.Hyafil F, Cornily JC, Rudd JH, Machac J, Feldman LJ, Fayad ZA. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: A comparison with 18F-FDG PET/CT and histology. J Nucl Med. 2009;50:959–965. doi: 10.2967/jnumed.108.060749. [DOI] [PubMed] [Google Scholar]
- 18.Cormode DP, Skajaa T, van Schooneveld MM, Koole R, Jarzyna P, Lobatto ME, Calcagno C, Barazza A, Gordon RE, Zanzonico P, Fisher EA, Fayad ZA, Mulder WJ. Nanocrystal core high-density lipoproteins: A multimodality contrast agent platform. Nano Lett. 2008;8:3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlomka JP, Roessl E, Dorscheid R, Dill S, Martens G, Istel T, Baumer C, Herrmann C, Steadman R, Zeitler G, Livne A, Proksa R. Experimental feasibility of multi-energy photon-counting k-edge imaging in pre-clinical computed tomography. Phys Med Biol. 2008;53:4031–4047. doi: 10.1088/0031-9155/53/15/002. [DOI] [PubMed] [Google Scholar]
- 20.Morawski AM, Lanza GA, Wickline SA. Targeted contrast agents for magnetic resonance imaging and ultrasound. Curr Opin Biotechnol. 2005;16:89–92. doi: 10.1016/j.copbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Hughes MS, McCarthy JE, Marsh JN, Arbeit JM, Neumann RG, Fuhrhop RW, Wallace KD, Znidersic DR, Maurizi BN, Baldwin SL, Lanza GM, Wickline SA. Properties of an entropy-based signal receiver with an application to ultrasonic molecular imaging. J Acoust Soc Am. 2007;121:3542–3557. doi: 10.1121/1.2722050. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Wang LV. Photoacoustic tomography and sensing in biomedicine. Physics in Medicine and Biology. 2009;54:R59. doi: 10.1088/0031-9155/54/19/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libby P, DiCarli M, Weissleder R. The vascular biology of atherosclerosis and imaging targets. J Nucl Med. 2010;51:33S–37. doi: 10.2967/jnumed.109.069633. [DOI] [PubMed] [Google Scholar]
- 24.El Aidi H, Mani V, Weinshelbaum KB, Aguiar SH, Taniguchi H, Postley JE, Samber DD, Cohen EI, Stern J, van der Geest RJ, Reiber JH, Woodward M, Fuster V, Gidding SS, Fayad ZA. Cross-sectional, prospective study of MRI reproducibility in the assessment of plaque burden of the carotid arteries and aorta. Nat Clin Pract Cardiovasc Med. 2009;6:219–228. doi: 10.1038/ncpcardio1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graebe M, Pedersen SF, Borgwardt L, Hojgaard L, Sillesen H, Kjaer A. Molecular pathology in vulnerable carotid plaques: Correlation with [18]-fluorodeoxyglucose positron emission tomography (fdg-pet) Eur J Vasc Endovasc Surg. 2009;37:714–721. doi: 10.1016/j.ejvs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Dobrucki LW, Dione DP, Kalinowski L, Dione D, Mendizabal M, Yu J, Papademetris X, Sessa WC, Sinusas AJ. Serial noninvasive targeted imaging of peripheral angiogenesis: Validation and application of a semiautomated quantitative approach. J Nucl Med. 2009;50:1356–1363. doi: 10.2967/jnumed.108.060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nchimi A, Defawe O, Brisbois D, Broussaud TK, Defraigne JO, Magotteaux P, Massart B, Serfaty JM, Houard X, Michel JB, Sakalihasan N. Mr imaging of iron phagocytosis in intraluminal thrombi of abdominal aortic aneurysms in humans. Radiology. 2010;254:973–981. doi: 10.1148/radiol.09090657. [DOI] [PubMed] [Google Scholar]
- 28.Pietila M, Malminiemi K, Ukkonen H, Saraste M, Nagren K, Lehikoinen P, Voipio-Pulkki LM. Reduced myocardial carbon-11 hydroxyephedrine retention is associated with poor prognosis in chronic heart failure. Eur J Nucl Med. 2001;28:373–376. doi: 10.1007/s002590000449. [DOI] [PubMed] [Google Scholar]
- 29.van den Borne SWM, Isobe S, Verjans JW, Petrov A, Lovhaug D, Li P, Zandbergen HR, Ni Y, Frederik P, Zhou J, Arbo B, Rogstad A, Cuthbertson A, Chettibi S, Reutelingsperger C, Blankesteijn WM, Smits JFM, Daemen MJAP, Zannad F, Vannan MA, Narula N, Pitt B, Hofstra L, Narula J. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. Journal of the American College of Cardiology. 2008;52:2017–2028. doi: 10.1016/j.jacc.2008.07.067. [DOI] [PubMed] [Google Scholar]
- 30.van den Borne SWM, Isobe S, Zandbergen HR, Li P, Petrov A, Wong ND, Fujimoto S, Fujimoto A, Lovhaug D, Smits JFM, Daemen MJAP, Blankesteijn WM, Reutelingsperger C, Zannad F, Narula N, Vannan MA, Pitt B, Hofstra L, Narula J. Molecular imaging for efficacy of pharmacologic intervention in myocardial remodeling. JACC: Cardiovascular Imaging. 2009;2:187–198. doi: 10.1016/j.jcmg.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Tung CH, Allport JR, Chen S, Weissleder R, Huang PL. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation. 2005;111:1800–1805. doi: 10.1161/01.CIR.0000160936.91849.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su H, Spinale FG, Dobrucki LW, Song J, Hua J, Sweterlitsch S, Dione DP, Cavaliere P, Chow C, Bourke BN, Hu XY, Azure M, Yalamanchili P, Liu R, Cheesman EH, Robinson S, Edwards DS, Sinusas AJ. Noninvasive targeted imaging of matrix metalloproteinase activation in a murine model of postinfarction remodeling. Circulation. 2005;112:3157–3167. doi: 10.1161/CIRCULATIONAHA.105.583021. [DOI] [PubMed] [Google Scholar]
- 33.Muzard J, Sarda-Mantel L, Loyau S, Meulemans A, Louedec L, Bantsimba-Malanda C, Hervatin F, Marchal-Somme J, Michel JB, Le Guludec D, Billiald P, Jandrot-Perrus M. Non-invasive molecular imaging of fibrosis using a collagen-targeted peptidomimetic of the platelet collagen receptor glycoprotein VI. PLoS One. 2009;4:e5585. doi: 10.1371/journal.pone.0005585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofstra L, Liem IH, Dumont EA, Boersma HH, van Heerde WL, Doevendans PA, DeMuinck E, Wellens HJJ, Kemerink GJ, Reutelingsperger CPM, Heidendal GA. Visualisation of cell death in vivo in patients with acute myocardial infarction. The Lancet. 2000;356:209–212. doi: 10.1016/s0140-6736(00)02482-x. [DOI] [PubMed] [Google Scholar]
- 35.Sosnovik DE, Schellenberger EA, Nahrendorf M, Novikov MS, Matsui T, Dai G, Reynolds F, Grazette L, Rosenzweig A, Weissleder R, Josephson L. Magnetic resonance imaging of cardiomyocyte apoptosis with a novel magneto-optical nanoparticle. Magnetic Resonance in Medicine. 2005;54:718–724. doi: 10.1002/mrm.20617. [DOI] [PubMed] [Google Scholar]
- 36.Sosnovik DE, Nahrendorf M, Panizzi P, Matsui T, Aikawa E, Dai G, Li L, Reynolds F, Dorn GW, II, Weissleder R, Josephson L, Rosenzweig A. Molecular mri detects low levels of cardiomyocyte apoptosis in a transgenic model of chronic heart failure. Circ Cardiovasc Imaging. 2009;2:468–475. doi: 10.1161/CIRCIMAGING.109.863779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanno S, Wu Y-JL, Lee PC, Dodd SJ, Williams M, Griffith BP, Ho C. Macrophage accumulation associated with rat cardiac allograft rejection detected by magnetic resonance imaging with ultrasmall superparamagnetic iron oxide particles. Circulation. 2001;104:934–938. doi: 10.1161/hc3401.093148. [DOI] [PubMed] [Google Scholar]
- 38.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christen T, Nahrendorf M, Wildgruber M, Swirski FK, Aikawa E, Waterman P, Shimizu K, Weissleder R, Libby P. Molecular imaging of innate immune cell function in transplant rejection. Circulation. 2009;119:1925–1932. doi: 10.1161/CIRCULATIONAHA.108.796888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swirski FK, Wildgruber M, Ueno T, Figueiredo J-L, Panizzi P, Iwamoto Y, Zhang E, Stone JR, Rodriguez E, Chen JW, Pittet MJ, Weissleder R, Nahrendorf M. Myeloperoxidase-rich Ly-6c+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. The Journal of Clinical Investigation. 2010;120:2627–2634. doi: 10.1172/JCI42304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J for the ASTRONOMER Investigators. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 42.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 43.Mummery CL, Davis RP, Krieger JE. Challenges in using stem cells for cardiac repair. Science Translational Medicine. 2010;2:27ps17. doi: 10.1126/scitranslmed.3000558. [DOI] [PubMed] [Google Scholar]
- 44.Lawall H, Bramlage P, Amann B. Stem cell and progenitor cell therapy in peripheral artery disease. A critical appraisal. Thromb Haemost. 2010;103:696–709. doi: 10.1160/TH09-10-0688. [DOI] [PubMed] [Google Scholar]


